Abstract
Purpose
Chronic lymphocytic leukemia (CLL) patients refractory to fludarabine based therapies have poor outcomes with currently available therapies. Early phase studies of alvocidib showed activity in relapsed CLL including patients with high risk genomic features and those refractory to fludarabine. A multi-center, international, phase II study of alvocidib in fludarabine refractory CLL was undertaken to validate these early results.
Patients and Methods
Patients with fludarabine refractory CLL or prolymphocytic leukemia arising from CLL were treated with single agent alvocidib. The primary outcome measure was overall response rate, with secondary outcomes including survival, toxicity, and response duration.
Results
One hundred and sixty five patients were enrolled at 34 centers, and 159 patients were treated. The median age was 61 years, the median number of prior therapies was 4, and 96% of patients were fludarabine refractory. The investigator-assessed overall response rate was 25%; the majority of responses were partial. Response rates were lower among patients with del(17p) (14%), but equivalent in patients with del(11q) or bulky lymphadenopathy. Median progression free and overall survival were 7.6 and 14.6 months respectively. Tumor lysis occurred in 39 patients (25%), and 13 received hemodialysis. Diarrhea, fatigue, and hematologic toxicities were common.
Conclusion
Alvocidib has clinical activity in patients with advanced, fludarabine refractory CLL. Clinical responses were observed in patients with high risk clinical and genomic features. With careful monitoring, alvocidib has a manageable safety profile. Future studies should focus on discovery of biomarkers of clinical response and tumor lysis, and enhanced supportive care measures.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia in the United States and Europe and is quite heterogeneous in clinical behavior1,2. Despite this variable clinical course, approximately two-thirds of affected patients will become symptomatic and require therapy. Treatment is not curative, and clinical outcomes among patients with relapsed CLL are poor, with disease related complications arising from bone marrow failure, lymphadenopathy, and immunosuppression. Patients with either no response or response shorter than 6 months to purine-nucleoside analogue based chemotherapy (“fludarabine refractory CLL”) have a dismal prognosis, with an anticipated survival of 10 months 3,4. Treatment options for such patients are currently limited 5. Alemtuzumab, no longer indicated for the treatment of CLL in the United States with label withdrawal by the manufacturer, induces response in approximately 35% of patients, but median progression free survival (PFS) is only 5 – 8 months 6,7. Among patients refractory to both alemtuzumab and fludarabine, ofatumumab showed an overall response rate (ORR) of 58%, though PFS was only 5.7 months8. Novel therapeutic approaches are needed for this high risk patient population.
Alvocidib (HMR1275; flavopiridol) is a semisynthetic flavonoid that inhibits cyclin-dependent kinases (CDKs) by competing with ATP for the kinase enzymatic site 9,10. Alvocidib induces apoptosis in primary CLL cells in vitro at pharmacologically achievable concentrations with a mechanism of action that is independent of TP5311-13. Early clinical studies of alvocidib utilizing a 24 to 72 hour continuous intravenous infusion (CIVI) failed to show clinical activity among a variety of malignancies including CLL 14-17. Subsequent preclinical studies showed high protein binding of alvocidib in human serum, and led to the development of a pharmacologically derived dosing schedule of a 30 minute intravenous bolus (IVB) followed by a 4 hour CIVI. Using this schedule, significant clinical activity was observed in a phase 1 study of patients with relapsed CLL, with an ORR of 40% including patients with bulky lymphadenopathy or high risk genetic features such as del(17p)18,19. Dose limiting toxicity was hyperacute tumor lysis syndrome (TLS). A subsequent single institution, phase 2 study utilizing this dosing strategy showed an ORR of 53% and median PFSl among responding patients of 12 months 20. Given the response rates observed among high risk patients, the EFC6663 study was initiated to assess the efficacy and safety of alvocidib in a multicenter, international phase 2 clinical study.
Patients and Methods
Study Design and Subjects
Patients had confirmed CLL as established by NCI 96 criteria or prolymphocytic leukemia (PLL) arising from CLL 21. Patients must have received at least one prior therapy including an alkylating agent(s) and be fludarabine refractory, either as asingle agent or in combination. Refractory to fludarabine was defined as a lack of response or relapse ≤ 6 months following completion of fludarabine-containing therapy3. Patients requiring treatment according to NCI 96 criteria were eligible for this phase II clinical trial. Additional eligibility criteria included: age ≥ 18 years, ECOG performance status 0 – 2, adequate hepatic function (AST and ALT < 2.5 × ULN), adequate renal function including normal potassium level and creatinine≤ 2 mg/dL, and WBC ≤ 150 × 109 / L. Institutional Review Boards at the participating institutions approved the protocol and all study participants provided written informed consent. This study was registered with ClinicalTrials.gov, numberNCT00464633.
Treatment Plan
Alvocidib was administered through a central venous catheter at a dose of 30 mg/m2 by 30 minute IVB followed by 30 mg/m2over 4 hoursby CIVI on day 1 of cycle 1. Patients who did not develop TLS underwent dose escalation to 30 mg/m2by 30 minute IVB followed by 50 mg/m2 CIVI onday 8of cycle 1 and for all subsequent treatments. Patients received alvocidib every week for 4 consecutive weeks, followed by a 2-week rest period (42 days per cycle) for up to a total of 6 cycles. Patients must have achieved at least a 25% reduction in lymphocytosis or nodal volume after completion of 2 cycles of therapy in order to continue. In the absence of meeting these criteria, treatment was discontinued. Supportive therapy consisted of acyclovir and trimethoprim / sulfamethoxazole for the duration of treatment. Ciprofloxacin was administered during periods of neutropenia; granulocyte colony stimulating factors were discouraged. Dexamethasone 20 mg orally was administered on days 1, 2, 8, and 9 of cycle 1 and day 1 of all subsequent cycles.
Prophylaxis and Management of TLS
Because severe TLS was observed in prior alvocidib studies in CLL 18,20, patients were closely observed for TLS and, when present, urgently managed. The first 2 doses of alvocidib were administered in an inpatient setting with frequent laboratory assessments and 24 hour observation post-dose. All patients received allopurinol 300 mg daily, and rasburicase 0.15 mg/kg was administered 2 hours before the first two doses of alvocidib. In the event of hyperkalemia, hemodialysis was immediately available. If TLS did not occur, patients were transitioned to outpatient therapy on day 15 of cycle 1 (dose 3). Patients who required hemodialysis for severe TLS were allowed to continue therapy, but continued at a dose of 30mg/m2 IVB followed by 30mg/m2 CIVI.
Assessment of Patient Outcomes
Clinical and biologic assessments performed at baseline included assessments of ECOG performance status;bone marrow aspirate and biopsy, cross sectional imaging of the chest, abdomen, and pelvis; and interphase cytogenetics (FISH). Clinical response was assessed after each treatment cycle using the NCI 96 Criteria as well as “hybrid” criteria, which incorporated bi-dimensional lymph node assessments by cross-sectional imaging into the NCI 96 criteria 21. Patients were followed for at least 6 months after the completion of therapy. PFS was calculated from the date of first treatment administration until the time of disease progression or death, whichever came first, censoring patients alive and relapse free at last follow-up. The safety profile was determined by the incidence of clinically significant adverse events (AEs) and serious adverse events (SAEs), graded according to the NCI CTCAE, version 3.0, and coded using the Medical Dictionary for Regulatory Activities(MedDRA) version 14.1.
Statistical Analysis
The primary endpoint was the overall objective response rate (ORR; CR + PR + nPR) as assessed by hybrid response criteria. Secondary endpoints included an evaluation of toxicity; determination of response duration (DR), PFS, and overall survival (OS). Based on an expected ORR of 20% among patients with fludarabine refractory CLL, the preplanned study size was 165 patients to yield a 2-sided 95% exact confidence interval of 14 – 27%. A preplanned interim futility analysis was performed after 66 evaluable patients received 2 cycles of therapy, and an independent data monitoring committee reviewed the safety data after treatment of 20 and 50 patients.
The intent-to-treat (ITT) population consisted of all patients who signed the consentform and were registered on the study. In the primary analysis, the ORRusing the hybrid criteriaand associated exact 95% confidence intervals (CI) were estimated. Similar calculations were performed using the NCI 96 criteria. For the secondary efficacy time-to-event variables PFS, objectiveDR, and OS, Kaplan-Meier estimates with corresponding 95% CI's were calculated for the AT(as-treated) population. Logistic regression analyses were performed modeling both the probability of observing a response (CR, PR, nPR) and the probability of developing TLS; these analyses were not pre-planned. Both univariate and multivariate analyses were performed.
Results
Patient Characteristics and Disposition
A total of 165 patients (ITT population) were enrolled and followed between March 2007 and December 2011 at 34 sites in 9 countries. The ITT patient characteristics are shown in Table 1. The median age was 61 years (range 29 - 82). The study population was 78% male, and 90% Caucasian. Mean creatinine clearance was 98.2 mL/min (range 41.1 - 275.2). One hundred twenty-one of 150 assessed patients (81%) were Rai Stage III or IV. Bulky lymphadenopathy (≥1 lymph node > 5 cm by cross sectional imaging) was present in 115 (70%). High risk cytogenetic feature del(17p) was present in 35% of patients, and del(11q) was present in 31%. The median number of prior therapies was 4 (range 1 – 12). All patients had received prior fludarabine, and 96% were fludarabine refractory. Additionally, 99% had received a prior alkylating agent, 87% had prior rituximab, 38% had prior alemtuzumab, and 29% were refractory to both fludarabine and alemtuzumab.
Table 1. Patient Characteristics in the ITT population.
| Characteristic (n) | ITT Population (n = 165) | |
|---|---|---|
| Number | % | |
| Age, years (standard deviation) | 60.8 (9.5) | |
| Male Gender | 128 | 78 |
| Race | ||
| Caucasian | 148 | 90 |
| Black | 10 | 6 |
| Asian | 1 | 1 |
| Other | 6 | 4 |
| Creatinine Clearance (mL/min, 159) | 98.2 (34.7) | |
| ≥ 30 - < 50 | 6 | 4 |
| ≥ 50 - ≤ 80 | 50 | 31 |
| > 80 | 103 | 65 |
| ECOG Performance Status (159) | ||
| 0 | 65 | 41 |
| 1 | 74 | 47 |
| 2 | 20 | 13 |
| Rai Stage (150) | ||
| I, II | 29 | 19 |
| III, IV | 121 | 81 |
| Binet Stage (109) | ||
| A | 3 | 3 |
| B, C | 106 | 97 |
| Bulky Lymphadenopathya | 115 | 70 |
| Cytogenetic Abnormalitiesb | ||
| del(17p) (141) | 49 | 35% |
| del(11q) (145) | 43 | 31% |
| Number of prior therapies (range) | 4 (1 – 12) | |
| Prior Therapies | ||
| Alkylating agent | 163 | 99 |
| Fludarabine | 165 | 100 |
| Rituximab | 143 | 87 |
| Alemtuzumab | 62 | 38 |
| Fludarabine refractory | 158 | 96 |
Defined as at least 1 lymph node > 5 cm in longest diameter by cross sectional imaging at study registration.
Classified according to Döhner, et al31.
One hundred fifty-ninepatients were treated with alvocidib, comprising the as-treated (AT) population. Thirty-five patients (22% of the ITT population) completed the planned 6 cycles of therapy. Seventy-nine patients (50%) received three or more cycles of therapy. The median number of treatment cycles administered was 2 and the mean number was 3.1 (S.D. = 1.9). Forty-nine patients discontinued therapy due to disease progression (30%), and 41 (25%) stopped due to an adverse event. Twenty-two patients (14%) did not dose escalate due to TLS. At the time of last study contact 57 patients (35%) were alive; no patients were lost to follow-up. Median follow-up for all treated patients was 19.8 months (95% CI: 19.1, 20.6).
Response to Therapy
All 165 patients in the ITT population were included in the response assessment (Table 2). Response was evaluated by the investigator and was reported as best response achieved after study registration. Using the hybrid response criteria, 41 patients (ORR 25%; 95% CI: 19 – 32) responded to therapy. By NCI 96 criteria, 50 patients responded (ORR 30%; 95% CI: 23 – 38). The best response by hybrid criteria included 3 CRs (2%), 2 nPRs (1%), and 36 PRs (22%). Fifty-three patients (32%) had SD, and 21 patients (13%) progressed on therapy. Fifty patients (30%) were not assessed for response by hybrid criteria. NCI 96 criteria responses were similar: 6 CRs (4%), 2 nPRs (1%), 42 PRs (26%), 66 (40%) patients with SD, 26 (16%) patients with PD, and 23 patients were not assessed (14%).
Table 2. Investigator-Reported Best Overall Responseto Alvocidib in the ITT Population.
| Hybrid Criteriaa | NCI-96 Criteriab | |||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| Overall Response Rate | 41 | 25 | 50 | 30 |
| Complete Response | 3 | 2 | 6 | 4 |
| Nodular Partial Response (nPR) | 2 | 1 | 2 | 1 |
| Partial Response | 36 | 22 | 42 | 26 |
| Stable Disease | 53 | 32 | 66 | 40 |
| Disease Progression | 21 | 13 | 26 | 16 |
| Not assessed | 50a | 30 | 23b | 14 |
| del(17p) (n = 49) | 7 | 14 | 13 | 27 |
| del(11q) (n = 43)c | 11 | 26 | 12 | 28 |
| Bulky lymphadenopathy (n = 115) | 29 | 25 | 33 | 29 |
| “Double refractory”d (n = 48) | 5 | 10 | 7 | 15 |
| TLS (n = 39) | 12 | 31 | 15 | 38 |
Of the 50 patients not assessed for best response, 6 were never treated with alvocidib and 28 receivedonly one treatment cycle. The remaining 16 patients received between 2 and 6 cycles of treatment, but the investigator failed to report a best response by hybrid criteria. No missing data imputation was performed.
Of the 23 patients who were not assessed for best response, 6 were never treated with alvocidib and 14 had only one treatment cycle. The remaining 3 patients received 2 or 3 cycles of treatment, but the investigator failed to report a best response by NCI 96 criteria. No missing data imputation was performed.
Classified according to Döhner, et al31.
Refractory to both fludarabine and alemtuzumab
Clinical and biologic factors associated with inferior response to cytotoxic-based therapies were overall not associated with response to alvocidib, though the study was not powered to detect potential differences (Tables 2 and 3). Among the 49 patients with del(17p) ORRs were statistically inferior by hybrid criteria on univariate, but not multivariate analysis (hybrid: 14% vs. 32%, univariateodds ratio of response (OR) = 0.36, p < 0.05; NCI 96: 27% vs. 33%, OR = 0.73, p = 0.6). As observed in prior studies of alvocidib in CLL, the presence of del(11q) or bulky lymphadenopathy were not associated with differences in ORR using either response criteria (p > 0.5 for all comparisonsl)20. Patients refractory to both fludarabine and alemtuzumab (“double refractory”) were statistically less likely to respond to therapy (hybrid: 10% vs. 31%, OR = 0.26, p< 0.01). Patients experiencing TLS were not more likely to respond to therapy (hybrid: 28% vs. 25%, OR = 1.2, p > 0.5. Multivariate logistic regression showed that Rai stage at study entry (I/II vs. III/IV) and number of prior regimens were associated with response using either hybrid (Table 3) or NCI 96 criteria (data not shown).
Table 3. Multivariate Logistic Regression Analysis of Hybrid Overall Response Rate.
| Univariate Model | Multivariate Modela | |||
|---|---|---|---|---|
| Factor | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| Age | 1.004 (0.967, 1.041) | 0.8353 | ||
| Male gender | 0.863 (0.376, 1.980) | 0.7279 | ||
| Rai Stage at entry:Stage III, IV | 0.265 (0.113, 0.623) | 0.0023 | 0.277 (0.108, 0.710) | 0.0075 |
| Presence of del(17p) | 0.362 (0.145, 0.902) | 0.0292 | 0.554 (0.203, 1.512) | 0.2490 |
| Presence of del(11q) | 1.017 (0.445, 2.325) | 0.9682 | ||
| Presence of bulky lymphadenopathy | 1.068 (0.493, 2.314) | 0.8679 | ||
| Baseline WBC | 0.589 (0.216, 1.609) | 0.3019 | ||
| Baseline LDH | 0.863 (0.711, 1.047) | 0.1379 | 0.895 (0.724, 1.106) | 0.3038 |
| Baseline β2-microglobulin | 0.478 (0.034, 6.672) | 0.5831 | ||
| Number of prior anti-cancer regimens | 0.843 (0.712, 0.995) | 0.0433 | 0.806 (0.655, 0.993) | 0.0427 |
| Refractory to fludarabine | 0.819 (0.153, 4.393) | 0.8161 | ||
A multivariate logistic regression analysis was run with all covariates for which p < 0.20 in aunivariate model. Using backward selection for covariates, low risk Rai stage and fewer prior regimens with final ORs of 0.252 (0.100, 0.636 p-value: 0.0035) and 0.813 (0.663, 0.998 p-value: 0.0483) respectively, were associated with a response.
Survival, Duration of Response, and Clinical Benefit
Secondary endpoints of this study included prospective assessments of PFS, OS, and DR. Median PFS for all patients in the AT population was 7.6 months (95% C.I. 4.5 – 9.8), with 100 patients (63%) having progressed during the period of study follow-up (Figure 1A). Among the 50 patients achieving a CR, nPR, or PR by NCI 96 criteria, 46 had adequate follow-up to assess DR. The median investigator-assessed DR in these patients was 13.7 months (95% C.I. 9.1 – 15.6), with 58% of patients maintaining a response to alvocidib for 12 months. Median OS for all patients in the AT population was 14.6 months (95% C.I. 9.8 – 17.2). There was no improvement in disease related fatigue, weight loss, or need for red blood cell or platelet transfusions with alvocidib therapy when compared to baseline.
Figure 1.
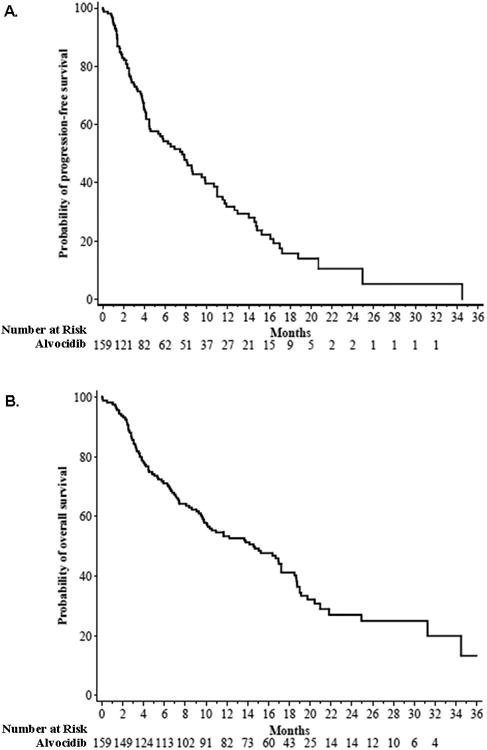
Progression free survival (A) and overall survival (B) among patients in the AT population treated with alvocidib.
Toxicity
Treatment-emergent adverse events (TEAE) occurred in all patients inthe ATpopulation. A total of 138 (87%) had a TEAE of Grade ≥3, and SAEs were reported in 117 (74%) patients. Anoverview of TEAE's is presented in Table 4. The side effect profile was similar to that observed in prior phase I and II studies of alvocidib 18,20. Gastrointestinal AEs were most common, with a majority of patients having diarrhea (82%), nausea (58%), or vomiting (41%). Infections occurred in 90 patients (57%), with 48 (30%) having severe (grade ≥ 3) infections. Constitutional symptoms including fatigue (60%), fever (38%), night sweats (30%), anorexia (16%), and weight loss (14%) were also common, though typically not severe.
Table 4. Common Toxicities by Severity in the AT Population.
| Toxicity | Grade | |
|---|---|---|
| All grades | Grade ≥ 3 | |
| Gastrointestinal | 141 (89%) | 39 (25%) |
| Diarrhea | 130 (82%) | 29 (18%) |
| Nausea | 92 (58%) | 1 (1%) |
| Vomiting | 65 (41%) | 3 (2%) |
| Fatigue | 96 (60%) | 27 (17%) |
| Infections | 90 (57%) | 48 (30%) |
| Febrile Neutropenia | 27 (17%) | 24 (15%) |
| Pneumonia | 12 (8%) | 10 (6%) |
| Catheter infection | 9 (6%) | 5 (3%) |
| Hematologica | 78 (49%) | 72 (45%) |
| Neutropenia | 57 (36%) | 54 (34%) |
| Thrombocytopenia | 30 (19%) | 30 (19%) |
| Anemia | 20 (13%) | 12 (8%) |
| Fever | 60 (38%) | 8 (5%) |
| Cough | 51 (32%) | 3 (2%) |
| Night Sweats | 48 (30%) | 7 (4%) |
| Musculoskeletal Pain | 44 (28%) | 5 (3%) |
| Edema | 41 (26%) | 1 (1%) |
| Tumor Lysis Syndrome | 39 (23%) | 33 (21%) |
| Headache | 34 (21%) | 2 (1%) |
| Decreased Appetite | 26 (16%) | 0 |
| Dizziness | 22 (14%) | 1 (1%) |
| Weight loss | 22 (14%) | 2 (1%) |
| Rash | 19 (12%) | 2 (1%) |
| Sleep disturbance | 17 (11%) | 1 (1%) |
Does not include baseline toxicity; only included if hematologic toxicity worsened by ≥ 1 grade level.
A total of 13 patients died within 30 days of the last study treatment. Seven of the deaths were attributed to disease progression. One death was from euthanasia, occurring at a site where state-sanctioned euthanasia was permitted. Four deaths were due to an infectious cause. One death was due to hyperacute TLS causing ventricular arrhythmia with a potassium level of 8.4 mmol/L measured 4.5 hours after initiation of alvocidib on day 1 of cycle 1.
Among the 159 alvocidib-treated patients, 39 (25%) developed TLS by Cairo-Bishop criteria ncluding 5 life-threatening events and 1 fatal event. TLS was managed with dialysis in 13 patients (8% of the entire AT population had hemodialysis), and the remaining patients were medically managed. In 37 of the 39 patients experiencing TLS, the onset occurred during the first cycle of therapy. Two patients had TLS onset during cycle 2. Six patients (4%) had TLS occur in more than 1 treatment cycle. Multivariate logistic regression showed that only baseline creatinine was significantly associated with risk of TLS (Supplemental Tables 1 and 2).
Discussion
The management of patients with fludarabine refractory CLL remains difficult due to several factors. Earlier studies of alvocidib showed promising response rates in heavily pretreated CLL patients and response rates that were independent of high risk cytogenetic features 19,20. The EFC6663 study was undertaken both to validate the observed response rates and to demonstrate that alvocidib could be safely administered in a multi-center, international clinical trial. This study is one of the largest undertaken in patients with fludarabine refractory CLL, and met the preplanned objective of showing an ORR of > 20% among this heavily pretreated patient population. The ORR of 30% is similar to that achieved with alemtuzumab 6,7, and median PFS of 7.6 months equaled the 5 to 8 months observed with either alemtuzumab and ofatumumab 8.
Both the overall response rate and toxicity reflected that observed in earlier alvocidib studies. The investigator assessed overall response rate of 30% (95% C.I. 23 – 38%) using the NCI 96 criteria is similar to the ORR of 41% observed in the pre-amendment cohort reported by Lin et al20 and 40% reported in a phase I study from the same institution by Phelps et al19. In the EFC6663 study, patients not achieving a 25% response after 2 cycles of therapy discontinued treatment, potentially decreasing the ORR and PFS when compared to prior alvocidib studies. Toxicities were also comparable, with TLS and neutropenic infections being the most common serious complications. Importantly, the single-site phase II study showed both improved response rates and tolerability with administration of alvocidib weekly for 3 weeks followed by a single week of rest: the ORR for the 4- and 6 – week treatment cycles were 63% and 41%, respectively. The 4 week treatment regimen improved treatment tolerance, patient compliance, and clinical response. Because the ORR and toxicity of the EFC6663 study mirrors the results of the pre-amendment phase II cohort, we conclude that future studies of alvocidib in CLL should utilize a 4 week treatment schedule.
In an effort to reduce the risk of TLS, study enrollment required a WBC of < 150 × 109 / L. Nonetheless, TLS occurred in 25% of treated patients. Though the protocol mandated frequent laboratory monitoring and required hemodialysis immediately available when needed, one episode of TLS resulted in a fatal outcome from severe hyperkalemia. Thirty-eight other episodes of TLS were successfully managed, with hemodialysis employed in 13 cases without additional sequelae. Approximately 1/3 of these patients subsequently responded to therapy. Because patients with fludarabine refractory CLL frequently cannot be cytoreduced prior to initiation of therapy, TLS may remain a risk of alvocidib therapy. Multivariate analysis showed that disease bulk and renal function were most predictive of TLS, suggesting that patient clinical features, rather than intrinsic sensitivity to alvocidib, best predict TLS. We conclude that alvocidib can be administered with careful monitoring during the first doses and subsequently transitioned to ambulatory outpatient administration.
Perhaps the most significant interval development in the clinical management of CLL since the initiation of the EFC6663 study is the clinical development of inhibitors of B cell receptor and related signaling pathways. Agents such as ibrutinib 23-25 and idelalisib26 have shown considerable clinical activity in relapsed CLL with favorable toxicity profiles in early phase clinical studies. The clinical responses, response duration, and toxicity profile with these novel agents exceed the outcomes with alvocidib reported herein. Nonetheless, these novel targeted therapies are ultimately not curative, and agents with novel mechanisms of action such as alvocidib will be needed for those patients who progress after B cell receptor signaling inhibitors. One potential benefit of alvocidib therapy is that responding patients have a period of disease quiescence of duration adequate for allogeneic transplantation.
The EFC6663 study validated the clinical activity of alvocidib observed in earlier clinical studies. Alvocidib yielded clinical responses of 9 to 16 months in patients with extensively pretreated, fludarabine refractory CLL. Plans for further clinical development of alvocidib are underway and include high-risk CLL as well as several other hematologic malignancies both as a single agent and as part of combination regimens27,28. The demonstrated clinical activity of alvocidib in refractory CLL and in genetically high-risk disease suggests a potential role for alvocidib as part of the treatment of these patients. Evaluation of the activity of alvocidib in combination with additional agents is planned as part of the further clinical development of alvocidib. Future studies should focus on identification of biomarkers of clinical response29 and risk for TLS30. Enhanced supportive care strategies may yield further improvement in patient adherence and response rates.
Supplementary Material
Acknowledgments
We thank our patients for their willingness to participate in this study and the hematology-oncology nurses and physician assistants for their special help. This work is supported in part by Leukemia and Lymphoma Society, D Warren Brown Foundation and P01 CA095426.
Footnotes
Prior Presentation: A pre-planned interim analysis was presented at the annual meeting of the American Society of Hematology, Orlando, FL, Dec 5, 2010 (oral presentation).
Conflicts of Interest: JRB served as a consultant for Sanofi after conclusion of this trial.
RAL received research support to the University of Chicago from Sanofi.
SS received honoraria and research support from Sanofi.
JD is an employee of Sanofi.
JCB and MRG have an unprosecuted use patent for the schedule of alvocidib utilized in this trial.
Authorship: All authors reviewed and approved the manuscript.
Contribution: Designed research: JCB, MRG
Collected, analyzed, and interpreted data: MCL, JD, SS, NH, AB, JCB, MRG
Referred patients and provided patient care: MCL, LA, JRB, JB, FCC, PG, RAL, TJK, VL, DWM, AJ, SS, AB, MH
Performed correlative analyses: NAH, SS, AJJ
Wrote the manuscript: MCL, JCB, MRG
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–15. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371:1017–29. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- 3.Keating MJ, O'Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:1755–62. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- 4.Perkins JG, Flynn JM, Howard RS, et al. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer. 2002;94:2033–9. [PubMed] [Google Scholar]
- 5.Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2009;115:2824–36. doi: 10.1002/cncr.24329. [DOI] [PubMed] [Google Scholar]
- 6.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 7.Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- 8.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–55. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losiewicz MD, Carlson BA, Kaur G, et al. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem Biophys Res Commun. 1994;201:589–95. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 10.Kaur G, Stetler-Stevenson M, Sebers S, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84:1736–40. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 11.Kitada S, Zapata JM, Andreeff M, et al. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–7. [PubMed] [Google Scholar]
- 12.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–16. [PubMed] [Google Scholar]
- 13.Konig A, Schwartz GK, Mohammad RM, et al. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B-cell leukemia lines. Blood. 1997;90:4307–12. [PubMed] [Google Scholar]
- 14.Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29:1253–7. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Lin TS, Howard OM, Neuberg DS, et al. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma. 2002;43:793–7. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GK, O'Reilly E, Ilson D, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Oncol. 2002;20:2157–70. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro GI, Supko JG, Patterson A, et al. A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clin Cancer Res. 2001;7:1590–9. [PubMed] [Google Scholar]
- 18.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–45. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–8. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7. [PubMed] [Google Scholar]
- 22.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 23.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR. Ibrutinib (PCI-32765), the First BTK (Bruton's Tyrosine Kinase) Inhibitor in Clinical Trials. Curr Hematol Malig Rep. 2013;8:1–6. doi: 10.1007/s11899-012-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiestner A. Emerging role of kinase-targeted strategies in chronic lymphocytic leukemia. Blood. 2012;120:4684–91. doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens DM, Ruppert AS, Maddocks K, et al. Cyclophosphamide, alvocidib (flavopiridol), and rituximab, a novel feasible chemoimmunotherapy regimen for patients with high-risk chronic lymphocytic leukemia. Leuk Res. 2013 doi: 10.1016/j.leukres.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 2010;28:418–23. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahoney E, Lucas DM, Gupta SV, et al. ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood. 2012;120:1262–73. doi: 10.1182/blood-2011-12-400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji J, Mould DR, Blum KA, et al. A pharmacokinetic/pharmacodynamic model of tumor lysis syndrome in chronic lymphocytic leukemia patients treated with flavopiridol. Clin Cancer Res. 2013;19:1269–80. doi: 10.1158/1078-0432.CCR-12-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–6. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


