Abstract
This study explores if children with fragile X syndrome (FXS) show advances, declines, or plateaus in adaptive behavior over time and the relationship of nonverbal cognitive abilities and autistic behavior on these trajectories. Parents of 55 children with FXS completed the Vineland Adaptive Behavior Scales between 3 and 6 times from 2 to 10 years of age. Using raw scores, results indicate that about half of the sample showed advances in adaptive behavior, while the other half showed declines, indicating a regression in skills. Children who were more cognitively advanced and had less autistic behaviors had higher trajectories. Understanding the developmental course of adaptive behavior in FXS has implications for educational planning and intervention, especially for those children showing declines.
Keywords: fragile X syndrome, adaptive behavior, developmental trajectory
The purpose of the present study is to longitudinally examine the trajectory of adaptive behavior in children with FXS from 2 to 10 years. Fragile X syndrome (FXS) is the most common inherited cause of intellectual disability (Crawford, Acuna, & Sherman, 2001; Sherman, Morton, Jacobs, & Turner, 1984; Turner, Webb, Wake, & Robinson, 1996), ranging from learning disabilities to severe intellectual disabilities (Loesch, Huggins, & Hagerman, 2004). FXS is caused by a mutation on the X chromosome that disrupts the expression of the FMR1 gene (Verkerk et al., 1991), which in turn leads to atypical brain development (Devys, Lutz, Rouyer, Bellocq, & Mandel, 1993; Tamanini, 1997). Because FXS is an X-linked disorder there is a higher incidence rate for males (Hagerman, 2007), affecting approximately 1 in 4,000 males and approximately 1 in 6,000 females (CDC, 2011).
Despite the wide range of within-syndrome variability, research into the developmental profile, or behavioral phenotype, associated with FXS has indicated a distinct pattern of relative strengths and weakness in different domains of development (i.e., language, cognition, etc.) for males and females with FXS (for a reivew see Dykens, Hodapp, & Finucane, 2000; Hagerman & Hagerman, 2002; Hagerman, 1999; Kau, Meyer, & Kaufmann, 2002; Keysor & Mazzocco, 2002). Other key phenotypic features of males and females with FXS are psychopathologies (especially social anxiety; Baumgardner, Reiss, Freund, & Abrams, 1995; Einfeld, Tonge, & Florio, 1994; Feinstein & Reiss, 1998; Freund et al., 1993; Mazzocco, Pennington, & Hagerman, 1994; Turk, 1992) and problem behaviors (i.e., restricted and repetitive behaviors, aggression, self-injurious behavior, etc.; Bailey, Raspa, Olmsted, & Holiday 2008; Bailey et al., 2012; Einfeld et al., 1994; Gabis et al., 2011; Hatton et al., 2006; Mazzocco, Kates, Baumgardner, Freund, & Reiss, 1997; Smith et al., 2012). Approximately 30% of individuals with FXS also have co-occurring autism (Demark, Feldman, & Holden, 2003; Kaufmann et al., 2004; Philofsky, Hepburn, Hayes, Hagerman, & Rogers, 2004). The combination of mild to severe intellectual disability, co-occurrence of autism, and increased rates of psychopathology and problem behaviors may lead to difficulties in adaptive behavior.
Why is Adaptive Behavior Important?
Adaptive behavior refers to the skills needed to function in everyday life within the domains of social interaction, communication, daily living, and motor skills (Ditterline & Oakland, 2009; Sparrow, Balla, & Cicchetti, 1984; Sparrow, Cicchetti, & Balla, 2005). Adaptive behavior influences an individual’s ability to participate in everyday activities including self-care and domestic skills, interacting with others in the community, and mobility (Daunhauer, 2011; WHO; 2001). Adaptive behaviors are assessed in a developmental sequence, such that as a child grows older, these important skills change to meet the increasing environmental demands associated with independence. Identifying patterns and profiles of adaptive behavior has important implications for understanding the nature of a specific disorder and the development and application of effective treatments.
Adaptive Behavior in FXS
Research on adaptive behavior in FXS has focused on identifying patterns of relative strength or weakness within specific behavioral domains. Table 1 provides a summary of this research. These studies primarily report static comparisons of one or two data points for each participant, with the exception of four developmental trajectory studies (Fisch, Carpenter, Holden, Simensen, et al., 1999b; Fisch et al., 2007; Hatton et al., 2003; Klaiman et al., 2014). Relative strengths have been reported in the domains of daily living (Burack et al., 1999; Dykens et al., 1996; Dykens, Hodapp, & Leckman, 1989; Dykens, Hodapp, Ort, & Leckman, 1993; Fisch et al., 1999b; Fisch et al., 2007; Hatton et al., 2003; Rogers, Wehner, & Hagerman, 2001) and motor skills (Hatton et al., 2003), while deficits were reported in the domain of socialization (Dykens et al., 1993; Fisch et al., 2007; Freund et al., 1993; Hatton et al., 2003). However, one recent study reported relative strengths in the domain of socialization (Klaiman et al., 2014). Mixed results were reported for adaptive communication (Burack et al., 1999; Dykens et al., 1996; Fisch et al., 1999b; Fisch et al., 2007; Hatton et al., 2003).
Table 1.
Patterns of Strength and Weakness in Adaptive Behavior Domains for Males and Females with FXS Reported in Past Studies
| Domain of Adaptive Behavior | Pattern | Reference | Sample |
|---|---|---|---|
| Communication | Strength | Burack et al., 1999 | Cross-sectional study of 17 males between 5 and 17 years |
| Fisch et al., 2007 | Longitudinal study of 44 males between 4.9 and 10.9 years at Time 1 | ||
| Hatton et al., 2003 | Longitudinal study of 10 females between 12 and 110 months at Time 1 | ||
| Weakness | Dykens et al., 1996 | Cross-sectional study of 132 males between 1 and 20 years and 21 males between 21 and 40 years | |
| Fisch et al., 1999b | Longitudinal study of 28 males between 4 and 14 years | ||
| Socialization | Strength | Klaiman et al., 2014 | Study of 186 males between 2 and 18 who had been observed between 1 and 4 time points |
| Weakness | Dykens, Hodapp, Ort, & Leckman, 1993 | Two-time point study of 17 males between 1 and 17 **Note: weakness only observed in 10–17 year olds |
|
| Fisch et al., 2007 | Longitudinal study of 44 males between 4.9 and 10.9 years at Time 1 | ||
| Freund et al., 1993 | Cross-sectional study of 17 females between 4 and 27 years | ||
| Hatton et al., 2003 | Longitudinal study of 55 males between 12 and 143 months and 10 females between 12 and 110 months at Time 1 | ||
| Daily Living | Strength | Burack et al., 1999 | Cross-sectional study of 17 males between 5 and 17 years |
| Dykens, Hodapp, & Leckman, 1989 | Cross-sectional study of 27 males between 9 and 51 years | ||
| Dykens, Hodapp, Ort, & Leckman, 1993 | Two-time point study of 17 males between 1 to 17 years **Note: Strength only observed in 10–17 year olds |
||
| Dykens et al., 1996 | Cross-sectional study of 132 males between 1 and 20 years and 21 males between 21 and 40 years | ||
| Fisch et al., 1999b | Longitudinal study of 28 males between 4 and 14 years | ||
| Fisch et al., 2007 | Longitudinal study of 44 males between 4.9 and 10.9 years at Time 1 | ||
| Hatton et al., 2003 | Longitudinal study of 55 males between 12 and 143 months and 10 females between 12 and 110 months at Time 1 | ||
| Rogers, Wehner, & Hagerman, 2001 | Cross-sectional study of 23 males and 1 female between 28 and 41 months | ||
| Motor Skillsa | Strength | Hatton et al., 2003 | Longitudinal study of 10 females between 12 and 110 months at Time |
Note: This table only includes studies that stated a domain was an area of strength or weakness. Studies that just indicated a domain as higher or lower in comparison to one another were not included.
Most studies of adaptive behavior using the VABS have not examined the motor skills domain because the samples were older than 60 months.
Research on the developmental trajectories of adaptive behavior, overall and within the different domains of functioning (i.e., communication, socialization, daily living, and motor skills), has identified three possible trajectories in individuals with FXS: (1) declines over time (Fisch et al., 1999a; Fisch et al., 2012; Fisch, Simensen, & Schroer, 2002; Freund, Peebles, Aylward, & Reiss, 1995; Klaiman et al., 2014), (2) positive trajectories until the age of 10 in males with FXS and then decline or stabilization after age 10 ( Dykens et al., 1996, 1993; Fisch et al., 1999b), or (3) a steady increase from early childhood through age 12 (Hatton et al., 2003).
Differential findings for these three trajectories may be influenced by the ages of the individuals in the samples, their gender, types of scores used (i.e., standard scores or age-equivalent scores), and number of time-points included in the respective studies using the Vineland Adaptive Behavior Scales (Sparrow et al., 1984; 2005). For example, Fisch and colleagues (2002; 2012), reported declines in adaptive behavior based on a pair of two-time-point analyses of males with FXS between the ages of 2 and 24 years. The first was completed with 18 males with FXS (Fisch et al., 2002) and the second included 28 males with FXS (Fisch et al., 2012). On the other hand, Hatton and colleagues (2003) used a longitudinal analysis with an average of 4.4 assessment points of 60 males and 10 females with FXS from 12 months through age 12 years. They reported increases in adaptive behavior overall, and across domains for males and females, with females showing higher trajectories (Hatton et al., 2003). Klaiman and colleagues (2014) also observed higher adaptive behavior trajectories for females (n= 89) than for males (n = 186) between 2 and 18 years, but both groups showed declines over time.
Previous studies examining the trajectory of adaptive behavior in FXS have used either standard scores that represents how discrepant performance is from the average performance in the normative sample, or age-equivalent scores which represent the average chronological age of the given raw score. Both types of scores are problematic when modeling growth over time for individuals with intellectual and developmental disabilities (IDD; Maloney & Larrivee, 2007; Mervis & Klien-Tasman, 2004; Kover, McDuffie, Hagerman, & Abbeduto, 2013). It is better to use raw scores instead of standard scores because individuals with IDD are not increasing in skills at the same rate as typically developing peers (Mervis & Klien-Tasman, 2004; Kover et al., 2013). Raw scores will show changes in actual skills while standard scores will show changes relative to typically developing peers and are likely to decline despite an increase in skills in individuals with IDD (Mervis & Klien-Tasman, 2004; Kover et al., 2013). Age-equivalent scores also compare performance to typically developing peers and have additional methodological issues, including having an ordinal scale of measurement, which limits the types of appropriate analysis methods available for use (Maloney & Larrivee, 2007). Therefore, using raw scores allows for the examination of whether there is a true decline in adaptive behavior in FXS because these scores are not influenced by the child’s chronological age and are measured in an interval level of measurement necessary for most statistical tests. To our knowledge, none of published studies on adaptive behavior, or its trajectory, in individuals with FXS has reported the data in raw scores.
Contributors to adaptive behavior in FXS
Although they are generally viewed as independent constructs, adaptive behavior is positively related to cognitive status (e.g., IQ or DQ) in both typical and atypically developing children (Ditterline & Oakland, 2009, Dykens et al., 1996, 1993; Freund et al., 1993; Hatton et al., 2003; Rogers, Wehner, & Hagerman, 2001). It is likely that similar relationships will be observed in children with FXS, despite past research suggesting that adaptive behavior scores may surpass nonverbal cognitive abilities in individuals with FXS, later in development (Hatton et al., 2003). Several studies have found that individuals with FXS begin to show a decline in IQ during adolescence (Bailey, Hatton, & Skinner, 1998; Hagerman et al., 1989; Hodapp et al., 1990; Lachiewicz, Gullion, Spiridigliozzi, & Aylsworth, 1987). This trend may also be mirrored in adaptive behavior (Fisch et al., 2012, 2002; Fisch, Carpenter, Holden, Howard-Peebles, et al., 1999; Freund et al., 1995), such that children with FXS with lower levels of cognitive ability will have lower levels of adaptive behavior, and vice versa.
In addition to cognitive abilities, the presence of autistic behavior appears to influence adaptive behavior scores in FXS, such that individuals with lower levels of autistic behavior have higher adaptive behavior scores (Hatton et al., 2003). This pattern is not surprising considering that the presence of autistic behavior in individuals with FXS tends to be related to more problem behaviors (Hatton et al., 2002) and poorer developmental outcomes (Bailey et al., 1998; Bailey, Hatton, Mesibov, Ament, & Skinner, 2000; Bailey, Hatton, Skinner, Mesibov, 2001). It is possible that elevated levels of autistic behavior may be related to a plateau or decline of adaptive behavior in FXS.
Present study
The purpose of the present study is to investigate the trajectory of adaptive behavior in children with FXS between ages of 2 and 10 years using a longitudinal, growth curve analysis of adaptive behavior raw scores. This study is aimed at determining if the trajectory of adaptive behavior in children with FXS shows true advances or declines overall, or in the particular domains of functioning, from early to middle childhood. Additionally, the present study explores the influence of cognitive development and autistic behavior on these trajectories.
Research Questions
-
What are the trajectories of adaptive behavior raw scores over time, including trajectories of adaptive behavior composite scores and scores within each domain?
What is the influence of cognitive development and autistic behavior on the trajectories of adaptive behavior in children with FXS?
What are the characteristics of children with FXS who show raw score declines in adaptive behavior?
Method
Participants
Participants were 55 children with a confirmed genetic test of full mutation FXS (44 males, 11 females). The age of the children ranged between 24 and 55 months at the first observation and between 67 and 121 months at the last observation (see Table 2 for developmental and demographic information). Over the course of the longitudinal study, children participated in 3 to 5 observations (M = 4.87, SD = .47) depending on the age of first observation and continued availability throughout the study. Participants were predominantly Caucasian (87.3%). Three mothers had full mutation FXS and the rest were pre-mutation carriers.
Table 2.
Participant Characteristics (N = 55)
| Characteristic | M/% | SD | Range |
|---|---|---|---|
| Age at First Observation (in months) | 34.11 | 5.58 | 24–55 |
| Age at Last Observation (in months) | 112.58 | 7.44 | 67–121 |
| MSEL Early Learning Composite at First Observation | 55.89 | 11.50 | 49–101 |
| MSEL Nonverbal Raw Score at First Observation | 44.31 | 8.95 | 29–73 |
| CARS at First Observationa | 26.07 | 5.78 | 15.5–42 |
| Number of Observations | 4.87 | .47 | 3–6 |
| Autism Diagnosis (%)b | 31 | ||
| Child gender (% male) | 80 | ||
| Child ethnicity (%) | |||
| Caucasian | 87.3 | ||
| Hispanic | 3.6 | ||
| Biracial | 7.3 | ||
| Maternal Education (%) | |||
| Some High School | 4 | ||
| High School Graduate | 4 | ||
| Some College | 29 | ||
| College Graduate | 29 | ||
| Post Graduate Training | 34 | ||
| Family Income (%) | |||
| Less than $15,000 | 5.4 | ||
| $15,000–$30,000 | 12.7 | ||
| $30,000–$50,000 | 12.7 | ||
| $50,000–$80,000 | 20.0 | ||
| $80,000–$100,000 | 14.5 | ||
| $100,000 or greater | 34.5 | ||
Note.
14 of the participants had CARS scores that exceeded 30 at the first observation. All presented means are prior to grand mean centering.
Autism diagnosis based on all available data from the CARS and parent report of diagnosis over the course of the study, which identified 17 children (15 males, 2 females).
Measures
Vineland Adaptive Behavior Scales–Interview Edition, Survey Form (Sparrow, Balla, & Cicchetti, 1984; Sparrow, Cicchetti, & Balla, 2005)
The Vineland Adaptive Behavior Scales (VABS) is a standardized semi-structured parent interview that assesses personal and social functioning of individuals from birth through adulthood (Sparrow et al., 1984; 2005). The VABS examines four domains of adaptive behavior: communication, socialization, daily living skills, and motor skills. These four domains together create the Adaptive Behavior Composite, which is a summative score of overall adaptive behavior. The Motor Skills domain is usually only administered to children under the age of 6. However, in the present study this domain was administered at each time point. The survey form contains 297 items and takes between 20 and 60 minutes to complete. A 3-point Likert scale (never, sometimes/partially, or usually) is used to score the items. However, items may also be scored as “parent does not know if the child can perform the activity” or as “the child has not had the opportunity to perform the activity”. For the overall Adaptive Behavior Composite, inter-rater reliability coefficients are in the moderately strong range and the test-retest reliability coefficients are in the good to excellent range. For the domains of the VABS, inter-rater reliability coefficients and the test-rest reliability coefficients are in the good to excellent range. For both the Adaptive Behavior Composite and the domains, the split-half reliability coefficients range from .70s to .90s. The VABS has established construct, criterion, and content validity and has satisfactory levels of internal consistency (Sparrow et al., 1984).
The Mullen Scales of Early Learning (Mullen, 1995)
The Mullen Scales of Early Learning (MSEL) is a standardized observational assessment for children from ages 3 to 68 months. The MSEL examines five domains of development (i.e., gross motor, fine motor, visual reception, expressive language, and receptive language) that are combined to create an overall standard score representing an estimate of overall developmental functioning. The MSEL has established content, construct, and predictive validity and strong concurrent validity with other well-known developmental assessments for young children (e.g., Bayley Scales of Infant Development [Bayley, 1993], Peabody Developmental Motor Scales [Folio & Fewell, 1983], Birth to Three Developmental Scale [Dodson & Bangs, 1979]). In addition, strong test-retest reliability (.82–.85), inter-rater reliability (.91–.99), and internal consistency coefficients have been established for the MSEL (.83–.95; Mullen, 1995).
Nonverbal cognitive abilities, as opposed to overall cognitive abilities, were used in this study to help control for the influence of language abilities on the Mullen Early Learning Composite. Nonverbal scores consist of combined scores from the visual reception and fine motor skills domains. Raw scores were used for the covariate because there was little variability in standard scores and many of the children had the minimum standard score (i.e., floor effects). The MSEL nonverbal raw score, obtained at the first observation, was used as a covariate because it is early in the trajectory and a time point when all 55 participants were observed. At the first observation, children had a mean MSEL nonverbal raw score of 44.31 (SD = 8.95).
The Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988)
The Childhood Autism Rating Scale (CARS) is a 15-item general rating scale of observed autistic behavior. Each item is scored on a 4-point Likert scale, where 1 is within normal limits for age and 4 is severely abnormal for age or developmental level. An overall score of autistic behavior is calculated based on these ratings. Total CARS scores of 30 to 37 indicate the presence of mild to moderate autism behavior symptoms and scores above 37 indicate more severe symptoms (Schopler et al., 1988). We used CARS scores from the child’s first observation as a predictor variable to describe each child’s level of autistic behavior. The mean CARS score at the first observation was 26.07 (SD = 5.78). This score is elevated compared to typically developing children of a similar age (Mayes et al., 2009), but similar to other reports of children with FXS (Bailey, Hatton, et al., 2001; Bailey, Mesibov et al., 2001; Hatton et al., 2006).
Procedure
Participants were recruited as part of an ongoing longitudinal study on family adaptation in FXS (see Brady et al., 2014; Warren et al., 2010 for details). Participants were recruited from 24 states across the United States from a national FXS research registry, parent list serves, family support groups, and advertisements at a national convention. Over an 8-year period, trained graduate research assistants visited families at their homes for data collection. During these visits, the MSEL was administered to the child and the VABS was administered to the child’s mother. The CARS was scored following the visits based on the research assistants’ experiences interacting with the child during the home visit.
Measurement schedule
The first three home visits were approximately 16 to 18 months apart. The fourth home visits occurred between 30 and 31 months after the third home visit, due to a change in project funding. The time between the fourth and fifth home visit was approximately 16 to 18 months. In addition, one child missed a second home visit, two children missed a fourth home visit, and 3 children missed a fifth home visit. All of the available VABS data collected between the ages of 2 and 10 years were used in the growth curve analysis described below, which allows for the modeling of trajectories with different numbers of observations and different assessment intervals (Signer & Willet, 2003). Therefore, no form of data imputation was used to account for missing data in this study.
Results
Modeling Approach
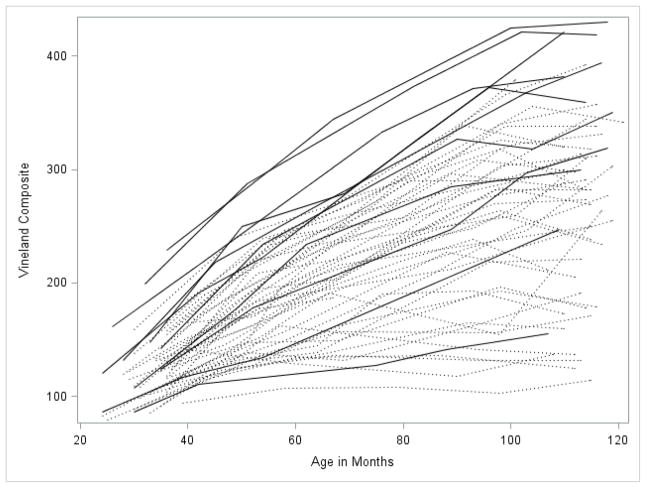
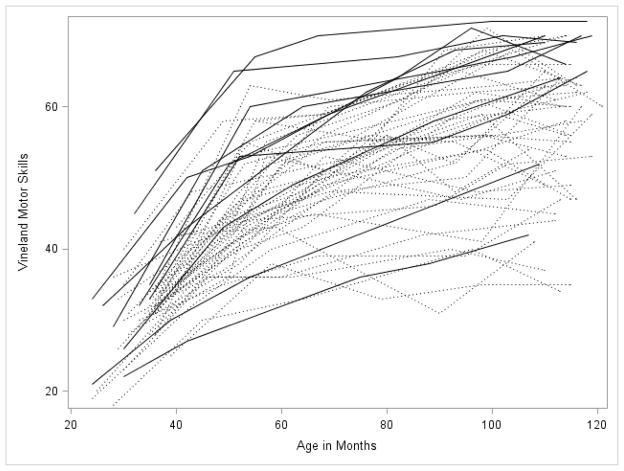
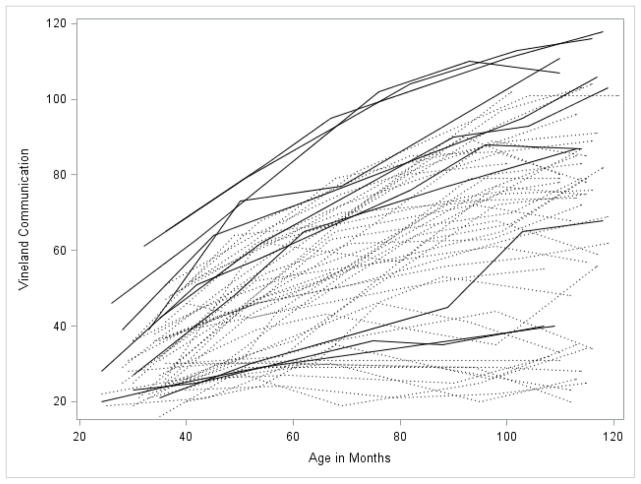
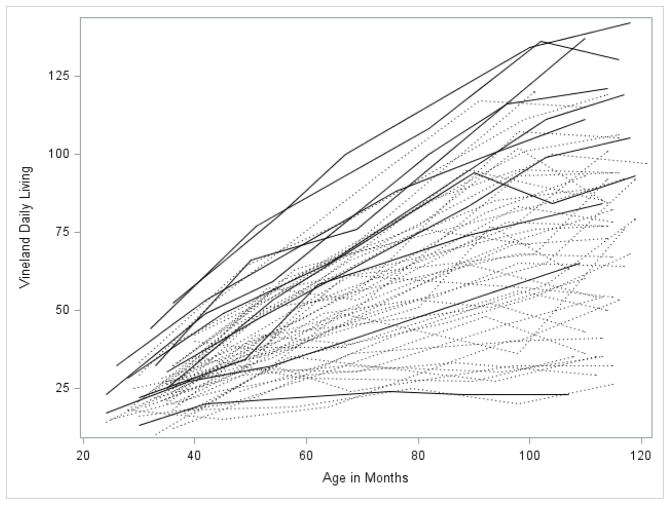
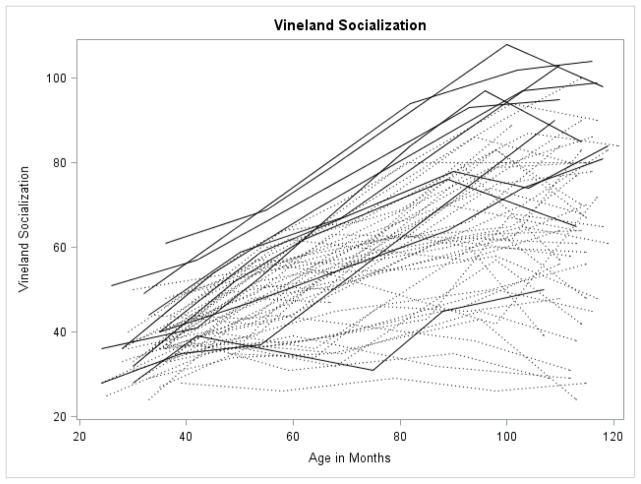
Figures 1 through 5 illustrate the trajectories in the observed data for each child over time on the VABS Adaptive Behavior Composite, communication, daily living, socialization, and motor skills domain scores. Solid lines denote females and dotted lines denote males. Low scores characterize most variables with limited variance at younger observation points followed by growth that is primarily linear with some decreasing of slope as the participant’s age. Variability in scores increased over time with some participants actually experiencing a decline in scores at the end of the observation period.
Figure 1.
Spaghetti plots for the Vineland Adaptive Behavior Composite over time. Dark lines are females with FXS; dotted lines are males with FXS.
Figure 5.
Spaghetti plots for the Motor Skills Domain over time. Dark lines are females with FXS; dotted lines are males with FXS.
Because of these observed trajectories, fixed effects for linear, quadratic, and cubic growth terms were evaluated initially in all growth models using SAS PROC MIXED. Age was centered at 30 months, a point early in the trajectory around which most participants were observed. Random intercepts, linear growth terms, and covariance between random intercepts and slopes were also included in the models. Model comparisons were made using two types of indices: the deviance statistic (change in the -2 log likelihood) and the Bayesian Information Criterion (BIC). Deviance statistics are distributed asymptotically as a Chi Square with degrees of freedom equal to the difference in the number of parameters estimated by the two models. Smaller BIC values indicate better fit. Once the best fitting growth models were established for each outcome, we added additional child predictors described below to the models.
Trajectory of adaptive behavior scores
The best fitting growth model for the VABS Adaptive Behavior Composite included significant fixed effects for linear and quadratic growth with an intercept at 30 months of 116, which was significantly different from zero. Scores increased at an average rate of 3.35 points per month of age and the rate of increase slowed over time as indicated by the significant negative quadratic estimate of -.02. There were significant random effects for intercepts, slopes, and the covariance between them indicating that individual trajectories were significantly different from the overall fixed trajectory for the group. A complete description of the parameter estimates can be found in Table 3.
Table 3.
Parameter Estimates for Growth (Top) and Predictor (Bottom) Models for Adaptive Behavior
| Variable | ABC | Communication | Daily Living | Socialization | Motor Skills | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||||
| Growth Variables | ||||||||||
| Intercept | 116.21*** | 4.38 | 29.49*** | 1.62 | 21.72*** | 1.33 | 36.01*** | 1.22 | 29.18*** | .94 |
| Linear | 3.35*** | .17 | .87*** | .06 | 1.05*** | .07 | .42*** | .11 | .98*** | .07 |
| Quadratic | −.017*** | .002 | −.005*** | .0005 | −.004*** | .0006 | .005 | .003 | −.01*** | .002 |
| Cubic | −.00006** | .00002 | .00006*** | .00002 | ||||||
| Random Effects | ||||||||||
| Intercept | 738.9*** | 174.3 | 111.50*** | 24.6 | 45.89*** | 14.65 | 27.16** | 9.97 | 22.63*** | 6.51 |
| Linear | .600*** | .13 | .06*** | .01 | .09*** | .02 | .04*** | .01 | .005** | .002 |
| Covariance | 13.07*** | 3.60 | 1.31*** | .43 | 1.58*** | .41 | .52* | .21 | .21** | .08 |
| Residual | 258.6*** | 29.29 | 26.27*** | 2.95 | 44.19*** | 5.06 | 36.71*** | 4.10 | 17.36*** | 2.01 |
| Model Fit | ||||||||||
| -2 log likelihood | 2523.1 | 1934.0 | 2005.08 | 1925.6 | 1689.4 | |||||
| BIC | 2551.2 | 1962.1 | 2033.8 | 1957.6 | 1721.5 | |||||
| Predictors Added
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | ABC | Communication | Daily Living | Socialization | Motor Skills | |||||
|
| ||||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Fixed Effects | ||||||||||
| Growth Variables | ||||||||||
| Intercept | 117.67*** | 3.23 | 29.83*** | 1.79 | 21.71*** | 1.14 | 36.65*** | 1.04 | 29.21*** | .80 |
| Linear | 3.27*** | .17 | .85*** | .05 | 1.04*** | .06 | .35** | .11 | .98*** | .07 |
| Quadratic | −.017*** | .002 | −.004*** | .0005 | −.004*** | .0006 | .006* | .003 | −.01*** | .002 |
| Cubic | −.00007** | .00002 | .00006*** | .00002 | ||||||
| Predictors | ||||||||||
| CARS intercept | −1.27*** | .58 | −.45 | .23 | −.46* | .19 | −.35 | .20 | −.16 | .12 |
| CARS linear | −.07*** | .02 | −.02** | .006 | −.02** | .007 | .02 | .02 | −.005* | .002 |
| CARS quadratic | −.001* | .0005 | ||||||||
| CARS cubic | .00001* | . 0000004 | ||||||||
| NV intercept | 1.91*** | .38 | .68*** | .15 | .24 | .15 | .48*** | .09 | .27** | .09 |
| NV linear | .02** | .007 | .01* | .004 | ||||||
| NV quadratic | −.0001* | .0001 | −.0001* | .00004 | ||||||
| Random Effects | ||||||||||
| Intercept | 253.61** | 84.5 | 50.44*** | 13.2 | 17.34* | 9.52 | 2.34 | 5.52 | 7.96* | 3.72 |
| Linear | .43*** | .10 | .05** | .01 | .05*** | .01 | .03*** | .01 | .004* | .002 |
| Covariance | 3.92*** | 2.03 | .40 | .27 | .53* | .24 | .16 | .14 | .09 | .06 |
| Residual | 258.9*** | 29.36 | 26.28*** | 2.96 | 43.28*** | 4.97 | 36.07*** | 4.05 | 16.48*** | 1.89 |
| Model Fit | ||||||||||
| -2 log likelihood | 2475.3 | 1895.1 | 1956.6 | 1874.6 | 1638.1 | |||||
| BIC | 2515.4 | 1935.1 | 2004.7 | 1957.6 | 1690.2 | |||||
Note. SE = Standard error. BIC = Bayesian information criterion
= < .001,
= < .01,
= < .05
For the communication and daily living domains, the best fitting model included significant fixed effects for both linear and quadratic growth. For the communication domain the intercept was 29 (which corresponds to standard score of 68 for a child at 30 months of age) with a positive slope of .87. For the daily living domain the intercept was 22 (which corresponds to a standard score of 64) with a slope of 1.05. The quadratic terms for both scales were small and negative (-.005 and -.004), indicating a slight slowing of the rate of growth over time. Once again all of the random effects were significant.
For the socialization and motor skills domains, the best fitting growth model included significant fixed effects for linear, quadratic, and cubic growth. For socialization, the intercept was 36 (which corresponds to a standard score of 74) with an average increase in scores of .42 per month. The positive quadratic term indicated that growth increased slightly as children aged. The significant negative cubic term indicated that, for some participants, there was a decline in socialization raw scores at the end of the observational period (see below for examination of participants that showed declines). The parameter estimate was significant, but very small. For motor skills, the intercept was 29 (which corresponds to a standard score of 64 for a child at 30 months) with a slope of .98 indicating that scores increased as children aged. The quadratic term was negative, indicating a general slowing of growth as the children aged, but the cubic term was positive indicating an uptick in scores for some children with FXS at the oldest observations.
Because the number of females in the sample was too small to statistically evaluate gender differences in growth, we performed the same models described above for males only, in order to determine if the results held after removing the female sample. The shapes of the trajectories were quite similar regardless of the sample used (i.e., total sample vs. males-only sample) with similar intercepts, slopes, and quadratic terms as can be observed in Figures 1 through 5. Generally, the intercept was slightly lower for each of the four subscales in the males-only model. Examination of the spaghetti plots (Figures 1–5) shows that the trajectories for females are similar to males with a few females’ scores consistently at the lower end of the distribution –showing limited growth – and other females’ scores at the upper ends of the distribution. There are a few females who consistently score higher than all of the males. This pattern is most clearly demonstrated in the socialization and communication domains.
Role of cognitive development and autistic behavior in adaptive behavior trajectories
The MSEL nonverbal scores significantly influenced intercepts in all models except for the daily living model, but only influenced slopes in daily living and motor skills, while the CARS significantly influenced intercepts for the Adaptive Behavior Composite and daily living models and slopes for all of the models except for socialization. MSEL nonverbal scores were positively related to outcomes with more cognitively advanced children scoring higher at 30 months and also increasing their rate of growth more quickly for daily living and motor skills. CARS scores were negatively related to outcomes with higher CARS scores indicating lower intercepts and smaller slopes (i.e., slower growth in skills) when significant.
The importance of a predictor in a multilevel model can be quantified by examining the variance explained, much like examining an R2 in a multiple regression model. Using this approach, the variance explained by the addition of MSEL nonverbal and CARS scores reduced intercepts by 66% in the Adaptive Behavior Composite, 55% in communication, 62% in daily living, 91% in socialization, and 64% in motor skills. The addition of these predictors to the Socialization model resulted in a variance estimate for the intercepts that was no longer significantly different from 0. In other words, the predictors in the model accounted for most of the variance in socialization scores at 30 months. Including nonverbal cognitive abilities and autistic behavior in the models further reduced the variance in slopes–variance in adaptive behavior composite slope was reduced 28%, communication slope was reduced 17%, daily living slope was reduced by 44%, socialization slope was reduced by 25%, and the variance in motor skills slope was reduced by 20%. A complete description of the parameter estimates can be found in Table 3.
Examination of children that showed declines in adaptive behavior
While the growth models characterized the overall trajectories of adaptive behavior for participants well, a subgroup of 30 participants (56% of the sample) showed declines in adaptive behavior raw scores starting around age 7 after initially demonstrating increasing skills. We defined a decline as a reduction of 5 points in the raw score or more on any of the four subscales (motor skills, communication, daily living skills, and socialization) over two or more time points. This represented a change, for example, from a score of a 2 “always does” to a score of 1 “sometimes/partially does” on a least 5 items on a domain of adaptive behavior; or a change from a 2 “always does” to a 0 “never does” on at least 3 items. Using this criterion, 24 participants (44%) did not decline by 5 points on any domain of adaptive behavior while 30 (56%) declined on at least one domain.
Further, we examined the slopes of these 30 individuals at the end of the study (centering age 110 months) to help us determine if their slopes were truly negative—as opposed to plateauing—when considered separately from the total sample. We found negative slopes for the socialization domain and for the Adaptive Behavior Composite raw score, indicating that these children were showing real declines.
A descriptive analysis was used to more fully characterize children who showed true declines in adaptive behavior in middle childhood. There were no significant differences between children who showed declines and children who never showed a decline on initial CARS scores and MSEL nonverbal cognitive scores (p values ranged from .72 to .96) collected in early childhood. Differential trends emerged when we examined gender for children who showed declines. Sixty-one percent of the males (26 of 43) showed declines, while only 36% of the females showed declines (4 of 11). Fisher’s exact test did not indicate that males were significantly more likely to show declines (p = .14), but the observed trends may be worth examining in a larger sample. Six male children (11% of the total sample) averaged a 5-point decline across all 4 domains. That is, they declined by 20 points or more on the Adaptive Behavior Composite. Three males declined on 3 domains, 8 children (7 males and 1 female) declined on 2 domains, and 19 children (16 male and 3 female) declined on only 1 domain.
Socialization was the domain where children most frequently showed a decline with 18 children (33% of the total sample, 15 males and 3 females) declining on this scale. Of these 18, 9 children (16%, 7 males and 2 females) showed declines only on the socialization domain and not on any other domain of the VABS. Eighteen percent of the total sample (9 males and 1 female) showed a decline in daily living, 15% (8 males) declined in communication, and 15% (7 males and 1 female) showed a decline in motor skills.
Summary of Results
Growth models indicated significant linear and quadratic growth from overall adaptive behavior, as measured by the Adaptive Behavior Composite, and within the different domains of adaptive behavior over time with a slowing in the rate of growth during the middle childhood years. Of particular note, our results indicate that 56% of our sample showed raw score declines in adaptive behavior raw scores by the end of the observation period. These declines were observed at a similar age period as previous studies reporting declines in adaptive behavior, but our approach of using raw scores allowed us to detect early advances in adaptive behavior prior to these declines. MSEL nonverbal cognitive abilities were positively related to outcomes with more cognitively advanced children scoring higher at 30 months on all subscales except for daily living and also increased their rate of growth more quickly for daily living and motor skills. CARS scores influenced 30-month intercepts for Adaptive Behavior Composite scores and daily living scores, and slopes for all outcomes of interest except socialization. CARS scores were negatively related to outcomes, such that higher CARS scores indicated lower intercepts and smaller slopes resulting in a slower growth in skills.
Discussion
The purpose of the present study was to examine the trajectory of adaptive behavior scores in children with FXS in order to determine if there were true raw score declines in skills, and to provide insight into variables associated with change in skills. Understanding whether or not individuals with FXS show declines in adaptive behavior during middle childhood is important for current, and future, education and treatment planning because these skills influence overall functioning and participation in everyday life.
Our findings suggest that middle childhood is a pivotal age period for adaptive behavior in FXS. While results for some children in our study (44%) were similar to past research findings that showed advances in adaptive behavior from toddlerhood through middle childhood (Dykens et al., 1993; 1996; Hatton et al., 2003), a substantial number of children with FXS (56% of our sample) showed declines within the different domains of adaptive behavior at or before the age of 10. Considering that we used raw scores, a decline means that these children, according to parent reports, had regressed, losing some of these important skills. Past studies have indicated declines or plateaus in overall adaptive behavior (as measured by the Adaptive Behavior Composite) and in individual domain scores either during childhood or after the age of 10 (Dykens et al., 1996, 1993; Fisch et al., 1999a; Fisch et al., 1999b; Fisch et al., 2012; Fisch et al. 2002; Freund, Peebles, Aylward, & Reiss, 1995; Klaiman et al., 2014). However, all of these studies used either standard scores or age-equivalent scores, which make it difficult to detect if true declines are being observed or if children with FXS are simply acquiring adaptive behavior skills at a slower rate than the normative sample. For example, the use of standard scores by Klaiman and colleagues (2014) allowed for comparison between individuals with FXS and individuals with typical development, which highlighted the fact that adaptive behavior skills are acquired more slowly by individuals with FXS. Our approach—longitudinal examination of raw scores–allowedus to identify more nuanced changes, such as early advances in adaptive behavior prior to a loss of skills from early to middle childhood. Together, these studies indicate that a substantial proportion of children with FXS lose adaptive behavior skills both in relation to their peers and in absolute terms.
Observing declines, or a regression, in skills tends to lead to the assumption that these children are lower functioning than children that did not show declines. Interestingly, no statistically significant differences were observed between children who showed advances in adaptive behavior and those that showed declines on initial nonverbal cognitive abilities or autistic behavior. However, the inclusion of both nonverbal cognitive abilities and autistic behavior, in the models of adaptive behavior significantly reduced the variance in the amount and rate of growth overtime. It seems that, similar to past studies of adaptive behavior, children with FXS who were more cognitively advanced, and had less autistic behaviors, had higher adaptive behavior trajectories (Dykens et al., 1996, 1993; Freund et al., 1993; Hatton et al., 2003; Rogers et al., 2001). Future studies should continue to examine the influence of cognitive abilities and autistic behavior on the development of adaptive behavior in FXS, especially for those who are showing declines.
Although past studies of adaptive behavior in FXS have reported declines in the Adaptive Behavior Composite and the different domains of functioning, no study to date has explored if these declines observed in overall adaptive behavior were due to declines in all domains or a drastic decline in only one or two domains. In the current study, 11% of the sample (six males) showed declines across all domains of the VABS, indicating a general decline in adaptive behavior. The remaining children showed declines only in some, or one, of the domains of the VABS. It is possible that the declines in adaptive behavior observed in other studies are not indicative of overall impairment in this cluster of skills, but instead are driven by impairments in just one or two domains of adaptive functioning. Nonetheless, the fact that a true decline in adaptive behavior skills—overall and within different domains—was observed in the present study is an undeniable source of concern. For the present sample, an obvious question is whether these declines plateau or increase during adolescence.
The declines we observed appear commensurate with the behavioral phenotype associated with FXS. Many individuals with FXS show deficits in social interactions including social anxiety, social avoidance, shyness, and poor eye contact (Hagerman, 2002; Kau et al., 2004; Roberts et al., 2007). In line with these phenotypic characteristics, we found that of children who showed any declines in adaptive behavior most often showed a decline in the socialization domain (i.e., 18 of the 30 children). These declines reflect potential impairments in important aspects of social interactions. For example, items on the socialization domain include has a group of friends, has a best friend, and ends conversations appropriately. Declines in skills such as these are likely to limit development of independence and influence interactions in the community and in future employment situations (Greenspan & Schoultz, 1981; Mueller, 1988). We also observed declines in adaptive communication for 15% of our sample, which is also in line with the phenotypic weakness in expressive and receptive language that has been noted in FXS (Abbeduto & Hagerman, 1997; Roberts, Mirrett, Anderson, Burchinal, & Neebe, 2002; Roberts, Mirrett, & Burchinal, 2001). Therefore, the declines we observed in both socialization and communication may have been influenced by the cumulative effect of limited early social interactions and/or poor receptive and expressive language skills. In other words, early phenotypic behaviors may cumulatively influence the development of adaptive social development in children with FXS, necessitating the identification and implementation of specific early interventions to promote social interactions and communication.
The domain of adaptive motor skills has received little research attention in FXS possibly because after the age of 6 this domain is not typically administered. Young children with FXS often experience delays in both gross and fine motor skills, although these delays are congruent with their overall developmental level (Bailey et al., 1998). However, motor skills influence other aspects of adaptive behavior (e.g., getting dressed, play behaviors, etc.). Our data indicate that continued monitoring of the motor skills domain is warranted because 15% of our sample showed declines in this domain during middle childhood. It is possible that some children with FXS who show declines in adaptive motor skills may, in turn, have lower levels of functioning in overall adaptive behavior, and in individual domains, because they are not able to readily perform the complex motor movements needed to successfully perform them (i.e., do not have the fine motor skills to button a shirt or gross motor skills to play on the playground with a friend). Our data shows that 4 males showed declines only in adaptive motor skills, while the other 4 children (3 males, 1 female) showed declines in adaptive motor skills and at least one other domain. Thus, declines in motor skills may have been associated with declines in other domains.
As its name implies, the domain of daily living reflects the practical skills individuals need to take care of one’s self and to live and interact independently in the community (Sparrow et al., 1984; 2005). The previously described social, language, and motor characteristics associated with FXS, in combination with problem behaviors and the level of autistic behavior, may influence the development of daily living skills. Eighteen percent (9 males and 1 female) of children in our sample showed declines in this domain during middle childhood. This finding stands in contrast to previous cross-sectional and longitudinal studies of children, adolescents, and adults indicating that daily living is an area of relative strength for individuals with FXS (Burack et al., 1999; Dykens et al., 1996; 1993; 1989; Fisch et al., 1999b; Fisch et al., 2007; Hatton et al., 2003; Rogers et al., 2001). Therefore, the daily living domain may not be spared from declines for all individuals with FXS.
Limitations and Strengths of the Current Study
The VABS is a standardized and well-accepted measure of adaptive behavior, but it is a parent report measure based on the parents’ interpretation of child behavior. Obtaining information from multiple informants—who have comprehensive information about the child’s adaptive functioning, such as teachers and clinicians—could contribute additional important information about adaptive behavior, especially declines, in other settings (e.g., school). The present study used a relatively small sample of convenience, with an even smaller sample of girls. Gender may also play a key role in understanding adaptive behavior in FXS. Because there were only 11 females in our sample, it was not possible to examine the trajectory of adaptive behavior in females with FXS. However, this limitation is offset to a degree by the longitudinal nature of the analysis.
One of the main strengths of this study is that we were able to obtain a sample of 55 families with FXS and maintain 53 of those families for an 8-year period, making the present study just one of two studies (i.e., Hatton et al., 2003) that have examined adaptive behavior at more than two-time points in children with FXS. Including more than two data points allows for more complex longitudinal analyses, such as those completed in our study. Also, our approach of using raw scores allowed us to examine the issue of whether or not children with FXS show true declines in adaptive behavior because these scores are not influenced by the child’s chronological age, which is present when using standard scores. Finally, our descriptive examination of children that showed declines in adaptive behavior is unique and provides insight into the characteristics associated with declines in adaptive behavior in FXS.
Conclusion and Future Directions
About half of the children in our study showed steady gains in adaptive behavior from toddlerhood through middle childhood, while the other half showed true declines—a regression in skills—in one or more domains of adaptive behavior in middle childhood (up to age 10). This is too our knowledge the earliest these declines have been documented. Middle childhood is a time when children start to seek more independence from their parents. A regression in adaptive behaviors skills at this time will negatively influence these children’s ability to function in everyday situations, and gain independence. These findings point to an important question for researchers and clinicians to investigate: can these declines in adaptive behavior be reversed or prevented?
In light of these findings, specific intervention strategies are needed to promote growth in adaptive behavior and, thereby, prevent declines in these areas prior to middle childhood. Our results point to areas in adaptive behavior that are at risk for a regression in skills, and that could be targeted for intervention. Using an additive risk model (Luthar, 1991; Sameroff, 1985), may also help to clarify how gender, presence of autism, nonverbal cognitive abilities, and environmental factors shape adaptive functioning in FXS, and should be considered for developing targeted interventions. In addition, our findings suggest that progress resulting from both behavioral and pharmaceutical interventions should be interpreted relative to the possibility of declines. That is, relatively small increases in adaptive behavior may be more clinically significant, given our findings.
In conclusion, our research suggests the importance of assessing the adaptive functioning of children with FXS annually as early as the beginning of middle childhood and targeting areas of real decline of raw scores for remedial intervention as soon as declines are discovered. Otherwise, those children experiencing declines may well become more dependent, not less, as they move into the challenging period adolescence.
Figure 2.
Spaghetti plots for the Communication Domain over time. Dark lines are females with FXS; dotted lines are males with FXS.
Figure 3.
Spaghetti plots for the Daily Living Domain over time. Dark lines are females with FXS; dotted lines are males with FXS.
Figure 4.
Spaghetti plots for the Socialization Domain over time. Dark lines are females with FXS; dotted lines are males with FXS.
Acknowledgments
This research was supported by NICHD grants T32 HD057844, P30 HD02528, and P30 HD003110.
This manuscript was presented as a poster at the proceedings of the 46th Annual Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disabilities in San Antonio, Texas
Laura Hahn is now at the University of South Carolina, Department of Psychology
Contributor Information
Laura J. Hahn, University of Kansas Life Span Institute 1000 Sunnyside Ave., Rm. 1052 Lawrence, KS, 66045, United States
Nancy C. Brady, University of Kansas, Speech-Language-Hearing: Sciences & Disorders, Lawrence, KS, 66045, United States
Steven F. Warren, University of Kansas, Life Span Institute, Lawrence, KS, 66045, United States
Kandace K. Fleming, University of Kansas, Life Span Institute, Lawrence, KS, 66045, United States
References
- Abbeduto L, Hagerman RJ. Language and communication in fragile X syndrome. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3(4):313–322. doi: 10.1002/(SICI)1098-2779(1997)3:4<313::AID-MRDD6>3.0.CO;2-O. [DOI] [Google Scholar]
- Bailey DB, Jr, Hatton DD, Mesibov G, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. Journal of Autism and Developmental Disorders. 2000;30(1):49–59. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton D, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103(1):29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Hatton D, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism & Developmental Disorders. 2001;31(2):165–174. doi: 10.1023/A:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark R, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism & Developmental Disorders. 1998;28(6):499. doi: 10.1023/A:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Bishop E, Mitra D, Martin S, Wheeler A, Sacco P. Health and economic consequences of fragile X syndrome for caregivers. Journal of Developmental & Behavioral Pediatrics. 2012;33:705–712. doi: 10.1097/DBP.0b013e318272dcbc. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant Development. 2. San Antonio, TX: The Psychological Corp; 1993. [Google Scholar]
- Baumgardner TL, Reiss AL, Freund LS, Abrams MT. Specification of the neurobehavioral phenotype in males with fragile X syndrome. Pediatrics. 1995;95(5):744–752. Retrieved from http://pediatrics.aappublications.org/content/95/5/744.short. [PubMed] [Google Scholar]
- Brady NC, Warren SF, Fleming K, Keller J, Sterling A. Effect of Sustained Maternal Responsivity on Later Vocabulary Development in Children With Fragile X Syndrome. American Journal of Speech, Language, and Hearing Research. 2014;57:212–226. doi: 10.1044/1092-4388(2013/12-0341)212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack A, Shulman C, Katzir E, Schaap T, Brennan J, Iarocci G, Amir N. Cognitive and behavioural development of israeli males with fragile X and Down syndrome. International Journal of Behavioral Development. 1999;23(2):519–531. doi: 10.1080/016502599383937. [DOI] [Google Scholar]
- Center for Disease Control (CDC) FMR1 and the fragile X syndrome. 2011 Retrieved January 31, 2013 from http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/fragile_x.pdf.
- Crawford DC, Acuna JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3(5):359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunhauer LA. The early development of adaptive behavior and functional performance in you childrne with Down syndrome: Current knowledge and future directions. International Review of Research in Intellectual Disabilities. 2011;40:109–137. [Google Scholar]
- Demark JL, Feldman MA, Holden JJA. Behavioral relationship between autism and fragile X syndrome. American Journal on Mental Retardation. 2003;108(5):314–326. doi: 10.1352/0895-8017(2003)108<314:BRBAAF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nature genetics. 1993;4(4):335–40. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- Ditterline J, Oakland T. Relationships between adaptive behavior and impairment. In: Goldstein S, Jack A, editors. Assessing Impairment: From Theory to Practice. New York, NY: Springer Science + Business Media, LLC; 2009. pp. 31–48. [Google Scholar]
- Dodson S, Bangs TE. Birth to Three Developmental Scale. Allen, TX: DLM Teaching Resources; 1979. [Google Scholar]
- Dykens EM, Hodapp R, Finucane B. Genetics and mental retardation syndromes: A new look at behavior and interventions. Baltimore, MD: Paul H Brookes; 2000. [Google Scholar]
- Dykens EM, Hodapp R, Leckman J. Adaptive and maladaptive functioning of institutionalized and noninstitutionlized fragile X males. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(3):427–430. doi: 10.1097/00004583-198905000-00021. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Hodapp RM, Ort S, Leckman J. Trajectories and profiles of adaptive behavior in males with fragile X syndrome. Jounral of Autism and Developmental Disorders. 1993;23(1):135–145. doi: 10.1007/BF01066423. [DOI] [PubMed] [Google Scholar]
- Dykens EM, Ort S, Cohen I, Finucane B, Spiridigliozzi G, Lachiewicz A, O’Conner R. Trajectories and profiles of adaptive behavior in males with fragile X syndrome. Journal of Autism & Developmental Disorders. 1996;(3):287–301. doi: 10.1007/BF02172475. [DOI] [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ, Florio T. Behavioural and emotional disturbance in fragile X syndrome. American journal of medical genetics. 1994;51(4):386–91. doi: 10.1002/ajmg.1320510417. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Reiss AL. Autism: The Point of View from Fragile X Studies. Journal of Autism and Developmental Disorders. 1998;28(5):393–405. doi: 10.1023/A:1026000404855. [DOI] [PubMed] [Google Scholar]
- Fisch G, Carpenter N, Holden JJ, Howard-Peebles PN, Maddalena A, Borghgraef M, Fryns JP. Longitudinal changes in cognitive and adaptive behavior in fragile X females: a prospective multicenter analysis. American journal of medical genetics. 1999a;83(4):308–12. [PubMed] [Google Scholar]
- Fisch G, Carpenter N, Howard-Peebles PN, Holden JJA, Tarleton J, Simensen R, Battaglia A. Developmental trajectories in syndromes with intellectual disability, with a focus on Wolf-Hirschhorn and its cognitive-behavioral profile. American journal on intellectual and developmental disabilities. 2012;117(2):167–79. doi: 10.1352/1944-7558-117.2.167. [DOI] [PubMed] [Google Scholar]
- Fisch G, Carpenter N, Howard-Peebles PN, Holden JJA, Tarleton J, Simensen R, Nance W. Studies of age-correlated features of cognitive-behavioral development in children and adolescents with genetic disorders. American Journal of Medical Genetics. Part A. 2007;143A(20):2478–89. doi: 10.1002/ajmg.a.31915. [DOI] [PubMed] [Google Scholar]
- Fisch G, Carpenter NJ, Holden JJ, Simensen R, Howard-Peebles PN, Maddalena A, Nance W. Longitudinal assessment of adaptive and maladaptive behaviors in fragile X males: growth, development, and profiles. American journal of medical genetics. 1999b;83(4):257–63. doi: 10.1002/(sici)1096-8628(19990402)83:4<257::aid-ajmg5>3.0.co;2-u. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10208158. [DOI] [PubMed] [Google Scholar]
- Fisch G, Simensen R, Schroer R. Longitudinal changes in cognitive and adaptive behavior scores in children and adolescents with the Fragile X mutation or autism. Journal of Autism and Developmental Disorders. 2002;32(2):107–114. doi: 10.1023/A:1014888505185. [DOI] [PubMed] [Google Scholar]
- Folio MR, Fewell RR. Peabody Developmental Motor Scales. Allen, TX: DLM Teaching Resources; 1983. [Google Scholar]
- Freund LS, Peebles C, Aylward E, Reiss AL. Preliminary report on cognitive and adaptive behaviors in preschool-ages males with fragile X. Developmental Brain Dysfunction. 1995;8:242–251. [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. Psychiatric Disorders Associated With Fragile X in the Young Female. Pediatrics. 1993;91(2):321–329. [PubMed] [Google Scholar]
- Gabis L, Baruch Y, Jokel A, Raz R. Psychiatric and autistic comorbidity in fragile X syndrome across ages. Journal of Child Neurology. 2011;26(8):940–948. doi: 10.1177/0883073810395937. [DOI] [PubMed] [Google Scholar]
- Greenspan S, Shoultz B. Why mentally retarded adults lose their jobs: Social competence as a factor in work adjustment. Applied Research in Mental Retardation. 1981;2(1):23–38. doi: 10.1016/0270-3092(81)90004-7. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Neurodevelopmental disorders: Diagnosis and treatment. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Hagerman RJ. Etiology, diagnosis, and development in fragile X syndrome. In: Roberts JE, Chapman RS, Warren SF, editors. Speech and language development and intervention in Down syndrome and fragile X syndrome. Baltimore, MD: Brookes; 2007. pp. 27–42. [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. Baltimore, MD: Johns Hopkins University Press; 2002. [Google Scholar]
- Hagerman RJ, Schreiner RA, Kemper MB, Wittenberger MD, Zahn B, Habicht K. Longitudinal IQ changes in fragile X males. American Journal of Medical Genetics. 1989;33(4):513–8. doi: 10.1002/ajmg.1320330422. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics Part A. 2006;1813:1804–1813. doi: 10.1002/ajmg.a. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Wheeler AC, Skinner ML, Bailey D, Sullivan K, Roberts J, Clark R. Adaptive behavior in children with fragile X syndrome. American Journal on Mental Retardation. 2003;108(6):373–390. doi: 10.1352/0895-8017(2003)108<373:ABICWF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hodapp R, Dykens E, Hagerman RJ, Schreiner R, Lachiewicz A, Leckman J. Developmental implications of changing trajectories of IQ in males with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(2):214–219. doi: 10.1097/00004583-199003000-00009. [DOI] [PubMed] [Google Scholar]
- Kau ASM, Meyer WA, Kaufmann WE. Early development in males with Fragile X syndrome: a review of the literature. Microscopy Research and Technique. 2002;57(3):174–8. doi: 10.1002/jemt.10069. [DOI] [PubMed] [Google Scholar]
- Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: Characteristics and specificity. American Journal of Medical Genetics. 2004;126A:9–17. doi: 10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A. 2004;129A(3):225–34. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Keysor CS, Mazzocco MMM. A developmental approach to understanding Fragile X syndrome in females. Microscopy Research and Technique. 2002;57(3):179–86. doi: 10.1002/jemt.10070. [DOI] [PubMed] [Google Scholar]
- Klaiman C, Quintin EM, Jo B, Lightbody aa, Hazlett HC, Piven J, Reiss aL. Longitudinal profiles of adaptive behavior in fragile X syndrome. Pediatrics. 2014;134(2):315–324. doi: 10.1542/peds.2013-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McDuffie AS, Hagerman RJ, Abbeduto L. Receptive vocabulary in boys with autism spectrum disorder: Cross-sectional developmental trajectories. Journal of Autism and Developmental Disorders. 2013:1–14. doi: 10.1007/s10803-013-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachiewicz AM, Gullion CM, Spiridigliozzi GA, Aylsworth AS. Declining IQs of young males with the fragile X syndrome. American Journal on Mental Retardation. 1987;92(3):272–278. [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Vulnerability and resilience: A study of high risk adolescents. Child Development. 1991;62:600–616. doi: 10.1111/j.1467-8624.1991.tb01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney ES, Larrivee LS. Limitations of age-equivalence scores in reporting results of norm-referenced tests. Contemporary Issues in Communication Sciences and Disorders. 2007;34:86–93. [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Morrow JD, Yurich KKL, Mahr F, Petersen C. Comparison of scores on the Checklist for Autism Spectrum Disorder, Childhood Autism Rating Scale, and Gilliam Asperger’s Disorder Scale for children with low functioning autism, high functioning autism, Asperger's disorder, ADHD, and typical development. Journal of Autism and Developmental Disorders. 2009;39(12):1682–93. doi: 10.1007/s10803-009-0812-6. [DOI] [PubMed] [Google Scholar]
- Mazzocco M, Kates WR, Baumgardner TL, Freund LS, Reiss AL. Autistic behaviors among girls with fragile X syndrome. Journal of Autism and Developmental Disorders. 1997;27(4):415–435. doi: 10.1023/A:1025857422026. [DOI] [PubMed] [Google Scholar]
- Mazzocco M, Pennington BF, Hagerman RJ. Social cognition skills among females with fragile X. Journal of Autism and Developmental Disorders. 1994;24(4):473–85. doi: 10.1007/BF02172129. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological issues in group-matching designs: α levels for control variable comparisons and measurement characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34(1):7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Mueller HH. Employer's reasons for terminating the employment of workers in entry-level jobs: Implications for workers with mental disabilities. Canadian Journal of Rehabilitation. 1988;1(4):233–240. [Google Scholar]
- Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and cognitive functioning and autism symptoms in young children with fragile X syndrome. American Journal on Mental Retardation. 2004;109(3):208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Anderson K, Burchinal M, Neebe E. Early communication, symbolic behavior, and social profiles of young males with fragile X syndrome. American Journal of Speech-Language Pathology. 2002;11(3):295–304. doi: 10.1044/1058-0360(2002/034). [DOI] [Google Scholar]
- Roberts JE, Mirrett PL, Burchinal M. Receptive vocabulary, expressive vocabulary, and speech production of boys with fragile X syndrome in comparison to boys with Down syndrome. American Journal on Mental Retardation. 2001;106(3):177–193. doi: 10.1352/0895-8017(2001)106. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, Kaufmann WE. Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2007;37(9):1748–60. doi: 10.1007/s10803-006-0305-9. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner E, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Developmental and Behavioral Pediatrics. 2001;22(6):409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ. Environmental factors in the early screening of children at risk. In: Frankeburg WK, Emde RN, Sullivan JW, editors. Early identification of children at risk: An international perspective. New York: Plenum Press; 1985. pp. 21–45. [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS) Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Sherman SL, Morton NE, Jacobs PA, Turner G. The marker (X) syndrome: a cytogenetic and genetic analysis. Annals of Human Genetics. 1984;48(1):21–37. doi: 10.1111/j.1469-1809.1984.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Smith L, Barker E, Seltzer M, Abbeduto L, Greenberg J. Behavioral phenotype of fragile X syndrome in adolescence and adulthood. American Journal on Intellectual and Developmental Disabilities. 2012;117(1):1–17. doi: 10.1352/1944-7558-117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti D. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland-II: Vineland adaptive behavior scales. 2. Minneapolis: Pearson; 2005. [Google Scholar]
- Tamanini F. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Human Molecular Genetics. 1997;6(8):1315–1322. doi: 10.1093/hmg/6.8.1315. [DOI] [PubMed] [Google Scholar]
- Turk J. The fragile-X syndrome. On the way to a behavioural phenotype. The British Journal of Psychiatry. 1992;160(1):24–35. doi: 10.1192/bjp.160.1.24. [DOI] [PubMed] [Google Scholar]
- Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. American Journal of Medical Genetics. 1996;64(1):196–7. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu Y-H, Kuhl DPA, Pizzuti A, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-H. [DOI] [PubMed] [Google Scholar]
- Warren SF, Brady N, Sterling A, Fleming K, Marquis J. Maternal responsivity predicts language development in young children with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2010;115(1):54–75. doi: 10.1352/1944-7558-115.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) The International Classification of Functioning (ICF) Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]