Abstract
Recent smoking cessation studies have shown that decreasing experiential avoidance (EA) (i.e., tendency to reduce or avoid internal distress) improves success, but to date none have examined the moderating effect of EA on the role of specific internal distress in smoking cessation. This study examined whether pre-quit general EA (Acceptance & Action Questionnaire) and smoking-specific EA (Avoidance and Inflexibility Scale) moderated the relations between four measures of post-quit internal distress (depressive symptoms, negative affect, physical withdrawal symptoms, craving), and smoking. Participates: 40 adult smokers who participated in a randomized controlled trial of Distress Tolerance treatment for smokers with a history of early lapse. Results: Multilevel models showed that pre-quit smoking-specific EA, but not general EA, significantly moderated the relationship between all measures of internal distress, except craving, and smoking over 13 weeks post-quit. When examined over 26 weeks, these relations remained unchanged for all, but the moderating effect became trend-level for depressive symptoms. Significant associations between post-quit internal distress and smoking were found only in those with high pre-quit smoking-specific EA. Moreover, pre-quit smoking-specific EA did not predict post-quit levels or changes in internal distress, suggesting that decreasing smoking-specific EA pre-quit may not reduce internal distress, but may instead reduce smoking risk in response to such distress during a quit attempt. Conclusions: Results mainly supported hypothesized relations, but only for smoking-specific EA. Smoking cessation interventions focusing on EA reduction may especially benefit those vulnerable to greater post-quit depressive and withdrawal symptoms, and those who smoke to regulate aversive internal states.
Keywords: smoking cessation, tobacco cessation, experiential avoidance, distress tolerance
The majority of cigarette smokers will lapse within a week of quitting (Japuntich, Piper, Leventhal, Bolt, & Baker, 2011; Zhu et al., 1996) and eventually return to smoking even with treatment (Fiore et al., 2008). Smokers report that lapses are most commonly triggered by affective and/or physical internal distress (e.g., nicotine withdrawal, stressful life event, psychiatric disorder such as depression) (Brandon, Tiffany, Obremski, & Baker, 1990; Kassel, Stroud, & Paronis, 2003; Shiffman et al., 1996; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). Established pharmacological and behavioral treatments have been developed to assist in cessation by reducing internal distress. However, research has revealed that beyond distress severity, the degree to which an individual is able to tolerate distress (i.e., distress tolerance, Brown, Lejuez, Kahler, & Strong, 2002; Brown et al., 2009) also plays an important role in cessation success.
A smoking lapse that serves the function of reducing internal distress can therefore be conceptualized as a form of experiential avoidance, defined as the tendency to engage in behaviors that result in reduction of or avoidance of internal distress (Hayes, Wilson, Gifford, Follette, & Strosahl, 1996). Distress tolerance and experiential avoidance are related but distinct constructs, with experiential avoidance serving as a strategy or function used by individuals low in distress tolerance (Boulanger, Hayes, & Pistorello, 2010). A recent innovation in behavioral smoking cessation treatment has been a shift in focus from reduction of distress severity to reduction of smokers’ EA (Hayes, et al., 1996), and their reactivity to such distress (Brown et al., 2008; Brown et al., 2013; Gifford et al., 2004; Gifford et al., 2011). Beyond smoking, EA has been found to be a central construct in changing mental and behavioral health problems (e.g., Hayes, Luoma, Bond, Masuda, & Lillis, 2006; Hayes, Masuda, Bissett, Luoma, & Guerrero, 2004).
Extant evidence supports the efficacy of behavioral treatments that target reducing EA to facilitate smoking cessation (Bricker, Wyszynski, Comstock, & Heffner, 2013; Brown, et al., 2013; Gifford, et al., 2004; Gifford, et al., 2011; Hernandez-Lopez, Luciano, Bricker, Roales-Nieto, & Montesinos, 2009). In particular, Gifford et al. (2004; 2011) demonstrated that cessation success was mediated by reduction in a self-reported measure of smoking-specific EA (i.e., tendency to smoke in order to reduce or avoid the specific physical, cognitive, and affective distress associated with nicotine withdrawal and craving). However, no studies have examined the theory-based (Hayes, Barnes-Holmes, & Roche, 2001; Hayes, et al., 1996) hypothesis that individuals high in EA are more likely to smoke in response to internal distress experienced during a quit attempt.
This study is a secondary analysis of data from a preliminary randomized controlled trial that compared a smoking cessation treatment focused on decreasing EA (distress tolerance treatment, DT) in the weeks leading up to quit date to standard behavioral smoking cessation treatment (ST) for smokers with a history of early lapse (Brown, et al., 2013). In this trial, participants who received the DT treatment were significantly more likely to be abstinent at the end of treatment (nicotine patch) at 8 weeks post-quit, relative to the ST group. In the current analyses, we tested the hypothesis that pre-quit (assessed on the day before quit date) levels1 of general EA (i.e., general tendency to avoid internal distress independent of the smoking cessation context) and smoking-specific EA would moderate the relationships between four measures of post-quit internal distress (depressive symptoms and withdrawal symptom subscales of negative affect, physical symptoms, and craving) and smoking after quit date, such that this relationship would be stronger among individuals with higher pre-quit general and/or smoking-specific EA.
Methods
Participants
Participants were 40 (out of 49) adult daily smokers who were enrolled in a randomized control trial of Distress Tolerance (DT) Treatment (Brown, et al., 2013). Nine (n = 9) RCT participants who did not complete the measures of pre-quit EA (see Measures below) were excluded. Inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Smoking at least 15 cigarettes per day for the past 3 years Motivated to quit smoking in the the next month |
Current DSM-IV Axis I disorder Psychoactive substance abuse or dependence (excluding nicotine dependence) within the past 6 months |
| Made at least one serious quit attempt in the past 10 years, but unable to quit for more than 72 hours | Current use of psychotropic medication A history of a significant medical condition Current use of pharmacotherapy for smoking cessation or other tobacco products |
Procedure
Procedures, including detailed descriptions of the treatments, have been reported previously (Brown, et al., 2008; Brown, et al., 2013). Briefly, after completing a baseline assessment, participants were randomly assigned to receive either Distress Tolerance Treatment (DT) or Standard Smoking Cessation Treatment (ST), which began 4 or 2 weeks prior to quit date, respectively. On quit date, all participants received 8 weeks of nicotine patch.
Measures
Nicotine Dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Abstinence
Self-reports of abstinence during the previous 7 days (i.e., 7-day point prevalence) were verified by expired carbon monoxide (CO, 5 ppm or less) at 1-, 2-, 3-, 4-weeks 8-, 13-, and 26-weeks post-quit, and by cotinine verification (cotinine, 10 ng/ml or less) at 13- and 26-weeks. Unverified reports of abstinence were coded as smoking.
Internal Distress
Depressive Symptoms were assessed with the Center for Epidemiological Studies-Depression scale (CES-D) (Radloff, 1977). Withdrawal Symptoms (negative affect, physical symptoms, and craving) were assessed with the Minnesota Nicotine Withdrawal Scale (Hughes & Hatsukami, 1986; Piasecki et al., 2000). Negative Affect included irritability/frustration/anger, anxiety/nervousness, sadness/depression; Physical Symptoms included difficulty concentrating, restlessness, appetite increase; and Craving was a single item. These measures are well-validated and frequently used in smoking cessation research (e.g., Kinnunen, Doherty, Militello, & Garvey, 1996; Toll, O'Malley, McKee, Salovey, & Krishnan-Sarin, 2007). We decided to use all three subscales of the withdrawal measure as smoking-specific EA assesses individual's tendency to avoid internal distress including negative affective (e.g., stress), craving, and physical sensations related to withdrawal symptoms.
Moderating Processes – Experiential Avoidance (EA)
General EA was assessed with the 9-item Acceptance and Action Questionnaire (AAQ-I) (Cronbach alpha = .70: (Hayes et al., 2004). Higher scores indicate greater EA. Smoking-specific EA was assessed with the 13-item Avoidance and Inflexibility Scale (AIS) (Cronbach's alpha = .93;(Gifford, Antonuccio, Kohlenberg, Hayes, & Piasecki, 2002), a measure of the degree to which an individual believes that smoking-related internal experiences (thoughts, feelings, and bodily sensations associated with craving and withdrawal symptoms) lead him/her to smoke, and the degree to which the individual believes that reduction in the frequency and intensity of these internal experiences (i.e., avoidance of them) is necessary in order not to smoke.
Analytic Plan
A series of multilevel models was used to test whether 1) internal distress was associated with smoking behavior, 2) pre-quit levels of general EA and smoking-specific EA moderated the relations between post-quit internal distress and smoking behavior, and 3) pre-quit levels of EA predicted increases in post-quit internal distress. Reports of depressive symptoms, withdrawal symptoms (negative affect, physical symptoms, craving) and smoking behavior (7-day point prevalence abstinence), assessed at the same time points (1, 2, 3, 4, 8, 13, 26 weeks post-quit date), and time (weeks since quit date) comprised the first level of data nested within individuals at the second level. We also examined these models with three levels where individuals were nested within treatment groups at the third level. However, since neither improvement in model fit nor changes in the results was found, we present the two level models in this paper. Pre-quit general EA (AAQ) and smoking-specific EA (AIS) were assessed on the day before quit quit date and used as level 2 variables.
First, the effect of each internal distress measure on smoking behavior was evaluated separately, controlling for time, gender, nicotine dependence, treatment condition, and baseline depressive symptoms. A “binomial” distribution was specified, as smoking was coded as a dichotomous outcome (abstinent = 1, smoking = 0). Next, we examined the moderating effect of pre-quit general EA and smoking-specific EA on the relations between internal distress and smoking separately. Cross-level interaction terms between pre-quit EA (level 2) and each internal distress measure (level 1) were included in the corresponding model. All models specified the intercept and the coefficient for the corresponding predictor (level 1) as random to allow them to vary across individuals. The coefficient for time (i.e., weeks since quit date) was also specified as random, and the correlation between random intercept and random coefficients was estimated if doing so improved model fit. Deviance statistics were used to compare model fit between two models (Raudenbush & Bryk, 2002). Analyses were run both over 1 to 13 weeks (3 months) and 1 to 26 weeks (6 months) since an optimal timeframe to examine the effects of pre-quit experiential avoidance on smoking reactivity remains unknown. Given that the aim of the current study was not to test treatment differences, all models were tested both with and without treatment condition as a control variable. The models including treatment condition are presented in this paper; however, removing treatment condition did not change the patterns of results.
Finally, we tested whether pre-quit levels of EA predicted average levels and /or changes in internal distress. These models controlled for gender, nicotine dependence, baseline scores of the corresponding variable, treatment condition, and smoking status during the same assessed period. In order to assess the moderating effects of experiential avoidance on post-quit changes in levels of each subscale of symptoms over time, an interaction term between time (level 1, centered) and pre-quit EA (level 2) was included in the model. All models specified the intercept (post-quit average level) and the coefficients for time (post-quit slope) to vary across individuals.
Results
Preliminary Analyses
No differences in baseline characteristics (see Table 2) were found between included and excluded participants. Pre-quit AAQ (M = 31.75, SD = 8.70) and AIS (M = 42.91, SD = 9.43), were significantly correlated (r = .34, p = .031). Results also showed no differences in post-quit EA (i.e., AIS or AAQ at 4, 13, or 26 weeks after quit date) across treatment conditions with or without controlling for smoking status and pre-quit EA, suggesting no continuing effects of treatment on EA after target quit date.
Table 2.
Demographic and Baseline Characteristics
| Included (n=40) | Excludeda (n=9) | |
|---|---|---|
| M (SD) /n (%) | M (SD)/ n (%) | |
| Age | 46.8 (10.66) | 48.88 (8.98) |
| Female | 22 (55%) | 3 (33%) |
| White | 36 (90%) | 8 (89%) |
| Nicotine Dependence (FTND) | 6.18 (1.81) | 6.89 (1.27) |
| Depressive symptoms (CES-D) | 8.88 (7.94) | 11.67 (7.68) |
| Experiential Avoidance | ||
| General (AAQ) | 31.23 (7.75) | 31.56 (8.71) |
| Smoking-specific (AIS) | 46.56 (10.24) | 50.56 (6.31) |
Note. No significant differences were found between included and excluded individuals. FTND: Fagerstrom Test for Nicotine Dependence; CES-D = Center for Epidemiological Studies-Depression Scale; AAQ: Acceptance & Action Questionnaire; AIS: Avoidance and Inflexibility Scale.
A total of 9 (out of 49) participants who did not complete the measures of pre-quit experiential avoidance (AAQ or AIS) were excluded from the analyses. Results of chi-square tests (for gender and race) and t-tests (for all other continuous variables) indicated that there were no statistical significant differences between included and excluded groups in this study (ps > .10).
Main Effects of Pre-quit Levels of Experience Avoidance and Post-quit Internal Distress
Results of multilevel model analyses showed that neither pre-quit levels of general EA (AAQ) nor smoking-specific EA (AIS) predicted smoking abstinence during the first 13 or 26 weeks post-quit. Similarly, depressive symptoms, negative affect and physical symptoms were not associated with 7-day point prevalence abstinence rates in the first 13 or 26 weeks of a quit attempt (Table 3, top panel)2. However, craving to smoke was significantly associated with decreased likelihood of abstinence over both 13 and 26 weeks.
Table 3.
Multilevel Analysis of the Moderating Effects of Pre-Quit Smoking-Specific Experiential Avoidance on Relations Between Post-Quit Internal Distress And Smoking Risk
| 1-13 weeks | 1-26 weeks | |||
|---|---|---|---|---|
| Predictor | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
|
Main effects
| ||||
| Female | 0.715 | (0.064, 8.001) | 0.847 | (0.096, 7.500) |
| Nicotine Dependence (FTND) | 0.578 | (0.290, 1.150) | 0.666 | (0.363, 1.223) |
| Baseline Depressive Symptoms (CES-D) | 1.011 | (0.868, 1.178) | 1.025 | (0.894, 1.176) |
| Treatment Condition | 2.510 | (0.218, 28.91) | 2.885 | (0.319, 26.11) |
| Pre-quit Experiential Avoidance (AIS) | 1.080 | (0.948 1,231) | 1.061 | (0.943, 1,194) |
| Time (weeks) since quit date | 0.719 | (0.640, 0.808)* | 0.790 | (0.738, 0.846)* |
| Post-quit Depressive Symptoms a | 0.925 | (0.827, 1.034) | 0.955 | (0.874, 1.044) |
| Post-quit Negative Affect a | 0.334 | (0.083, 1.336) | 0.492 | (0.151, 1.597) |
| Post-quit Physical Withdrawal Symptoms a | 0.499 | (0.099, 2.506) | 1.473 | (0.314, 6.917) |
| Post-quit Craving to Smoke a | 0.323 | (0.172, 0.608)** | 0.284 | (0.158, 0.511)** |
|
Interactions | ||||
| Post-quit Depressive Symptoms b | 0.968 | (0.899, 1.044) | 0.988 | (0.924, 1.057) |
| c Pre-quit Experiential Avoidance (AIS) | 0.992 | (0.984, 0.999)* | 0.994 | (0.987, 1.000)° |
| Post-quit Negative Affect b | 0.315 | (0.086, 1.165) | 0.412 | (0.128, 1.326) |
| c Pre-quit Experiential Avoidance (AIS) | 0.862 | (0.748, 0.993)* | 0.877 | (0.774, 0.995)* |
| Post-quit Physical Withdrawal Symptoms b | 0.379 | (0.099, 1.457) | 1.250 | (0.282, 5.536) |
| c Pre-quit Experiential Avoidance (AIS) | 0.828 | (0.713, 0.963)* | 0.844 | (0.719, 0.991)* |
| Post-quit Craving to Smoke b | 0.335 | (0.175, 0.642)** | 0.286 | (0.158, 0.518)** |
| c Pre-quit Experiential Avoidance (AIS) | 1.024 | (0.951, 1.102) | 1.010 | (0.946, 1.078) |
Note:
Separate models was run for each predictor. The main effect model for baseline covariates and AIS without predictors is shown.
The effect of the level 1 predictor on abstinence when pre-quit experiential avoidance is at its overall mean (pre-quit AIS mean = 42.91).
Interaction terms evaluated in a subsequent model for each predictor variable. All other terms are derived from the main effects models. Intercept was specified as random and all other predictors were treated as fixed. FTND: Fagerstrom Test for Nicotine Dependence; CES-D: Center for Epidemiological Studies-Depression scale; AIS: Avoidance and Inflexibility Scale. All within-subject predictors and moderators were assessed at the same as smoking status.
p < .10, p < .05
p < .01
Moderating Effects of Pre-quit Levels of Experiential Avoidance
General Experiential Avoidance
No moderating effect of general EA (AAQ) on the relations between smoking and internal distress during the first 13 or 26 weeks was found.
Smoking-Specific Experiential Avoidance
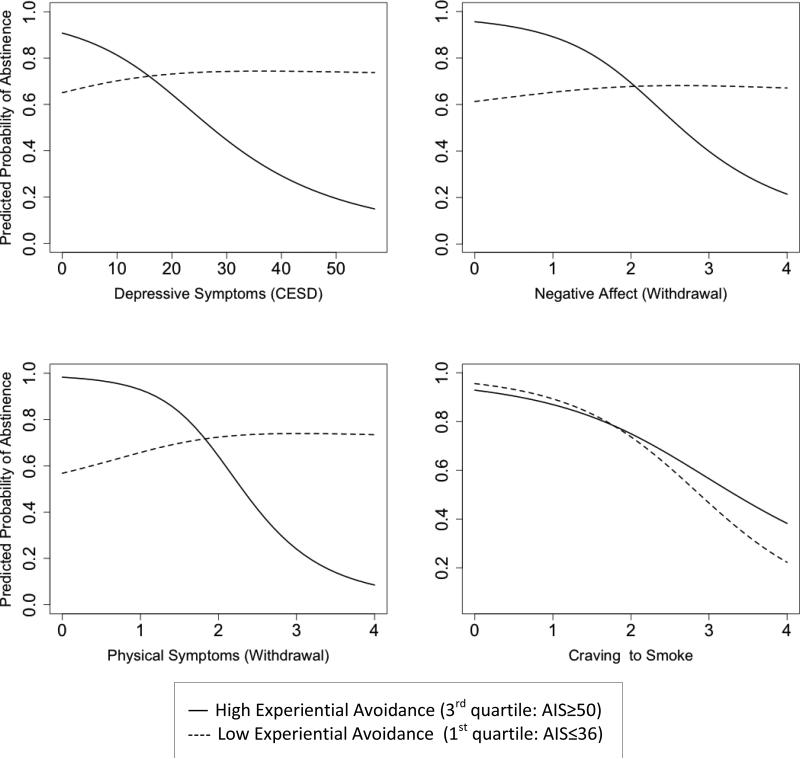
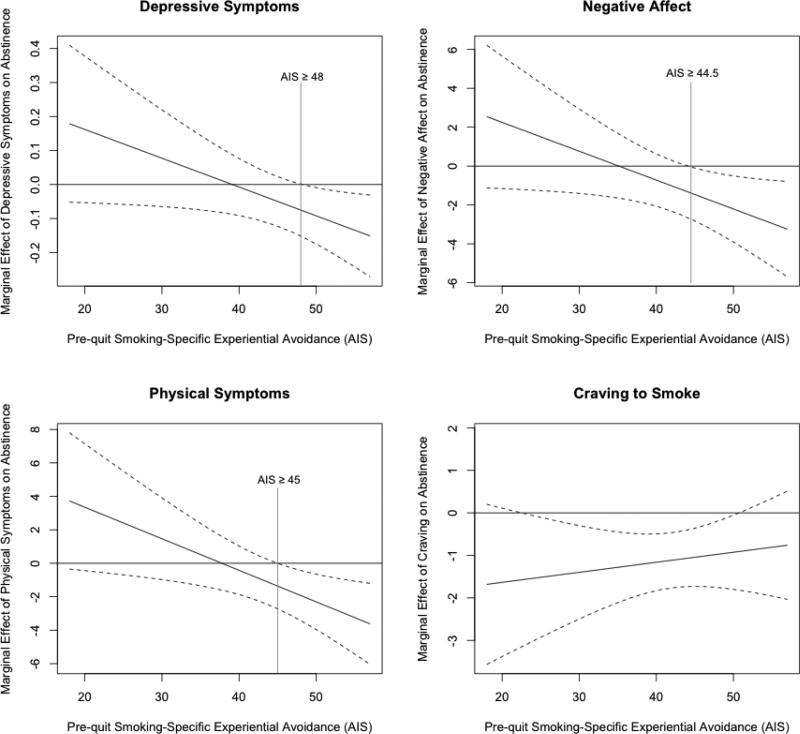
Multilevel models revealed that smoking-specific EA (AIS) significantly moderated the relations between smoking and all internal distress measures except craving (i.e., depressive symptoms, negative affect, and physical symptoms), in the hypothesized direction, for the first 13 weeks (Table 3, bottom panel, and Figure 1). Marginal effect plots that capture the changes in the coefficient estimate and its 95% confidence intervals as a level of the moderator (AIS) changes revealed that higher post-quit depressive symptoms were significantly associated with lower likelihood of abstinence only among those with pre-quit AIS scores of ≥ 48 (a threshold value of AIS where the distress-smoking relationships became significant). Similar patterns were observed in negative affect and physical symptoms such that higher scores were significantly related to lower likelihood of abstinence only in those with pre-quit AIS of ≥ 44.5 or ≥ 45, respectively (Figure 2).
Figure 1.
Predicted probability of abstinence over 13 weeks post-quit for smokers with high (3rd quartile: AIS ≥ 50) and low pre-quit smoking-specific experiential avoidance (1st quartile: AIS ≤ 36) for each of the predictors (i.e., depressive symptoms, negative affect, physical symptoms, and craving).
Figure 2.
Marginal effects of depressive symptoms, negative affect, physical withdrawal symptoms and craving on abstinence rates over 13 weeks post-quit by pre-quit levels of smoking-specific experiential avoidance. Each plot shows the threshold value of the moderator (i.e., pre-quit smoking-specific experiential avoidance - AIS) where the effects of the predictor on outcome (i.e., smoking risk) become significant. The changes in the coefficient estimate and its 95% confidence intervals as a level of the moderator changes are captured in these marginal effect plots.
The moderating effects of AIS on the relationship between negative affect and smoking as well as physical withdrawal and smoking remained significant through 26 weeks post-quit; however, the moderating effects of AIS on the relationships between depressive symptoms and smoking became trend-level (p = .075) (Table 3, bottom panel).
Pre-quit Levels of Experiential Avoidance and Post-quit Internal Distress
Results revealed no association between pre-quit levels of general EA (AAQ) (ps > .10) or smoking-specific EA (AIS) (ps > .32) and post-quit average levels or changes in any internal distress measure over 13 weeks post-quit. Exclusion of treatment condition in the models did not change significance or direction of the findings. This suggests that post-quit levels of internal distress did not differ by levels of pre-quit EA (general or smoking-specific) and that the observed impact of smoking-specific EA on the relationships between smoking and internal distress over 13 weeks were not due to a higher likelihood that those with high AIS experienced greater internal distress post-quit.
Discussion
Results of the current analyses revealed that significant positive relationships between post-quit depressive symptoms, negative affect, physical withdrawal symptoms, and smoking were found only among those with higher pre-quit smoking-specific experiential avoidance (EA) while level of post-quit craving significantly predicted smoking regardless of pre-quit levels of smoking-specific EA. These findings indicate that affective and physical distress, but not craving, pose a higher risk of smoking for those with higher pre-quit levels of smoking-specific EA during a quit attempt. This may suggest that although craving is experienced as distressing in most smokers trying to quit smoking, craving may qualitatively differ in subjective experience from other affective and physical withdrawal symptoms.
In addition, similar patterns emerged with regard to a threshold level of smoking-specific EA for all three measures of internal distress (reduction of AIS scores from above to below 44.5, 45 or 48, for depressive symptoms, negative affect, and physical symptoms, respectively). In fact, the threshold found in this study was close to the baseline mean and median AIS score (mean = 46.56, median = 48). Past studies with different inclusion criteria (Gifford, et al., 2011; ‘at least 1 cigarette per day,’ Schloss & Haaga, 2011) also reported similar mean levels of baseline smoking-specific EA (means = 48.47, 49.3, respectively). It is possible that the threshold found in this study may be generalizable to smokers in general, but this should be tested in future studies. Assuming a simple linear effect of EA on the relations between internal distress and smoking outcome may obscure the associations between reduced smoking-specific EA and smoking behavior.
The observed moderating effects of smoking-specific EA on relationships between negative affect and physical symptoms and smoking remained significant for 6 months, whereas the moderating effect of smoking-specific EA on the relationship between depressive symptoms and smoking was weakened after 3 months post-quit. This suggests that the effects of pre-quit smoking-specific EA on reactivity (i.e., smoking) to depressive symptoms were short-lived after a quit attempt and may differ from that on reactivity to negative affect more generally and physical withdrawal. It is possible that lasting (or increasing) depressive symptoms longer than 6 months post-quit may reflect greater relapse vulnerability that is qualitatively distinct from heightened relapse risk related to withdrawal-related distress. In addition, smoking-specific EA is designed to assess individuals' tendency to avoid specific internal distress related to withdrawal, suggesting that reduction in EA specific to withdrawal may not necessarily be generalized to other more pervasive distress such as depressive symptoms.
We did not find moderating effects of general EA on post-quit internal distress-smoking relations, which is consistent with previous findings that problem-specific EA, compared to general EA, is more relevant to changes in targeted behavior (Gifford, et al., 2004; Gregg, Callaghan, Hayes, & Glenn-Lawson, 2007; Lundgren, Dahl, & Hayes, 2008). Furthermore, recent studies have shown that general EA (AAQ) does not correlate significantly with behavioral indices of distress tolerance that are assessed in the laboratory (e.g., Mirror Tracing Persistence Task, MTPT Schloss & Haaga, 2011) and have been shown to predict successful smoking cessation (Brandon et al., 2003). Together these findings underscore the importance of context when examining the role of general or problem-specific EA and similar concepts such as distress intolerance or emotion regulation in the process of behavioral change (see Aldao, 2013 for emotion regulation; McHugh & Otto, 2011 for distress intolerance).
Furthermore, pre-quit levels of smoking-specific EA did not predict levels of post-quit depressive symptoms, negative affect, physical withdrawal symptoms, or craving, supporting the hypothesis that EA does not impact levels of distress, but rather one's reactions to such internal distress. Taken together, these findings suggest that reducing smoking-specific EA prior to a quit attempt may not reduce internal distress, but may instead reduce a smoker's response (i.e., smoking) to such distress during a quit attempt, thereby increasing cessation success.
Limitations
There are a few limitations of this study that warrant caution when interpreting the results. First, internal distress and smoking behavior were assessed at the same assessment points. In addition, internal distress (e.g., negative affect) was not manipulated. As such, interpretation of causal relations has to be tempered. Second, there were only 7 assessment points in this study. More frequent assessments (e.g., such as ecological momentary assessment) in future studies would allow us to prospectively examine the effects of internal distress on smoking behavior and its relationship to EA without reducing data points. Third, given the small sample size in this study, the reliability of the current findings is unknown. As such, replication with a larger sample is needed. In addition, although no significant difference in post-quit AIS or AAQ were found between treatments, it is possible that those who received the DT treatment qualitatively differed from those in the standard treatment. Future studies with larger sample sizes that allow us to directly investigate the potential continuing effects of post-quit treatment on the moderating role of pre-quit EA on distress-smoking relationship is needed. Finally, all participants had never been abstinent from smoking for more than 3 days in the past 10 years. Consequently, especially with additional inclusion and exclusion criteria in this study, generalizability may be limited.
Conclusion
Results mainly supported the hypothesized relations, but only for smoking-specific EA. Reducing smoking-specific EA below a certain threshold may be crucial to reduce lapse risk associated with internal distress among early-lapsers. Smoking cessation interventions focusing on reduction in smoking-specific EA may be especially beneficial for those who are vulnerable to greater post-quit depressive and withdrawal symptoms, and who smoke to regulate aversive internal states, such as female smokers (Berlin et al., 2003; DeBernardo et al., 1999; File, Fluck, & Leahy, 2001) or smokers with elevated depressive symptoms (Lerman et al., 1996; Schleicher, Harris, Catley, & Nazir, 2009).
Acknowledgments
Funding: The project described was supported in part by Award Number DA017332 from the National Institute on Drug Abuse awarded to Richard A. Brown. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse.
Footnotes
The treatment began 4 weeks prior to quit date in order to prepare smokers for their quit date (i.e., reducing EA before smokers face withdrawal-related distress on quit date). Pre-quit level of EA (regardless of prior treatment effects) was used to test whether reducing pre-quit EA, targeted by treatments, would actually impact the relationships between internal distress and lapse/relapse risk after quit date in a theory consistent direction.
Only in the model for negative affect, the coefficient for time, in addition to the intercept and the coefficient for negative affect, was allowed to vary across individuals because a statistically significant reduction in deviance (change in deviance = 6.98, df = 2, p = .03) was observed, indicating a better fit to the data. For the other three models, only the intercept and the coefficient for corresponding level 1 predictor (i.e., depressive symptoms, craving, and physical symptom) were specified as random since setting additional parameter (i.e., time) to random did not significantly reduce deviance (ps > .08), indicating no improvement to model fit.
Contributor Information
Haruka Minami, Alpert Medical School of Brown University and Butler Hospital, Providence, RI.
Erika Litvin Bloom, Alpert Medical School of Brown University and Butler Hospital, Providence, RI.
Kathleen M. Palm Reed, Clark University.
Steven C. Hayes, University of Nevada, Reno
Richard A. Brown, Alpert Medical School of Brown University and Butler Hospital, Providence, RI
References
- Aldao A. The future of emotion regulation research: capturing context. Perspectives on Psychological Science. 2013;8:155–172. doi: 10.1177/1745691612459518. [DOI] [PubMed] [Google Scholar]
- Berlin I, Singleton EG, Pedarriosse AM, Lancrenon S, Rames A, Aubin HJ, Niaura R. The modified reasons for smoking scale: factorial structure, gender effects and relationship with nicotine dependence and smoking cessation in French smokers. Addiction. 2003;98(11):1575–1583. doi: 10.1046/j.1360-0443.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- Boulanger J, Hayes SC, Pistorello J. Experiential avoidance as a functional contextual concept. In: Kring A, Sloan D, editors. Emotion Regulation and Psychopathology. Guilford Press; New York: 2010. [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112(3):448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine & Tobacco Research. 2013;15(10):1756–1764. doi: 10.1093/ntr/ntt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111(1):180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behavior Modification. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Hayes SC. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research. 2013 doi: 10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBernardo RL, Aldinger CE, Dawood OR, Hanson RE, Lee SJ, Rinaldi SR. An E-mail assessment of undergraduates' attitudes toward smoking. Journal of American college health. J of ACH. 1999;48(2):61–66. doi: 10.1080/07448489909595675. [DOI] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2001;4(4):371–376. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: May, 2008. Treating Tobacco Use and Dependence: 2008 Update. 2008. [Google Scholar]
- Gifford EV, Antonuccio DO, Kohlenberg BS, Hayes SC, Piasecki MM. Combining Bupropion SR with acceptance and commitment-based behavioral therapy for smoking cessation: Preliminary results from a randomized controlled trial.. Paper presented at the Association for Advancement of Behavioral Therapy; Reno, NV. 2002. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki M, Rasmussen- Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behav Ther. 2004;35:689–706. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behav Ther. 2011;42(4):700–715. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Gregg JA, Callaghan GM, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2007;75(2):336–343. doi: 10.1037/0022-006X.75.2.336. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Barnes-Holmes D, Roche B. Relational frame theory: A post- Skinnerian account of human language and cognition. Kluwer Academic/Plenum Publishers; New York: 2001. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behaviour Research and Therapy. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Masuda A, Bissett R, Luoma J, Guerrero LF. DBT, FAP, and ACT: How empirically oriented are the new behavior therapy technologies? Behav Ther. 2004;35(35- 54) [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG, Bissett RT, Pistorello J, Toarmino D, McCurry SM. Measuring experiential avoidance: A preliminary test of a working model. The Psychological Record. 2004;54:553–578. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experimental avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. Journal of Consulting and Clinical Psychology. 1996;64(6):1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2009;23(4):723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. Journal of Consulting and Clinical Psychology. 2011;79(1):34–42. doi: 10.1037/a0022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology. 1996;64(4):791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N. Investigation of mechanisms linking depressed mood to nicotine dependence. Addictive Behaviors. 1996;21(1):9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Lundgren T, Dahl J, Hayes SC. Evaluation of mediators of change in the treatment of epilepsy with acceptance and commitment therapy. Journal of Behavioral Medicine. 2008;31(3):225–235. doi: 10.1007/s10865-008-9151-x. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Otto MW. Domain-general and domain-specific strategies for the assessment of distress intolerance. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2011;25(4):745–749. doi: 10.1037/a0025094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology. 2000;109(1):74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2nd ed. SAGE Publications, Inc.; Thousand Oaks, CA: 2002. [Google Scholar]
- Schleicher HE, Harris KJ, Catley D, Nazir N. The role of depression and negative affect regulation expectancies in tobacco smoking among college students. Journal of American college health : J of ACH. 2009;57(5):507–512. doi: 10.3200/JACH.57.5.507-512. [DOI] [PubMed] [Google Scholar]
- Schloss HM, Haaga DA. Interrelating Behavioral Measures of Distress Tolerance with Self-Reported Experiential Avoidance. J Ration Emot Cogn Behav Ther. 2011;29(1):53–63. doi: 10.1007/s10942-011-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996;64(5):993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Toll BA, O'Malley SS, McKee SA, Salovey P, Krishnan-Sarin S. Confirmatory factor analysis of the Minnesota Nicotine Withdrawal Scale. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors. 2007;21(2):216–225. doi: 10.1037/0893-164X.21.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. Journal of Consulting and Clinical Psychology. 1996;64(1):202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]