Abstract
Background
Concentration of care has been promoted as fostering superior outcomes. This study was undertaken to determine if the concentration of care is occurring in Florida for a pancreaticoduodenectomy, and if so, is it having a salutary effect.
Methods
The data for a pancreaticoduodenectomy were obtained from the Florida Agency for Health Care Administration for three 3-year periods:1992–1994, 2001–2003, 2010–2012; data were sorted by surgeon volume of pancreaticoduodenectomy during these periods and correlated with post-operative length of stay (LOS), in-hospital mortality and hospital charges (adjusted to 2012 dollars).
Results
Relative to 1992–1994, in 2010–2012 46% fewer surgeons performed 115% more pancreaticoduodenectomies with significant reductions in LOS and in-hospital mortality, and higher charges (P < 0.001 for each). From 1992–1994 to 2010–2012 there was an 18-fold increase in the number of pancreaticoduodenectomies by surgeons completing ≥ 12 per year (n = 45 to n = 806, respectively). During 2010–2012, the more frequently surgeons performed a pancreaticoduodenectomy, the shorter LOS, the lower in-hospital mortality, the greater the likelihood of discharge home and the lower the hospital charges (P < 0.03 for each).
Conclusions
Over the last 20 years, the concentration of care has occurred in Florida with substantially fewer surgeons undertaking many more pancreaticoduodenectomies with dramatic improvements in LOS and in-hospital mortality, albeit with increased hospital charges.
Introduction
An analysis of centralization from the Netherlands that was first initiated in 1994 and demonstrated a volume–mortality relationship.1 The Leapfrog Group released a statement in 2000 projecting that as many 98 000 American deaths were caused by medical errors.2 One of the proposed safety initiatives to prevent medical errors recommended that patients should go to high-volume hospitals for complex treatments. Since then, centralization of complex care has been a steadily increasing subject in the medical literature. Pancreatic resections are among one of the ‘complex treatments’ recommended to be carried out at high-volume centres. A pancreaticoduodenectomy is a formidable pancreatic resection that requires a sound approach and technique. Many previous studies have demonstrated a clear benefit for patients who undergo a pancreaticoduodenectomy at a high-volume centre.3–9 As much as a 61% decline in in-hospital mortality has been attributed to centralization of a pancreaticoduodenectomy.8
However, what does current practice reflect? Are trends beginning to reflect what the Leapfrog criteria suggest? A study in 2003 demonstrated that 77% of pancreaticoduodenectomies were completed at low-volume institutions.10 A different study, published in 2004, reported that 60% of pancreatic resections were operated at low-volume hospitals.11 We have previously shown that pancreaticoduodenectomies were performed in low-volume hospitals in Florida.3,4
Now, 13 years after the first Leapfrog statement was published, an update on the volume-outcome relationship with a pancreaticoduodenectomy is needed. As well, an update on our previous reports on where pancreaticoduodenectomies are undertaken and how the frequency with which surgeons perform a pancreaticodudoenectomy determines the length of stay (LOS), hospital charges and in-hospital mortality seems warranted. The purpose of this study was to determine if the concentration of care for a pancreaticoduodenectomy was occurring and, as well, we sought to determine if high-volume surgeons achieve best results as would be purported by many of these same studies. Our hypotheses in undertaking this study were that a greater number of pancreaticoduodenectomies are being done at high-volume centres by high-volume surgeons (i.e. centralization of care was occurring) and that this is positively impacting patient outcomes.
Patients and methods
The database for the State of Florida Agency for Healthcare Administration (AHCA) was queried to identify all patients who underwent a pancreaticoduodenectomy over three 3-year periods 9 years apart: 1992–1994, 2001–2003 and 2010–2012. The ICD-9 procedure code 57.2 was utilized to identify patients whose principle procedure while hospitalized was a pancreaticoduodenectomy.12 Where pancreaticoduodenectomies were performed was determined by the physician (i.e. surgeon) ID number. Surgeons were stratified by the number of pancreaticoduodenectomies that they undertook over a 36-month period: one to three pancreaticoduodenectomies (≤ 1 per year), four to nine pancreaticoduodenectomies (> 1 to ≤ 3 per year), 10–18 pancreaticoduodenectomies (> 3 to ≤ 6 per year), 19–36 pancreaticoduodenectomies (> 6 to ≤ 12 per year) and greater than 36 pancreaticoduodenectomies (≥12 per year). Leapfrog criteria designate hospitals at which 12 or more pancreaticoduodenectomies are undertaken yearly as ‘high-volume’. This definition was applied to surgeons; therefore, high-volume surgeons were defined as surgeons who undertook at least 36 pancreaticoduodenectomies within a 3-year span.13 Under a separate data analysis, very high-volume surgeons were also identified. Very high-volume surgeons were defined as surgeons that undertook more than 30 pancreaticoduodenectomies per year. This volume was based on a paper that identified that 31 pancreaticoduodenectomies per year was the ideal number to reduce in-hospital mortality.14
In the years 1992–1994 and 2001–2003, up to 10 diagnoses codes were collected in the AHCA database for each patient; whereas in years 2010–2012, up to 30 diagnoses were collected in the AHCA database. Thereby, to keep the number of diagnoses analysed consistent, only the first 10 diagnoses codes were evaluated in years 2010–2012 to determine the number and variety of comorbidities. The following comorbidities were tallied for each patient: Old Myocardial Infarction (ICD-9: 412), Diabetes (ICD-9 250), Chronic Obstructive Pulmonary Disorder (ICD-9 496), Congestive Heart Failure (ICD-9 428), Chronic Kidney Disease (ICD-9 585), Emphysema (ICD-9: 492), AIDS (ICD-9: 42), Cirrhosis (ICD-9: 571) and/or Peripheral Vascular Disease (ICD-9: 443).12 Older editions of ICD coding were searched to ensure that ICD-9 codes were consistent throughout the periods investigated. These comorbidities were chosen from the list of comorbidities utilized to calculate the Charlson Comorbidity Index.15
Data were collected and evaluated on post-operative LOS, hospital charges and in-hospital mortality. Hospital charges were adjusted to 2012 dollars according the Bureau of Labor Statistics.16 The patients’ discharge statuses were identified as ‘discharged to home with self-care’, ‘home with home health care’, ‘hospice’, ‘skilled nursing facility’, or ‘expired’. This was collated by ‘discharge to home’ (regardless of with self-care or with home healthcare) or ‘discharge to a facility’ (hospice or skilled nursing facility). The discharge data were stratified by whether the pancreaticoduodenectomy was performed by a high-volume surgeon or a low-volume surgeon.
Data were maintained on a spreadsheet (Excel, Microsoft®, Redmond, WA, USA) and analysed using GraphPad InStat, version 3.06 (GraphPad InStat®, GraphPad Software, Inc, San Diego, CA, USA). The mean data are presented where appropriate unless otherwise noted. Significance was accepted with 95% probability.
Results
A total of 3531 pancreaticoduodenectomies were completed over the years 1992–1994, 2001–2003 and 2010–2012 (Table 1). From 1992–1994 through to 2010–2012, there was a significant decrease (46% fewer) in the number of surgeons performing significantly more (115% more) pancreaticoduodenectomies (Table 1).
Table 1.
The number of pancreaticoduodenectomies (PDs) 21 per surgeon
| PD per surgeon over 36 months | Time period | No. surgeons | No. PDs over 36 months |
|---|---|---|---|
| 1–3 (≤1 per year) | 1992–1994 | 332 | 458 |
| 2001–2003 | 248 | 372 | |
| 2010–2012 | 127 | 206 | |
| 4–9 (>1 & ≤3 per year) | 1992–1994 | 24 | 125 |
| 2001–2003 | 51 | 273 | |
| 2010–2012 | 38 | 225 | |
| 10–18 (>3 & ≤6 per year) | 1992–1994 | 4 | 58 |
| 2001–2003 | 26 | 146 | |
| 2010–2012 | 13 | 160 | |
| 19–36 (>6 & ≤12 per year) | 1992–1994 | 2 | 43 |
| 2001–2003 | 3 | 81 | |
| 2010–2012 | 7 | 172 | |
| 36–90 (12–30 per year) | 1992–1994 | 1 | 45 |
| 2001–2003 | 6 | 248 | |
| 2010–2012 | 11 | 534 | |
| >90 (>30 per year) | 1992–1994 | 0 | 0 |
| 2001–2003 | 1 | 113 | |
| 2010–2012 | 2 | 272 | |
| Total | 1992–1994 | 363 | 729 |
| 2001–2003 | 334 | 1233 | |
| 2010–2012 | 196 | 1569 | |
| Overall Total | 3531 | ||
In 1992–1994, 63% of the pancreaticoduodenectomies were carried out by surgeons who were undertaking ≤ 1 per year, whereas 6% of the pancreaticoduodenectomies were performed by surgeons who undertook > 12 per year, and thereby met the Leapfrog criteria for a ‘high-volume’ surgeon (Table 1). In 2001–2003, 30% of pancreaticoduodenectomies were done by surgeons who were completing ≤ 1 per year and 29% of the pancreaticoduodenectomies were performed by surgeons who undertook > 12 per year (Table 1). In 2010–2012, 13% of the pancreaticoduodenectomies were done by surgeons who were undertaking ≤ 1 per year, whereas 51% of the pancreaticoduodenectomies were performed by surgeons who completed > 12 per year (Table 1). From 1992–1994 to 2010–2012, there was an 18-fold increase in the number of pancreaticoduodenectomies done by surgeons completing ≥ 12 per year (45–806) whereas the number of surgeons undertaking ≥ 12 per year increased from 1 to 11 (Table 1).
As fewer surgeons did more pancreaticoduodenectomies over the studied time periods, there were significant reductions in LOS. In-hospital mortality significantly decreased from 1992–1994 to 2010–2012; this is a 64% decrease in in-hospital mortality (P < 0.001). From 1992–1994 to 2001–2003, in-hospital mortality decreased by 35% and from 2001–2003 to 2010–2012 in-hospital mortality decreased by 45%. Hospital charges significantly increased by $40 495 from 1992–1994 to 2010–2012 (P < 0.001); this is a 44% increase from 1992–1994 to 2010–2012. From 1992–1994 to 2001–2003, the increase was 11% and from 2001–2003 to 2010–2012 the increase was 30%. Each of these data points is depicted in Table 2 and Fig. 1. The table is stratified by number of pancreaticoduodenectomies per surgeon. The figure displays totals over the three different time periods for each data point.
Table 2.
Surgeon volume, time period, post-operative length of stay (LOS), hospital charges and in-hospital mortality
| No. PDs per surgeon over 36 months | Period | Post-op LOS (days) median (mean ± standard deviation) | Hospital charges (2012 dollars) median (mean ± standard deviation) | In-hospital mortality (%) |
|---|---|---|---|---|
| 1–3 ≤1 per year | 1992–1994 | 16 (20 ± 15.5) | 99 644 (125 882 ± 102091.4) | 15.9 |
| 2001–2003 | 14 (18 ± 12.6) | 126 539 (158591 ± 118119.0) | 13.7 | |
| 2010–2012 | 14 (18 ± 15.2) | 206 014 (264 991 ± 207391.7) | 9.2 | |
| 4–9 >1 & ≤3 per year | 1992–1994 | 15 (17 ± 10.9) | 86 112 (104 405 ± 79602.8) | 6.4 |
| 2001–2003 | 13 (16 ± 10.3) | 111 935 (140 473 ± 88545.9) | 8.4 | |
| 2010–2012 | 13 (16 ± 12.7) | 163 789 (225 451 ± 203748.1) | 4.4 | |
| 10–18 >3 & ≤6 per year | 1992–1994 | 15 (18 ± 11.5) | 74 495 (97 566 ± 85363.24) | 6.9 |
| 2001–2003 | 12 (15 ± 9.5) | 129 468 (161 840 ± 138467.7) | 8.2 | |
| 2010–2012 | 12 (15 ± 10.7) | 189 949 (225 259 ± 170791.8) | 8.1 | |
| 19–36 >6 & ≤12 per year | 1992–1994 | 17 (22 ± 15.3) | 72 496 (96 074 ± 61187.8) | 4.7 |
| 2001–2003 | 11 (17 ± 21.0) | 101 398 (148 404 ± 145713.6) | 9.9 | |
| 2010–2012 | 9 (12 ± 8.2) | 93 514 (121 828 ± 114409.3) | 1.7 | |
| 36–90 >12 & ≤30 per year | 1992–1994 | 13 (19 ± 13.9) | 101 460 (161238 ± 148582.1) | 11.1 |
| 2001–2003 | 12 (14 ± 8.9) | 86 237 (113 748 ± 90 956) | 3.2 | |
| 2010–2012 | 10 (13 ± 9.9) | 115 364 (151 364 ± 115 015) | 3.0 | |
| >90 >30 per year | 1992–1994 | N/A | N/A | N/A |
| 2001–2003 | 10 (12 ± 7.7) | 88 238 (96 003 ± 40 452) | 0 | |
| 2010–2012 | 9 (12 ± 11) | 121 342 (162 120 ± 151 633) | 3.3 | |
| Total | 1992–1994 | 15 (20 ± 14.4) | 92 605 (120 317 ± 100187.5) | 12.6 |
| 2001–2003 | 12 (16 ± 11.6) | 102 947 (139 540 ± 108889.1) | 8.2 | |
| 2010–2012 | 11 (14 ± 11.5) | 133 550 (183 104 ± 162977.6) | 4.5 | |
PDs, pancreaticoduodenectomies.
Figure 1.
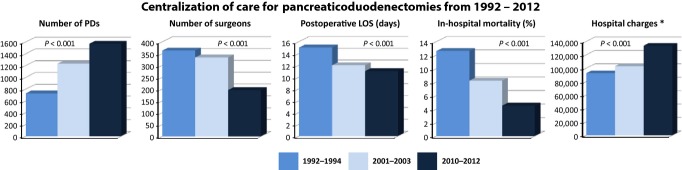
Changes in outcomes and charges of a pancreaticoduodenectomy over 20 years. LOS, length of stay
The variety and number of comorbidities, including the number of patients with major comorbidities, were not different across the three time periods of 1992–1994, 2001–2003 and 2010–2012 (Table 3). As well, the presence of major comorbidities among the patients was similar across all time periods and was independent of surgeon volume across all time periods (Table 3).
Table 3.
Pre-operative comorbidity status
| Frequency of pancreaticoduodenectomy | Time frame | Percent of patients with a major comorbidity |
|---|---|---|
| 1–3 (≤1 per year) | 1992–1994 | 35% |
| 2001–2003 | 36% | |
| 2010–2012 | 31% | |
| Overall | 34% | |
| 4–9 (>1 & ≤3 per year) | 1992–1994 | 26% |
| 2001–2003 | 33% | |
| 2010–2012 | 40% | |
| Overall | 23% | |
| 10–18 (>3 & ≤6 per year) | 1992–1994 | 24% |
| 2001–2003 | 40% | |
| 2010–2012 | 31% | |
| Overall | 34% | |
| 19–36 (>6 & ≤12 per year) | 1992–1994 | 40% |
| 2001–2003 | 38% | |
| 2010–2012 | 34% | |
| Overall | 36% | |
| >36 (>12 per year) | 1992–1994 | 22% |
| 2001–2003 | 33% | |
| 2010–2012 | 34% | |
| Overall | 33% | |
Across all time periods, patients were more likely to be discharged home than to a facility if they had their operation performed by a high-volume surgeon compared with a low-volume surgeon (Table 4, P < 0.001). In 1992–1994, the patients that were operated on by a high volume surgeon (and survived) were all discharged home.
Table 4.
Location to where patients were discharged to
| Surgeon volume | Discharge status | 1992–1994 | 2001–2003 | 2010–2012 |
|---|---|---|---|---|
| High | Discharged to home | 100% | 94% | 88% |
| High | Discharged to facility | 0% | 6% | 12% |
| Low | Discharged to home | 0% | 83% | 81% |
| Low | Discharged to facility | 100% | 17% | 19% |
A subgroup analysis was done for 2010–2012, the greater the surgeon frequency of pancreaticoduodenectomy, the lower the LOS; a linear regression of LOS versus surgeon frequency of pancreaticoduodenectomy resulted in R² = 0.8565 with a negative, significantly non-zero slope (P = 0.029) (Fig. 2). Also in 2010–2012, an increased frequency of a pancreaticoduodenectomy was associated with lower in-hospital mortality; a linear regression of in-hospital mortality versus surgeon frequency of a pancreaticoduodenectomy resulted in R² = 0.5329 with a negative, significantly non-zero slope (P < 0.001) (Fig. 3). Furthermore, in 2010–2012, hospital charges decreased with increasing surgeon frequency of a pancreaticoduodenectomy; a linear regression of hospital charges versus surgeon frequency of pancreaticoduodenectomy resulted in R² = 0.6195 with a negative, significantly non-zero slope (P < 0.001) (Fig. 4).
Figure 2.
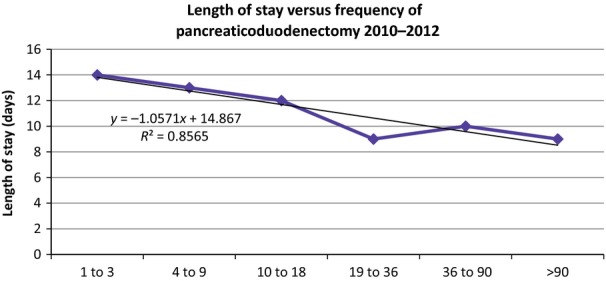
The length of stay in days compared to frequency in which surgeons perform pancreaticoduodenectomy
Figure 3.
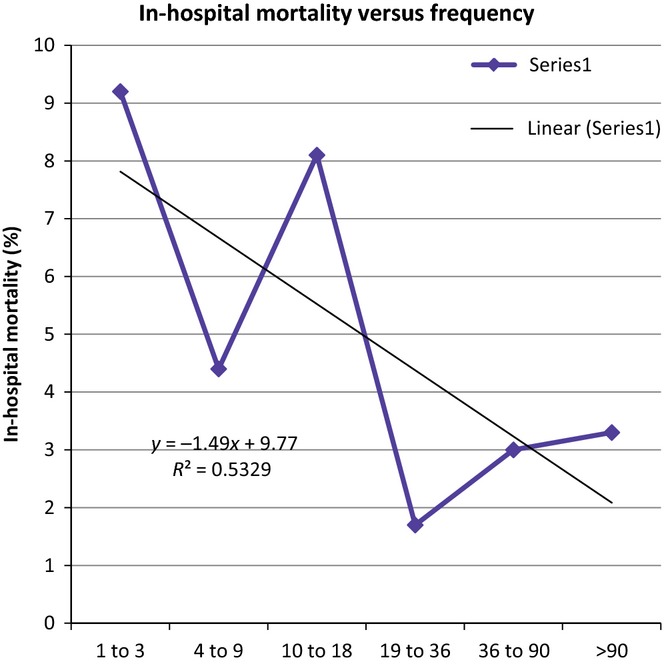
The in-hospital mortality rate is correlated to frequency in which surgeons perform a pancreaticoduodenectomy
Figure 4.
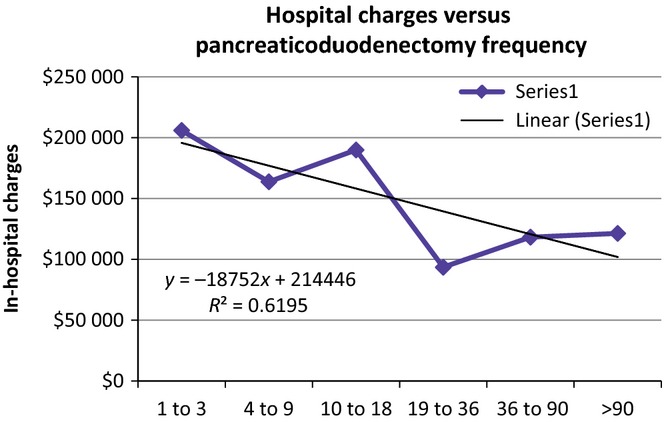
The hospital charges compared to the frequency in which surgeons undertake pancreaticoduodenectomy
There were only two surgeons that met the criteria of the very high-volume surgeon in 2010–2012. One surgeon met the criteria in both the 2001–2003 and 2010–2012 periods.
Multivariate analysis revealed that in 1992–1994, increased frequency contributed to patients having a lower acuity discharge status, increased charges and a decreased LOS. In the 2001–2003 time period, surgeons that completed more pancreaticoduodenectomies were associated with a lower acuity discharge status and had a decreased in-hospital mortality. The frequency did not contribute to LOS or hospital charges. For the most current time period (2010–2012), increased frequency was related to lower hospital charges and a decreased in-hospital mortality.
Discussion
This study documents the long-term trends of centralization of care for a pancreaticoduodenectomy, and that centralization is yielding salutary benefits. In 2001 and 2008, using the statewide AHCA database from Florida, we reported that a pancreaticoduodenectomy was not centralized to major centres or undertaken primarily by high-volume surgeons.3,4 Nonetheless, when those reports were written, it was evident that patient outcomes, measured by LOS and in-hospital mortality rates, were superior with high-volume surgeons. Notably, between those reports of time periods 1995–1997 and 2003–2005 overall state-wide in-hospital mortality did not change and seemed unacceptably high in each of the periods. Implied in these reports was the notion that centralization of care would improve outcomes with a pancreaticoduodenectomy. This report shows that over the past 20 years in Florida, substantially fewer surgeons are undertaking significantly more pancreaticoduodenectomies with reductions in LOS and in-hospital mortality, albeit with greater charges. Centralization of care has occurred in Florida, and it seems to be continuing with a salutary effect. As well, the more frequently surgeons performed a pancreaticoduodenectomy in Florida in 2010–2012 the lower the LOS, the lower the hospital charges, the lower the in-hospital mortality and the higher the percentage of patients discharged home. This again supports the concept of centralization of care for a pancreaticoduodenectomy.
The patients in this study are representative of the population in which a pancreaticoduodenectomy is performed across America; there is nothing about these patients that is unique. The data set used is complete and robust allowing for a complete, thorough, and comprehensive analysis. The results and conclusions drawn within can be taken with confidence.
This report involves over 3500 pancreaticoduodenectomies undertaken in three different 3-year periods each 9 years apart. From 1992–1994 to 2010–2012, less than half as many surgeons were undertaking more than twice as many pancreaticoduodenectomies. Now, for the first time, high-volume surgeons perform more than half of the pancreaticoduodenectomies performed in Florida. With this change, there was a notable decrease in in-hospital mortality, as it decreased by more than half. As well, post-operative LOS decreased by a median of more than a quarter, more than 4 days. These improvements were not a result of better patient selection, as there was no difference in the percentages of patients with major comorbidities among the time periods studied. However, there was a ‘cost’ to these significant improvements as total hospital charges, even when adjusted to 2012 dollars, significantly increased.
There were dramatically more high-volume surgeons in 2010–2012 than in prior time periods. High-volume surgeons did more pancreaticoduodenectomies, as we noted an 18-fold increase in the pancreaticoduodenectomies undertaken by high-volume surgeons. As expected, high-volume surgeons had better clinical outcomes; however, they might be measured. If a patient had their pancreaticoduodenectomy completed by a high-volume surgeon, they were more likely to be discharged home. Being discharged home probably reflects a host of issues and seems a fair surrogate marker of quality of care, given that the patients were similar among time periods for their comorbidities. Interestingly, the percentage being discharged to home has declined from the 100% seen from 1992–1994 to 88% seen in the most current time frame. This decrease in the percentage discharged to home most probably reflects surgeons’ attempt to reduce LOS and readmission rates, as well as the higher prevalence of facilities (i.e. skilled nursing facilities, rehabilitation centres, etc.).
Our data adds to the body of literature that supports the concentration of care for pancreaticoduodenectomy. With the dissemination of well-trained surgeons, this may not always be true. For example, a carotid endarterectomy is an operation that was recently removed from the ‘complex treatments’ recommended to be carried out at high-volume institutions based on available data.17 While the same may occur in the future for a pancreaticoduodenectomy, we are not there yet as the concentration of care that has occurred over the past 20 years has translated into real, definable and a meaningful benefit for patients. Our data supports the redirection of patients in need of a pancreatic resection to high-volume centres. These data are not totally unique; a 2007 study out of Texas, which reviewed Texan Inpatient Data, demonstrated that 58% of pancreaticoduodenectomies were undertaken at facilities that undertook more than 10 resections per year.5 While, their in-hospital mortality was similar to the in-hospital mortality obtained by high-volume surgeons in Florida in the most recent time span (3.3% versus 3.1%, respectively), 10 resections per hospital per year seem to be on the low side.
This study provides the most current snapshot of the centralization of care and demonstrates that the majority of pancreatic resections are being done by high-volume surgeons in high-volume centres. This centralization is associated with a salutary decrease of in-hospital mortality across all patients in the sample, primarily because of the impact of the large number of patients receiving care from higher volume surgeons. Prior studies, including one from Gouma and colleagues published in 2000, found there was a marked difference in the post-operative death rate (16% versus 1.5%) between low-volume hospitals and high-volume hospitals, respectively.6 Certainly, high-volume hospitals have high-volume surgeons. Notably, a high percentage of patients in that series were still having resections by surgeons that performed less than five pancreatic resections per year (including a pancreaticoduodenectomy).6 When our group published in 2008, we demonstrated that higher volume surgeons had a significantly lower in-hospital mortality, but it did not demonstrate an ‘across the board’ overall lower in-hospital mortality rate than the previously time period analysed because so many patients were still cared for at low-volume centres.4
The centralization of care for a pancreaticoduodenectomy has possibly been influenced by the training of General Surgeons by accredited residency training programmes. Recently, General Surgery trainees reported a median of 12 pancreatic operations of all types and varieties during their entire 5-year residency. That does not seem like much, particularly given the potential variety of pancreatic operations over such an extended period of time. This low level of experience gained through residency training supports further specialized training programmes for surgeons interested in undertaking a pancreaticoduodenectomy, and HPB surgery as a whole.18 It follows that surgeons who complete such dedicated post-residency training would seek to focus on pancreatic surgery, including a pancreaticoduodenectomy, further encouraging centralization of care. Further, we plan to do a study that analyses how educational centres are possibly influencing referral patterns.
Centralization of care is dictated by referral patterns, both physician-to-physician referrals and patient self-referrals. These referrals have increased because of the plethora of information on the need for a ‘high volume surgeon’ to perform the pancreaticoduodenectomy. This thought is not uniquely ours.19 Also, we have had patients travel from out of state to receive care from an experienced pancreatic surgeon. For the most part, this was a result of research done on the Internet. A study that looked at online surveys of physicians found that 76% of individuals viewed an online medical review prior to meeting with a physician had an impact on whom they saw.20 As the millennial generation ages, the percentage of those searching for information on their physicians prior to seeking care should rise.
Centralization of care potentially leads to challenging patient care scenarios, such as the operative candidate who cannot transfer to a high-volume centre for a pancreatic resection. If a pancreaticoduodenectomy is attempted only at high-volume centres, then low-volume centres will have even lower volumes going forward. This action would seemingly work to further the schism between high-volume and low-volume centres. Thus, how does our healthcare system guarantee access to care for patients living in rural or low population density areas that cannot relocate for care?
At what point is there a cap where frequency no longer demonstrates benefits? Does the law of diminishing return apply to pancreatic surgery? Arguably, the highest volume providers would be taking on higher-risk patients or patients with more advanced disease. Joseph et al. published that 31 pancreatic resections per year provided the optimal outcome.14 Two of the high volume surgeons in our practice undertook 117 and 155 operations in the most recent time period, averaging to 39 and 52 pancreaticoduodenectomies per year. Their volume helped contribute to an in-hospital mortality rate of 3.1%, which is less than half the national average of 6.6%.7 A systematic review of the literature by van Heek and colleagues demonstrated that a surgeon volume of a minimum of 24 pancreaticoduodenectomies per year achieved less than 3% in-hospital mortality.1 If there is such a cap, it is unknown.
It remains important to identify opportunities to improve mortality, morbidity, LOS, hospital charges and access to care. A pancreaticoduodenectomy, a complex and specialized operation, provides an opportunity to analyse patterns of centralization of care. In Florida, a pancreaticoduodenectomy has been undertaken in fewer centres by more high-volume surgeons over the past 20 years, and a salutary benefit has been demonstrated with this centralization of care.
Conflicts of Interest
None declared.
References
- 1.van Heek N, Kuhlmann K, Scholten R, de Castro S, Busch O, van Gulik T, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–790. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milstein A, Galvin R, DelBlanco S, Salber P, Buck C. Improving the safety of health care: the leapfrog initiative. Eff Clin Pract. 2000;3:316–317. [PubMed] [Google Scholar]
- 3.Rosemurgy A, Bloomston M, Serafini F, Coon B, Murr M, Carey L. Frequency with which surgeons undertake pancreaticoduodenectomy determines length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg. 2001;5:21–26. doi: 10.1016/s1091-255x(01)80009-3. [DOI] [PubMed] [Google Scholar]
- 4.Rosemurgy A, Cowgill S, Coe B, Thomas A, Al-Saadi S, Goldin S, et al. Frequency with which surgeons undertake pancreaticodudoenectomy continues to determine length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg. 2008;12:442–449. doi: 10.1007/s11605-007-0442-2. [DOI] [PubMed] [Google Scholar]
- 5.Riall T, Eschbach K, Townsend C, Nealon W, Freeman J, Goodwin J. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007;11:1242–1252. doi: 10.1007/s11605-007-0245-5. [DOI] [PubMed] [Google Scholar]
- 6.Gouma D, van Geenen R, van Gulik T, de Haan R, de Wit L, Busch O, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPhee J, Hill J, Whalen G, Zayaruzny M, Litwin D, Sullivan M, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon T, Bowman H, Tielsch J, Bass E, Burleyson G, Cameron J. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birkmeyer J, Finlayson S, Tosteson A, Sharp S, Warshaw A, Fisher E. Effect of hospital volume on in-hospital mortality with pancreaticoduodnectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 10.Ho V, Heslin M. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237:509–514. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimick J, Finlayson S, Birkmeyer J. Regional availability of high-volume hospitals for major surgery. Health Aff. 2004 doi: 10.1377/hlthaff.var.45. Web Exclusive:var45–53. [DOI] [PubMed] [Google Scholar]
- 12.Buck CJ. 2012 ICD-9-CM Volumes 1, 2, & 3 For Hospitals. St. Louis, Missouri: Jeanne R. Olsen; 2012. [Google Scholar]
- 13.Birkmeyer J, Dimick J. 2003. The Leapfrog Group's Patient Safety Practices: The Potential Benefits of Universal Adoption. Available at http://leapfroggroup.org/media/file/Leapfrog-Birkmeyer.pdf: 2004 (last accessed 6 February 2014)
- 14.Joseph B, Morton J, Hernandez-Boussard T, Rubinfeld I, Faraj C, Velanovich V. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208:520–527. doi: 10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 15.D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32:382–387. [PubMed] [Google Scholar]
- 16.Swisher S, Maish M, Erasmus J, Correa A, Ajani J, Bresalier R, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Christian C, Gustafson M, Betensky R, Daley J, Zinner M. The leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg. 2003;238:447–457. doi: 10.1097/01.sla.0000089850.27592.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daee S, Flynn J, Jacobs M, Mittal V. Analysis and implications of changing hepatopancreatobiliary (HPB) case loads in general surgery residency training for HPB surgery accreditations. HPB. 2013;15:1010–1015. doi: 10.1111/hpb.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finks J, Osborne N, Birkmeyer J. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black EW, Thompson LA, Saliba H, Dawson K, Paradise Black NB. An analysis of healthcare providers’ online ratings. Inform Prim Care. 2009;17:249–253. doi: 10.14236/jhi.v17i4.744. [DOI] [PubMed] [Google Scholar]
- 21.Enestvedt C, Diggs B, Cassera M, Hammill C, Hansen P, Wolf R. Complications nearly double the cost of care after pancreaticoduodenectomy. Am J Surg. 2012;204:332–338. doi: 10.1016/j.amjsurg.2011.10.019. [DOI] [PubMed] [Google Scholar]


