Abstract
Background
A variety of techniques have been described for portal vein (PV) and/or superior mesenteric vein (SMV) resection/reconstruction during a pancreatectomy. The ideal strategy remains unclear.
Methods
Patients who underwent PV/SMV resection/reconstruction during a pancreatectomy from 2005 to 2014 were identified. Medical records and imaging were retrospectively reviewed for operative details and outcomes, with particular emphasis on patency.
Results
Ninety patients underwent vein resection/reconstruction with one of five techniques: (i) longitudinal venorrhaphy (LV, n = 17); (ii) transverse venorrhaphy (TV, n = 9); (iii) primary end-to-end (n = 28); (iv) patch venoplasty (PV, n = 17); and (v) interposition graft (IG, n = 19). With a median follow-up of 316 days, thrombosis was observed in 16/90 (18%). The rate of thrombosis varied according to technique. All patients with primary end-to-end or TV remained patent. LV, PV and IG were all associated with significant rates of thrombosis (P = 0.001 versus no thrombosis). Comparing thrombosed to patent, there were no differences with respect to pancreatectomy type, pre-operative knowledge of vein involvement and neoadjuvant therapy. Prophylactic aspirin was used in 69% of the total cohort (66% of patent, 81% of thrombosed) and showed no protective benefit.
Conclusions
Primary end-to-end and TV have superior patency than the alternatives after PV/SMV resection and should be the preferred techniques for short (<3 cm) reconstructions.
Introduction
A pancreatectomy with venous reconstruction is increasingly being performed to offer the benefits of surgical resection to patients with locally advanced disease. Several single-centre reports have established that a pancreaticoduodenectomy with venous resection/reconstruction can be performed with comparable morbidity, mortality and long-term survival to those with standard resections.1–3 The 2009 expert consensus statement advocated for a pancreaticoduodenectomy with vein resection/reconstruction as a recommended standard of practice for pancreatic adenocarcinomas locally invading the portal vein (PV)/ superior mesenteric vein (SMV) or the superior mesenteric-portal vein (SM-PV) confluence in institutions experienced and capable of doing these technical operations.4 The role of surgery with vascular reconstruction in pancreatic neuroendocrine (pNET) tumours is somewhat less defined than pancreatic adenocarcinoma; however, the retrospective data available supports an aggressive approach to surgical resection in carefully selected patients.5,6
At present, venous resection/reconstruction during a pancreaticoduodenectomy is performed in up to 20-25% of patients in some centres.1 Despite being increasingly common, a vascular resection during pancreatic surgery is non-standardized. Although a variety of techniques have been described,7–9 the ideal strategy remains unclear. Outside of the basic tenants to create a tension-free anastomosis and optimize size match when interposition grafting is used, there is little in the literature relating the technical aspects and outcomes specific to each procedure. In addition, there is significant heterogeneity in the use of anticoagulation/antiplatelet therapy after PV/SMV reconstruction; use is at the discretion of the surgeon with no published guidelines that exist for the type or duration of anticoagulation/antiplatelet after venous reconstruction.10 The aim of this study was to define the rate and predictors of thrombosis after a pancreatectomy and concomitant venous resection/reconstruction, with particular attention to the influences of operative technique and post-operative pharmacological management.
Patients and methods
Patients
Patients who underwent any pancreatic resection for any pathology where a resection and reconstruction of the portomesenteric venous system was performed from 2005 to 2014 were identified through a prospectively maintained database. Surgical resection types included a Whipple pancreaticoduodenectomy, total pancreatectomy and subtotal pancreatectomy. Venous reconstruction was performed by one of three surgeons (one hepato-pancreato-biliary and two vascular) surgeons. Specific patient data were retrospectively collected using the hospital electronic medical record after Institutional Review Board approval was obtained. Data abstracted included demographics, neoadjuvant and treatment history, intra-operative variables, type of vascular reconstruction, and pathological staging. Post-operative imaging studies were reviewed to determine patency or occlusion of the venous reconstruction. All patients underwent radiographic surveillance follow-up at 1, 3, then at 3-month intervals with computed tomography (CT) or magnetic resonance imaging (MRI). Those patients that underwent venous reconstruction by a vascular surgeon had several other follow-up appointments and additional imaging by vascular ultrasound. Acute thrombosis was defined as occlusion of the portal venous system confirmed by imaging within 30 days of the operation; late thrombosis was defined as lack of patency on follow-up imaging after 30 days from surgery.
Operative technique
Patients with CT evidence of tumour vessel abutment or occlusion at the PV, SMV or SM-PV confluence had the right neck or one upper thigh prepped and draped for access to the right internal jugular or the superficial femoral vein according to surgeon preference. The right internal jugular vein takes a more superficial course in the neck and has a larger diameter than the left internal jugular vein.11 Venous reconstruction was categorized into one of five techniques. Those without use of a conduit include (i) longitudinal venorrhaphy (LV – either by using a Satinsky clamp with a longitudinal closure of the vein, Fig. 1a–b or by performing a sleeve resection of the vein with a TA30 stapler pulled close to the tumour, Fig. 1c–d), (ii) transverse venorrhaphy (TV) where a longitudinal ellipse of the vein is excised; however, a transverse closure of the vein is performed (Fig. 1e–f), and (iii) segmental resection of the vein (with or without splenic vein preservation) and primary end-to-end closure (primary) using a running 6-0 Prolene suture (Fig. 2a–b). Venous reconstruction that included use of a conduit was performed by (iv) patch venoplasty (patch) with a native vein harvested from another location, a cryopreserved vein or Bovine pericardium used to fill a tangential resection of the vein (Fig. 3a) and (v) segmental resection of the vein and interposition graft (IG) reconstruction through a number of native conduits, primarily including the internal jugular vein, renal vein, saphenous vein and superficial femoral vein (Fig. 3b–c). Splenic vein resection was not performed routinely; the vein was divided when tumour invasion involved this confluence, additional venous length was needed to perform primary end-to-end closure, or to facilitate exposure to the proximal superior mesenteric artery (SMA) if required. Prior to venous reconstruction, in most cases, the arterial dissection was completed first so that the specimen was left attached only at the site of vein encasement or abutment. In those instances where chronic venous occlusion had resulted in numerous varices, early decompression was accomplished by creating a mesocaval shunt or early venous reconstruction with internal jugular grafting prior to pancreatic dissection. Systemic heparinization was not routinely used for venous reconstruction.
Figure 1.
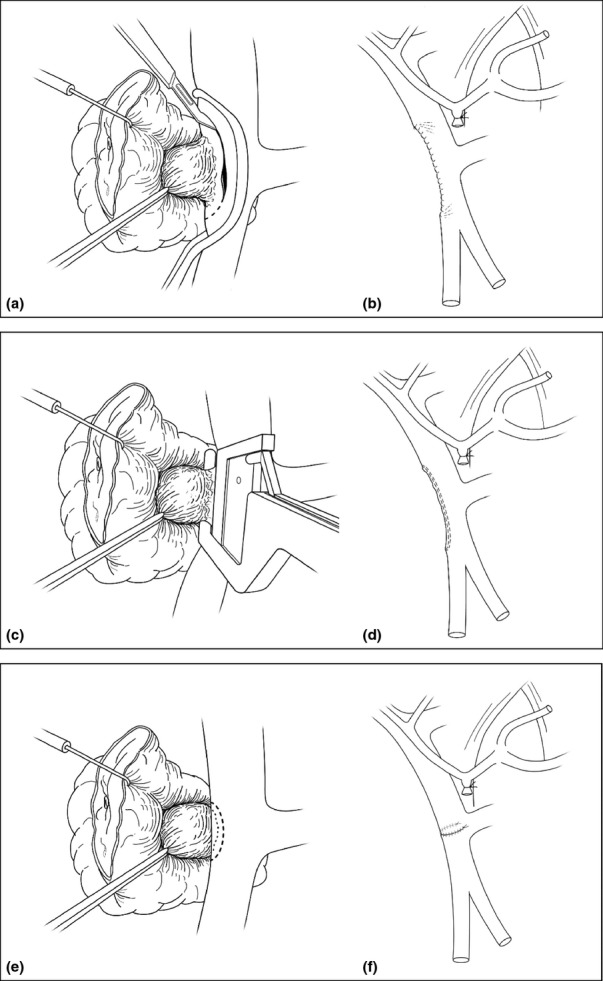
Technical illustrations of before and after images of a longitudinal venorrhaphy performed via Statisnksy clamp (a, b), via a TA 30 stapler (c, d), and transverse venorrhaphy (e, f). Note that the transverse venorrhapy is the least likely to compromise the vein lumen diameter
Figure 2.
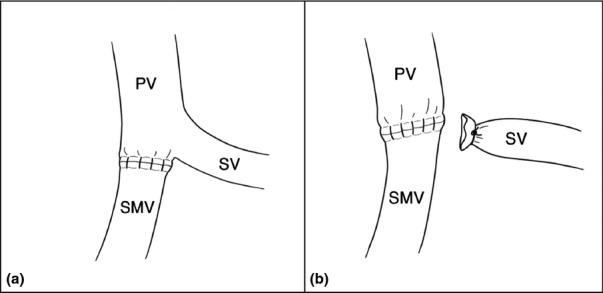
Technical illustrations of venous reconstruction for shorter segmental resections with primary end-to-end closure with (a) or without (b) splenic vein preservation. PV, portal vein; SMV, superior mesenteric vein; SV, splenic vein
Figure 3.
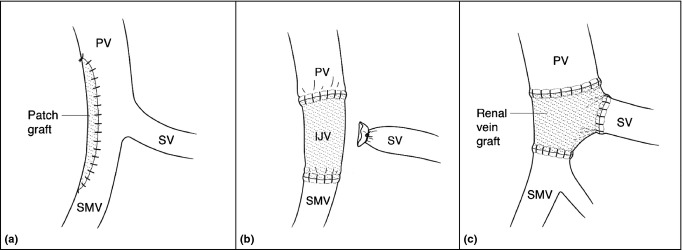
Technical illustration of venous reconstructions for longer segmental resections using a patch (a) or interposition graft conduit such as the internal jugular vein (IJV) (b) or a renal vein graft (c). PV, portal vein; SMV, superior mesenteric vein; SV, splenic vein
Statistical analysis
Discrete categorical variables were compared using the chi-square test or Fisher's exact test, where applicable. Continuous variables were expressed as median with an interquartile range (IQR) and means were compared using the Mann–Whitney U-test. Kaplan–Meier estimations were used to analyse venous patency from the time of surgery. All tests were two-tailed, and statistical significance was set at a P-value < 0.05. Statistical analyses were performed using STATA version 13.1 (StataCorp, College Station, TX, USA).
Results
Venous reconstruction
A pancreatectomy requiring venous reconstruction was performed in 90 patients during the study period out of 665 total pancreatectomies. Patient characteristics of the cohort, stratified by the occurrence of thrombosis, are shown in Table 1. Operative and post-operative characteristics are shown in Table 2. In patients in which a Patch or IG reconstruction was performed, an autologous conduit or patch was used in 24 (27%) reconstructions (2 gonadal, 4 internal jugular, 2 renal, 6 saphenous and 10 superficial femoral veins) and a preserved conduit or patch such as cryovein (n = 2) or bovine pericardium (n = 10) was used in 12 (13%). There was one peri-operative death within 30-days (1%) in a patient from the thrombosed group who underwent a Whipple for pancreatic adenocarcinoma. The patient suffered a post-operative bile leak and an episode of intraabdominal bleeding requiring transfusion on post-operative day 11; however, no etiology of the bleed was found on CT angiogram. The scan demonstrated new splenic and SMV/PV thrombosis as it entered the reconstructed portal vein. The patient ultimately sustained a post-operative aspiration event leading to cardiac arrest and multi-organ failure with the eventual withdrawal of care. Mortality within 90-days, including the above patient, was 4.4%.
Table 1.
Characteristics of the patients who underwent venous reconstruction
| Variable | All patients (n = 90) | Patent (n = 74) | Thrombosed (n = 16) | P-value |
|---|---|---|---|---|
| Age, median (IQR) | 67 (55–73) | 68 (59–74) | 63 (51–70) | 0.108 |
| Male, n (%) | 54 (60) | 43 (58) | 11 (69) | 0.576 |
| Pre-operative vein invasion, n (%) | 58 (64) | 46 (62) | 12 (75) | 0.399 |
| Neoadjuvant chemotherapy | 20 (22) | 15 (20) | 5 (31) | 0.337 |
| Histology, n (%) | ||||
| Adenocarcinoma | 67 (74) | 56 (76) | 11 (68.5) | 0.582 |
| Neuroendocrine | 17 (19) | 14 (19) | 3 (19) | |
| Other | 6 (7) | 4 (5) | 2 (12.5) | |
| Operation, n (%) | ||||
| Whipple | 73 (81) | 60 (81) | 13 (81) | 0.556 |
| Subtotal | 12 (6) | 9 (12) | 3 (19) | |
| Total | 5 (13) | 5 (7) | 0 | |
| Lymphovascular invasion, n (%) | 50 (56) | 42 (57) | 8 (50) | 0.782 |
| Nodal disease, n (%) | 65 (72) | 55 (74) | 10 (63) | 0.365 |
IQR, interquartile range.
Table 2.
Venous reconstruction operative and postoperative characteristics
| Variable | All patients (n = 90) | Patent (n = 74) | Thrombosed (n = 16) | P-value |
|---|---|---|---|---|
| Vascular reconstruction type, n (%) | ||||
| LV | 17 (19) | 13 (18) | 4 (25) | 0.001 |
| TV | 9 (10) | 9 (12) | 0 | |
| Patch | 17 (19) | 12 (16) | 5 (31) | |
| IG | 19 (21) | 12 (16) | 7 (44) | |
| Primary | 28 (31) | 28 (38) | 0 | |
| Conduit, n (%) | ||||
| None | 54 (60) | 50 (68) | 4 (25) | 0.007 |
| Autologous | 24 (27) | 16 (22) | 8 (50) | |
| Preserved | 12 (13) | 8 (10) | 4 (25) | |
| Vein resected, n (%) | ||||
| PV | 20 (22) | 17 (23) | 3 (18.75) | 0.715 |
| SMV | 47 (52) | 37 (50) | 10 (62.5) | |
| PV/SMV confluence | 23 (26) | 20 (27) | 3 (18.75) | |
| Operating time, min, median (IQR) | 420 (356–486) | 401 (351–468) | 480 (400–546) | 0.003 |
| Blood loss, ml, median (IQR) | 750 (400–1238) | 600 (400–1200) | 1000 (775–1625) | 0.128 |
| Transfusion, n (%) | 43 (48) | 32 (43) | 11 (69) | 0.096 |
| Shunt/temporary graft, n (%) | 3 (3.3) | 2 (2.7) | 1 (6.25) | 0.448 |
| Length of venous resection, cm, median (IQR) | 2.5 (2–3.75) | 2.5 (2–3.25) | 3 (2.4–4) | 0.203 |
| Anticoagulation type, n (%) | ||||
| Intravenous heparin | 6 (7) | 3 (4) | 3 (19) | 0.066 |
| Coumadina | 4 (4) | 4 (5) | 0 | 1.000 |
| Low molecular weight heparin | 7 (8) | 4 (5) | 3 (19) | 0.103 |
| Aspirin | 63 (70) | 50 (68) | 13 (81) | 0.374 |
| Anticoagulation duration, n (%) | ||||
| ≤3 months | 28 (31) | 25 (34) | 3 (19) | 0.373 |
| >3 months | 36 (40) | 26 (35) | 10 (63) | 0.052 |
| Pancreatic fistula, n (%) | 10 (11) | 7 (9) | 3 (19) | 0.374 |
| Post-operative ICU, n (%) | 65 (72) | 50 (68) | 15 (94) | 0.035 |
| Length of stay, median (IQR) | 11 (9–18) | 10 (9–16) | 18 (11–27) | 0.006 |
All four patients on Coumadin were on Coumadin prior to venous reconstruction for other indications.
LV, longitudinal venorrhaphy; TV, transverse venorrhaphy; IG, interposition graft; PV, portal vein; SMV, superior mesenteric vein; IQR, interquartile range; ICU, intensive care unit.
Patency and post-operative pharmacological management
With a median follow-up of (last available imaging to assess patency) 316 days (IQR 173–679), thrombosis occurred in 16 of the 90 patients (18%). The median time to thrombosis was 43 days (IQR 12–73). The Kaplan–Meier estimate of venous patency for each of the reconstruction types is demonstrated in Fig. 4. The overall estimate of venous patency for all types was 78% at 1000 days.
Figure 4.
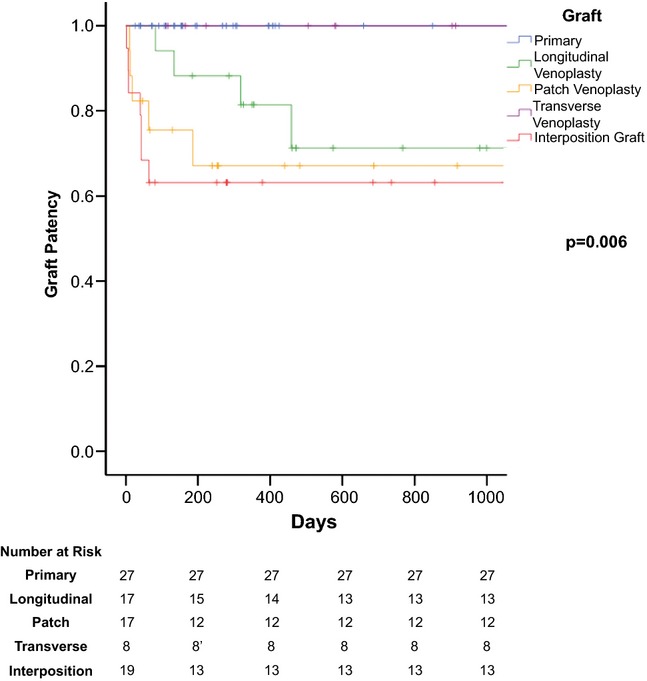
Patency of venous reconstructions
The use of anticoagulation (warfarin, low-molecular-weight heparin) varied within the cohort, in some patients being used for pre-existing conditions (Table 2). With regard to antiplatelet therapy, prophylactic aspirin was given to patients in 70% of the total cohort (66% of patent and 81% of thrombosed patients) but neither aspirin use (yes/no) or duration of therapy (≤3 months or >3 months) showed any protective benefit between the groups (Table 2).
Thrombosis l
Of the 16 patients that developed thrombosis, acute thrombosis (within 30 days of surgery) occurred in six patients. Three patients underwent patch reconstruction and the other three underwent IG reconstruction. Five of the six patients had early CT scans done for individual reasons (one patient for of a haemorrhage as noted above, two patients for ileus, one patient for delayed gastric emptying and one patient for a suspected colon anastomotic leak after concomitant transverse colectomy (in addition to a subtotal pancreatectomy owing to mesenteric invasion) during which the thrombosis was noted but there was no significant clinical sequelae related to the thrombosis. All of these patients were out of the intensive care unit and haemodynamically stable at the time of CT scan. Of the remaining 10 patients that had late thrombosis, the median time to thrombosis was 73 days (IQR 48–173). Three of the ten patients were found on the same scan as an overt cancer recurrence in the pancreatic resection bed. Six of the remaining seven patients with late thrombosis were still within 64 days post-operative.
Discussion
In experienced centres, a resection of pancreatic tumours with SM-PV involvement can be achieved with acceptable morbidity and mortality. With proper patient selection, the need for a vascular resection in patients with pancreatic adenocarcinoma does not significantly impact survival duration if a complete resection (R0/R1) has been performed.1,12 A recent review of outcomes of 1000 pancreatectomies (of which almost a quarter underwent vascular reconstructions) found that vascular complications, including both thrombosis and a haemorrhage, were the leading cause of early death.13 Patency rates between 70% and 90% after vascular reconstruction are reported using a wide variety of conduits – PTFE,14 left renal vein,15 saphenous vein,7 allografts,16 as well as different tangential or segmental resections with patch, primary anastomosis or interposition grafting.9 However, in contrast to a grouped patency rate for all types of venous reconstruction, to better examine the effect of technique on long-term patency of the SMV/PV confluence after reconstruction, patients were classified by type of reconstruction and individual patency rates were calculated.
The present findings demonstrated that patients who underwent a pancreatectomy with vascular reconstruction by means of a segmental resection with a primary end-to-end technique or tangential resection with TV closure had 100% patency rates compared with those undergoing LVclosure (71.3%), IG (63.2%) or patch venoplasty (67.1%) carried out to 1000 days. These two types of reconstruction demonstrated better patency rates than the alternatives after venous resection and reconstruction and should be the preferred techniques for short (<3 cm) segments of the involved vein. Tumour abutment of the lateral or posterolateral wall of the SMV or SM-PV confluence may not always be appreciated on pre-operative imaging.17 A subtle deformity of the vein wall at the tumour interface can often indicate tumour adherence on an adequate venous phase of a contrast-enhanced CT scan. Therefore, surgeons performing these operations should have a strategy to deal with venous adherence discovered at the time of surgery. Within this series, a third of patients were not suspected pre-operatively to require vein resection – in retrospect, these patients primarily underwent an LV or TV reconstruction. Seventy-six percent of LVs were performed in patients where pre-operative vein involvement was unsuspected. In these situations, the TA stapler or longitudinal suture closure are easy and expeditious reconstructions, but can lead to narrowing of the vein (compared to closure in a transverse fashion) and this reduction in diameter, can over time lead to patency failure. There was a change throughout the study period where 88% of the LV had been performed in the early part of the study (2005–2011); however, this transitioned to 78% of the TV performed in the later part of the study (2012–2014) with this latter technique frequently used (and replacing the LV) for unsuspected preoperative vein involvement. Primary end-to-end is another option for reconstruction, often used in the setting of known vein involvement with planned reconstruction. Even in the case of subtotal pancreatectomies, complete mobilization of the right colon mesentery, detachment of the transverse mesocolon from the anterior surface of the duodenum and pancreas, division of the ligament of Treitz, mobilization of the distal duodenum and proximal jejunum and ligation of the splenic vein are all manoeuvers which brings the SMV stump cephalad to facilitate primary anastomosis in shorter resected segments.18
Both the IG and the patch were intended for longer length defects; however, the patch venoplasty was preferred for tumours that only involved the lateral or anterior wall of the vein or in some patients, when there were two separate areas of vein adherence to the tumour so as not to create too long of an IG. These two types of reconstruction had the lowest patency rates of the cohort and all patients with early thrombosis had reconstructions done via these two types. Of the 17 patch venoplasties, 14 operative reports recorded the length of the patch used, and five patches were noted to be under 3.5 cm. Of the 19 interposition grafts, 13 reports recorded length of the graft placed, and five were noted to be under 3.5 cm. In retrospect, perhaps in some of these instances, more mobilization might have resulted in a primary end-to-end reconstruction based on length reported (although it is also very possible that a greater length of PV/SMV was resected than the ultimate length of the interposition graft used, so it is very difficult to assess this retrospectively). The other eight cases had reported vein involvement at a length between 4 and 6 cm, probably indicating bulkier tumours and more technically difficult resections. Of our 16 total cases that thrombosed, 44% consisted of IG reconstructions and, as a result, this type of reconstruction demonstrated the lowest patency rate of all five techniques. For longer segment resections, we feel there is still an important role for the use of IG reconstructions, and this has been demonstrated in other series to have high patency rates when autologous venous conduits are used.9,19 Of the IGs that thrombosed, there was one case done by the HPB surgeon using an IJ vein conduit. A mesocaval shunt was used for a cystic tumour with SMV and splenic vein thrombosis (with resulting hypersplenism, varices and thrombocytopenia). The graft was patent on a routine scan 2 months after surgery but thrombosed at month 3 when the patient was admitted with urospesis (with no clinical sequelae of the thrombosis at that time nor with three additional years of follow-up). We suspect that the presence of well-developed collaterals in these patients with complete occlusion pre-operatively may contribute to their risk of thrombosis because of reduced flow through the graft.
A limitation of this study is that the remaining patients were done by vascular surgeons and varied in technique and conduit use. Two of the IG reconstructions were done with cadaveric vein grafts, and one reconstruction had a size mismatch with the saphenous vein, elements that may also have contributed to post-operative thrombosis. The remaining three cases were done with a superficial femoral vein – one case represents the only case reconstructed in an emergent fashion owing to an injury to the SMV during a pancreaticoduodenectomy. While two attempts at a primary reconstruction were performed, there was still significant tension on the repair in the setting of poor proximal and distal control. An IG was eventually performed but there was note of Fogarty embolectomy clot extraction prior to completion of the proximal anastomosis, given the increased length of time for reconstruction. This case ultimately resulted in one of the acute thromboses. Therefore, in many of these IG cases, there were certainly identifiable factors (beyond the technique in its most basic form) that led to a higher risk of thrombosis. A significantly longer operative time was noted in patients with thrombosis, suggesting an increased difficulty in the operative procedure as well. We continue to believe that IG with a well-matched graft (we favour the internal jugular) is the preferred reconstruction when >3–4 cm of PV/SMV is resected.
Most institutional studies incorporate various forms of anticoagulation/antiplatelet therapy at the discretion of the surgeon with aspirin being a common minimum therapy for prophylaxis (as was the case in this series). Within this cohort, 70% of patients were placed on aspirin post-operatively and 58% of the patients were on aspirin for >3 months duration. There was no protective benefit in prophylactic use of aspirin or duration of use in the prevention of thrombosis. In another reported series of 64 patients who underwent a pancreaticoduodenectomy with vascular reconstruction where 53% of patients received anticoagulation with warfarin or antiplatelet therapy with aspirin or clopidogrel (based on surgeon preference), there was no difference in thrombosis rates between those receiving anticoagulation or not.14 One factor that motivates the use of anticoagulation in patients with venous reconstruction done for pancreatic adenocarcinoma is the fact that malignancy is a clear risk factor for thrombosis and that even among cancer patients, thromboembolic events are most common after esophagogastric and hepatopancreaticobiliary procedures.20 However, this needs to be carefully weighed against the risk of post-operative bleeding: reported to be present in 2–17% of pancreatectomies and has been reported to be a leading cause of death of patients that died within 90 days of a pancreaticoduodenectomy.13 A haemorrhage secondary to an eroded or pseudoaneurysmal visceral vessel already has a reported mortality rate of 23% and typically presents with an initial sentinel bleed that in the setting of anticoagulation would be lethal. Therefore, routine post-discharge subcutaneous heparin or Lovenox are infrequently used for prophylaxis. A recent meta-analysis incorporating 13 studies and 361 patients with both benign and malignant disease (eight studies with an anticoagulation policy including aspiring, clopidogrel, heparin, or warfarin and five studies without an anticoagulation policy) found no difference in morbidity, mortality, or incidence of early portal vein thrombosis in pancreatic resections with venous reconstruction.10 Despite these data, given the technical complexity and heterogeneity of these operations and the consequences of thrombosis, it would be difficult to standardize practice in the absence of a randomized prospective trial.
In conclusion, our data demonstrate that a pancreatectomy with venous resection/reconstruction can be performed safely. The technique of reconstruction strongly affects patency. For short segment resections (<3 cm), although often more time consuming, primary end-to-end reconstructions and transverse venorrhaphies provide superior outcomes to the alternatives (longitudinal venorrhaphies and patch venoplasty). For longer segmental resections (>3.5 cm), where the options are either patch venoplasty or interposition grafting, there is still an important role for interposition grafting when done with an appropriate size match and autologous vein to achieve greater long-term patency.
Funding sources
None.
Conflict of interest
None declared.
References
- 1.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- 2.Toomey P, Hernandez J, Morton C, Duce L, Farrior T, Villadolid D, et al. Resection of portovenous structures to obtain microscopically negative margins during pancreaticoduodenectomy for pancreatic adenocarcinoma is worthwhile. Am Surg. 2009;75:804–809. doi: 10.1177/000313480907500911. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- 3.Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 5.Haugvik SP, Labori KJ, Waage A, Line PD, Mathisen O, Gladhaug IP. Pancreatic surgery with vascular reconstruction in patients with locally advanced pancreatic neuroendocrine tumors. J Gastrointest Surg. 2013;17:1224–1232. doi: 10.1007/s11605-013-2221-6. [DOI] [PubMed] [Google Scholar]
- 6.Norton JA, Harris EJ, Chen Y, Visser BC, Poultsides GA, Kunz PC, et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146:724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DY, Mitchell EL, Jones MA, Landry GJ, Liem TK, Sheppard BC, et al. Techniques and results of portal vein/superior mesenteric vein reconstruction using femoral and saphenous vein during pancreaticoduodenectomy. J Vasc Surg. 2010;51:662–666. doi: 10.1016/j.jvs.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Chu CK, Farnell MB, Nguyen JH, Stauffer JA, Kooby DA, Sclabas GM, et al. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: a multicenter analysis. J Am Coll Surg. 2010;211:316–324. doi: 10.1016/j.jamcollsurg.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Krepline AN, Christians KK, Duelge K, Mahmoud A, Ritch P, George B, et al. Patency rates of portal vein/superior mesenteric vein reconstruction after pancreatectomy for pancreatic cancer. J Gastrointest Surg. 2014;18:2016–2025. doi: 10.1007/s11605-014-2635-9. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasegaram MD, Eslick GD, Lee W, Brooke-Smith ME, Padbury R, Worthley CS, et al. Anticoagulation policy after venous resection with a pancreatectomy: a systematic review. HPB. 2014;16:691–698. doi: 10.1111/hpb.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizuka M, Nagata H, Takagi K, Kubota K. Right internal jugular vein is recommended for central venous catheterization. J Invest Surg. 2010;23:110–114. doi: 10.3109/08941930903469342. [DOI] [PubMed] [Google Scholar]
- 12.Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg. 2008;247:300–309. doi: 10.1097/SLA.0b013e31815aab22. [DOI] [PubMed] [Google Scholar]
- 13.Clark W, Silva M, Donn N, Luberice K, Humphries LA, Paul H, et al. Targeting early deaths following pancreaticoduodenectomy to improve survival. J Gastrointest Surg. 2012;16:1869–1874. doi: 10.1007/s11605-012-1958-7. [DOI] [PubMed] [Google Scholar]
- 14.Smoot RL, Christein JD, Farnell MB. Durability of portal venous reconstruction following resection during pancreaticoduodenectomy. J Gastrointest Surg. 2006;10:1371–1375. doi: 10.1016/j.gassur.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Smoot RL, Christein JD, Farnell MB. An innovative option for venous reconstruction after pancreaticoduodenectomy: the left renal vein. J Gastrointest Surg. 2007;11:425–431. doi: 10.1007/s11605-007-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meniconi RL, Ettorre GM, Vennarecci G, Lepiane P, Colasanti M, Laurenzi A, et al. Use of cold-stored vein allografts for venous reconstruction during pancreaticoduodenectomy. J Gastrointest Surg. 2013;17:1233–1239. doi: 10.1007/s11605-013-2201-x. [DOI] [PubMed] [Google Scholar]
- 17.Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: persistent controversy and current recommendations. Ann Surg Oncol. 2009;16:789–791. doi: 10.1245/s10434-009-0322-y. [DOI] [PubMed] [Google Scholar]
- 18.Cloyd JM, Dua MM, Visser BC. Early vein reconstruction and right-to-left dissection for left-sided pancreatic tumors with portal vein occlusion. J Gastrointest Surg. 2014;18:2034–2037. doi: 10.1007/s11605-014-2616-z. [DOI] [PubMed] [Google Scholar]
- 19.Hirono S, Kawai M, Tani M, Okada K, Miyazawa M, Shimizu A, et al. Indication for the use of an interposed graft during portal vein and/or superior mesenteric vein reconstruction in pancreatic resection based on perioperative outcomes. Langenbecks Arch Surg. 2014;399:461–471. doi: 10.1007/s00423-014-1182-x. [DOI] [PubMed] [Google Scholar]
- 20.Merkow RP, Bilimoria KY, McCarter MD, Cohen ME, Barnett CC, Raval MV, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254:131–137. doi: 10.1097/SLA.0b013e31821b98da. [DOI] [PubMed] [Google Scholar]


