Abstract
De novo differntiation of CD4+Foxp3+ regulatory T cells [induced (i) Tregs] occurs preferentiatlly in the gut-associated lymphoid tissues (GALT). We addressed the contribution of background genetic factors in affecting the balance of iTreg, Th1, and Th17 cell differentiation in GALT in vivo following the transfer of naïve CD4+CD45RBhigh T cells to strains of RAG2-deficient mice with differential susceptibility to inflammatory colitis. iTreg represented up to 5% of CD4+ T cells in mesenteric lymph nodes (MLN) of less-susceptible C57BL/6 RAG2−/− mice compared to <1% in highly-susceptible C57BL/10 RAG2−/− mice 2 weeks following T cell transfer before the onset of colitis. Early Treg induction was correlated inversely with effector cell expansion and the severity of colitis development, was controlled primarily by host and not T cell-dependent factors, and was strongly associated with IL-12/23 production by host CD11c+CD103+ dentritic cells. These data highlight the importance of genetic factors regulating IL-12/23 production in the regulation of the balance between iTreg differentiation and TE expansion in lymphopenic mice, and indicate a direct role for iTreg in the regulation of colonic inflammation in vivo.
INTRODUCTION
Although the pathogenesis of human Crohn’s disease (CD) is still poorly understood, it is now widely believed that the intestinal inflammation of this devastating condition represents a dysregulated immune response to commensal bacterial antigens.1 Furthermore, data from mouse models and human disease support a primary role for unrestrained CD4+ T cell responses in driving this inflammation. This is likely due to some combination of IFN-γ producing Th1 cells and pathogenic Th17 cells induced by the production of IL-12 and/or IL-23 by dendritic cells (DCs) and macrophages,2–6 although the colitogenic potential of Th17 cells has recently been challenged by experimental data in mice showing a protective role for IL-17A in colitis development.7 Experimental colitis using p19 knock out mice has shown a critical role for IL-23 in driving these pathogenic intestinal immune responses,6, 8–10 and restraining regulatory T cell (Treg) differentiation in mice.11
To maintain intestinal homeostasis, such pathogenic immune responses in the gut are constantly restrained by a number of factors, including CD4+CD25+Foxp3+ Tregs.1 Co-transfer or subsequent transfer of Tregs is able to prevent or cure experimental colitis following transfer of CD4+CD45RBhigh naïve T cells to RAG2−/− mice, respectively.12, 13 Aside from thymically derived “natural” Tregs (nTregs), CD4+Foxp3+ T cells can also be induced outside the thymus (iTregs),14 particularly in the gut-associated lymphoid tissues (GALT) following interactions with CD103+ DCs in a TGFβ and retinoic acid-dependent manner.15–17 iTregs can have suppressive functions similar to nTregs in vitro,15, 16, 18, 19 and may represent a high proportion of the total CD4+Foxp3+ Tregs in peripheral compartments.20 However, the precise role for iTregs in regulating intestinal homeostasis is not yet clear, as recent studies have come to contradictory conclusions regarding their importance in experimental colitis models.11, 21
Inbred mouse strains that differ in their susceptibility to autoimmune diseases offer an important tool for understanding complex disorders like IBD, because it allows the study of disease evolution in the context of minimal genetic heterogeneity and controlled environmental influences. The C57BL/6 (B6) and C57BL/10 (B10) mouse strains were derived from the same parental C57BL strain and therefore have been thought to possess a very close genetic relationship. However, we have previously shown that B10-RAG2-deficient mice were more susceptible to colitis induced by CD4+CD45RBhigh T cell adoptive transfer in comparison to B6-RAG2-deficient mice housed under the same conditions.22 In the current manuscript, we explored the causes for unequal disease development in these two closely related mouse strains in the T cell transfer model of colitis. Our results support an important role of iTregs in the regulation of immune homeostasis and demonstrate that genentic control of DC-derived IL-12/23 controls the balance of iTregs/TE early in disease evolution and determines disease outcome.
RESULTS
Increased susceptibility of B10 RAG2-deficient mice to colitis is associated with an enhanced colonic accumulation of Th1 and Th17 cells
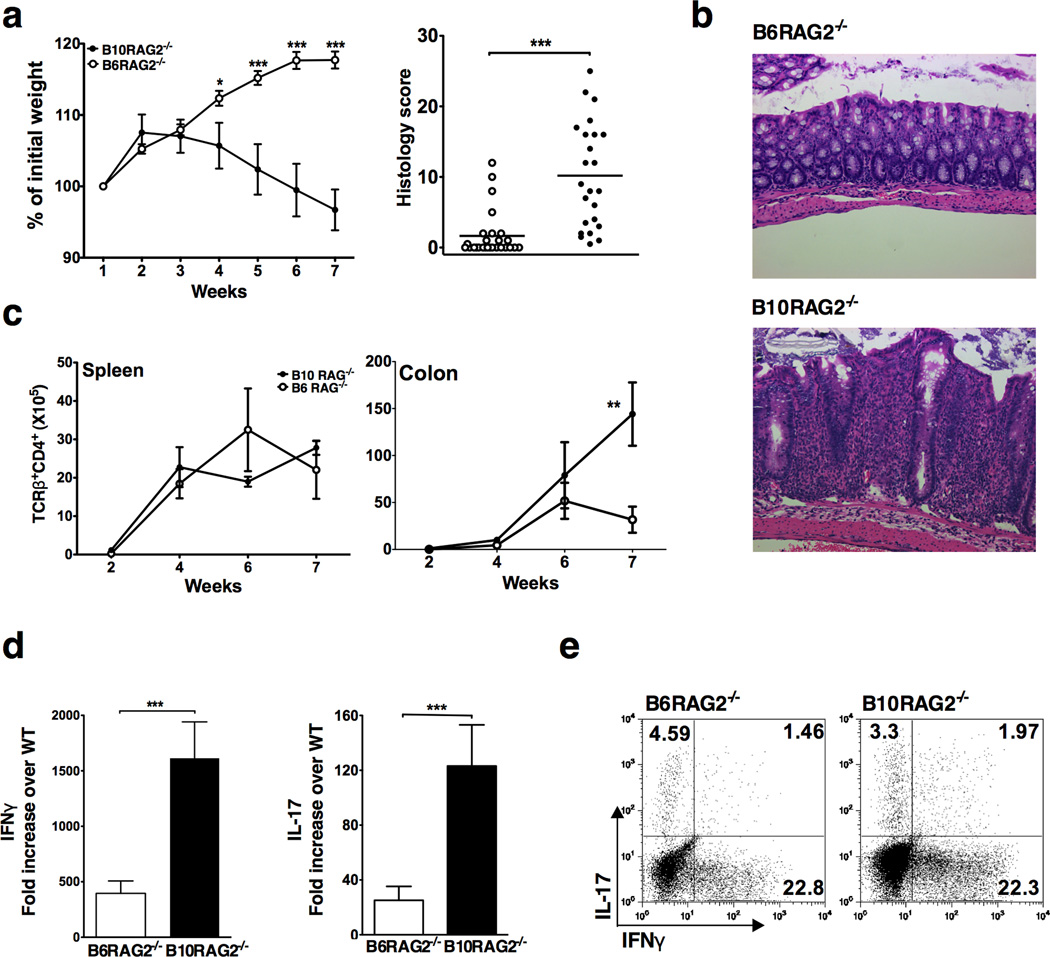
Adoptive transfer of naïve CD4+CD25−CD45RBhigh T cells to B10-RAG2−/− recipients resulted in significantly more weight loss and higher colitis score compared to B6-RAG2−/− recipients housed under the same conditions (Figure 1a and b). Consistent with our previously published studies, disease incidence and severity was significantly greater in the B10-RAG2−/− strain. 22 This was accompanied by a higher rate of CD4+ T cell accumulation in the colon of the B10-RAG2−/− recipients (Figure 1c), whereas no difference in T cell accumulation rates was detected in the spleen (Figure 1c).
Figure 1.
B10-RAG2−/− recipients are more susceptible to T cell transfer colitis due to enhanced colonic accumulation of pathogenic T cells. (a) Weight change (Left panel) and colonic histology scores (right panel) at study end-point (7 weeks) following T cell adoptive transfer to B6-RAG2−/− and B10-RAG2−/− mice. Data shown are mean ± SD of 25 mice per group and representative of at least 3 independent experiments with 25–30 mice per group in each. (b) Representative colon histology in B6-RAG2−/− (score=2) and B10-RAG2−/− (score=25) mice at the study end-point (H&E-staining with original magnification 40X). (c) Total numbers of CD4+TCRβ+ cells isolated from the indicated organs at the indicated time points after T cell transfer. Data are mean ± SD of 5 mice per group per time point and representative of at least 3 independent experiments showing similar results with 20 mice per group in each experiment. (d) Real time RT-PCR analysis of IFNγ and IL-17 gene expression in the colonic tissue at the study end-point. Data are generated as mean fold increase ± SD, normalized to GAPDH (2ΔΔCt) of 38 and 33 animals per group in duplicate from 2 independent experiments. (e) Cytokine production by CD4+TCRβ+ lymphocytes isolated from the colonic lamina propria of B6 and B10-RAG2−/− mice at the study end-point and stimulated ex vivo for 6 hours in the presence of PMA/Ionomycin. Plots are representative of at least 3 independent experiments with at least 20 pooled colons per group in each experiment. Asterisks indicate statistically significant difference between the two strains (**P<0.01, ***P<0.001).
Analysis of cytokine expression in whole colonic tissue at the study end-point (7 weeks) showed that mRNA for both IFNγ and IL-17 was very highly expressed in the B10-RAG2−/− recipients compared to the B6-RAG2−/− recipients (Figure 1d). Similar proportions of infiltrating CD4+ T cells in the two strains expressed IFNγ (20−25%), IL-17 (3–5%) or both (1–2%) despite higher absolute CD4+ T cell numbers infiltrating the colon of the B10-RAG2−/− strain (Figure 1c and e). Therefore, the B10-RAG2−/− recipients accumulated more effector T cell populations that have been previously shown to be colitogenic in this model.8, 23, 24
Imbalance of pathogenic and regulatory T cells early after T cell transfer correlates with enhanced disease severity in B10-RAG2−/− recipients
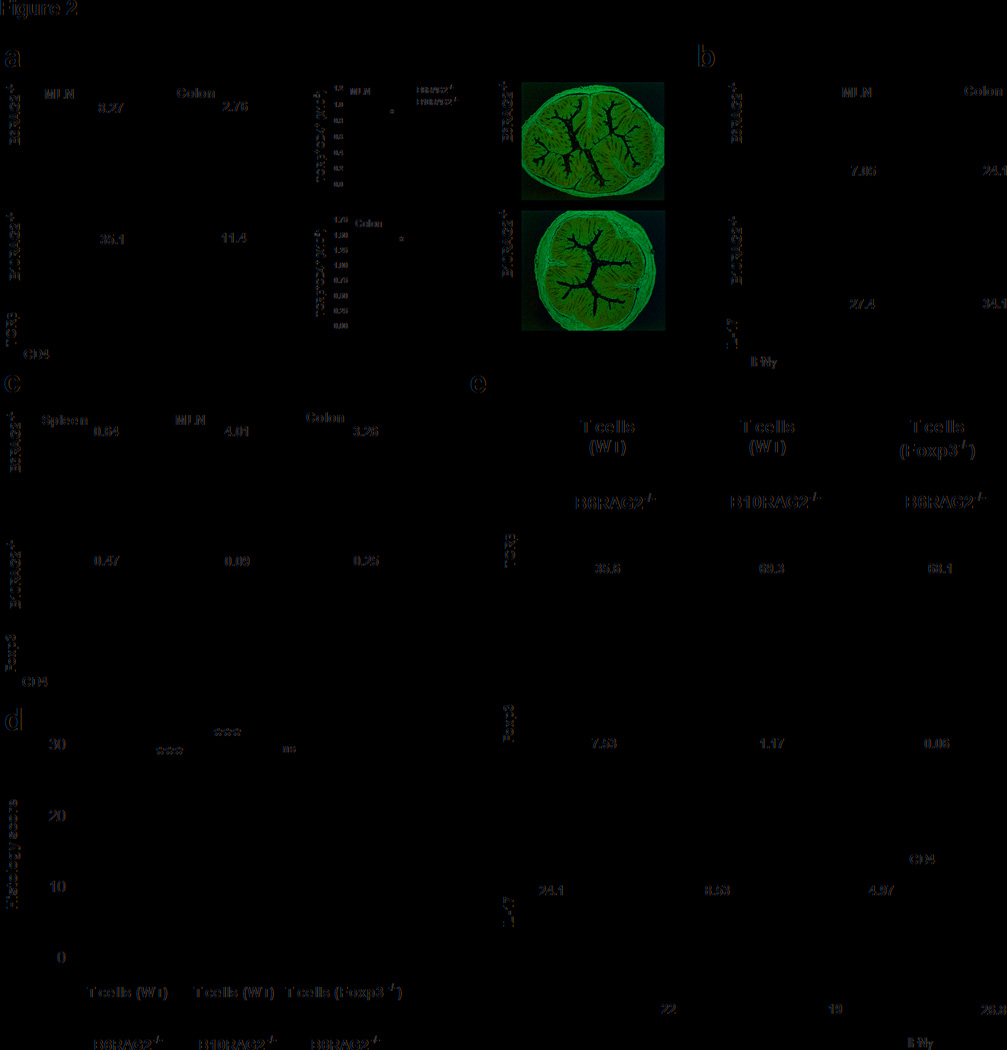
We next evaluated whether the genetic difference in disease susceptibility was determined at the level of T cell priming by studying T cell differentiation in mesenteric lymph nodes (MLNs) at early time points after T cell transfer. CD4+ T cell expansion in the MLNs was significantly greater in the B10-RAG2−/− recipients as early as 2 weeks following transfer indicated by the higher frequency and absolute numbers of CD4+TCRβ+ T cells. This occurred despite the absence of histological evidence of colonic inflammation at this time point in either strain (Figure 2a). In addition, accumulation of CD4+ T cells in the colon was already evident by week 2, and the differences in the frequency and absolute numbers of CD4+TCRβ+ T cells were similar to those seen in the MLNs (Figure 2a). Furthermore, we observed that larger proportions of CD4+TCRβ+ cells in both the MLNs and the colon were capable of producing IFNγ in the B10 strain at this early time point (Figure 2b).
Figure 2.
Disease severity associates with the early local balance of CD4+Foxp3+ T cells and Th1/17 cells, which depends on the ability to generate iTregs (a) Flow cytometry analysis (left panel) of lymphocytes, gated based on characteristic light-scatter properties, isolated from the indicated organs of B6 and B10-RAG2−/− recipients at week 2 after T cell transfer. Plots are representative of 5 independent experiments with 3–5 pooled mice in each. Middle panel: Total numbers of CD4+TCRβ+ T cells isolated from the indicated organs at 2 weeks after T cell transfer. Results are mean ± SEM of 3 independent experiments with 3 pooled mice in each. Right panel: Representative colon histology from at least 3 independent experiments with 3 mice in each of B6 and B10-RAG2−/− recipients at 2 weeks after T transfer (H&E-staining with original magnification 10X). (b) Cytokine production by CD4+TCRβ+ lymphocytes isolated from the indicated organs of B6 and B10-RAG2−/− mice at 2 weeks after T transfer and stimulated ex vivo for 6 hours in the presence of PMA/Ionomycin. (c) Percentage of Foxp3+ cells gated on CD4+TCRβ+ T cells isolated from the indicated organs at 2 weeks after T transfer. Plots (b and c) are representative of 5 independent experiments with 3–5 pooled mice per group in each experiment. (d) In situ expression of Foxp3 in MLNs and colonic isolated lymphoid follicles from B6 and B10-RAG2−/− at 2 weeks after T transfer (Hoechst counter-stain, original magnification 20X). Images are representative of 2 independent experiments with 3 mice in each. (e) Histology scores of Rag-deficient mice that were given Foxp3−/− or WT CD4+CD25CD45RBhigh T cells. Scores are mean ± SD of 9–15 mice in each group and representative of 2 independent experiments with similar number of mice in each. (f) Percentage of CD4+TCRβ+ (upper panel), and Foxp3+ (middle panel) lymphocytes, gated based on characteristic light-scatter properties, isolated from the MLNs of B6 and B10-RAG2−/− recipients given Foxp3−/− or WT CD4+CD25CD45RBhigh T cells at study end point (7 weeks). Lower panel: cytokine production by CD4+TCRβ+ lymphocytes isolated from the MLNs of B6 and B10-RAG2−/− mice at the study end-point and stimulated ex vivo for 6 hours in the presence of PMA/Ionomycin. Plots are representative of 2 independent experiments with 9–15 mice per group in each. Asterisks indicate statistically significant difference between the two strains (*P<0.05, ***P<0.001, ns: P>0.05).
In contrast to these findings, as shown in Figure 2c, we observed significantly higher frequencies of CD4+ Foxp3+ Tregs in the MLNs and colons of B6-RAG2−/− compared to the B10-RAG2−/− mice two weeks after adoptive transfer, both by flow-cytometry (Figure 2c) and by in situ staining of MLNs and isolated lymphoid follicles (Figure 2d). The generation of CD4+Foxp3+ cells in the spleen was inefficient in both mouse strains and no differences in CD4+Foxp3+ T cell numbers were found at this site (data not shown). At time points later than 2 weeks, the frequency of Foxp3+ cells observed early after transfer decreased in both strains most likely due to a differential accumulation of CD4+ effector cells during the course of disease development. Despite these changes over time, the frequencies of CD4+ Foxp3+ cells were consistently higher in the MLNs and colons of B6-RAG2−/− compared to the B10-RAG2−/− mice (Supplementary Figure 2a). Therefore, the differences in disease susceptibility between B10 and B6-RAGs−/− mice were manifest early after adoptive transfer as indicated by the higher accumulation of TE and lower accumulation of CD4+ Foxp3+ Tregs in the B10-RAG2−/− mice.
Importantly, for these studies, transferred naïve T cells contained negligible Treg contamination, since only CD4+CD25− cells with the brightest CD45RB expression were sorted and used for transfers (Supplementary Figure 1a). This suggested that the CD4+Foxp3+ population observed in the recipient mice represent peripherally generated iTregs rather than expansion of contaminating nTregs of the donor. To address this question more directly, we transferred to RAG2−/− mice small numbers of genetically marked Foxp3+ T cells (CD4+ GFP+ cells from Foxp3-eGFP mice), at equivalent or higher numbers to those that potentially contaminate our starting CD4+CD45RBhigh T cell populations (0.02–2%), together with CD4+CD45RBhigh T cells from WT mice. We then assessed the expansion of CD4+Foxp3-eGFP+ cells over time. Two weeks following the transfer, negligible frequencies of CD4+Foxp3-eGFP+ cells were found in the MLNs (Supplementary Figure 1b). We also examined the Foxp3 expression 2 weeks after transfer of CD4+CD45RBhighFoxp3− cells from Foxp3-eGFP mice and found an equivalent percentage of CD4+Foxp3-eGFP+ cells to those found following transfer of CD4+CD25−CD45RBhigh cells from WT mice (Supplementary Figure 1b). These data strongly argue that Foxp3+ T cells found after transfer of CD4+CD45RBhigh T cells represent Tregs induced de novo from transferred naïve T cells.
To address the possibility that the induced Foxp3+ Tregs are important for the control of proliferation and differentiation of effector T cells in this model, we transferred WT or Foxp3-deficient CD4+CD45RBhigh T cells (isolated from wild-type/scurfy-mixed bone marrow chimeric mice) into B6-RAG2−/− recipients. Mice that received Foxp3-deficient naïve T cells developed worse disease than those transferred with WT CD4+CD45RBhigh T cells, and equivalent to that seen in the B10-RAG2−/− mice transferred with WT cells (Figure 2e). Consistent with these findings, lack of de novo Treg differentiation following the transfer of Foxp3-deficient CD4+CD45BRhigh T cells into in B6-RAG2−/− mice resulted in enhanced Th1 effector cells within inflamed tissues (Figure 2f). These data indicate that defective peripheral generation of iTregs is able to alter disease severity.
Treg/TE differentiation and colitis severity is due to host and not T cell-dependent strain differences
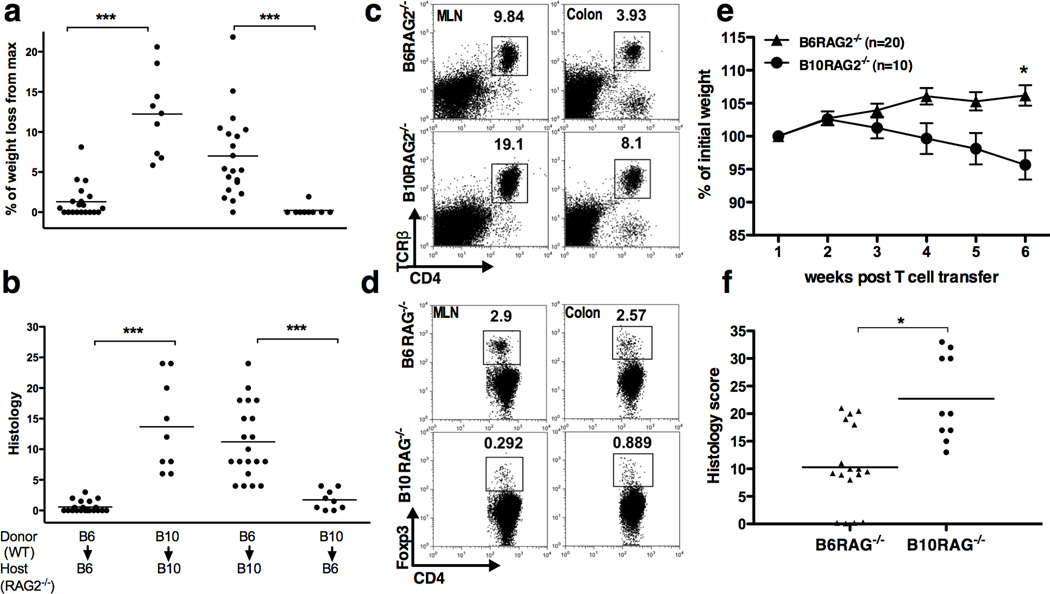
We next explored whether the differential induction of TE and Tregs and disease outcome in the two strains was due to T cell- or host-dependent factors. To address this issue, we transferred CD4+CD25−CD45RBhigh T cells from either B10 or B6 WT animals to either B6-RAG2−/− or B10-RAG2−/− hosts. As shown in Figure 3, B10-RAG2−/− recipients suffered maximum weight loss (Figure 3a) and developed severe colitis (Figure 3b) regardless the origin of the donor T cells. In contrast, B6-RAG2−/− recipients transferred with CD4+CD25−CD45RBhigh T cells from either B6 or B10 WT mice had significantly less weight loss and intestinal inflammation than B10-RAG2−/− hosts (Figure 3a and b). Of note, B6 and B10 mice share MHC haplotypes, and no mice in this study developed signs of graft versus host (GVH) disease, including skin lesions and hair loss. Furthermore, even if GVH disease did occur to some degree in these experimental settings, it is unlikely to have affected only the B10 but not the B6 strain. Therefore, these data indicate that the differential disease outcome in these mouse strains primarily depends on host and not T cell-intrinsic factors.
Figure 3.
Differential iTreg/pathogenic T cell balance and disease severity is host-dependent and T cell- and antigen-independent. (a) Weight change and (b) histology scores 7 weeks post-transfer of naïve T cells from B6 donors to B10-RAG−/− recipients and vise versa. One representative of two independent experiments with similar results is shown (9–20 mice per group) (c, d) Flow cytometry analysis of lymphocytes, gated based on characteristic light-scatter properties, isolated from the indicated organs of B6 and B10-RAG2−/− recipients of OT-II CD4+CD45RBhigh T cells followed by OVA feeding. One representative of two independent experiments is shown with 5 pooled mice per group per experiment. (e, f) Timed pregnant female mice from breeding pairs of B6 and B10 RAG−/− were co-housed together with their pups 3 days after giving the birth. Following weaning, the pups of each strain were separated, housed under the same conditions, and transferred CD4+CD45RBhigh T cells. Data are mean ± SD of 10–20 mice per group and representative of 2 independent experiments with similar number of mice in each. (e) Weight change after T cell transfer, (f) Colonic histology scores at study end-point (week 7). Asterisks indicate statistically significant difference between the two strains (*P<0.01, ***P<0.001).
To confirm these findings, we transferred CD4+ T cells from OT-II/RAG1−/− to B6 or B10-Rag-deficient hosts that were then fed ovalbumin. In this experimental setting, potential effects of T cell receptor specificity and other differences intrinsic to the B6 or B10 strains in the starting T cell population are eliminated.25 Furthermore, because CD4+ T cells from OT-II/RAG1−/− mice do not contain Foxp3+ Tregs,15, 16, 26 Foxp3+ Tregs developing after adoptive transfer can be more clearly attributed to differences in iTreg generation rather than differences in the expansion rates of contaminating Foxp3+ populations. As shown in Figure 3, fourteen days after T cell transfer, OT-II T cells expanded in both hosts but the frequencies of CD4+TCRβ+ T cells were two fold higher in the B10- than in the B6-RAG2−/− hosts (Figure 3c). This was accompanied by higher absolute numbers of CD4+TCRβ+ cells isolated from the MLN (10–20-fold) or colon (2–3 fold) of B10- compared to B6-RAG2−/− mice (Supplementary Figure 2b). In contrast, the frequencies of Foxp3+ Tregs were substantially lower in the B10- than in the B6-RAG2−/− hosts (Figure 3d).
Differences in IL-12/23 production by host CD103+ dendritic cells regulate the ratio of pathogenic and regulatory T cells
In addressing the host-specific factors that influence these differential effects on effector and regulatory T cell expansion and differentiation, we first began to address the possibility that the differences in intestinal commensal bacteria may influence disease development. Since the acquisition of intestinal commensal bacteria occurs at weaning, and is primarily affected by early environmental rather than genetic factors27, co-nursing, during which newborn mice suckle from both mothers, should minimize commensal microbial differences between the two strains. This is particularly true for segmented filamentous bacteria and Helicobacter sp. that are horizontally transferred and can affect colitis induction. As shown in Figure 3e and f, co-weaning (and co-housing) did not affect the severity of colonic inflammation in the two strains.
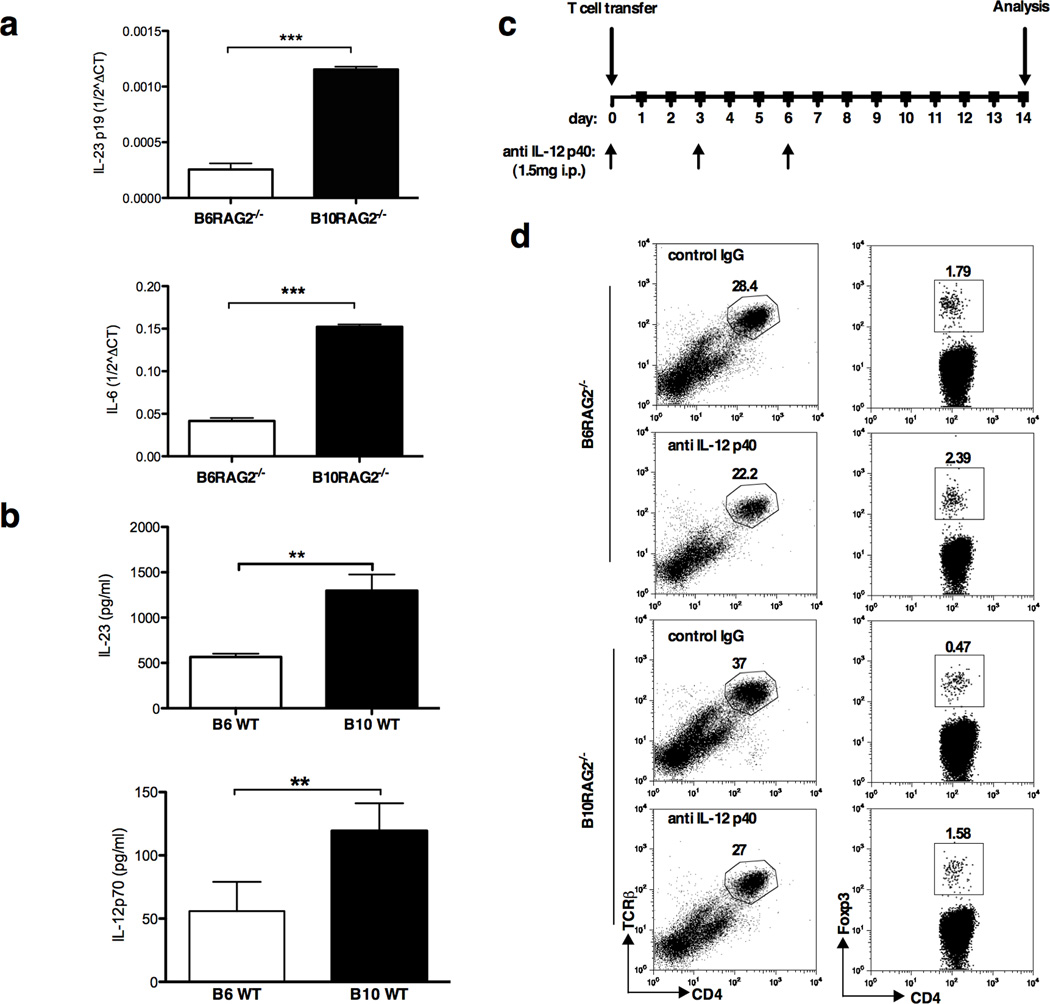
We next addressed the possibility that differences in CD103+ or CD103− DC number or function could account for the different disease patterns. Previous studies have shown that in steady-state conditions, CD4+ Foxp3+ Treg cells are preferentially induced in the GALT by CD103+ dendritic cells (DCs) via their production of TGF-β and retinoic acid.15–17 In contrast, CD103− DCs from GALT drive Th1 cell differentiation and have been associated with IL-23 production.15 However, during colitis CD103+ DCs become more inflammatory and can drive effector rather than Treg differentiation.28 Therefore, we examined the phenotype and function of DC populations in the MLNs of B6- and B10-RAG2−/− mice two weeks after T cell transfer. We found no difference in the percentage of total CD11c+MHCIIhigh cells or of CD11c+MHCIIhigh CD103− or CD103+ cells in the MLNs between the two strains (Supplementary Figure 3). In contrast, when we examined cytokine production by qRT-PCR, FACS-purified CD11c+CD103+ DCs from MLNs of B10-RAG2−/− recipients expressed higher levels of IL-6 and IL-23p19 compared to those from B6-RAG2−/− hosts (Figure 4a). Furthermore, in response to systemic LPS challenge, WT B10 mice produced substantially higher amount of serum level of IL-12p70 and IL-23 compared to WT B6 mice (Figure 4b), consistent with prior studies.29, 30
Figure 4.
Host CD103+ dendritic cell differences in IL-12/23 production can affect the proportions of pathogenic and regulatory T cells. (a) IL-23 and IL-6 mRNA expression by unstimulated (not sure we can say it is unstimulated because they are after T cell transfer, only if you want to emphasize it is not stimulated in vitro, maybe it is not necessary) FACS sorted MHCIIhighCD11c+CD103+ DCs freshly isolated from the MLNs of B6 and B10-RAG2−/− mice 2 weeks following adoptive transfer of CD4+CD25CD45RBhigh T cells. Data shown are mean ± SD of 2 independent experiments (IL-23p19) with pooled cells from 10 mice per group in each; and mean ± SD of Ct triplicates (IL-6) representative of 3 independent experiments with 10 mice per group in each. (b) Serum IL-12p70 and IL-23 following intraperitoneal injection of LPS. Results represent mean ± SD of 3 mice per group from 2 independent experimets (IL-23), and mean ± SD of five mice per group from at least 3 independent experiments (IL-12p70). (c) Study design of anti-IL-12/23p40 treatment. (d) Flow cytometry analysis of lymphocytes, gated based on characteristic light-scatter properties, isolated from the MLNs of B6 and B10-RAG2−/− recipients of CD4+CD25CD45RBhigh T cells treated with intraperitoneal injection of 1.5mg of neutralizing anti-IL-12/23p40 or control IgG. Data shown are representative of 2 independent experiments with similar results. Cells for analysis are pooled from 5 mice per group per experiment. Asterisks indicate statistically significant difference between the two strains (**P<0.01, ***P<0.001).
These data suggested that host DC-derived IL-12 and/or IL-23 production might be involved in controlling the balance of pathogenic to regulatory T cells observed in the two strains. To address this possibility, we treated B6 and B10-RAG2−/− mice with anti-IL-12/23p40 antibody on days 0, 3 and 6 after transfer of CD4+CD25−CD45RBhigh T cells (Figure 4c). Two weeks post T cell transfer, neutralization of IL-12/23 resulted in an increased percentage of iTregs in the B10-RAG2−/− MLN accompanied with an decreased percentage of CD4+TCRβ+ TE (Figure 4d), thus resulting in a phenotype similar to the B6-RAG2−/− strain which is less susceptible to colitis.
Taken together, the data presented in this manuscript indicate that disease outcome in the T cell transfer model of colitis depends on the balance of iTreg and TE cell differentiation from naïve T cells in the gut-associated lymphoid tissues (GALT) early following T cell transfer. The balance of iTregs and TE induction is affected by the genetic background of the mice, and is likely linked to the intrinsic ability of local DCs and especially the MLNs CD103+ DCs to guide T cell differentiation via the production of IL-12 and IL-23 axis (Figure 5).
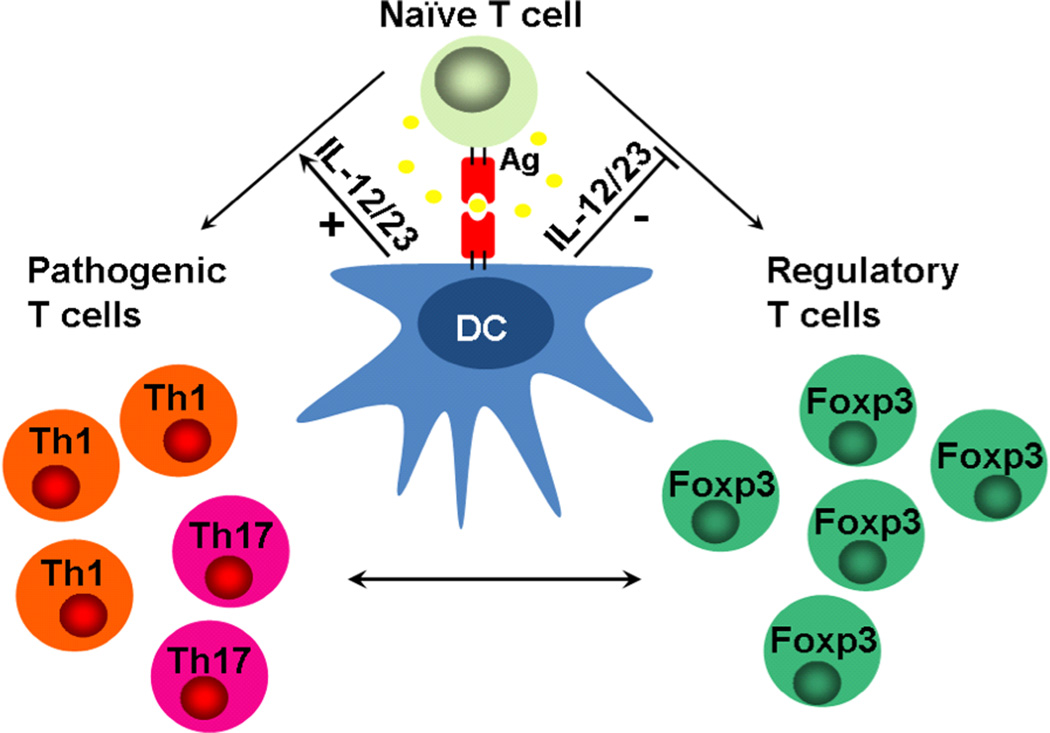
Figure 5.
Proposed hypothesis. In the setting of chronic lymphopenia, adoptively transferred naïve CD4+CD45RBhigh T cells differentiate into regulatory T cells (iTreg) or pathogenic/effector T cells (Th1 or Th17 ) . The process is controlled by genetic background-dependent host dendritic CD103+ cell production of IL-12/23 that restrains the differentiation of iTregs and enhances the generation of pathogenic T cells. iTreg/Teffector cell balance early after T cell transfer can significantly affect intestinal immune homeostasis and determine colitis severity.
DISCUSSION
In the present manuscript we confirmed the differential outcome of experimental colitis in two genetically similar RAG2-deficient mouse strains given CD4+CD45RBhigh T cells22 By closely monitoring the fate of the injected naïve T cells, we showed that severe disease observed in B10-RAG2−/− mice correlated with the enhanced accumulation of effector/pathogenic CD4+ T cells (TE) in the MLNs and colon lamina propria compared to less susceptible B6-RAG2−/− mice.
Inflammation in this T cell-mediated model of inflammatory bowel disease is dependent on host IL-23, and T cell expression of IFNγ and T-bet. However, the precise role of Th17 cells and IL-17A/F is more controversial. While the disease is also dependent on T cell expression of RORγt, the “master regulator” for Th17 cell differentiation, blocking IL-17 signaling pathways with antibodies or T cells from KO mice, can result in equivalent, less, or more severe disease.5–7 Furthermore, IL-17-producing RORγt positive cells may give rise to IL-17+/IFN-g+, or even IL-17−/IFNg+ cells in this model under the influence of IL-23, implicating Th17 cells as possible precursors for pathogenic IFNγ-producing T cells in vivo.24 Similarly, CD in humans has been associated with exacerbated Th1 and Th17 responses, with a clear role for Th1 cells and IFNγ and less clear role for Th17 cells.1, 31–33 Interestingly, in the current studies, enhanced disease in the B10-Rag2−/− mice was associated not with a skewed TE response, but with an equivalently enhanced accumulation of IFNγ+ and IL-17+, and IFNγ+IL-17+ TE cells.
To understand whether the enhanced TE cell accumulation in the B10-Rag2−/− mice was due to intrinsic effects on T cell priming, we measured T cell differentiation in the MLNs early after T cell transfer, and before the development of colitis in either strain. We found that TE cell differentiation was enhanced and iTreg differentiation was inhibited in the MLN of B10-Rag2−/− when compared to the B6-Rag2−/− mice. Furthermore, using Foxp3-deficient CD4+CD45RBhi T cells, we demonstrated that defects in peripheral iTreg induction were sufficient to modify disease outcome in the less susceptible B6-RAG2−/− mice, consistent with a prior report.21 These studies implicated strain-specific differences in T cell priming of TE and iTregs between B10 and B6 strains, and provided essential support for the ability of iTregs to directly control immunological homeostasis to commensal bacteria.
Naïve T cells can differentiate into Treg,, Th1, or Th17 cells depending on the cytokine milieu present during T cell priming. In this regard, TGF-β signals play a primary regulatory role. Specifically, in Th1 polarizing conditions, TGF-β can restrain Th1 differentiation and induce Foxp3 expression possibly via the S1P1-mTOR axis and the direct inhibition of T-bet expression.34, 35 In contrast, in the presence of IL-6, TGFβ drives Th17 rather than Foxp3+ Treg differentiation,36 which is enhanced by IL-23. IL-6 inhibits Foxp3 expression,37, 38 desensitizes T cells to Treg mediated suppression,39 and preconditions naïve T cells to respond to IL-23 through induction of the IL-23R during T cell activation.40 Furthermore, despite its direct inhibitory effect on Th1 differentiation,41 IL-6 may indirectly enhance Th1 responses through the restriction of iTreg induction, similar to IL-23.42 In our model, we found higher levels of IL-6 and IL-23p19 expression by CD103+ DCs isolated from the MLNs of the B10-RAG2−/− strain as compared to the less susceptible B6-RAG2−/− hosts, implicating genetic control of CD103+ DC function as an important determinant of disease outcome.
Since IL-23 has been implicated in promoting IL-17 production, Th17 cells have been considered to play an important role in driving IL-23-dependent diseases. However, , IL-23 has also been linked to enhanced pathogenic Th1 responses in studies of experimental autoimmune encephalomyelitis,43 and murine adoptive transfer colitis,11 and has been found to enhance Th1 anti-tumour responses.44 Our findings associate high levels of IL-23 with enhanced Th1, as well as Th17 and IFN-g+ IL17+ T cell responses in the more susceptible B10-strain. In this regard, Izcue et al, have shown that transfer of naive T cells to IL23A−/−RAG1−/− mice has failed to elicit colitis and has been associated with an increased frequency of CD4+Foxp3+ cells in the intestine.11 Consistent with these findings, inadequate generation of iTregs observed in our highly susceptible B10-RAG−/− mice was likely due to higher levels of IL-23 produced by this mouse strain, which contributed to the enhanced TE responses resulting in more severe colitis in B10-RAG−/− mice. However, since anti-IL-12/23p40 treatment was able to restore the iTreg population in the most susceptible B10-RAG−/− strain and to dampen the enhanced Th1 response, we cannot, at the present time, rule out an independent role for IL-12p70 in these studies, especially since IL-12 is also able to skew the TGF-β-dependent iTreg or Th17 developmental program into a Th1-like direction.45
Our findings are also consistent with studies showing that CD103+ DCs can lose their tolerogenic properties during colitis development. Thus, “inflammatory” CD103+ DCs generated in the setting of colitis are impaired in their ability to induce Foxp3+ Tregs and instead favour the emergence of IFNγ+ and IL-17+ CD4+ T cells.28 The findings presented here support this conclusion, and highlight a key role for CD103+ DCs in gut homeostasis by regulating the fate of naïve T cell differentiation towards either iTregs that promote tolerance or Th1/17 effector cells that drive inflammatory responses.
Finally, the current studies provide important information regarding the more generalized susceptibility of B10 (vs. B6) mouse strains to inflammatory disease. Thus, it has been shown that B10 mice are not only highly sensitive to colitis induction in the T cell-transfer model shown here, but also in the TNBS-colitis model in Rag2+/+ mice.29, 46 Furthermore, B10 mice are highly sensitive to models of experimental autoimmune uveitis and experimental skin inflammation, when compared to B6 mice.22, 47
Despite their common origin, the B6 and B10 mouse strains have substantial genetic differences most likely caused by residual allogenicity present in the parental C57 stock at the time of separation44. B6 and B10 strains differ by as much as 20cM in segments across the genome.48 With regard to susceptibility to autoimmune disease, one potentially important difference has been located on Chromosome 11, which contains the ortholog of human IBD5 locus that has been associated with an “extensive and complicated” phenotype of Crohn’s disease.49, 50 This region contains a susceptibility locus (Tnbs2) for colitis induced by intra-rectal administration of trinitrobenzene sulfonic acid (TNBS) in both the B6 and SJL/J mouse strains, and harbors the IL-12p40 gene.29, 30 Furthermore, increased susceptibility to TNBS-colitis of WT B10 (vs. B6) mice was associated with enhanced production of IL-12p70, and enhanced disease in SJL/J mice with enhanced production of both IL-12 and IL-23 in response to systemic LPS administration.29, 30 Recently, the genetic polymorhism in the IL-12p40 chain of SJL/J mice was found to directly affect the binding affinity of IL-12p40 to IL-12p35 and IL-23p19 most likely due to conformational changes following differential glycosylation as a consequence of the polymorphism. Zwiers et al, suggested that genetic regulation of IL-12 or IL-23 could be relevant to genetic susceptibility of B10 mice.29, 30 The current studies support this hypothesis by providing a plausible mechanism connecting the genetic regulation of IL-12 and IL-23 with the differential induction of TE and iTreg cells by CD103+ DCs.
METHODS
Animals
C57BL/6 (B6), C57BL/10SgSnAi (B10), C57BL/6-RAG2−/− (B6-RAG2−/−), C57BL/10-RAG2−/− (B10-RAG2−/−), TCR-OT-II.2a/RAG1−/− transgenic mice were purchased from Taconic Farms. Scurfy mice were kindly given by Dr. Ethan Shevach (NIH). For equalizing gut microflora in relevant experiments timed pregnant female mice from breeding pairs of B6 and B10-RAG2−/−, were co-housed together with their pups 3 days after giving the birth. Future B6-RAG2−/− and B10-RAG2−/− recipients freely suckled from mothers of both stains until weaning. All mice were housed under specific pathogen free conditions at an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at the NIAID. Experiments conducted were under animal study protocol LMI11E approved by the NIAID Animal Care and Use Committee.
Induction of colitis by adoptive T cell transfer
Total CD4+ T cells were isolated from spleens of 6- to 8-week-old female C57BL/6 or C57BL/10 mice via negative selection using the magnetic activation cell sorter (MACS®) CD4 T cell isolation kit (Miltenyi Biotec, Inc., Auburn, CA). Enriched cells were subsequently sorted for CD4+CD25−CD45RBhigh naïve T cells using fluorescence-activated cell sorter (FACSAriaTM, BD Biosciences, San Jose, CA). 2 x 105 CD4+CD25−CD45RBhigh cells were transferred intraperitoneally (i.p.) to recipient 6- to 8-week-old female RAG2-deficient mice. The development of intestinal inflammation was monitored by weight loss, which correlates with histological evidence of colitis. The study-end point was defined by a mean weight loss of 5–10% from initial weight of B10-RAG2−/− mice, which usually occurred 6–8 weeks after adoptive transfer. Colonic inflammation was assessed by previously published scoring systems modified as follows.23 In brief, hematoxylin and eosin (H&E) stained longitudinal and cross-sections of colons were graded semi-quantitatively for crypt length (0–2), lamina propria cellularity (0–3) and epithelial abnormalities including goblet cell loss, epithelial hyperplasia, erosions and ulcerations (0–3). Changes were also scored as to the percent area of involvement according to the scale 1=25%, 2=50%, 3=75%, and 4=100%. For each analysis, cells collected and pooled from 20–30 animals per group.
Generation of scurfy/WT chimeric mice
B6 SJL mice with congenic marker CD45.1 (Taconic Farms, Inc., Germantown, NY) were irradiated with 900 RAD and subsequently injected with combined bone marrow (BM) cells (5x106) from 3 weeks old scurfy mice and wild type (WT) B6 SJL mice at 1:1 ratio intravenously. Eight weeks after reconstitution, CD4+CD45RBhighCD45.1−CD45.2+ T cells (Foxp3-deficient) and CD4+CD45RBhighCD45.1+CD45.2− T cell (Foxp3-sufficient) were FACS sorted from spleen cells, and used for transfers.
Adoptive transfer of OT-II T cells and ovalbumin feeding
FACS sorted CD4+CD45RBhigh T cells from 6- to 8-week-old TCR-OT-II.2a/RAG1−/− transgenic mice were transferred i.p. to 6- to 8-week-old female RAG2-deficient recipient mice at 4 x 105 cells/mouse. 24 hours after adoptive transfer, mice were given drinking water containing 1.5% OVA for 5 consecutive days. On day 14, total lymphocytes from MLN and colon were analysed by flow cytometry. For each analysis, cells collected and pooled from 3 animals per group.
Cell preparation
Single cell suspensions from spleens and MLNs were made by homogenizing the tissue through 100- then 40µm strainers (BD Falcon) in HBSS (Invitrogen, Carlsbad, CA). Desired cell population, T cells or DCs, was enriched using CD4 T cell isolation kit for T cells or anti CD11c microbeads for DCs by MACS® (Miltenyi Biotec, Inc., Auburn, CA). After enrichment, CD11c+ cells were subsequently sorted for CD11c+MHCIIhiCD103+ subset using FACSAria™ (BD Biosciences, San Jose, CA). To obtain lamina propria mononuclear cells (LPMCs), colon tissue was washed in Ca++ and Mg++ free HBSS and cut into 0.5 cm pieces. The epithelium was stripped by incubation in HBSS supplemented with 15mM HEPES, 5mM EDTA (Invitrogen, Carlsbad, CA), 10% FCS (Gemini, West Sacramental, CA), 1mM DTT (Sigma-Aldrich, St. Louis, MO) for 15 min under vigorous shaking at 37°C. Remaining tissue was digested in complete Iscove’s DMEM containing 0.5mg/ml liberase CI and 30ug/ml Dnase I (Roche, Indianapolis, IN) in a shaking incubator at 37°C for 1 hour. Single-cell suspensions were made by passing digested tissue through 100- and 40µm strainers.
Flow cytometry
Cells were stained with the following directly conjugated monoclonal antibodies (mAb): CD4 (RM4-5), TCRβ (H57-597), CD25 (7D4), IFNγ, IL-17 and Foxp3 (FJK-16s) (eBioscience, San Diego, CA) and analysed on a FACSCaliber™ flow cytometer (BD Biosciences, San Jose, CA) using FlowJo software (FlowJo; Tree Star, Inc.). For intracellular cytokine analysis, cells were stimulated with 0.1 µM PMA and 1 µM Ionomycin (Sigma-Aldrich, St. Louis, MO), plus 10 µg/ml Brefeldin A (BD Bioscience, San Jose, CA), for 6 h at 37°C and stained for cytokine production using the BD Cytofix/Cytoperm™Plus fixation/permeabilization Kit (BD Biosciences, San Jose, CA). Foxp3 expression was performed using the mouse regulatory T cell staining kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
Real-time RT-PCR
Freshly harvested mouse MLN and colon tissues were stored in RNAlater (Ambion, Austin, TX). Total RNA extraction from individual tissue sample was performed using the RNeasy mini kitTM (QIAGEN, Valencia, CA). cDNA was reverse-transcribed using Superscript III first-strand synthesis kit (Invitrogen) and real time PCR analysis was performed on the 7900HT Fast Real-Time PCR instrument (Applied Biosystems, Carlsbad, CA) using FAM-labeled gene-specific probes and primers (Applied Biosystems, Carlsbad, CA). Relative target gene expression is expressed as 1/2ΔΔCt with GAPDH as endogenous control and non-transferred mice as reference, Ct is threshold cycle.
LPS challenge
B6 and B10 WT mice were injected (i.p.) with a sub-lethal (300 µg) dose of Salmonella enteritidis LPS (Sigma-Aldrich, St. Louis, MO) dissolved in phosphate-buffered saline. Serum level of IL-12p70 or IL-23 was measured 6 hours after injection by ELISA (R&D Systems, Minneapolis, MN) according to manufacturer instructions. Five mice per group were analysed.
Anti-IL12/23-p40 treatment
B6- and B10-RAG2−/− mice were injected with anti-IL-12/23 p40 or control IgG (1.5mg/mouse/dose) intraperitoneally (i.p.) on the day prior to CD4+CD25−CD45RBhigh naïve T cells transfer, then subsequently these mice were given the same dose i.p. on day 3 and day 6 post T cell transfer. Two weeks after T cell transfer, MLNs were taken out from these mice and Foxp3+ expressing T cells were assessed by FACS.
Statistical analysis
Comparisons between groups were performed using Student’s T test for independent samples or Mann-Whitney U test when appropriate. For the experiments with multiple time points the two-way ANOVA was used for the analysis of differences between the two strains followed by Bonferroni post-hoc tests. Statistical significance was established at p<0.05. GraphPad Prism 4.03 software was used for analysis.
Supplementary Material
ACKNOWLEDGEMENTS
Finding for these studies was provided by the Division of Intramural Research, NIAID, NIH, Bethesda, MD, USA
The authors would like to thank the Flow Cytometry Unit, Research Technilogies Branch, NIAID for their help with flow cytometric sorting.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Elson CO, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 5.Leppkes M, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Yen D, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor W, Jr, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullberg MC, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlig HH, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 13.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 15.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 18.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 19.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J. Exp. Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haribhai D, et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J. Immunol. 2009;182:3461–3468. doi: 10.4049/jimmunol.0802535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon F, et al. Antibodies to complement receptor 3 treat established inflammation in murine models of colitis and a novel model of psoriasiform dermatitis. J. Immunol. 2006;177:6974–6982. doi: 10.4049/jimmunol.177.10.6974. [DOI] [PubMed] [Google Scholar]
- 23.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahern PP, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohr J, Knoechel B, Abbas AK. Regulatory T cells in the periphery. Immunol. Rev. 2006;212:149–162. doi: 10.1111/j.0105-2896.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 26.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 27.Friswell MK, et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One. 2010;5:e8584. doi: 10.1371/journal.pone.0008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouma G, Kaushiva A, Strober W. Experimental murine colitis is regulated by two genetic loci, including one on chromosome 11 that regulates IL-12 responses. Gastroenterology. 2002;123:554–565. doi: 10.1053/gast.2002.34752. [DOI] [PubMed] [Google Scholar]
- 30.Zwiers A, et al. A polymorphism in the coding region of Il12b promotes IL-12p70 and IL-23 heterodimer formation. J. Immunol. 2011;186:3572–3580. doi: 10.4049/jimmunol.1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, Hibi T. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn’s disease. Gastroenterology. 2009;137:1736–1745. doi: 10.1053/j.gastro.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 33.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neurath MF, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 38.Doganci A, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 40.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 41.Diehl S, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 42.Serada S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U S A. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- 44.Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J. Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 45.Prochazkova J, Pokorna K, Holan V. IL-12 inhibits the TGF-beta-dependent T cell developmental programs and skews the TGF-beta-induced differentiation into a Th1-like direction. Immunobiology. 2012;217:74–82. doi: 10.1016/j.imbio.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 46.Meylan F, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal. Immunol. 2011;4:172–185. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun B, et al. Genetic susceptibility to experimental autoimmune uveitis involves more than a predisposition to generate a T helper-1-like or a T helper-2-like response. J. Immunol. 1997;159:1004–1011. [PubMed] [Google Scholar]
- 48.Slingsby JH, Hogarth MB, Simpson E, Walport MJ, Morley BJ. New microsatellite polymorphisms identified between C57BL/6, C57BL/10, and C57BL/KsJ inbred mouse strains. Immunogenetics. 1996;43:72–75. doi: 10.1007/BF00186607. [DOI] [PubMed] [Google Scholar]
- 49.Rioux JD, et al. Genetic variation in the 5q31 cytokine gene cluster confers susceptibility to Crohn disease. Nat. Genet. 2001;29:223–228. doi: 10.1038/ng1001-223. [DOI] [PubMed] [Google Scholar]
- 50.Brescianini S, Trinh T, Stoll M, Schreiber S, Rioux JD, Daly MJ. IBD5 is associated with an extensive complicated Crohn’s disease feature: implications from genotype-phenotype analysis. Gut. 2007;56:149–150. doi: 10.1136/gut.2006.102723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.