Abstract
Background
The incidence of hepatocellular carcinoma (HCC) is increasing, but surgical management continues to be underutilized. This retrospective review investigates treatment decisions and survival for early stage HCC.
Methods
The National Cancer Database (NCDB) was queried for all patients with curable HCC (Stage I/II) from 1998 to 2011 (n = 43 859). Patient and tumour characteristics were analysed to determine predictors of having surgery and of long-term survival.
Results
Only 39.7% of patients received surgery for early stage HCC. Surgical therapies included resection (34.6%), transplant (28.7%), radiofrequency ablation (27.1%) and other therapies. Surgery correlated with improved median survival (48.3 versus 8.4 months), but was only performed on 42% of stage I patients and 50% of tumours smaller than 2 cm. Patients were more likely to receive surgery if they were Asian or white race, had private insurance, higher income, better education, or treatment at an academic centre (P < 0.05). However, private insurance and treatment at an academic centre were the only variables associated with improved survival (P < 0.05).
Conclusion
Fewer than half of patients with curable HCC receive surgery, possibly as a result of multiple socioeconomic variables. Past these barriers to care, survival is related to adequate and reliable treatment. Further efforts should address these disparities in treatment decisions.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common solid organ tumour and the third leading cause of cancer-related death worldwide.1 The incidence of HCC has been increasing for years, but incidence-based mortality has slowed, probably owing to earlier detection and intervention.2 However, there is a large population of patients with curable disease who do not receive surgical management. A recent meta-analysis found an average rate of curative intent surgery of 22%, ranging from 14 to 51%.3 Rates were higher in single-centre studies, and the rate for early stage HCC was 59%. Rates of surgical management in other studies range from 20 to 57%.4–7 While the phenomenon of underutilized surgical care is known, the reasons for this disparity remain unclear.
The present study aims to provide a comprehensive understanding of surgical management for early stage HCC. This analysis utilized a nationally validated, prospectively gathered, cancer database to investigate how many patients with potentially curable disease receive surgical management and what variables are associated with treatment decisions and survival.
Patients and methods
Data source
Data for this study were drawn from the American College of Surgeons National Cancer Data Base (NCDB) liver Participant User File (PUF) for the years 1998–2011. This is a nationwide, facility-based, clinical data set that captures 70% of all diagnosed malignancies in the US.8 The NCDB collects de-identified patient-level data from nationally accredited cancer programme registries using standardized data items and coding definitions. These data include patient demographics as well as detailed information regarding cancer staging, tumour histology, treatment types and courses, short-term surgical outcomes, and long-term survival.
Patient cohort
The liver PUF was queried for all patients with clinical stage I/II HCC (n = 43 859), according to the AJCC Cancer Staging Manual edition in use during the year in which the case was diagnosed. Subtypes of HCC were excluded. Patients were separated into groups who did and did not receive surgical management, defined as surgical resection, liver transplant, radiofrequency ablation and other liver-directed therapies. The following patient information was collected for all patients: age (years), gender, race (Asian, black, white or other), primary insurance, income (median household income for patient zip code based on 2000 US Census data, in quartiles), education (median percentage of adults in the patient's zip code without a high school degree [HSD] based on 2000 US Census data, in quartiles), tumour grade, tumour size (<2 cm, 2–5 cm, >5 cm), AJCC clinical stage (I or II), patient Charlson/Deyo comorbidity score (0,1,2), facility type (academic: > 500 new cancer diagnoses annually, at least four postgraduate training programmes; comprehensive community: >500 new cancer diagnoses annually, postgraduate training optional; community: 100–500 new cancer diagnoses annually, postgraduate training optional; and other), Urban/Rural status (metropolitan, urban, rural), great circle distance (distance in miles between patient's residence and reporting hospital), surgical procedure (resection, transplant, radiofrequency ablation, other/unknown) for patients who had surgery and AJCC pathological stage (I–IV) for patients who had surgery.
Statistical analysis
The two cohorts were compared with respect to the variables above using chi-squared tests for categorical variables and rank-sum tests for continuous variables. Kaplan–Meier survival analysis was used to compare survival between the two groups. For survival analyses, data were limited to the years 1998–2006 to ensure appropriate follow-up. As a result of this and limitations based on missing data, only 15 235 patients (10.2%) were included in the survival analysis. Multiple logistic regression models were created to analyse predictors of having surgery and 30-day mortality; Cox regression was used to model long-term survival. The following variables of clinical interest were included in the models: clinical stage, age, gender, race, primary insurance, income, education, Charlson–Deyo score and facility type. An alpha level of 0.05 was used for all significance tests. The data were analysed using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient, tumour and facility characteristics
Table S1 (available online as Supporting Information) describes the cohorts of patients who did and did not receive surgical management for the stage I/II HCC. Overall, not quite 40% of patients received surgical management. The rates of surgical management over time in this cohort are presented in Fig. 1. Patients who received surgery were younger, less likely to be black and more often privately insured. Income and education were directly correlated with increasing rates of surgical management.
Figure 1.
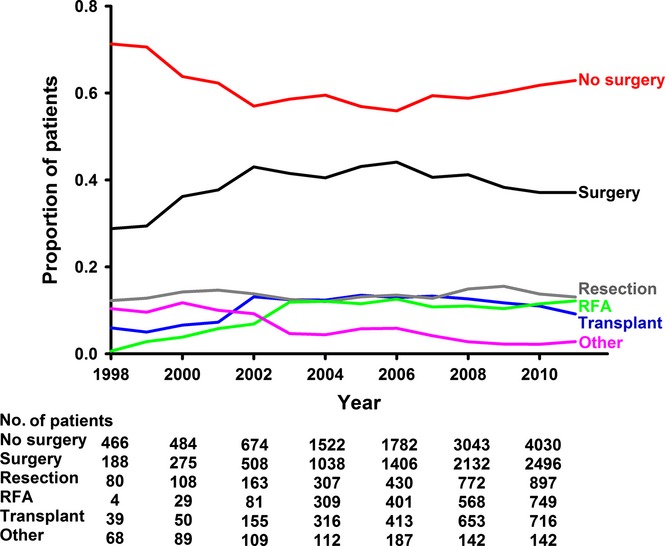
Percentage of patients per year with stage I/II hepatocellular carcinoma (HCC) who have surgical management. RFA, radiofrequency ablation
Surgical therapy was more common for smaller tumours. However, tumours graded as ‘moderately differentiated’ were most likely to receive surgery. Post-operatively, only 5.9% of tumours were upstaged to stage III or IV, and none were downstaged to stage 0. Also of note, patients with a Charlson–Deyo score of 0 were less often managed surgically.
Surgical therapy was much more common in academic centres than community centres. There was little variation based on urban/rural designation, but patients who ended up having surgery travelled further for their care than patients who did not.
Predictors of surgery and post-operative mortality
Results from the multivariate analysis for predictors of receiving surgical management are in Table 1. Asian patients were more likely than whites to receive surgery, and black patients were less likely. Lower odds of surgery were associated with clinical stage II versus I, increasing age and male gender. Also less likely to have surgery were patients without private insurance, with low income, with less education and those not treated at academic centres.
Table 1.
Multivariate analysis of predictors of having surgery for stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB)
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Clinical Stage 2 (versus 1) | 0.73 | 0.70–0.76 | <0.001 |
| Age (1 year increase) | 0.98 | 0.98–0.98 | <0.001 |
| Male | 0.92 | 0.87–0.97 | 0.001 |
| Race | |||
| White | 1.00 | ||
| Asian | 1.34 | 1.23–1.45 | <0.001 |
| Black | 0.82 | 0.77–0.88 | <0.001 |
| Other | 0.99 | 0.85–1.15 | 0.888 |
| Insurance | |||
| Private | 1.00 | ||
| Medicare | 0.83 | 0.78–0.88 | <0.001 |
| Medicaid | 0.53 | 0.50–0.57 | <0.001 |
| Not Insured | 0.32 | 0.28–0.36 | <0.001 |
| Other | 0.78 | 0.65–0.92 | 0.003 |
| Income | |||
| >$46 000 | 1.00 | ||
| $35 000–46 000 | 0.96 | 0.90–1.03 | 0.238 |
| $30 000–35 000 | 0.88 | 0.81–0.95 | 0.001 |
| <$30 000 | 0.92 | 0.84–1.00 | 0.048 |
| Education | |||
| <14% | 1.00 | ||
| 14–20% | 0.87 | 0.81–0.93 | <0.001 |
| 20–29% | 0.86 | 0.80–0.93 | <0.001 |
| >29% | 0.72 | 0.66–0.78 | <0.001 |
| Charlson–Deyo Score | |||
| 0 | 1.00 | ||
| 1 | 1.31 | 1.24–1.38 | <0.001 |
| 2 | 1.21 | 1.14–1.27 | <0.001 |
| Facility | |||
| Academic Programme | 1.00 | ||
| Community Cancer Programme | 0.31 | 0.28–0.35 | <0.001 |
| Comprehensive Community Cancer Programme | 0.53 | 0.50–0.55 | <0.001 |
| Other Cancer Programme | 0.16 | 0.10–0.25 | <0.001 |
Of patients who received surgical management, several factors were associated with increased odds of post-operative mortality (Table 2): increasing age, government insurance and treatment at a comprehensive community cancer programme.
Table 2.
Multivariate analysis of predictors of 30-day mortality for stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB) who received surgical management
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Clinical Stage 2 (versus 1) | 1.00 | 0.79–1.27 | 0.98 |
| Age (1 year increase) | 1.04 | 1.02–1.05 | <0.001 |
| Male | 1.09 | 0.85–1.40 | 0.491 |
| Race | |||
| White | 1.00 | ||
| Asian | 0.74 | 0.47–1.16 | 0.193 |
| Black | 0.84 | 0.58–1.22 | 0.363 |
| Other | 1.64 | 0.84–3.17 | 0.145 |
| Insurance | |||
| Private | 1.00 | ||
| Medicare | 1.37 | 1.02–1.83 | 0.037 |
| Medicaid | 2.30 | 1.59–3.33 | <0.001 |
| Not Insured | 2.07 | 0.99–4.35 | 0.054 |
| Other | 1.63 | 0.69–3.83 | 0.262 |
| Income | |||
| >$46 000 | 1.00 | ||
| $35 000–46 000 | 1.23 | 0.89–1.70 | 0.208 |
| $30 000–35 000 | 1.19 | 0.81–1.76 | 0.372 |
| <$30 000 | 1.47 | 0.95–2.27 | 0.088 |
| Education | |||
| <14% | 1.00 | ||
| 14–20% | 1.24 | 0.89–1.75 | 0.202 |
| 20–29% | 1.16 | 0.80–1.68 | 0.424 |
| >29% | 1.22 | 0.80–1.87 | 0.352 |
| Charlson–Deyo Score | |||
| 0 | 1.00 | ||
| 1 | 1.05 | 0.81–1.38 | 0.711 |
| 2 | 1.19 | 0.91–1.57 | 0.212 |
| Facility | |||
| Academic Programme | 1.00 | ||
| Community Cancer Programme | 0.96 | 0.44–2.08 | 0.910 |
| Comprehensive Community Cancer Programme | 1.34 | 1.04–1.72 | 0.021 |
| Other Cancer Programme | 1.51 | 0.20–11.7 | 0.691 |
CI, confidence interval; education, percentage of people in the patient's zip code without a high school degree.
Long-term survival
Figure 2 shows the survival benefit for patients who had surgical. Multivariate Cox analysis (Table 3) confirmed that surgery was associated with a survival benefit, as was Asian race. Clinical stage II and increasing age were associated with inferior survival. Other correlates of inferior survival included non-private insurance as well as treatment of a comprehensive community cancer.
Figure 2.
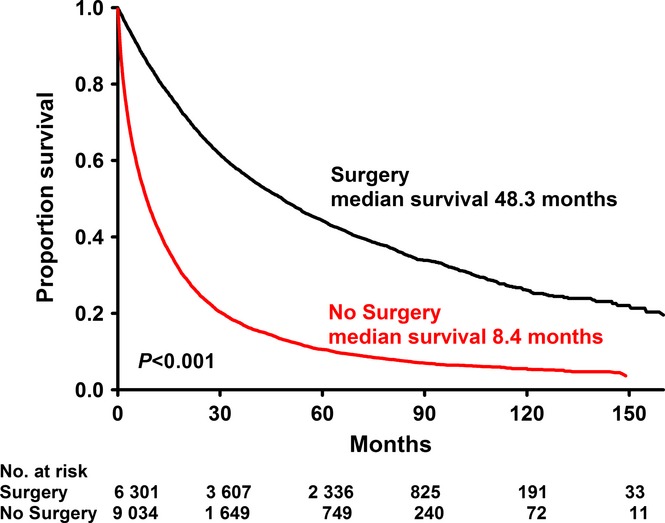
Kaplan–Meier survival analysis of all stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB) based on whether or not they received surgical management
Table 3.
Multivariate analysis of predictors of survival for all stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB)
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Having surgery | 0.74 | 0.70–0.78 | <0.001 |
| Clinical Stage 2 (versus 1) | 1.06 | 1.02–1.11 | 0.001 |
| Age | 1.01 | 1.00–1.01 | <0.001 |
| Male | 1.04 | 0.99–1.10 | 0.121 |
| Race | |||
| White | 1.00 | ||
| Asian | 0.84 | 0.77–0.91 | <0.001 |
| Black | 1.05 | 0.98–1.12 | 0.216 |
| Other | 0.95 | 0.80–1.13 | 0.572 |
| Insurance | |||
| Private | 1.00 | ||
| Medicare | 1.11 | 1.05–1.18 | 0.001 |
| Medicaid | 1.15 | 1.06–1.24 | 0.001 |
| Not Insured | 1.14 | 1.01–1.28 | 0.037 |
| Other | 1.10 | 0.90–1.36 | 0.355 |
| Income | |||
| >$46 000 | 1.00 | ||
| $35 000–46000 | 1.04 | 0.97–1.11 | 0.262 |
| $30 000–35000 | 1.05 | 0.97–1.13 | 0.257 |
| <$30000 | 1.06 | 0.97–1.16 | 0.234 |
| Education | |||
| <14% | 1.00 | ||
| 14–20% | 1.03 | 0.96–1.11 | 0.383 |
| 20–29% | 1.03 | 0.96–1.11 | 0.416 |
| >29% | 1.01 | 0.93–1.10 | 0.821 |
| Charlson–Deyo Score | |||
| 0 | 1.00 | ||
| 1 | 1.02 | 0.96–1.07 | 0.542 |
| 2 | 1.02 | 0.96–1.08 | 0.494 |
| Facility | |||
| Academic Programme | 1.00 | ||
| Community Cancer Programme | 1.07 | 0.98–1.17 | 0.154 |
| Comprehensive Community Cancer Programme | 1.07 | 1.02–1.13 | 0.009 |
| Other Cancer Programme | 0.92 | 0.74–1.15 | 0.463 |
CI, confidence interval; education, percentage of people in the patient's zip code without a high school degree.
Subgroups of non-surgical patients
In an effort to better understand the types of patients with curable HCC who did not receive surgical management, we performed a subset analysis on three groups of non-surgical patients: those with small tumours (<2 cm), stage I cancer or no comorbidities (Charlson–Deyo score = 0). As seen in Table S2 (available online as Supporting Information) these patients differ in several ways from the patients in Table S1 (available online as Supporting Information) who had surgery. Patients in these groups were more often of black race, lower income, less education and treated at a community centre. They were also less likely to have private insurance and more likely to be uninsured.
Discussion
This updated analysis of the treatment of early stage HCC demonstrates that surgical treatment is still quite underused in a potentially curable cancer. Surgery was associated with significantly increased survival among patients with stage I/II HCC, and yet < 40% of patients were managed surgically. Moreover, non-medical patient factors appear to be associated with this treatment decision. While reported rates of resectability for HCC are lower than 40%, this analysis focuses on early stage HCC and is the most nationally representative study to do so.
Several previous studies have identified possible explanations for this underutilization of surgical therapy. Some have suggested that demographic factors, such as race or socioeconomic status, influence the use of surgery for HCC.3,5,9–11 While socioeconomic factors may influence treatment, it is unclear if they affect survival.4,9,12,13 It has also been suggested that the location and type of hospital are important factors affecting treatment patterns.6,7 However, many of these studies are limited by a reliance on data from a single institution or Medicare, and may not accurately represent national treatment patterns for all patients with HCC.
In this study, several patient characteristics were associated with a less likelihood of surgical management, higher 30-day mortality and inferior long-term survival. Not surprisingly, older patients and those with stage II (compared to stage I) fared worse in all three of these categories. Patients on Medicare, Medicaid or without insurance were less likely to have surgery, and those that did have worse outcomes. Insurance status has previously been shown to affect treatment and survival.5,9,14 While these studies also showed that treatment was affected by increased stage at presentation, with privately insured patients presenting earlier, this study has shown that mortality and survival are different even among early stage HCC. Also, as government insurance is being expanded nationally,15 it is important to have a thorough understanding of how Medicaid and Medicare outcomes compare to those of privately insured patients.
In contrast to insurance status, patient socioeconomic status played a different role. Patients who were black had lower income or less education were significantly less likely to receive surgical treatment. However, when considering HCC patients who received surgery, these factors did not impact 30-day mortality or survival. These contrasting findings highlight the importance of access to care. Socioeconomic status matters for a variety of reasons, but perhaps a patient's race, education or income affects his/her cancer survival much less than the availability of appropriate and reliable treatment. This was further supported by the sub-analysis of non-surgical patients (Table S2, available online as Supporting Information); while race, income and education of these patients were different from surgically managed patients, the most striking difference between the groups was the rate of uninsured patients.
Another variation in care and outcomes was based on treatment facilities. In this analysis, academic cancer centres performed vastly more surgery on early stage HCC than did community cancer centres. Also, comprehensive community cancer centres, as designated by the NCDB, had worse 30-day mortality and inferior survival compared with academic centres. Prior studies have shown that hospital type can influence treatment decisions16,17 and outcomes.18–20 Although internationally accepted consensus treatment, guidelines are available, such as the BCLC staging system,21 they do not account for disparities in patient and hospital resources. For example, hospitals without adequate radiofrequency ablation (RFA) or transplant services, or patients without reliable financial and social support, are unable to adhere to such guidelines. These data suggest that academic hospitals may be better suited to handle the type of complicated, multi-disciplinary care that HCC requires than their community counterparts.
This study has limitations owing to its retrospective and administrative nature. The results only identify correlations and were not able to define causation. Also, some data fields are vague or possibly incorrectly populated. For example, the documented reason for most patients who did not receive surgery was ‘not part of first planned treatment’ without any further explanation. Because of this, we are unable to understand truly how comorbidities, patient and provider preference, hospital resources and other specific variables affect treatment decisions. For example, we could not evaluate the role of or patient preference or degree of liver disease. Survival analyses are also limited owing to missing values in some data fields. The NCDB does not provide specific treatment centre characteristics other than geographic area and academic/community designation, so further investigations into nuanced differences between hospitals and what affects treatment decisions are limited. Also, the ‘other’ surgical category represents a variety of interventional techniques, including transarterial therapy and cryosurgery, which are quite different from surgical resection and transplantation. Finally, while the use of patient zip codes and census data is a validated method to compare socioeconomic measures,20,22,23 it is a surrogate for more granular patient data and potentially confounds the results.
In conclusion, this analysis has shown that surgery improves survival for patients with early stage HCC, but it is vastly underutilized in the United States. Socioeconomic factors, as well as type of facility, seem to affect these treatment decisions. These data suggest that a key barrier is getting patients to the appropriate centre for cancer treatment, as race and socioeconomic status were not associated with differences in survival. In order to improve survival for the growing number of patients with HCC, further research is necessary to understand the specific barriers to care for patients with curable disease.
Funding sources
Funding was received from the University of Cincinnati Department of Surgery.
Conflict of interest
None to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Univariate analysis of all stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB) based on whether or not they received surgical management.
Table S2. Univariate analysis of hepatocellular carcinoma (HCC) patients with small tumors (<2 cm), stage I disease or no comorbidities (Charlson–Deyo score = 0) who did not have surgery.
References
- 1.Mikhail S, Cosgrove D, Zeidan A. Hepatocellular carcinoma: systemic therapies and future perspectives. Expert Rev Anticancer Ther. 2014;14:1205–1218. doi: 10.1586/14737140.2014.949246. [DOI] [PubMed] [Google Scholar]
- 2.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38:703–712. doi: 10.1111/apt.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaki P, Wong RJ, Marupakula V, Nangia S, Nguyen L, Ditah IC, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer. 2014;120:1725–1732. doi: 10.1002/cncr.28639. [DOI] [PubMed] [Google Scholar]
- 5.Zak Y, Rhoads KF, Visser BC. Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg. 2011;146:778–784. doi: 10.1001/archsurg.2011.37. [DOI] [PubMed] [Google Scholar]
- 6.Hyder O, Dodson RM, Nathan H, Herman JM, Cosgrove D, Kamel I, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J Am Coll Surg. 2013;217:896–906. doi: 10.1016/j.jamcollsurg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SA, Smith JK, Li Y, Ng SC, Carroll JE, Tseng JF. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117:1019–1026. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 8.Mohanty S, Bilimoria KY. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. 2014;109:629–630. doi: 10.1002/jso.23568. [DOI] [PubMed] [Google Scholar]
- 9.Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer. 2010;116:1801–1809. doi: 10.1002/cncr.24936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins AS, Cox DD, Johnson LB, Ward EM. Persistent disparities in liver transplantation for patients with hepatocellular carcinoma in the United States, 1998 through 2007. Cancer. 2011;117:4531–4539. doi: 10.1002/cncr.26063. [DOI] [PubMed] [Google Scholar]
- 11.Nathan H, Hyder O, Mayo SC, Hirose K, Wolfgang CL, Choti MA, et al. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Ann Surg. 2013;258:1022–1027. doi: 10.1097/SLA.0b013e31827da749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong RJ, Corley DA. Survival differences by race/ethnicity and treatment for localized hepatocellular carcinoma within the United States. Dig Dis Sci. 2009;54:2031–2039. doi: 10.1007/s10620-008-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 14.Zaydfudim V, Whiteside MA, Griffin MR, Feurer ID, Wright JK, Pinson CW. Health insurance status affects staging and influences treatment strategies in patients with hepatocellular carcinoma. Ann Surg Oncol. 2010;17:3104–3111. doi: 10.1245/s10434-010-1181-2. [DOI] [PubMed] [Google Scholar]
- 15.Jones DK, Singer PM, Ayanian JZ. The changing landscape of Medicaid: practical and political considerations for expansion. JAMA. 2014;311:1965–1966. doi: 10.1001/jama.2014.3700. [DOI] [PubMed] [Google Scholar]
- 16.Nathan H, Segev DL, Bridges JF, Massie AB, Cameron AM, Hirose K, et al. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol. 2013;20:448–456. doi: 10.1245/s10434-012-2619-5. [DOI] [PubMed] [Google Scholar]
- 17.Parsons HM, Harlan LC, Stevens JL, Ullmann CD. Treatment of small cell lung cancer in academic and community settings: factors associated with receiving standard therapy and survival. Cancer J. 2014;20:97–104. doi: 10.1097/PPO.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell MC, You YN, Hu CY, Cormier JN, Feig BW, Skibber JM, et al. A novel risk-adjusted nomogram for rectal cancer surgery outcomes. JAMA Surg. 2013;148:769–777. doi: 10.1001/jamasurg.2013.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassig AA, Joseph AM, Lindgren BR, Fernandes P, Cooper S, Schotzko C, et al. The effect of treating institution on outcomes in head and neck cancer. Otolaryngol Head Neck Surg. 2012;147:1083–1092. doi: 10.1177/0194599812457324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khullar OV, Gillespie T, Nickleach DC, Liu Y, Higgins K, Ramalingam S, et al. Socioeconomic risk factors for long-term mortality after pulmonary resection for lung cancer: an analysis of more than 90,000 patients from the National Cancer Data Base. J Am Coll Surg. 2015;220 doi: 10.1016/j.jamcollsurg.2014.10.009. 156–168.e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoehn RS, Wilson GC, Wima K, Hohmann SF, Midura EF, Woodle ES, et al. Comparing living donor and deceased donor liver transplantation: a matched national analysis from 2007 to 2012. Liver Transpl. 2014;20:1347–1355. doi: 10.1002/lt.23956. [DOI] [PubMed] [Google Scholar]
- 23.Martin JM, Handorf EA, Kutikov A, Uzzo RG, Bekelman JE, Horwitz EM, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer. 2014;120:2114–2121. doi: 10.1002/cncr.28697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate analysis of all stage I/II hepatocellular carcinoma (HCC) patients in the National Cancer Data Base (NCDB) based on whether or not they received surgical management.
Table S2. Univariate analysis of hepatocellular carcinoma (HCC) patients with small tumors (<2 cm), stage I disease or no comorbidities (Charlson–Deyo score = 0) who did not have surgery.


