Abstract
Mast cells are proposed to be one of the targets for mucosal vaccine adjuvants. We previously demonstrated that mucosal adjuvants containing IgG immune complexes could activate connective tissue mast cells enhancing immune responses. Here we suggest that mucosal mast cells (MMC) may also contribute to augmentation of antigen-specific immune responses following treatment with antigens complexed with IgG. We demonstrated that both bone marrow-derived cultured MMC and tissue resident MMC incorporated ovalbumin (OVA) at a greater level in the presence of anti-OVA IgG. Co-culture of OVA/IgG-pulsed bone marrow-derived MMC with splenocytes from OT-II mice promoted OVA-specific activation and proliferation of T cells, a process known as cross-presentation. Furthermore, bone marrow-derived cultured MMC underwent apoptosis following treatment with IgG immune complexes, a feature that has been described as favouring phagocytosis of mast cells by professional antigen-presenting cells.
Keywords: antigen presentation/processing, apoptosis, IgG immune complexes, mast cells, phagocytosis
Introduction
The mucosal immune system has been suggested to be the portal of entry for many pathogens, highlighting the need for the development of potent mucosal vaccines that provide better local protection in addition to systemic defence against infection. Mucosal vaccination strategies that intentionally target specific cell populations involved in the innate and adaptive immune responses have been developed that are mainly dependent on the appropriate selection of an adjuvant to be included in the formulation of mucosal vaccines. These adjuvants mainly target cells of the innate immune system including dendritic cells, macrophages, neutrophils, eosinophils, natural killer cells and natural killer T cells. These cells express various pattern recognition receptors that recognize danger signals including pathogen-associated molecular patterns typical to microorganisms.1 More recently, mast cells have also been depicted in their potential to boost mucosal vaccination as mast cell activators are demonstrated to be powerful mucosal adjuvants.2 We have previously shown robust adjuvanticity exerted by Fcγ receptor-mediated mast cell activation.3,4
Mast cells represent a heterogeneous family of bone marrow (BM) -derived tissue resident cells. There are two phenotypically distinct rodent mast cell phenotypes, namely connective tissue mast cells (CTMC) and mucosal mast cells (MMC), which are classified depending on tissue distribution, histochemical staining and mediator composition.5,6 MMC are predominantly located in mucosal tissues, whereas CTMC are widespread in connective tissues such as skin, submucosa, muscularis propria and serosal sites including the peritoneal cavity. Besides the high-affinity IgE receptor, i.e. FcεRI, mouse mast cells all express constitutively the low-affinity, inhibitory IgG receptor FcγRIIB. In addition to FcεRI and FcγRIIB, CTMC express FcγRIIIA, an activating IgG receptor.4 Although CTMC and MMC are predicted to respond differently to microenviromental stimuli, experimental models dissecting their functional differences are scarce. We have previously shown that CTMC contribute to mucosal immunization in the presence of an adjuvant formulation that includes IgG immune complexes, which is dependent on FcγRIIIA, whereas MMC cannot be activated by IgG immune complexes because of the lack of FcγRIIIA.4 This is consistent with previous findings regarding the responsiveness of CTMC and MMC to IgG immune complexes.7,8 Interestingly, we demonstrated that MMC develop apoptosis as a result of IgG immune complex treatment, probably as a result of their expression of only one Fcγ receptor which is FcγRIIB. In contrast, balanced expression of FcγRIIB and FcγRIIIA protects CTMC from ovalbumin (OVA)/IgG immune complex-mediated apoptosis.9
Mast cells are reported to participate in antigen cross-presentation.10 This is a mechanism whereby professional antigen-presenting cells (APC) engulf mast cells that have previously internalized antigens, and the antigens can be further processed and presented by the professional APC to T lymphocytes. Morphological changes of mast cells as a result of apoptosis facilitate the ingestion of mast cells by APC. Indeed, mast cells have been implicated in the phagocytosis of various types of antigens and can serve as an antigen reservoir.11–14 Induction of mast cell apoptosis is documented to be critical for the efficiency of cross-presentation.10 Given the fact that MMC undergo apoptosis in response to treatment with IgG immune complexes, we speculated that IgG-bound antigens could be incorporated into MMC by a unique mechanism, which raises the intriguing possibility that MMC may take part in cross-presentation of the antigens in the perspective of mucosal immunization with a vaccine formulation containing IgG-complexed antigens.
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan Laboratories (Horst, the Netherlands). KitW-sh/W-sh mice that are deficient in mast cells15 were provided by Prof. G. Nilsson (Karolinska Institute, Stockholm, Sweden). The OT-II mice, whose T cells have transgenic T-cell receptors specific for the 323–339 peptide of OVA (OVA323–339) were obtained from the MIVAC breeding unit, University of Gothenburg. All animal procedures were carried out with the approval of the Ethics Committee for Laboratory Animals in Gothenburg, Sweden.
Mast cell culture
Mouse BM cells were obtained from the femurs and tibias of C57BL/6 mice. MMC were cultured from BM cells as described previously.16 Briefly, MMC were obtained by culturing mouse BM cells in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, Steinheim, Germany) containing 5% conditioned medium from X63/0 myeloma cells transfected with an interleukin-3 expression vector (from J. Halgren-Martinsson, Uppsala, Sweden) and recombinant mouse cytokines including 25 ng/ml stem cell factor (PeproTech, Rocky Hill, NJ) and 5 ng/ml interleukin-9 (ImmunoTools Friesoythe, Germany), as well as 1 ng/ml recombinant human transforming growth factor-β1 (PeproTech). The cell culture medium was supplemented with 10% fetal bovine serum and other supplements as described.16
Preparation of single-cell suspension from mouse nasal tissues
Mouse nasal tissues together with the turbinates were cut into small pieces followed by digestion with 400 U/ml DNase I (Roche Applied Science, Mannheim, Germany) and 25 μg/ml Liberase TM (Roche) at 37° for 20 min. Digested cells were passed through a 70-μm nylon net followed by red blood cell lysis.
Treatment of mast cells by IgG immune complexes
Ovalbumin and IgG immune complexes were generated as described previously.9 Briefly, mouse IgG1 anti-OVA (Sigma-Aldrich), or in some experiments an isotype control antibody, were mixed together with OVA (Sigma-Aldrich) or Alexa Fluor 488-conjugated OVA (Invitrogen Life Technologies, Carlsbad, CA) followed by incubation at 37° for 20 min. Immune complexes composed of CTA1-DD (provided by Prof. N. Lycke, University of Gothenburg; a fusion protein comprising the Al subunit of cholera toxin linked to a synthetic dimer of fragment-D of Staphylococcus aureus protein A) and IgG were produced similarly. The final concentrations of OVA, CTA1-DD and IgG for cell incubation were 130, 110 and 90 μg/ml, respectively. The molar ratio of antigen/antibody or CTA1-DD/IgG was roughly 5 : 1 in both cases. In some experiments, different concentrations or ratios of OVA and IgG were used as indicated. Mast cells were incubated with the immune complexes in a 96-well plate at 37° in 5% CO2 overnight or as indicated. Cells were washed thoroughly with medium and were used further for flow cytometric analysis, microscopy imaging, ImageStream analysis, or mast cell/T-cell co-culture.
Confocal microscopy
Bone marrow-derived MMC were treated with PBS or Alexa Fluor 488-conjugated OVA in the presence or absence of anti-OVA IgG overnight followed by staining with biotin-conjugated anti-mouse c-Kit (clone 2B8; eBioscience, San Diego, CA). Cells were next washed and stained with streptavidin-conjugated Texas Red (Invitrogen Life Technologies). Cells were fixed with 4% formaldehyde and spun onto cytospin slides. Next, the slides were mounted with ProLong® Gold anti-fade reagent (Invitrogen Life Technologies), and confocal images were acquired using an LSM 700 Axio Observer.Z1 under Plan-Apochromat 63 ×/1·4 Oil differential interference contrast immersion objective (Carl Zeiss, Jena, Germany).
ImageStream analysis
Bone marrow-derived MMC were treated with Alexa Fluor 488-conjugated OVA in the presence or absence of anti-OVA IgG overnight followed by staining with Pacific Blue-conjugated anti-mouse FcεRIα (Clone MAR-1; Biolegend, San Diego, CA). Cells were fixed similarly as for confocal microscopy. The Alexa Fluor 488 and Pacific Blue fluorescence signals were analysed using an ImageStreamX MarkII (Amnis Corporation, Seattle, WA). The images were evaluated by IDEAS software (Amnis).
Mast cell and T-cell co-culture
Bone marrow-derived MMC were incubated with PBS or OVA (130 μg/ml) in the presence or absence of anti-OVA IgG (90 μg/ml) for 48 hr. A total of 105 control or antigen-pulsed MMC were co-cultured in 96-well round-bottom plates with 4 × 105 unsorted splenocytes from OT-II mice. The co-cultures were incubated at 37° for 72 hr followed by measurement of the expression of surface CD69, which represents cell activation, and intracellular Ki-67, which indicates cell proliferation, in CD4+ T cells using flow cytometry.
Flow cytometric analysis
The differentiation of BM-derived mast cells was determined by phycoerythrin-conjugated anti-mouse c-Kit (Clone 2B8; eBioscience) and allophycocyanin-conjugated hamster anti-mouse FcεRIα (Clone MAR-1; eBioscience). Cell surface expression of FcγRIIIA was measured by FITC-conjugated rat anti-mouse FcγRIIIA (Clone 275003; R&D Systems, Minneapolis, MN). Apoptosis was determined by staining the cells with FITC-conjugated annexin V (ImmunoTools) and 7-aminoactinomycin D (7-AAD; Sigma-Aldrich). Alternatively, cell apoptosis was measured by FITC-conjugated lectin from Narcissus pseudonarcissus (ImmunoTools) according to instructions from the manufacturer. Narcissus pseudonarcissus-derived lectin recognizes changes in the structures of glycoconjugates that correlate with apoptosis.17 In some experiments, apoptosis was determined by detecting mitochondrial membrane potential disruption. For this purpose, cells were stained with MitoTracker Red (Invitrogen Life Technologies) according to the manufacturer’s protocol. For identifying nasal tissue mast cells, the digested cells were stained for two mast cell markers by FITC-conjugated anti-mouse IgE (Clone R35-72; BD Biosciences, San Jose, CA) and phycoerythrin-conjugated anti-mouse c-Kit (Clone 2B8; eBioscience), as well as for non-mast cell markers by Horizon V450-conjugated anti-mouse CD3 (Clone 17A2), anti-mouse CD19 (Clone 1D3), and anti-mouse Gr-1 (Clone RB6-8C5) antibodies (all from BD Biosciences). For measuring T-cell activation and proliferation, cells were stained by phycoerythrin-Cy7-conjugated anti-CD4 (Clone GK1.5; eBioscience) and phycoerythrin-conjugated anti-CD69 (Clone H1.2F3; eBioscience) followed by fixation and permeabilization using the Intracellular Fixation & Permeabilization Buffer Set (eBioscience) and intracellular staining for the nucleoprotein Ki-67 using the Ki-67 Set (clone B56; BD Biosciences). The fluorescence intensity was analysed with an LSR-II (BD Biosciences) and FlowJo software (FlowJo, Ashland, OR).
Statistical analysis
Data were analysed for statistical significance using an unpaired t-test with GraphPad Prism software (San Diego, CA).
Results
Antigen internalization in MMC is enhanced by antigen-specific IgG
Mast cells have classically been described as a cell type that is capable of phagocytosis.11–14 In this study, we demonstrated first by conventional flow cytometry that BM-derived MMC could efficiently phagocytose OVA and internalization efficiency was increased with prolonged incubation time (Fig.1a). Furthermore, these MMC substantially increased the internalization when OVA was complexed with anti-OVA IgG (Fig.1b). This was true over a wide range of OVA and IgG concentrations (Table1). The enhancement was still apparent when the concentrations of both OVA and IgG were proportionally reduced by fivefold or 25-fold, or when OVA concentration was reduced by fivefold with the IgG concentration unchanged. However, the enhancement was abrogated by antibody excess when OVA concentration was reduced 25-fold (Table1). As conventional flow cytometry cannot differentiate between intracellular internalization and adherence of OVA to the cell surface, the internalization was confirmed by confocal microscopy (Fig.1c). Consistent with the flow cytometry data (Fig.1b), almost all the cells incorporated fluorescent OVA following overnight incubation with OVA either alone or in immune complexes (Fig1c). To quantify the enhanced intracellular incorporation of OVA by the cells following treatment with IgG immune complexes, BM-derived MMC were analysed by ImageStream cytometry. IgG-complexed OVA indeed was engulfed by these MMC at a greater level (Fig.1d). Next, we assessed OVA incorporation by mouse nasal tissue mast cells. We have developed a flow cytometry-based technique to identify mouse nasal CTMC and MMC.4 Hence, single cell suspensions obtained from mouse nasal tissues were incubated overnight with fluorescent OVA with or without anti-OVA IgG. Nasal CTMC and MMC were identified (Fig.2a,c) in contrast to the absence of a mast cell population in KitW-sh/W-sh mice (Fig.2b) Furthermore, both MMC and CTMC in the nasal tissues could engulf fluorescent OVA and such incorporation was increased if the cells were incubated with OVA/IgG complexes (Fig.2d–g). Taken together, these results strongly suggest that MMC could efficiently incorporate antigens in IgG immune complexes.
Figure 1.
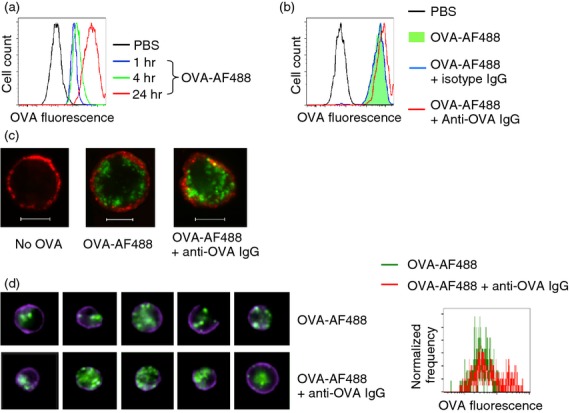
Augmented antigen internalization by mouse bone marrow-derived cultured mucosal mast cells (MMC) in the presence of antigen-specific IgG. (a) MMC were treated with PBS (black), Alexa Fluor 488-conjugated ovalbumin (OVA; 130 μg/ml) for 1 hr (blue), 4 hr (green), or 24 hr (red) followed by flow cytometric analysis. (b) MMC were incubated with PBS (black), Alexa Fluor 488-conjugated OVA (130 μg/ml) in the absence (green filled) or presence of anti-OVA IgG (90 μg/ml, red) or isotype control IgG (blue) overnight followed by flow cytometric analysis. (c) MMC were essentially treated as described in (b) followed by staining with Texas Red-labelled anti-c-Kit to mark the cell periphery (red fluorescence). Cells were fixed and cytospins were prepared followed by confocal microscopic analysis. Scale bar: 5 μm. (d) MMC were initially treated as described in (b) followed by staining with Pacific Blue-labelled anti-FcεRIa to mark the cell periphery (blue fluorescence). Cells were next fixed and analysed by ImageStream cytometry. Representative individual cell images of each treatment (left panel) and accumulative events shown as a histogram (right panel) were displayed. Data represent two (d) or at least three (a–c) separate experiments.
Table 1.
Ovalbumin (OVA) incorporation by mucosal mast cells incubated with varying doses of OVA and IgG1
| OVA dilution | IgG dilution | Alexa Fluor 488 GEM | P-value | |
|---|---|---|---|---|
| OVA | – | n.a. | (3·0 ± 0·2) × 104 | 0·030 |
| OVA + IgG | – | – | (4·7 ± 1·3) × 104 | |
| OVA | 5× | n.a. | (9·7 ± 2·4) × 103 | 0·015 |
| OVA + IgG | 5× | – | (14·3 ± 3·3) × 103 | |
| OVA | 25× | n.a. | (2·3 ± 0·2) × 103 | 0·77 |
| OVA + IgG | 25× | – | (2·4 ± 0·4) × 103 | |
| OVA | 5× | n.a. | (8·8 ± 2·3) × 103 | 0·0058 |
| OVA + IgG | 5× | 5× | (11·8 ± 2·5) × 103 | |
| OVA | 25× | n.a. | (2·6 ± 0·5) × 103 | 0·0046 |
| OVA + IgG | 25× | 25× | (3·3 ± 0·5) × 103 |
Mouse bone marrow-derived cultured mucosal mast cells were incubated overnight with Alexa Fluor 488-conjugated OVA (130 μg/ml) alone, or together with anti-OVA IgG (90 μg/ml). In some experiments OVA and/or IgG were diluted as indicated. Incorporation of OVA by the cells was analysed by flow cytometry. Data are expressed as geometric mean (GEM) fluorescence ± SD. “–” denotes not diluted; n.a., not applicable.
Figure 2.
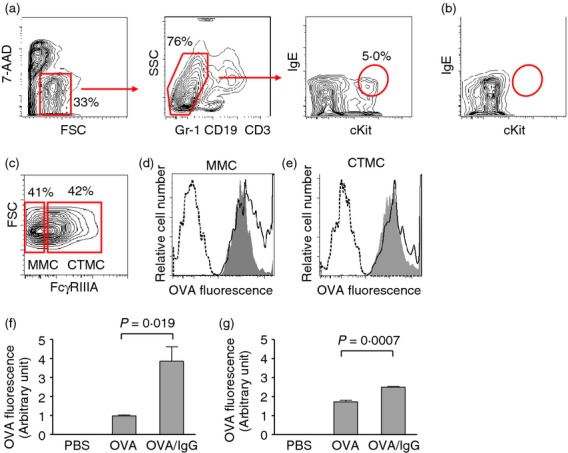
Augmented antigen internalization by mouse nasal tissue mast cells in the presence of antigen-specific IgG. (a) The strategy for gating nasal mast cells is shown. Single cell suspensions were obtained by digesting nasal tissue from wild-type mice followed by flow cytometric analysis. c-Kit+ IgE+ mast cells were gated among live (7-AAD−) Gr-1− CD19− CD3− cells. 7-AAD, 7-aminoactinomycin D; SSC, side scatter; FSC, forward scatter. (b) Nasal tissue cells from a mast cell-deficient KitW-sh/W-sh mouse were similarly processed and gated as in (a), which demonstrates the lack of c-Kit+ IgE+ mast cells. (c) Mast cells as gated in (a) were further analysed for FcγRIIIA expression and were gated into two sub-populations: The FcγRIIIA-low subset and the FcγRIIIA-high subset, representing mucosal mast cells (MMC) and connective tissue mast cells (CTMC), respectively. (d, e) Mouse nasal tissues were digested and single cell suspensions were prepared, followed by overnight incubation of the cells with PBS (dashed line), or Alexa Fluor 488-ovalbumin (OVA; 130 μg/ml) in the absence (filled) or presence (solid line) of anti-OVA IgG (90 μg/ml). The internalization of OVA by MMC (d) and CTMC (e) was examined by flow cytometry using the gating strategy shown in (a, c) and representative histograms were shown. (f, g) Quantification of the data obtained in (d, e) is expressed as geometric mean fluorescence of three replicate cultures (mean ± SEM) out of pooled nasal cells from nine mice. Numbers adjacent to outlined areas indicate per cent cells in each gate.
MMC develop apoptosis following treatment by IgG immune complexes
It has long been observed that homotypic cross-linking of FcγRIIB by IgG immune complexes, not engaging any activating receptors, induces apoptosis of B-lineage cells.18–20 We have recently shown that MMC undergo apoptosis following treatment with IgG immune complexes.9 Here, we measured IgG immune complex-mediated apoptosis of BM-derived MMC by binding of Narcissus pseudonarcissus-derived lectin as a result of cell membrane structural changes (Fig.3b) and reduced Mito Tracker dye staining as a result of the mitochondrial membrane potential collapse (Fig.3c), in addition to annexin V/7-AAD staining profiles (Fig.3a) as previously reported.9 Next, we examined the ability of the IgG immune complexes composed of a mucosal adjuvant CTA1-DD and IgG in apoptosis induction. CTA1-DD is a fusion protein consisting of the Al subunit of cholera toxin (CT) linked to a synthetic dimer of fragment-D of S. aureus protein A (DD).21 The DD domain unspecifically binds immunoglobulins and as a result, CTA1-DD and IgG can form complexes.22 Similar to the OVA/IgG complexes, CTA1-DD complexed with IgG also induced apoptosis of MMC (Fig.3d). These experiments confirmed our previous findings and further suggest that vaccine formulations containing IgG immune complexes may induce apoptosis of MMC.
Figure 3.
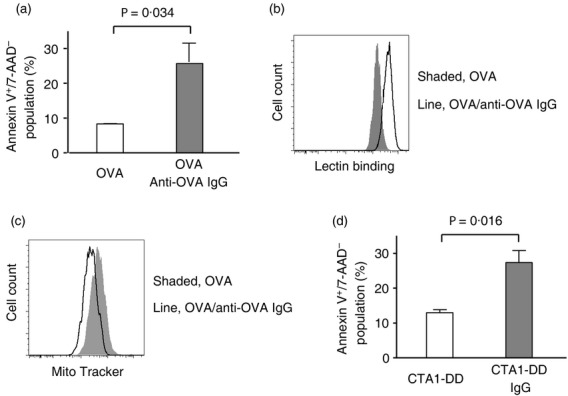
Induction of apoptosis in mouse bone marrow-derived cultured mucosal mast cells (MMC) following treatment with IgG immune complexes. (a–c) MMC were treated overnight with ovalbumin (OVA; 130 μg/ml) or immune complexes composed of OVA and anti-OVA IgG (90 μg/ml). Apoptotic cells were revealed as annexin V+/7-AAD− (a), lectin binding as a result of cell membrane structural changes (b), or reduced Mito Tracker dye staining as a result of the loss of mitochondrial membrane potential (c) by flow cytometry. (d) MMC were treated overnight with either CTA1-DD (a fusion protein comprising the Al subunit of cholera toxin linked to a synthetic dimer of fragment-D of Staphylococcus aureus protein A; 110 μg/ml) or CTA1-DD/IgG complexes and cell apoptosis was examined as explained in (a). Data are expressed as mean + SEM of three separate experiments (a, d) or representative flow cytometric histograms (b, c).
T cells can be activated in an antigen-specific manner by antigen-incorporated mast cells
The BM-derived MMC were incubated with OVA or OVA/IgG to allow for incorporation of OVA followed by co-culture with splenocytes from the OT-II mice. Expression of CD69 and Ki-67 on CD4+ T cells in the co-culture was measured as markers for cell activation and proliferation, respectively. In the absence of OVA, minimal CD69 and Ki-67 expression was observed on the co-cultured CD4+ T cells. The priming of MMC with OVA before the co-culture did not markedly enhance the activation of CD4+ T cells as determined by CD69 and Ki-67 expression. However, incubation of MMC with OVA complexed with anti-OVA IgG resulted in pronounced up-regulation of CD69 and Ki-67 expression (Fig.4).
Figure 4.
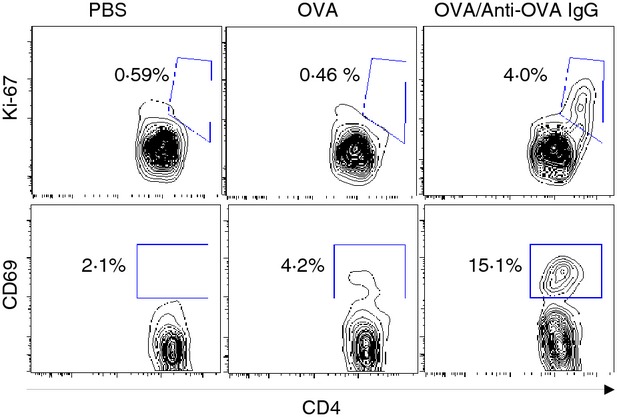
Antigen-specific activation of T cells by antigen/IgG complex-pulsed mucosal mast cells (MMC). Mouse bone marrow-derived cultured MMC were incubated with PBS, ovalbumin (OVA), or OVA/anti-OVA IgG complexes for 48 hr followed by rigorous washing. The antigen-pulsed MMC were next co-cultured with splenocytes from OT-II mice for 3 days. Activation and proliferation of CD4+ T cells were analysed by the expression of CD69 and Ki-67, respectively, by flow cytometry. The numbers adjacent to outlined areas represent the percentages of Ki-67+ CD4+ T cells (upper panels) or CD69+ CD4+ T cells (lower panels) among total CD4+ T cells. Data represent one of three independent experiments.
Discussion
Mast cells are classically known as one of the major effector cells in allergic pathology especially in IgE-mediated disorders. However, over the last few decades it has become evident that mast cells through their ability to orchestrate both innate and adaptive immune responses play important roles in both health and disease beyond the scope of allergy.23 Mucosal surfaces, especially those of the nasorespiratory, gastrointestinal and genitourinary tracts, provide critical functional barriers between the body and the external environment. The fact that mast cells are distributed at these mucosal surfaces suggests that these cells may form the first line of defence against microorganisms and other exogenous insults.24
In the present study, we demonstrated that MMC that had previously incorporated IgG-complexed antigen were able to activate antigen-specific T cells through a process known as cross-presentation. Two mechanisms may underlie such IgG-dependent activation. First of all, MMC internalized with increased efficiency an antigenic protein complexed with IgG. Second, treatment of MMC by IgG immune complexes promoted cell death, a phenomenon that is described as facilitating cross-presentation.10 The most likely receptor through which MMC can interact with IgG immune complexes is the inhibitory Fc receptor, FcγRIIB, the only IgG receptor that is expressed by these cells.9 Given the findings reported by Kambayashi et al.10 we may well speculate that professional APC, e.g. spleen-derived dendritic cells that were present in the co-culture, could engulf apoptotic MMC and present the antigens that had been previously incorporated by MMC to T cells. Although it has been previously reported that mast cells may phagocytose antigens and act as APC directly following activation,25,26 more recent investigation revealed that mast cells lack constitutive expression of MHC-II and co-stimulatory molecules (e.g. CD86), and mast cells may up-regulate, following activation, these key molecules required for activation of T cells.27,28 Therefore, we tend to suggest that MMC apoptosis and engulfment of MMC by professional APC may mechanistically account for the antigen presentation and T-cell activation, as MMC cultured using our protocol expressed MHC-II or CD86 neither constitutively nor following treatment with IgG immune complexes (data not shown).
Several lines of evidence have revealed the close physical proximity between mast cells and other immune effectors in inflamed tissues following infection. Through elaboration of a broad array of cytokines and chemokines, mast cells may efficiently promote the recruitment of various subsets of leucocytes, including dendritic cells and T cells, to the site of infection.29,30 Furthermore, compared with CTMC, MMC are located closer to the mucosal surface,4,31 and they are strategically positioned to access antigens introduced through the mucosal portal of entry. Therefore, such cross-presentation of antigens to T cells by MMC with the help of APC may happen in vivo.
Recently the roles of mast cells have been extended to providing adjuvanticity for vaccination.2–4,32–34 We have come to recognize that greater levels of antigen-specific immune responses could be obtained when the mucosal adjuvant CTA1-DD, by virtue of its unspecific binding to immunoglobulins, was complexed with IgG forming immune complexes that could activate nasal CTMC.4 In contrast, MMC failed to be activated by CTA1-DD/IgG immune complexes.4 Instead, MMC could be induced to undergo apoptosis following treatment with immune complexes composed of OVA and IgG.9 In the current study, we further demonstrated that CTA1-DD/IgG immune complexes could induce apoptosis of MMC. We have previously provided evidence that CTMC were most likely the target population for CTA1-DD/IgG complexes.4 In the current study, we further revealed a role of MMC in enhancing immune responses by cross-presenting antigens to T cells contributing to the establishment of antigen-specific T-cell responses. In practical immunization, this may happen when IgG immune complex-containing vaccine formulations are used. For example, if the adjuvant complexes composed of CTA1-DD/IgG are linked with vaccine antigens, MMC may also be exploited as the targets for primary mucosal vaccination. Alternatively, cross-presentation of antigens to T cells mediated by MMC may occur generally after secondary infection or secondary immunization when both antigen-specific IgG and antigens are available.
An intriguing question is whether CTMC could also mediate cross-presentation of IgG-complexed antigen to T cells. Many features distinguish CTMC and MMC,5,6 and perhaps the most relevant contrast between the two subtypes in this setting is the differential expression of Fcγ receptors. CTMC express both FcγRIIB, an inhibitory Fcγ receptor, and FcγRIIIA, an activating Fcγ receptor. In contrast, MMC only express FcγRIIB but not FcγRIIIA. It is most likely that both receptors can mediate engulfment of antigens. Although we previously demonstrated that expression of both FcγRIIB and FcγRIIIA by CTMC protects the cells from IgG immune complex-mediated apoptosis,9 this protection may depend on the balanced expression of the two receptors and death will occur if the balance is lost through changes in the growth/culture environment. Therefore, CTMC may possibly participate in cross-presentation of antigens to T cells under favourable conditions.
In conclusion, in this study we provide evidence for a role of MMC in the activation of T cells and support the concept that these cells have a major role in the first line of defence against natural infection or in boosting vaccination.
Acknowledgments
The authors would like to thank A. Stensson and Y. Wang for technical support with confocal microscopy and ImageStream analysis, respectively. This work was supported by funding from the Mucosal Immunobiology and Vaccine Research Centre (MIVAC), University of Gothenburg, and grants from the Ministry of Human Resources and Social Security of China, the National Natural Science Foundation of China (No. 81102281), Nanjing Medical Science and Technique Development Foundation, China (QRX11039), Stiftelsen Clas Groschinskys Minnesfond, Sweden and Konsul Berghs stiftelse, Sweden.
Glossary
- 7-AAD
7-aminoactinomycin D
- APC
antigen-presenting cells
- BM
bone marrow
- CTMC
connective tissue mast cells
- MMC
mucosal mast cells
- OVA
ovalbumin
Author contributions
JD and YF performed the experiments; ZX designed the study; JD, YF and ZX wrote the paper.
Disclosure
The authors have no conflicts of interest to disclose.
References
- Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines. 2011;10:539–50. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Shelburne CP, Hart JP, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–41. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- Fang Y, Larsson L, Mattsson J, Lycke N, Xiang Z. Mast cells contribute to the mucosal adjuvant effect of CTA1-DD after IgG-complex formation. J Immunol. 2010;185:2935–41. doi: 10.4049/jimmunol.1000589. [DOI] [PubMed] [Google Scholar]
- Fang Y, Zhang T, Lidell L, et al. The immune complex CTA1-DD/IgG adjuvant specifically targets connective tissue mast cells through FcγRIIIA and augments anti-HPV immunity after nasal immunization. Mucosal Immunol. 2013;6:1168–78. doi: 10.1038/mi.2013.16. [DOI] [PubMed] [Google Scholar]
- Enerback L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66:303–12. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Befus AD. Mast cells at mucosal frontiers. Curr Mol Med. 2005;5:573–89. doi: 10.2174/1566524054863915. [DOI] [PubMed] [Google Scholar]
- Katz HR, Arm JP, Benson AC, Austen KF. Maturation-related changes in the expression of FcγRII and FcγRIII on mouse mast cells derived in vitro and in vivo. J Immunol. 1990;145:3412–7. [PubMed] [Google Scholar]
- Katz HR, Raizman MB, Gartner CS, et al. Secretory granule mediator release and generation of oxidative metabolites of arachidonic acid via Fc-IgG receptor bridging in mouse mast cells. J Immunol. 1992;148:868–71. [PubMed] [Google Scholar]
- Fang Y, Larsson L, Bruhns P, Xiang Z. Apoptosis of mouse mast cells is reciprocally regulated by the IgG receptors FcγRIIB and FcγRIIIA. Allergy. 2012;67:1233–40. doi: 10.1111/j.1398-9995.2012.02878.x.. [DOI] [PubMed] [Google Scholar]
- Kambayashi T, Baranski JD, Baker RG, et al. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–96. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer SS, Simson JA, Farrington JE. Mast cell phagocytosis of red blood cells. Am J Pathol. 1975;80:481–98. [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Ross EA, MacGregor JI, et al. Mast cell phagocytosis of FimH-expressing enterobacteria. J Immunol. 1994;152:1907–14. [PubMed] [Google Scholar]
- Della RF, Granata A, Monaco M, Basile G. Phagocytosis of cancer cells by mast cells in breast cancer. Anticancer Res. 2009;29:3157–61. [PubMed] [Google Scholar]
- Sher A, Hein A, Moser G, Caulfield JP. Complement receptors promote the phagocytosis of bacteria by rat peritoneal mast cells. Lab Invest. 1979;41:490–9. [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, et al. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekoff M, Strasser A, Nilsson G. FcεRI aggregation promotes survival of connective tissue-like mast cells but not mucosal-like mast cells. J Immunol. 2007;178:4177–83. doi: 10.4049/jimmunol.178.7.4177. [DOI] [PubMed] [Google Scholar]
- Heyder P, Gaipl US, Beyer TD, et al. Early detection of apoptosis by staining of acid-treated apoptotic cells with FITC-labeled lectin from Narcissus pseudonarcissus. Cytometry A. 2003;55:86–93. doi: 10.1002/cyto.a.10078. [DOI] [PubMed] [Google Scholar]
- Pearse RN, Kawabe T, Bolland S, et al. SHIP recruitment attenuates FcγRIIB-induced B cell apoptosis. Immunity. 1999;10:753–60. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Cutler AJ, Brownlie RJ, et al. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–29. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- Tzeng SJ, Bolland S, Inabe K, Kurosaki T, Pierce SK. The B cell inhibitory Fc receptor triggers apoptosis by a novel c-Abl family kinase-dependent pathway. J Biol Chem. 2005;280:35247–54. doi: 10.1074/jbc.M505308200. [DOI] [PubMed] [Google Scholar]
- Lycke N, Schon K. The B cell targeted adjuvant, CTA1-DD, exhibits potent mucosal immunoenhancing activity despite pre-existing anti-toxin immunity. Vaccine. 2001;19:2542–8. doi: 10.1016/s0264-410x(00)00487-4. [DOI] [PubMed] [Google Scholar]
- Agren LC, Ekman L, Lowenadler B, Lycke NY. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997;158:3936–46. [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol. 2013;190:4458–63. doi: 10.4049/jimmunol.1203420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandji P, Oskeritzian C, Cacaraci F, et al. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J Immunol. 1993;151:6318–28. [PubMed] [Google Scholar]
- Fox CC, Jewell SD, Whitacre CC. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994;158:253–64. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]
- Kambayashi T, Allenspach EJ, Chang JT, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol. 2009;182:4686–95. doi: 10.4049/jimmunol.0803180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudenzio N, Espagnolle N, Mars LT, et al. Cell–cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–88. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- Shelburne CP, Nakano H, St John AL, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–42. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine SM, Issekutz TB, Marshall JS. Virus stimulation of human mast cells results in the recruitment of CD56+ T cells by a mechanism dependent on CCR5 ligands. FASEB J. 2012;26:1280–9. doi: 10.1096/fj.11-188979. [DOI] [PubMed] [Google Scholar]
- Gersch C, Dewald O, Zoerlein M, et al. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–9. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–52. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayamuro H, Yoshioka Y, Abe Y, et al. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol. 2010;84:12703–12. doi: 10.1128/JVI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John AL, Chan CY, Staats HF, Leong KW, Abraham SN. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat Mater. 2012;11:250–7. doi: 10.1038/nmat3222. [DOI] [PMC free article] [PubMed] [Google Scholar]


