Abstract
Chromophobe renal cell carcinoma (ChRCC) and oncocytoma present with a perplexing overlap of morphologic and immunohistochemical features. ChRCC have deletions in the 1p21.1 region including the amylase α-1A gene (AMY1A). No such deletions are found in oncocytoma. Instead, oncocytomas shared other deletions on chromosome 1: 1p31.3, 1q25.2, and 1q44. We performed AMY1A immunostaining on 75 oncocytomas (57 tissue microarray [TMA] cores, 18 whole slides) and 54 ChRCCs (20 TMA cores, 34 whole slides). Staining was assessed using the H-score method. The intensity was graded as follows: no staining=0, weak=1, moderate=2, and strong=3. The AMY1A immunostain preferentially stained the distal tubules and collecting ducts of normal kidney. All oncocytomas (100%) expressed AMY1A with an H-score that varied from 100 to 300 (mean 205). Mild to moderate heterogeneity in staining intensity was noted within a given oncocytoma. For oncocytomas, 87% (65/75) cases had H-scores of at least 120 with a mean score of 221. Notably, the 13% (10/75) of oncocytoma cases that had an H-score of 100 were derived from the TMA. A total of 87% (47/54) of the ChRCC cases were negative for the AMY1A immunostain. Of the ChRCC cases, 4% (2/54) showed very weak cytoplasmic staining (H-score of 70 each), which was less than the lowest H-score of oncocytoma cases. All 5 cases of ChRCC, which showed an H-score of 100 or more, were referred to as eosinophilic variants of ChRCC. Three of these 5 cases showed a very nondescript, diffuse staining of the cytoplasm. Two of these 5 cases showed an H-score of 130. We think that as the staining pattern of these 2 cases is similar to that of oncocytoma, they should be put in a category of renal oncocytic neoplasms favoring oncocytoma. This result shows that AMY1A staining could be very helpful in further classifying even a subset of the eosinophilic variants of ChRCC. The difference between ChRCC and oncocytoma was statistically significant (χ2 test, P<0.0001). All cases of clear cell RCC and papillary RCC were negative for AMY1A expression. Overall, sensitivity and specificity of AMY1A staining for oncocytoma was 100% (95% confidence interval, 0.95–1.00) and 96.75% (95% confidence interval, 0.93–0.99), respectively. Similarly, the sensitivity and specificity for distinguishing oncocytoma from ChRCC was 100% (95% confidence interval, 0.95–1.00) and 90.74% (95% confidence interval, 0.80–0.97), respectively. These data show that the novel marker AMY1A can be of great diagnostic utility when trying to differentiate ChRCC (classic and eosinophilic variant) and oncocytoma.
Keywords: chromophobe, renal cell carcinoma, oncocytoma, AMY1A, amylase 1A, immunohistochemistry
Chromophobe renal cell carcinoma (ChRCC) and oncocytoma are distinct renal tumors with a proposed common cell of origin: the intercalated cell of the collecting duct. Classic histopathology of ChRCC and oncocytoma are readily distinguishable; however, not uncommonly, some of these renal tumors may present with a perplexing overlap of morphologic and immunohistochemical (IHC) features. The eosinophilic variant of ChRCC is one such example in which the abundance of smaller, eosinophilic cells mimics oncocytoma. Despite the pathologic overlap of these 2 tumors, their biological behavior and clinical outcomes are significantly different, which is why it is important to distinguish them. Oncocytoma is a benign tumor and despite microscopic extension into perinephric adipose tissue and vascular invasion, which occur infrequently, has a low mortality of 0%.1–3 ChRCC is a malignant tumor with a higher mortality rate. The majority of ChRCC cases present with stage T1 and T2 disease (86%). Only 10% of ChRCC cases show extracapsular extension, and only 4% show renal vein involvement.4
Several IHC markers have been investigated to distinguish these 2 tumors such as LMP2, parvalbumin, cytokeratin 7 (CK7), MOC-31, cadherin, caveolin-1, c-kit, claudin-7 and 8, MAGE-A3/4, NYES0-1, and S100A1.5–13 Unfortunately, no single marker or panel of biomarkers conclusively aids in this distinction. In a recent study from our institution, copy number variations across different types of renal neoplasms were analyzed using high-resolution single nucleotide polymorphism arrays.14 Interestingly, all ChRCC cases were found to exclusively share common deletions in the 1p21.1 region that includes the AMY1A gene. No such deletions were found in oncocytoma. Instead, oncocytomas shared other deletions on chromosome 1: 1p31.3, 1q25.2, and 1q44. Four of 5 clear cell tumors had deletions of the entire coding region of the amylase 1A gene. Two of the papillary tumors had a complete deletion as well, whereas the remaining had deletions dispersed throughout the AMY1A gene domain; a single exon deletion can prevent assembly of a functional transcript. Human α-amylases (α-1, 4-glucan 4-glucanohydrolase, E. C. 3. 2.1.1) are mainly produced in the salivary gland and pancreas. Among the several amylase genes that are expressed at high levels in either the salivary gland or the pancreas, AMY1A gene encodes the salivary gland–type amylase isoenzyme that hydrolyzes the 1,4-α-glucoside bonds in oligosaccharides and polysaccharides to produce maltose, which is cleaved to 2 molecules of glucose by enzyme maltase.15 Amylase enzyme (mainly salivary type) is also produced in some malignant tumors, viz., lung cancer, ovarian cancer, plasmacytoma, normal thyroid tissue, thyroid adenomas, and cancer.16–19 The aim of this study was to examine the utility of Amy1A in distinguishing between oncocytoma and ChRCC.
MATERIALS AND METHODS
Case Cohort Selection
Following the approval by our institutional review board, of the total 229 surgically resected renal tumors, 210 were retrieved from the case archives of the Department of Pathology at the University Of Pittsburgh Medical Center from 2005 through 2012 and 14 from the Department of Pathology, East Carolina University. Renal tumor subtypes included oncocytoma (n=75), ChRCC (n=54), clear cell RCC (n=60), and papillary RCC (n=40) cases (Table 1). These cases were reviewed at the time of diagnosis and assessed for tumor type, grade, and pathologic stage. A panel of IHC markers including but not limited to CK7, carbonic anhydrase 9, CD117, parvalbumin, RCC antigen, vimentin, and AMACR were used for confirmation of diagnosis. Any case with diagnostic ambiguity was excluded from this study. Both whole slides (WS) and tissue microarrays (TMA) were used for assessing AMY1A IHC.
TABLE 1.
Case Cohort by Renal Tumor Subtype
| Tumor Subtype | Total Cases | WS Sections | TMA Cores |
|---|---|---|---|
| Oncocytoma | 75 | 18 | 57 |
| Chromophobe RCC | 54 | 34 | 20 |
| Clear cell RCC | 60 | 9 | 51 |
| Papillary RCC | 40 | 15 | 25 |
TMA Construction and Design
The TMA was constructed using the following tissues: oncocytoma, ChRCC, clear cell RCC, papillary RCC, and normal kidney tissue adjacent to the tumor (n=47). The Beecher instrument manual arrayer was used to obtain cores from paraffin-embedded tissues, each core being 0.6mm in diameter. Various normal organs (liver, adrenal, heart, testis, brain, ovary, prostate, spleen, and lung) were also included in the TMA.
IHC for AMY1A
Sections were cut at 5 μm thickness and picked up on Superfrost Plus glass slides, dried overnight at room temperature, and subsequently baked in a 60°C oven for 45 minutes before staining. IHC staining for AMY1A antibody was developed on a Dako Autostainer Plus instrument using a streptavidin horseradish peroxidase detection protocol. The slides were first deparaffinized and rehydrated and then treated for 20 minutes in Borg (Biocare Medical) antigen retrieval buffer in the Biocare Decloaking Chamber. Sections were then treated with 3% hydrogen peroxide to block endogenous peroxidase. After washing with TBS buffer for 5 minutes, slides were then incubated with 5% normal goat serum (Vector Labs) for 20 minutes. Slides were then washed and incubated with avidin/biotin blocking kit (Vector Labs). After washing, slides were incubated with the primary antibody AMY1A, clone 2D4 (Cat# H0000276-M04; Abnova, Walbut, CA) at 1:100 dilution (diluted in Dako diluent) for 60 minutes. Slides were incubated with biotinylated mouse IgG (Vector Labs) for 30 minutes. Slides were then incubated with a streptavidin 4+ HRP label (Biocare Medical) for 30 minutes. Slides were developed with DAB+substrate chromogen (Dako) for 10 minutes and counterstained with hematoxylin. All incubations were carried out at room temperature.
Scoring System
Staining was assessed using the H-score method (stain intensity × percentage of cells positive for each intensity score). Staining intensity was graded as follows: no staining=0, weak=1, moderate=2, and strong=3. At least 10% positive staining (any intensity) of tumor cells was considered positive staining with AMY1A. This corresponded to an H-score of at least 10.
Statistical Analysis
Statistical analysis was performed using IBM SPSS statistics version 20 (IBM Corp., Armonk, NY). Univariate analysis was performed using the χ2 test, and a P-value <0.05 was considered significant.
RESULTS
Clinical and pathology data were found in 74 of 75 oncocytoma cases and 36 of 54 ChRCC cases. Demographic data are summarized in Table 2. Of the 74 oncocytoma cases, 49 were from male patients and 25 from female patients (male to female ratio 1.96:1), with an age range of 25 to 89 years. In 27/74 (36%) cases patients were between 45 and 64 years of age, whereas in 42/74 (56%) cases they were between 65 and 84 years. The mean tumor size was 4 cm (range, 1 to 15 cm). In 46 cases, oncocytomas involved the right kidney, and 28 involved the left kidney. In 33 cases patients underwent partial nephrectomy, and in 41 cases they underwent radical nephrectomy.
TABLE 2.
Case Cohort by Demographics
| Oncocytoma (n=74/75) | ChRCC (n=36/54) | |
|---|---|---|
| Sex | ||
| Male | 49 | 17 |
| Female | 25 | 19 |
| Male:female ratio | 1.96:1 0.89:1 | |
| Laterality | ||
| Right | 46 | 11 |
| Left | 28 | 25 |
| Surgery | ||
| Partial | 33 | 14 |
| Complete | 41 | 22 |
| Tumor size (cm) | ||
| Range | 1–15 | 1–14.5 |
| Mean | 4 | 8.36 |
In the ChRCC group, 17 patients were male and 19 were female (male to female ratio 0.94: 1), with an age range of 25 to 89 years. In 18/36 (50%) cases individuals were between 45 and 64 years of age, whereas in 16/36 (44%) cases they were between 65 and 84 years. The mean tumor size was 8.33 cm (range, 1 to 14.5 cm). In 11 cases tumors involved the right kidney, and in 25 they involved the left kidney. There were 22 cases resected by radical nephrectomy, and in 14 cases patients only underwent partial nephrectomy.
The result of IHC staining of AMY1A in all renal tumors is summarized in Table 3. All oncocytomas (100%) expressed AMY1A protein by IHC (Figs. 1D, 2B–D). The staining pattern was granular with diffuse immunoreactivity of the cytoplasm. Mild to moderate heterogeneity in staining intensity was noted within a given oncocytoma (Fig. 2E). Immunostaining varied from mild to strong intensity with the majority of tumors showing moderate to strong staining (68%). The H-score varied from 100 to 300 with a mean of 205. An H-score of 100 was seen in 13% (10/75) of oncocytomas, all of which were interpreted on the TMA. The remainder (87%, 65/75) of these cases had H-scores of at least 120 with a mean score of 221.
TABLE 3.
Staining Profile of Renal Tumors
| AMY1A H-Score
|
||||
|---|---|---|---|---|
| 0 | 1–99 | 100–199 | 200–300 | |
| Renal tumor type (n [%]) | ||||
| Oncocytoma (n=75) | 0 (0) | 0 (0) | 24 (32) | 51 (68) |
| ChRCC (n=54) | 47 (87) | 2 (4) | 5 (9) | 0 (0) |
| Clear cell RCC (n=60) | 60 (100) | 0 (0) | 0 (0) | 0 (0) |
| Papillary RCC (n=40) | 40 (100) | 0 (0) | 0 (0) | 0 (0) |
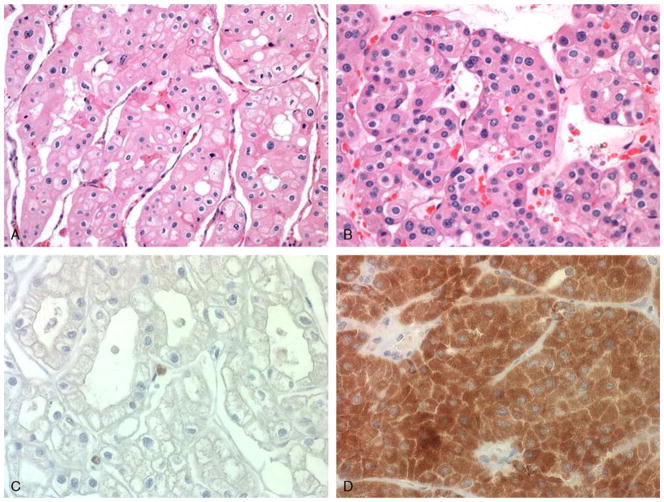
FIGURE 1.
ChRCC (A) and oncocytoma (B) (hematoxylin and eosin). ChRCC (C) is negative for IHC staining by AMY1A (H-score 0), whereas oncocytoma (D) shows diffuse, strong staining (H-score 300).
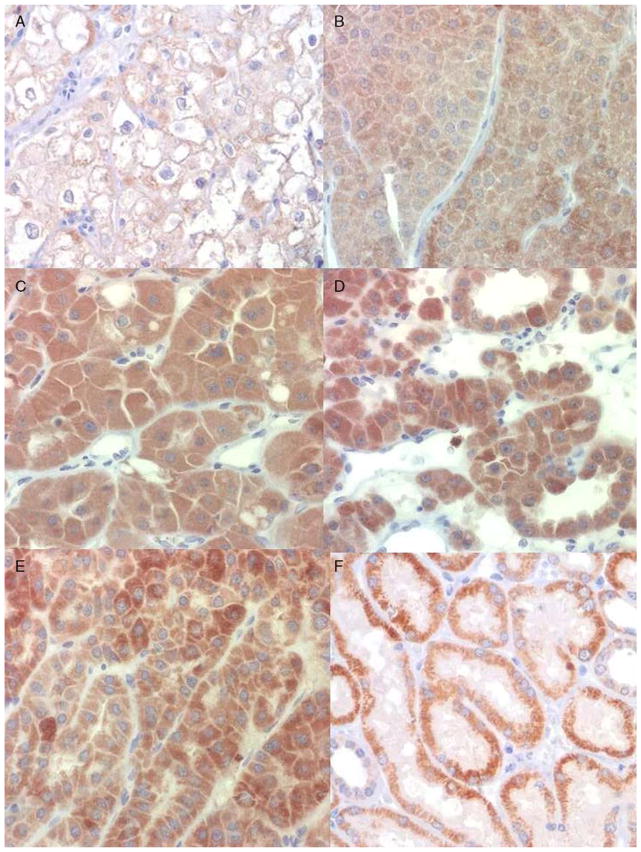
FIGURE 2.
IHC staining of ChRCC and oncocytoma by AMY1A. A, ChRCC showing very weak staining (H-score 70). Oncocytoma showing (B) weak staining (H-score 100), (C) moderate staining (H-score 200), and (D) strong staining (H-score 300). E, Heterogeneity of AMY1A staining intensity in an oncocytoma. F, Preferential staining of distal convoluted tubules and collecting duct in normal kidney tissue.
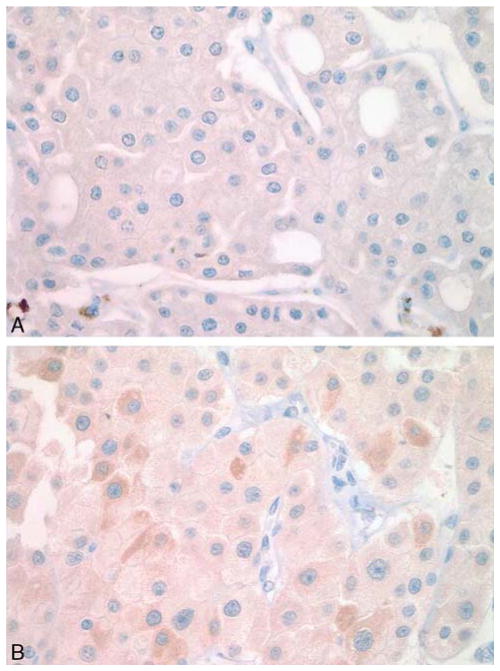
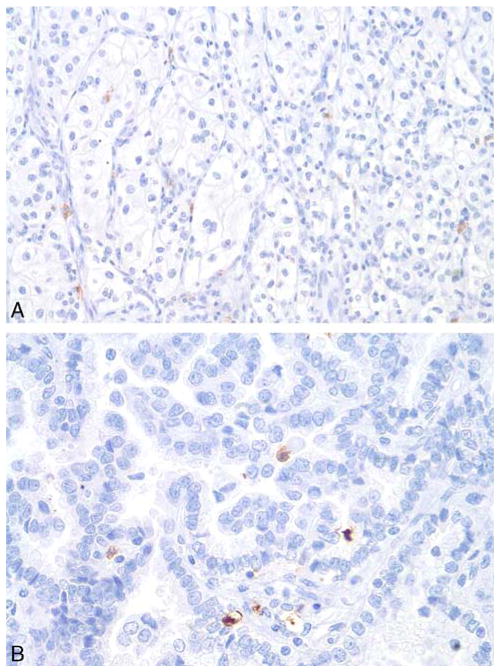
The majority (87%, 47/54) of ChRCC cases were negative for the AMY1A stain (Fig. 1C). Of the ChRCC cases 4% (2/54) showed very weak, albeit diffuse, staining (H-score of 70 each), which was less than the lowest Hscore of the oncocytoma cases (Fig. 2A). Five cases of eosinophilic variants of ChRCC were also included in this study: 3 of these 5 cases showed a diffuse weak staining (1+ intensity) in almost all tumor cells, resulting in an Hscore of 100 (Fig. 3A), and 2 cases showed a heterogenous staining with some cells staining stronger than others (1+ to 2+), with an H-score of 130 each (Fig. 3B). The observed difference in the staining profile between oncocytoma and ChRCC was statistically significant (χ2 test, P<0.0001). All cases of clear cell RCC and papillary RCC were negative for AMY1A expression (Fig. 4). However, AMY1A stained some stromal cells and benign- looking cells of undetermined origin. The AMY1A preferentially stained the distal tubules and the collecting ducts of normal kidney tissue (Fig. 2F).
FIGURE 3.
IHC staining by AMY1A of the eosinophilic variant of ChRCC. A, A weak, diffuse staining with an H-score of 100 and (B) heterogenous staining with an H-score of 130.
FIGURE 4.
IHC staining by AMY1A of (A) clear cell RCC and (B) papillary RCC was negative.
On the basis of the aforementioned staining pattern observed in these renal tumors, the overall sensitivity and specificity of AMY1A staining for oncocytoma was 100% (95% confidence interval, 0.95–1.00) and 96.75% (95% confidence interval, 0.93–0.99), respectively. Similarly, the sensitivity and specificity for distinguishing oncocytoma from ChRCC was 100% (95% confidence interval, 0.95– 1.00) and 90.74% (95% confidence interval, 0.80–0.97), respectively.
DISCUSSION
ChRCC and oncocytoma share a presumed common cell of origin, the intercalated cells of the collecting duct. Although in most instances ChRCC and oncocytoma can be clearly distinguished on morphologic and IHC features, some renal tumors, particularly the eosinophilic variant of ChRCC, may demonstrate overlapping features making it difficult to distinguish them on the basis of hematoxylin and eosin staining alone. Therefore, additional special stains such as the Hale colloidal iron stain and IHC analysis are frequently used to aid in making this distinction. The Hale colloidal iron stain shows a diffuse and strong, reticular staining in ChRCC, whereas it has focal and weak, fine dust–like positivity in oncocytoma.20 Unfortunately, this histochemical staining is technically challenging to perform, and consistency of staining is poor. In a study reported by Abrahams et al,21 only 37% of their ChRCC cases showed characteristic flocculent to reticular staining with the Hale colloidal iron. ChRCC examined under the electron microscope shows intracytoplasmic 160 to 300 nm microvesicles, tubulovesicular mitochondrial cristae, hyaline globules, microvilli, and a paucity of glycogen particles. In contrast, an abundance of mitochondria is a characteristic feature of oncocytoma, and these tumors lack the ultrastructural features of ChRCC.21 Unfortunately, electron microscopy is an expensive and laborious process and has limitations on using paraffin-embedded tissues. Therefore, IHC is the preferred ancillary testing modality used to try distinguishing these 2 renal tumors. To the best of our knowledge, there has been no single IHC marker available that reliably distinguishes ChRCC from oncocytoma.
CK7 has been shown to have pattern-specific staining in ChRCC and oncocytoma. ChRCC has a strong peripheral accentuation of CK7, whereas oncocytoma has patchy, weak to moderate cytoplasmic expression. In a study by Pan et al,8 10 of 36 ChRCC cases did not stain with CK7. Other authors also had similar findings with CK7.22 These published data show that CK7 cannot be used as a reliable marker to differentiate between oncocytoma and ChRCC. In a study performed by Martignoni et al,6 all their cases (32/32) of ChRCC were positive for parvalbumin with granular cytoplasmic staining and marked peripheral accentuation. However, 11/16 of their oncocytoma cases also showed variable granular cytoplasmic and nuclear parvalbumin expression. More recently, Zheng et al5 suggested the utility of nuclear expression of LMP2 as a useful indicator to differentiate oncocytoma and the eosinophilic variant of ChRCC. In their study, 7 of 7 cases of ChRCC (eosinophilic variant) showed nuclear LMP2 staining, as opposed to only 2 of 56 cases of oncocytoma. These authors also stated that 79% of oncocytomas and 100% of classic ChRCCs showed cytoplasmic positivity. Although some studies have shown that S100A1 can be useful in distinguishing ChRCC from oncocytoma, it needs to be verified.23
Some authors have suggested the utility of IHC panels in distinguishing ChRCC from oncocytoma. In a study performed by Liu et al,24 the authors suggested that if the tumor is Hale colloidal iron+/CK7+/EpCAM+ (epithelial cell adhesion molecule) it is probably a ChRCC. However, these authors also mentioned that oncocytoma can demonstrate moderate staining with Hale colloidal iron and focal staining for CK7 and EpCAM. Ohe et al25 found a significant difference between the percentage of ChRCC and oncocytoma cells that stained positively for KAI1 (100% vs. 10%), ESA (83% vs. 10%), and ERA (83% vs. 10%). However, these panels were not 100% sensitive nor were they very specific.
The amylase genes exist in a multigene cluster, consisting of 6 kinds of isogene, 3 salivary amylase isogenes (AMY1A, AMY1B, AMY1C), 2 pancreatic amylase isogenes (AMY2A and AMY2B), and a truncated pseudogene (AMYP1).26 AMY1A gene and the mRNA transcripts were detected in the thyroid gland, normal lung tissue, tracheal epithelium, ovary, fallopian tube, and uterine cervix.27 However, AMY1A expression was neither evaluated in normal renal tissue nor in renal neoplasms in humans. The expression of the α-amylase gene has been evaluated in normal dog kidney tissue. Although the enzyme activity was detected in the normal renal tissue, the AMY1A or AMY2 gene expression was undetected by reverse transcription polymerase chain reaction.28
We investigated the use of AMY1A IHC in distinguishing between ChRCC and oncocytoma. In summary, our findings showed that AMY1A is an excellent marker for oncocytoma, with a sensitivity of 100% and specificity of 96.75%. The AMY1A immunostain was positive in 100% cases of oncocytoma. AMY1A showed moderate to strong diffuse staining in 87% (65/75) of oncocytoma cases (mean H-score 221). The remaining 13% cases (10/75) of oncocytoma, all of which were derived from the TMA, showed a mean H-score of 100. This can be explained by the heterogenous staining seen in oncocytomas on WS, with up to 10% to 50% of areas showing weak staining (1+). It is to be noted that despite this heterogeneity in oncocytoma, none of the tumor cells were negative. This heterogeneity of AMY1A expression should be taken into account when interpreting WS. None of the oncocytoma cases had an H-score of <100. This was in huge contrast to the staining of ChRCC in which 87% of cases were negative for AMY1A. Two cases showed weak, patchy staining for AMY1A with an H-score of 70 each. Three of the 5 cases of eosinophilic variant of ChRCC demonstrated homogenous weak (1+) staining in all tumor cells as seen in other cases of ChRCC. The remaining 2 cases with an H-score of 130 demonstrated a more heterogenous staining profile (1+ to 2+). As the staining profile of the latter 2 cases was similar to that of oncocytoma, we classified them as a renal oncocytic neoplasm, favoring to be oncocytoma.
The presented evidence shows that AMY1A is a very reliable marker to help differentiate oncocytoma from ChRCC. Although the eosinophilic variant of ChRCC can show immunoexpression of AMY1A, the distribution and staining intensity is helpful in appropriate classification. None of the neoplastic cells in clear cell RCC (n=60) and papillary RCC (n=40) was positive for AMY1A staining. On the basis of our study, moderate to strong, diffuse AMY1A staining is a highly sensitive and specific novel marker for oncocytoma that can be used to reliably distinguish oncocytoma from ChRCC. Larger studies will be of interest to further substantiate the applicability of this immunostain in diagnostically challenging cases of renal oncocytic neoplasms.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Amin MB, Crotty TB, Tickoo SK, et al. Renal oncocytoma: a reappraisal of morphologic features with clinicopathologic findings in 80 cases. Am J Surg Pathol. 1997;21:1–12. doi: 10.1097/00000478-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Ordonez B, Hamed G, Campbell S, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. 1997;21:871–883. doi: 10.1097/00000478-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dvorakova M, Dhir R, Bastacky SI, et al. Renal oncocytoma: a comparative clinicopathologic study and fluorescent in-situ hybridization analysis of 73 cases with long-term follow-up. Diagn Pathol. 2010;5:32–37. doi: 10.1186/1746-1596-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty TB, Farrow GM, Lieber MM. Chromophobe cell renal carcinoma: clinicopathological features of 50 cases. J Urol. 1995;154:964–967. doi: 10.1016/s0022-5347(01)66944-1. [DOI] [PubMed] [Google Scholar]
- 5.Zheng G, Chaux A, Sharma R, et al. LMP2, a novel immunohistochemical marker to distinguish renal oncocytoma from the eosinophilic variant of chromophobe renal cell carcinoma. Exp Mol Pathol. 2013;94:29–32. doi: 10.1016/j.yexmp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martignoni G, Pea M, Chilosi M, et al. Parvalbumin is constantly expressed in chromophobe renal carcinoma. Mod Pathol. 2001;14:760–767. doi: 10.1038/modpathol.3880386. [DOI] [PubMed] [Google Scholar]
- 7.Mathers ME, Pollock AM, Marsh C, et al. Cytokeratin 7: a useful adjunct in the diagnosis of chromophobe renal cell carcinoma. Histopathology. 2002;40:563–567. doi: 10.1046/j.1365-2559.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- 8.Pan CC, Chen PC, Ho DM. The diagnostic utility of MOC31, BerEP4, RCC marker and CD10 in the classification of renal cell carcinoma and renal oncocytoma: an immunohistochemical analysis of 328 cases. Histopathology. 2004;45:452–459. doi: 10.1111/j.1365-2559.2004.01962.x. [DOI] [PubMed] [Google Scholar]
- 9.Adley BP, Gupta A, Lin F, et al. Expression of kidney-specific cadherin in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol. 2006;126:79–85. doi: 10.1309/JFE2-B57Y-QFPW-PL10. [DOI] [PubMed] [Google Scholar]
- 10.Garcia E, Li M. Caveolin-1 immunohistochemical analysis in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Am J Clin Pathol. 2006;125:392–398. [PubMed] [Google Scholar]
- 11.Carvalho JC, Wasco MJ, Kunju LP, et al. Cluster analysis of immunohistochemical profiles delineates CK7, vimentin, S100A1 and C-kit (CD117) as an optimal panel in the differential diagnosis of renal oncocytoma from its mimics. Histopathology. 2011;58:169–179. doi: 10.1111/j.1365-2559.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 12.Osunkoya AO, Cohen C, Lawson D, et al. Claudin-7 and claudin-8: immunohistochemical markers for the differential diagnosis of chromophobe renal cell carcinoma and renal oncocytoma. Hum Pathol. 2009;40:206–210. doi: 10.1016/j.humpath.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Demirovic A, Dzombeta T, Tomas D, et al. Immunohistochemical expression of tumor antigens MAGE-A3/4 and NY-ESO-1 in renal oncocytoma and chromophobe renal cell carcinoma. Pathol Res Pract. 2010;206:695–699. doi: 10.1016/j.prp.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Krill-Burger JM, Lyons MA, Kelly LA, et al. Renal cell neoplasms contain shared tumor type-specific copy number variations. Am J Pathol. 2012;180:2427–2439. doi: 10.1016/j.ajpath.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [Accessed December 22, 2012.];AMY1A amylase, alpha 1A (salivary) [Homo sapiens (human)] 2012 Available at: http://www.ncbi.nlm.nih.gov/gene/276.
- 16.Grove A. Amylase in lung carcinomas. An ultrastructural and immunohistochemical study of two adenocarcinomas, and a review of the literature. Apmis. 1994;102:135–144. [PubMed] [Google Scholar]
- 17.Corlette MB, Dratch M, Sorger K. Amylase elevation attributable to an ovarian neoplasm. Gastroenterology. 1978;74(pt 1):907–909. [PubMed] [Google Scholar]
- 18.Fujii H, Yashige H, Kanoh T, et al. Amylase-producing multiple myeloma. Arch Pathol Lab Med. 1991;115:952–956. [PubMed] [Google Scholar]
- 19.Doi S, Tomita N, Higasiyama M, et al. Expression of alpha-amylase isozymes in human thyroid tissues. Cancer Res. 1991;51:3544–3549. [PubMed] [Google Scholar]
- 20.Tickoo SK, Amin MB, Zarbo RJ. Colloidal iron staining in renal epithelial neoplasms, including chromophobe renal cell carcinoma: emphasis on technique and patterns of staining. Am J Surg Pathol. 1998;22:419–424. doi: 10.1097/00000478-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Abrahams NA, MacLennan GT, Khoury JD, et al. Chromophobe renal cell carcinoma: a comparative study of histological, immunohistochemical and ultrastructural features using high throughput tissue microarray. Histopathology. 2004;45:593–602. doi: 10.1111/j.1365-2559.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu SL, Kothari P, Wheeler TM, et al. Cytokeratins 7 and 20 immunoreactivity in chromophobe renal cell carcinomas and renal oncocytomas. Mod Pathol. 2002;15:712–717. doi: 10.1097/01.MP.0000017566.29755.8A. [DOI] [PubMed] [Google Scholar]
- 23.Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92–109. doi: 10.5858/2010-0478-RAR.1. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Qian J, Singh H, et al. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch Pathol Lab Med. 2007;131:1290–1297. doi: 10.5858/2007-131-1290-IAOCRC. [DOI] [PubMed] [Google Scholar]
- 25.Ohe C, Kuroda N, Takasu K, et al. Utility of immunohistochemical analysis of KAI1, epithelial-specific antigen, and epithelial-related antigen for distinction of chromophobe renal cell carcinoma, an eosinophilic variant from renal oncocytoma. Med Mol Morphol. 2012;45:98–104. doi: 10.1007/s00795-011-0546-3. [DOI] [PubMed] [Google Scholar]
- 26.Groot PC, Bleeker MJ, Pronk JC, et al. The human alpha-amylase multigene family consists of haplotypes with variable numbers of genes. Genomics. 1989;5:29–42. doi: 10.1016/0888-7543(89)90083-9. [DOI] [PubMed] [Google Scholar]
- 27.Seyama K, Nukiwa T, Takahashi K, et al. Amylase mRNA transcripts in normal tissues and neoplasms: the implication of different expressions of amylase isogenes. J Cancer Res Clin Oncol. 1994;120:213–220. doi: 10.1007/BF01372559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocharla H, Mocharla R, Hodes ME. Alpha-amylase gene transcription in tissues of normal dog. Nucleic Acids Res. 1990;18:1031–1036. doi: 10.1093/nar/18.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]