Abstract
Peripheral blood mononuclear cells (PBMC) of untreated, HIV-infected patients contain HIV-specific CD8 T cells as well as their corresponding targets, HIV-infected CD4 T cells. To determine if CD4 T-cell depletion in HIV-infected patients may result from autologous CD8–CD4 T-cell interaction, CD8 and CD4 T cells procured from PBMC of acute and chronic untreated HIV-infected patients were sorted and co-incubated. Formation of CD8-CD4 T-cell conjugates was observed by fluorescence microscopy. Apoptosis of CD4 T cells in conjugation was recorded by digitized images and was further observed and measured by FACS using Annexin staining. Perforin expression in the CD8 T cells was measured using intracellular monoclonal perforin antibody staining. HIV DNA in the conjugated CD4 T cells was detected by in situ PCR. We found that 6·1 ± 0·5% of CD4 T cells from acute HIV-infected patients and 3·0 ± 0·5% from chronic HIV-infected patients formed CD8–CD4 T-cell conjugates. Annexin binding and cell morphology typical of apoptosis were observed in the conjugated CD4 T cells. The majority of CD8 T cells that had conjugated to CD4 T cells expressed perforin. The conjugated CD4 T cells exhibited nuclear HIV DNA. CD8 T cells and HIV-infected CD4 T cells, both procured from the PBMC of untreated HIV-infected patients, form conjugates. Apoptotic lytic activity has been observed in the conjugated CD4 T cells. We propose that CD4 T-cell annihilation in HIV-infected patients results, at least in part, from the interactions of perforin-rich CD8 T cells with autologous, HIV-infected CD4 T cells.
Keywords: apoptosis, CD4 T cells, CD8 T cells, HIV
Introduction
The appearance of HIV-1-specific CD8 T cells in acute HIV-1 infection correlates with the decline in both viral load and CD4 T cells.1–5 The mechanism(s) that brings about progressive decline of CD4 T cells in HIV-infected individuals is controversial.6–9 A number of processes have been proposed to account for the depletion of CD4 T cells.10–15 The majority of HIV-infected CD4 T cells are resting memory cells in a latent state of infection, probably expressing only minute amounts of viral proteins, making their actual contribution to CD4 T-cell depletion unknown.10,16,17
The aim of this study was to delineate the role that CD8 T cells play in CD4 T-cell depletion during HIV infection. Depletion of CD8 T cells in simian immunodeficiency virus-infected macaques has resulted in a rapid rise in plasma viral load.2,3,18 Recognition of viral peptides by CD8 T cells has been demonstrated to be specific and to eventually result in the killing of target cells by cytotoxic T-cell lymphocytes (CTL).14,4,5,19,20 CD8 T cells can suppress viral spread by the production of interferon-γ, by the induction of apoptosis via the cell surface receptors Fas–Fas ligand, and by secreted perforin and granzymes. Several studies have also demonstrated that expression of perforin can serve as a marker of ongoing activation and killing capacity of CD8 T cells.21,22 Apart from their positive effects of controlling viral spread, CTL have been shown to cause tissue damage in viral infections, such as in viral myocarditis23,24 and viral hepatitis.25 These observations suggest that the gradual elimination of CD4 T cells observed in HIV-infected patients could be attributed, at least in part, to HIV-specific CD8 T cells. Between 3 and 10% of all CD8 T cells in the peripheral blood mononuclear cells (PBMC) of chronically HIV-1-infected patients are HIV-1-specific,26–28 which can target HIV-1-infected CD4 T cells in the peripheral blood.16,26,29 The major HIV reservoir resides in a pool of latently infected resting memory CD4 T cells carrying an integrated form of the viral genome that does not produce virions.16,17 A suggested approach for the eradication of HIV-1 involves reversing latency in patients on anti-retroviral therapy.30,31 Cells harbouring induced proviruses could then be lysed by HIV-1-specific CTL,32 while new rounds of infection are blocked by anti-retroviral therapy.
Here, we report on the interaction of peripheral blood CD8 T cells with autologous, HIV-infected CD4 T cells from HIV-infected patients, with the aim of testing the hypothesis that HIV-specific CD8 T cells can recognize and kill autologous, HIV-infected CD4 T cells.
Materials and methods
Study population
Twenty-two untreated HIV-1-infected male patients from the Kobler AIDS Centre at the Tel-Aviv Medical Centre (Tel-Aviv, Israel) were enrolled in the study along with 22 age-matched healthy male controls. HIV-1 antibodies were detected by ELISA kit (AxSYM HIV-1, 2gO, Abbott Laboratories, Wiesbaden, Germany) and confirmed by Western blot (Inno-LIA HIV1+2 Score, Innogenetics, Gent, Belgium). Acute HIV-1-infected patients were identified by clinical presentation and sero-conversion analysis. The 22 study patients were divided into two groups: acute HIV-1-infected patients (n = 11, 3–12 weeks after an acute HIV-1 syndrome) (Table1) and chronic HIV-1-infected patients (n = 11, 6–40 months after an acute HIV-1 syndrome) (Table2). All patients provided written informed consent to participate according to the guidelines of the institutional Human Subjects Protection Committee. The experiments were conducted on the day blood was drawn.
Table 1.
Conjugations and cytolytic activity of CD4–CD8 T cells in acute HIV infection
| Case | Time since acute HIV syndrome (weeks) | Laboratory tests | Conjugate formation (%) | Cytolytic activity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | Viral load | HIV | Control | HIV | Control | ||
| 1 | 3 | 961 | 1395 | 42 100 | 7·9 | 1·4 | 25 | 13 |
| 2 | 3 | 975 | 1100 | 22 800 | 8·1 | 1·6 | 31 | 8 |
| 3 | 3 | 580 | 1160 | 31 474 | 6·1 | 1·0 | 30 | 14 |
| 4 | 4 | 1035 | 4278 | 780 610 | 4·5 | 0·6 | 18 | 5 |
| 5 | 8 | 780 | 860 | 2407 | 4·4 | 0·6 | 19 | 8 |
| 6 | 8 | 1260 | 2296 | 454 | 6·5 | 1·0 | 13 | 5 |
| 7 | 12 | 735 | 1242 | 19 300 | 3·6 | 1·0 | 14 | 5 |
| 8 | 12 | 550 | 1350 | 125 974 | 3·3 | 0·6 | 20 | 8 |
| 9 | 4 | 368 | 1610 | 1 000 000 | 5·6 | 0·6 | 12 | 6 |
| 10 | 3 | 469 | 2263 | 177 517 | 8·2 | 1·3 | 16 | 5 |
| 11 | 5 | 420 | 540 | 49 416 | 8·2 | 1·3 | 8 | 5 |
| Average | 739 | 1645 | 6·1 | 0·9 | 19 | 8 | ||
| SE | 69 | 362 | 0·58 | 0·06 | 2·4 | 1·09 | ||
Table 2.
Conjugation and cytolytic activity of CD4–CD8 T cells in chronic HIV infection
| Case | Time since acute HIV syndrome (weeks) | Laboratory tests | Conjugate formation (%) | Cytolytic activity | ||||
|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | Viral load | HIV | Control | HIV | Control | ||
| 1 | 73 | 340 | 1020 | 96 318 | 3·8 | 1·1 | 23 | 23 |
| 2 | 363 | 480 | 1416 | 5218 | 0·6 | 0·5 | 11 | 8 |
| 3 | >52 | 960 | 1376 | 6300 | 1·2 | 0·6 | 2 | 2 |
| 4 | 64 | 400 | 800 | 8685 | 3·5 | 1·0 | 13 | 12 |
| 5 | 60 | 672 | 798 | 71 000 | 2 | 0·8 | 14 | 12 |
| 6 | >52 | 720 | 2160 | 2498 | 5·5 | 1·0 | 12 | 13 |
| 7 | 130 | 323 | 850 | 189 | 2·8 | 1·0 | 9 | 13 |
| 8 | 26 | 456 | 1146 | 85 117 | 2·2 | 0·6 | 5 | 8 |
| 9 | 104 | 520 | 580 | 694 | 5·6 | 0·6 | 10 | 8 |
| 10 | 31 | 870 | 1537 | 30 923 | 3·6 | 0·6 | 17 | 6 |
| 11 | 130 | 574 | 1160 | 30 690 | 4 | 0·9 | 3 | 5 |
| Average | 574 | 1077 | 3·1 | 0·9 | 11 | 8 | ||
| SE | 63 | 130 | 0·48 | 0·06 | 2·05 | 1·09 | ||
Plasma viral load and CD4 and CD8 T-lymphocyte counts
Plasma HIV-1 RNA levels were determined by the COBAS AmpliPrep/COBAS TaqMan HIV-1 test (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Levels of RNA in plasma were considered undetectable when they were lower than 40 copies/ml. CD4 and CD8 T-cell counts were assessed using Tritest CD3 FITC/CD4 PE/CD45 PerCP and Tritest CD3/FITC/CD8 PE/CD45 PerCP reagents (Becton Dickinson, Franklin Lakes, NJ) according to the manufacturer’s instructions. Analysis was carried out on a FACSCaliber machine, using CellQuest 3.3 software (Becton Dickinson).
Sorting of CD4 and CD8 T cells from the PBMC of HIV-infected patients
Thirty millilitres of whole blood was collected into vacutainer tubes containing EDTA. The PBMC were separated over a Ficoll-Hypaque density gradient. CD8 and CD4 T cells were sorted: positive CD4 T cells were separated using Dynabeads FlowComp Human CD4 (Invitrogen 113.61D; Invitrogen, Carlsbad, CA), followed by positive selection for CD8 T cells using Dynabeads FlowComp Human CD8 (Invitrogen 113·62D) according to the manufacturer’s protocol, resulting in isolated cells that were bead-free and antibody-free, phenotypically unaltered and suitable for flow cytometry and functional studies. The purity of the isolated cells was determined by FACS analysis to be 89–92%.
Conjugate formation of CD8–CD4 T cells
Isolated CD4 T cells were labelled with the fluorescent dye calcein, 1–2 μm (Molecular Probes #C-1430; Molecular Probes, Eugene, OR) in PBS for 1 hr at 37°.33 Conjugates were formed by mixing 106 CD8 T cells with an equal number of calcein-labelled CD4 T cells suspended in 1 ml PBS–fetal calf serum. The suspension was allowed to stand for 10 min after which the cells were co-centrifuged at 185 g for 10 min at room temperature, re-suspended and placed on ice. The number of conjugates formed was recorded by a blinded reviewer using a haemocytometer under fluorescence microscopy.34 At least 1000 cells were scored for each patient. The per cent conjugation is the number of conjugated CD4 T cells divided by the total number of CD4 T cells × 100. Counting CTL target cell conjugates under a microscope has been shown to be both accurate and specific.21,34,35
Quantification of CD4 T cells in apoptosis following CD8–CD4 T-cell interaction
Sorted CD4 and CD8 T cells were allowed to form conjugates that were incubated for 1·5 hr at 37°. The cells were then allowed to adhere to poly-l-lysine-coated glass slides, stained with 5 μl annexin V-FITC (Calbiochem, San Diego, CA) and anti-CD8 allophycocyanin-conjugated antibody (BioLegend, San Diego, CA) for 15 min at room temperature, and recorded by fluorescence microscopy. Binding of annexin V-FITC was used to measure CD4 T-cell apoptosis. In viable cells, phosphatidyl serine is located on the cytoplasmic surface of the cell membrane. During apoptosis, phosphatidyl serine is exposed on the outer cell surface, which enables binding of annexin V-FITC. Binding of propidium iodide (PI) to nucleic acid has been used to detect advanced apoptotic cells. The annexin V-FITC apoptosis detection kit (Calbiochem PF032) was employed following conjugate formation between sorted CD8 and CD4 T cells compared with stand-alone CD8 and CD4 T cells as control, according to the manufacturer’s instructions. Briefly, equal numbers of sorted CD8 and CD4 T cells were mixed and allowed to form conjugates, followed by incubation at 37° for 2 hr. The apoptotic activity of CD8 effectors against conjugated CD4 cells was measured using FACS: annexin-positive and PI-positive cells represented cells in different stages of apoptosis. The cytolytic activity was the percentage of total cells in apoptosis. The difference between the average of isolated live CD4 and CD8 T cells compared with CD8–CD4 T cells in conjugation reflected CD8 T cell-induced killing.
Recording CD4 T-cell apoptosis following conjugation with CD8 T cells
Isolated CD4 T cells were labelled with 1–2 μm fluorescent dye calcein. Conjugates were formed by mixing half a million CD8 T cells with an equal number of calcein-labelled CD4 T cells suspended in 1 ml RPMI–10% FCS. Then, 2 × 105 cells suspended in 300 μl medium were plated in eight-well flat-bottomed plates, culture area 0·8 cm²/well (Lab-Tek™, Swedesboro, NJ) and placed on an inverted microscope. i (Cell R, Olympus, Tokyo, Japan). Ten different regions of interest for a single HIV patient were recorded simultaneously with 10 different regions of interest for one healthy control in each experiment. The temperature was maintained at 37° in a 5% CO2 environment throughout the experiments. The region of interest was photographed under fluorescence microscopy before and after the recording to recognize the calcein-labelled CD4 T cells.
Cell staining with surface and intracellular monoclonal antibodies
For cell surface staining, the cells were incubated with anti-CD8 allophycocyanin-conjugated antibody (BioLegend) in 50 μl PBS, 2% fetal calf serum for 20 min on ice. For intracellular perforin staining, cells were first stained with a cell surface antibody as described above, washed, then permeabilized using FACS-Perm2 (BD Biosciences, San Jose, CA) and incubated with an anti-perforin-FITC antibody (BD Pharmingen, San Diego, CA) for 30 min at room temperature. The cells were then washed and seeded on poly-l-lysine-coated slides and recorded by fluorescence microscopy.
Cytometric analysis
CellQUEST software on a FACSCalibur (Becton Dickinson) was used for the cytometric analysis. A total of 100 000 events were acquired for each analysis. Lymphocytes were identified by gating on forward and side scatter of the CD8 T cells, and the percentage of perforin-expressing cells was determined (Table3).
Table 3.
Percent perforin-expressing CD8 T cells
| Case | Acute stage (%) | Chronic stage (%) | Control (%) |
|---|---|---|---|
| 1 | 75 | 48 | 16 |
| 2 | 35 | 22 | 14 |
| 3 | 49 | 33 | 16 |
| 4 | 34 | 26 | 6 |
| 5 | 35 | 18 | 12 |
| 6 | 20 | 45 | 13 |
| 7 | 24 | 14 | |
| 8 | 24 | 18 | |
| 9 | 10 | 16 | |
| 10 | 7 | ||
| 11 | 17 | ||
| Average | 41·3 | 27·7 | 12·8 |
| SE | 4·4 | 3·6 | 2·8 |
Identification of HIV-infected CD4 T cells by in situ PCR
We used in situ PCR of HIV DNA to test if CD4 T cells in the conjugates are infected by HIV. This method was previously described by Bagasra for PBMC of HIV-infected patients.26,36
Primers
The PCR primers were designed to recognize integrated HIV DNA in the CD4 T cells of clinical samples. The primer pair from the HIV long terminal repeats was: ltr-f- GCCTCAATAAAGCTTGCCTTGA, ltr-r: GGCGCCACTG CTAGAGATTTT.
Sequencing of the PCR product
DNA from CD4 T cells of HIV-infected patients was amplified and sequenced. We selected a sequence of 56 nucleotides from the amplified DNA shared by several patients and synthesized and tagged it with a fluorescin dye to be used as a probe. The selected sequence 5′-CAC AAC AGA CGG GCA CAC ACC TAC TTT AAG CAC TCA AGG CAA GCT TTA TTG AGG CA-3′.
In situ PCR
Following conjugation, CD4 and CD8 T cells were fixed on slides in 4% paraformaldehyde in 1× PBS, amplified and hybridized with the fluorescein-tagged probe using frame-seal incubation chambers.
Statistical analysis
All data were analysed by using one-way analysis of variance. Statistical differences among means were considered significant at P < 0·05. A post hoc test (Tukey–Kramer) was performed when the interaction between treatments was significant. The JMP version 5.1 was used for all analyses. Values are presented as mean ± standard error of the mean.
Results
CD8 and CD4 T cells from the PBMC of HIV-infected patients form conjugates
The ability of CD8 T cells procured from HIV-1-infected patients to form conjugates with autologous CD4 T cells was determined (Fig.1; Tables1 and 2). Conjugate formation between CD8 T cells and their target cells was previously studied and found to be specific.21,34,35 The present experiments included cells from healthy controls, acute HIV-1-infected patients and chronic HIV-1 infected patients. PBMC were sorted for CD4 and CD8 T cells, the CD4 T cells were labelled with the green fluorescent tag calcein. Equal numbers of CD8 and CD4 T cells were interacted, recorded and counted (Fig.1). A mean of 6·1 ± 0·5% of the CD4 T cells from acute HIV-1-infected patients and 3·0 ± 0·5% from chronic HIV-infected patients formed CD8–CD4 T-cell conjugates, compared with 0·9 ± 0·06% from HIV-negative controls (P < 0·0001) (Tables1 and 2; Fig. 3).
Figure 1.
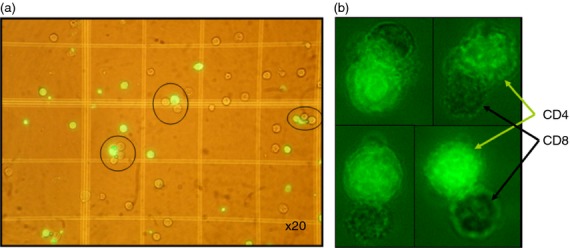
Conjugate formation: CD4 and CD8 T cells were sorted from peripheral blood mononuclear cells (PBMC) of HIV-1-infected patients. Calcein-labelled CD4 T cells (green florescence) and non-labelled autologous CD8 T cells (one million each) were allowed to form conjugates by co-centrifugation followed by re-suspension. (a) Conjugates were counted in a haemocytometer chamber under fluorescence microscopy, – magnification × 20. (b) CD4 (green) and CD8 cells in conjugates, magnification × 100.
Figure 3.
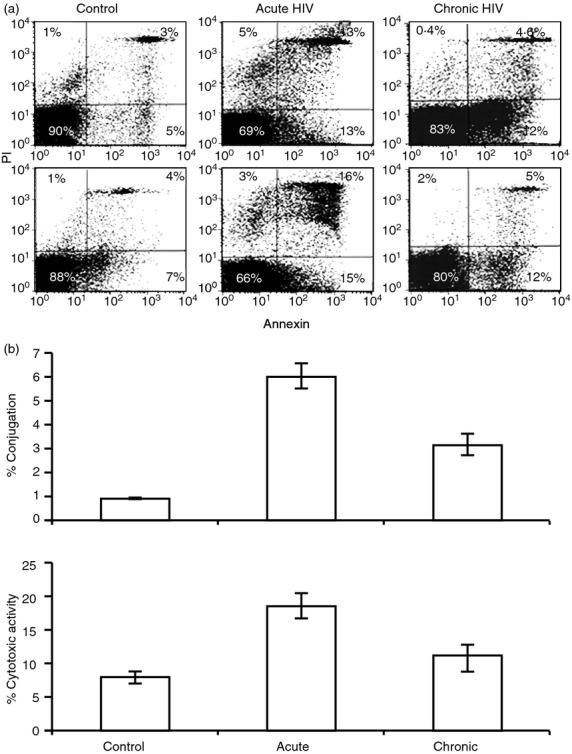
Conjugate formation and lytic activity. Killing activity was measured with an annexin V-(FITC) apoptosis detection kit and analysed by FACS where annexin and PI-positive cells represent cells in different stages of apoptosis. (a) Two different experiments are presented: 90% and 88% of the cells in the non-infected control group are intact compared with 69% and 66% in the acute HIV-infected patients and 83% and 80% in the chronic HIV-infected patients. The main difference between the acute and chronic HIV-infected patients stems from the increased late apoptosis occurring in the former. (b) Statistical analysis of the CD8-CD4 T-cell conjugate formation and cytotoxic activity between 11 acute and 11 chronic HIV-infected patients and healthy controls. Average ± SE; bars with different letters differ at P < 0·05 (Tukey–Kramer test).
Apoptosis of conjugated CD4 T cells
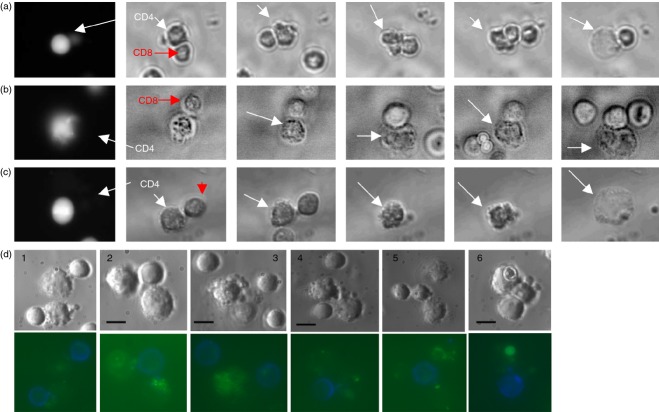
We continuously recorded the apoptotic process in conjugated CD4 T cells in tissue culture under a microscope (Fig.2a–c, see Supporting information, Videos. S1–S3). Digitized images were acquired every 2 min for up to 3 hr using a live cell imaging interface. The CD4 T cells were identified by the green fluorescence of calcein. We recorded cells derived from six HIV-infected patients as well as six healthy controls. Three representative films and snapshots thereof are shown in Fig.2(a–c). Figure2(a) illustrates conjugation of CD4–CD8 T cells, eventually leading to apoptosis of CD4 T cells from the PBMC of an acute HIV-infected patient. Figure2(b,c) illustrate similar events in two chronic HIV-infected patients. CD4 target cells can be seen to have undergone apoptosis even after the CD8 T cell had detached from it (Fig.2c; see Supporting information, Video S3) as previously demonstrated.21 No comparable events of conjugation of CD4–CD8 T cells resulting in CD4 T-cell apoptosis were observed in healthy controls.
Figure 2.
CD8-mediated killing of autologous conjugated CD4 T cells during acute and chronic HIV infection. (a–c, video S1–S3) We continuously recorded the apoptotic process of the conjugated calcein-labelled CD4 T cells in tissue culture under a microscope. Three representative films and snapshots from the films are shown. (a) CD4 and CD8 T cells conjugate, eventually leading to CD4 T-cell apoptosis from the peripheral blood mononuclear cells (PBMC) of an acute HIV-infected patient. (b and c) Events similar to (a) in two chronic HIV-infected patients. (c, video S3) The CD4 target cells were able to undergo apoptosis even after the CD8 T cell had detached from it. (d 1–6) CD8 T cell (blue) and the apoptosis marker annexin V (green) staining of conjugates. Only CD4 T cells that had conjugated to CD8 T cells demonstrate apoptosis (annexin staining, cell shrinkage, plasma membrane blebs and formation of apoptotic bodies) during both the acute (d 1–3) and chronic (d 4–6) stages of HIV infection. Each scale bar is 10 μm.
To verify that the observed apoptosis was indeed of CD4 T cells that had conjugated to CD8 T cells, the cells were labelled with an anti-CD8 antibody (blue) and the apoptosis marker Annexin V-FITC (green). Annexin binding and morphology typical of apoptosis (i.e. cell shrinkage, plasma membrane blebs and formation of apoptotic bodies) were observed under the microscope. Apoptosis was detected in CD4 T cells that had conjugated to CD8 T cells during the acute (Fig.2d; 1–3) as well as the chronic stages of HIV infection (Fig.2d; 4–6).
Conjugation of CD8–CD4 T cells and cytolytic activity in acute and chronic HIV-1 infection
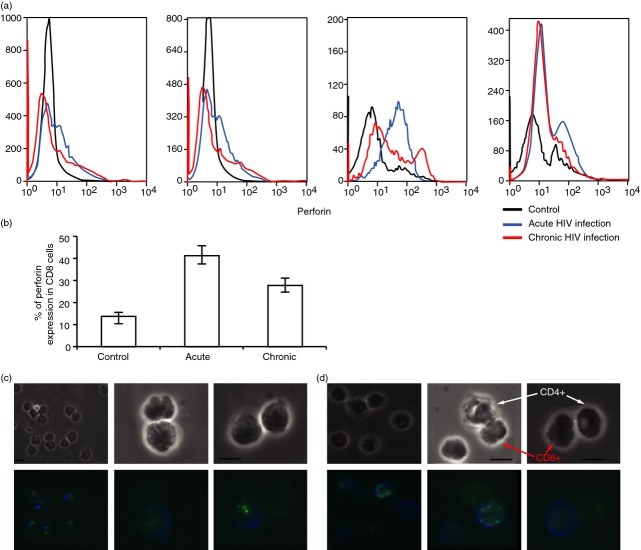
We applied FACS analysis to quantify the apoptosis of conjugated CD4 T cells from HIV-1-infected patients during both the acute and chronic phases of HIV-1 infection using the Annexin V-FITC apoptosis detection kit and compared their findings with those of healthy controls (Fig.3a). We observed differences between both the numbers of intact CD4 and CD8 T cells (Annexin-negative, PI-negative) and the number of cells in early apoptosis (Annexin-positive, PI-negative) with the number of cells in late apoptosis (Annexin-positive, PI-positive). The cytolytic activity is the percentage of cells in apoptosis out of the total number of cells (two different experiments in Fig.3a). It emerged that 90% and 88% of the cells of the control were intact compared with 69% and 66% of the acute HIV-infected patients and 83% and 80% of the chronic HIV-infected patients. It appeared that the main difference between the acute and chronic HIV-infected patients stemmed from the increased late apoptosis within the acute HIV-infected patients (Fig.3a).
Figure3(b) and Tables1 and 2 compare CD4 T cells, CD8 T cells, viral load, conjugate formation and cytolytic activity observed in 11 acute (Table1) and 11 chronic (Table2) HIV-1 infected patients with cells of healthy controls. Conjugate formation in the acutely infected patients was 6·1 ± 0·5%, significantly higher compared with the 3·1 ± 0·5% of the chronically infected patients, and the 0·9 ± 0·06% for the healthy controls (P < 0·0001 for each) (Fig.3b). Cytotoxic activity in the acutely infected patients was 18·6 ± 2·4%, significantly higher compared with 10·9 ± 2·0% in the chronically infected patients and 8·8 ± 1·0% in healthy controls (P = 0·0008) (Fig.3b).
Perforin expression in sorted and conjugated CD8 T cells from HIV-1-infected patients
Perforin expression was determined in sorted CD8 T cells procured from PBMC of HIV-1-infected patients and from the healthy controls. CD8 T cells were labelled with CD8 and perforin antibodies, and 105 cells were analysed by FACS for perforin expression (Table3). There were no significant differences in perforin expression between the acute (41 ± 4·4%) and chronic (27 ± 3·6%) groups of infected patients: both were significantly different from the healthy controls (12 ± 2·8%; P < 0·0001 for each) (Fig.4a,b). Immunofluorescent staining was applied to determine perforin expression in individual conjugated CD8 T cells. Perforin expression (green) in conjugated CD8 T cells (blue) was found in 60% of CD8 T cells found in conjugation with CD4 T cells in both the acute (Fig.4c) and chronic HIV-1-infected patients (Fig.4d).
Figure 4.
Perforin expression in CD8 T cells from peripheral blood mononuclear cells (PBMC) of acute and chronic HIV-infected patients and healthy controls. (a) FACS analysis of perforin expression in CD8 T cells in acute HIV infection (blue), chronic HIV infection (red), and non-infected controls (black) (four different experiments are presented). (b) Statistical analysis of perforin expression in sorted CD8 T cells of acute and chronic HIV-infected patients and healthy controls (average ± SE), bars with different letters differ at P < 0·05 (Tukey–Kramer test). (c) Immunofluorescence of CD8 (blue) and perforin (green) in conjugated CD8–CD4 T cells of acute and (d) chronic HIV-infected patients. Each scale bar is 10 μm.
Conjugated CD4 T cells are HIV-infected
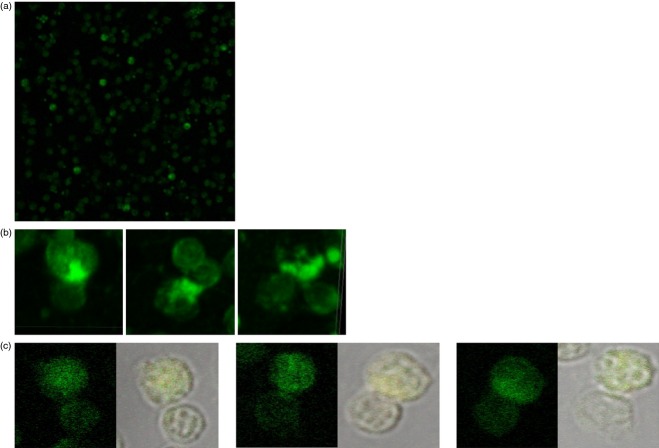
In situ PCR was used to identify integrated HIV DNA in conjugated CD4 T cells in four HIV-infected patients. Previous studies employing in situ PCR to identify chronic HIV-infected CD4 T cells within the PBMC of HIV-infected patients found an average of 6–8% infected cells.26,29 Figure5(a, b) illustrates conjugation of CD4–CD8 T cells from the PBMC of a 42-year-old patient, 2 years after infection, with a viral load of 25 471 copies/ml and CD4 count of 666/mm3. Figure5(a) demonstrates a mixture of CD8 and CD4 T cells in which 10% of the CD4 T cells have integrated HIV DNA in their nucleus. All conjugates demonstrated an HIV DNA within them (Fig.5b). Figure5(c) illustrates conjugation of CD4–CD8 T cells from the PBMC of another chronic HIV-infected patient.
Figure 5.
The CD4 T cells in the conjugates are HIV- infected. We demonstrated that the CD4 T cells in the conjugates are HIV-infected with in situ PCR for identifying the integrated HIV DNA in the cells (green). The conjugation of CD4–CD8 T cells are from the peripheral blood mononuclear cells (PBMC) of a 42-year-old patient, 2 years after infection, with a viral load of 25 471 copies/ml and CD4 count of 666/mm3. (a) A mixture of CD8 and CD4 T cells in which 10% of the CD4 T cells have integrated HIV DNA in their nucleus. (b) Conjugated CD4 T cells with integrated HIV DNA in their nucleus (c) Conjugated CD4 T cells with integrated HIV DNA in their nucleus from the PBMC of another patient. All conjugates demonstrated an HIV-infected cell within them
Discussion
AIDS is characterized by a failure of the immune system to overcome HIV-1 infection, resulting in persistent viral infection with an insidious disease progression. This process occurs despite a strong HIV-1-specific CD8 T-cell response.1–3 The precise role of CD8 T cells during the clinical course of HIV-1 infection has not yet been defined. CD8 T cells are an essential component of an effective host immune response to viral infections.5,37 There are HIV-specific CD8 T cells as well as latently infected CD4 T cells in the PBMC of untreated HIV-1-infected patients. The biology of latent HIV-infected CD4 T cells is still an enigma. The latent reservoir in resting CD4 T cells is the major barrier to HIV-1 eradication. Studies that used sensitive in situ PCR to identify chronic HIV-infected CD4 T cells within the PBMC found that between 0·1–13·5% of those CD4 T cells are positive for HIV DNA (average of 6 and 8%).26,29 Recently, Siliciano and Siliciano published a study that provides a molecular basis for understanding measures of the latent reservoir.17 Through an analysis of proviruses that did not give rise to infectious virus following a single round of T-cell activation (non-induced proviruses), they provided a definitive explanation for the large discrepancy between results of PCR and culture assays of latent reservoir size. The identification of intact, non-induced proviruses indicates that the size of the latent reservoir may be much greater than previously thought. They reconstructed full-length, intact non-induced proviruses from multiple patients, and all showed growth kinetics comparable to induced proviruses from the same patient and a reference isolate. These intact non-induced proviruses are not detected in standard culture assays.
The frequency of cells harbouring HIV pro-viral DNA in lymph nodes and gut biopsies is comparable to the frequency of chronic HIV-infected memory CD4 T cells in PBMC.16 Studies of CD8 T cells and chronically HIV-infected CD4 T cells in the PBMC are important to understand the events taking place in the lymphoid tissue of HIV-infected patients.
The activity of HIV-specific CD8 T cells against HIV-1 infected autologous CD4 T cells as it occurs in vivo has never been successfully tested. The published studies that attempted to assess the function of CD8 T cells had involved the use of target cells that were either infected in vitro by HIV or coated with HIV proteins, and so did not authentically represent the in vivo setting.27 Assays employing epitope-specific CTL lines involve pre-incubation with interleukin-2 that up-regulates perforin expression, creating an artificially vigorous cytotoxic activity.21
To overcome those limitations, we studied CD8-CD4 T-cell interaction with cells procured from the blood of untreated HIV-infected patients. Those cells were neither manipulated nor stimulated in vitro. We demonstrated that CD8 T cells procured from the blood of untreated HIV-1-infected patients express perforin and form conjugates with autologous CD4 T cells during both the acute and chronic phases of the infection. Using annexin PI, we measured significant apoptotic lytic activity in conjugated CD4 T cells during the acute period of the infection but less so during the chronic period (Tables1 and 2). We were able to follow and document apoptosis of CD4 T cells while conjugated with CD8 T cells in both the acutely and chronically infected patients (Fig.2a–c). Annexin staining enabled us to observe the apoptosis of the CD4 T cells conjugated to the CD8 T cells (Fig.2d). The results of in situ PCR of HIV DNA confirmed that CD4 T cells in the conjugates are indeed infected by HIV (Fig.5).
The decline in CD4 T cells in the peripheral blood of HIV-infected patients during the chronic stage of infection is slow, a feature that may be indicative of controlled elimination of CD4 T cells. We demonstrate that HIV-specific CD8 T cells can recognize HIV-infected CD4 T cells (Fig.1; Tables1 and 2). These results are in accord with the number of latent HIV-infected CD4 T cells found by others in the PBMC of HIV-infected patients by sensitive in situ PCR.26,29 The frequency of latently infected cells detected in the viral outgrowth assay is 300-fold lower than the frequency of resting CD4+ T cells that harbour proviruses detectable by PCR.38 Of the integrated DNA, 12% has a complete genome of the virus.17,38
We demonstrate that perforin expression in conjugated CD8 T cells was considerably higher during the acute and chronic phases of HIV infection compared with the CD8 T cells of uninfected controls (Table3; Fig. 4). The high conjugate formation and cytotoxic activity we have observed during acute HIV infection correlated well with the known concomitant decline in the number of CD4 T cells during this stage of infection. We demonstrate that the killing of CD4 T cells by CD8 T cells continues during the chronic period of HIV infection as well, albeit at a much slower pace. In addition, the majority of CD8 T cells conjugated to CD4 T cells during both the acute and chronic phases of HIV infection expressed high levels of perforin (Fig.4).
Based on these results, we propose that the annihilation of CD4 T cells in HIV-1-infected patient results, at least in part, from an ongoing autologous interaction of CD8 T cells with HIV-infected CD4 T cells initiated by conjugate formation and culminating in gradual elimination of the conjugated CD4 T cells. We assume that a dynamic balance is established between HIV-infected CD4 T cells and HIV-specific CTL. It is likely that the virus manipulates the immune system to maintain a low-grade infection, so achieving prolonged survival combined with efficient virus spread.
Acknowledgments
This work was supported by a joint grant from the Weizmann Institute of Science and Tel Aviv Sourasky Medical Centre. Esther Eshkol is thanked for editorial assistance. Professor Gil Ziv is thanked for scientific assistance.
Glossary
- AICD
activation-induced cell death
- Ab
antibody
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- FCS
fetal calf serum
- PS
phosphatidyl serine
- PI
propidium iodide
- CTL
cytotoxic T lymphocyte
- TRAIL
TNF-related apoptosis- inducing ligand
- MHC
major histocompatibility complex
Disclosures
The authors have no financial or commercial conflicts of interest.
Supporting Information
Video S1. Illustrates conjugation of CD4–CD8 T cells, eventually leading to apoptosis of CD4 T cells from the PBMC of an acute HIV-infected patient.
Video S2 and S3. Illustrate similar events in two chronic HIV-infected patients. In video S3 the CD4 T cell can be seen to have undergone apoptosis even after the CD8 T cell had detached from it.
References
- Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–61. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- Davenport MP, Petravic J. CD8+ T cell control of HIV – a known unknown. PLoS Pathog. 2010;6:e1000728. doi: 10.1371/journal.ppat.1000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon ML, Piacentini M. New insights on the role of apoptosis and autophagy in HIV pathogenesis. Apoptosis. 2009;14:501–8. doi: 10.1007/s10495-009-0314-1. [DOI] [PubMed] [Google Scholar]
- Thomas C. Roadblocks in HIV research: five questions. Nat Med. 2009;15:855–9. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- Cummins NW, Badley AD. Mechanisms of HIV-associated lymphocyte apoptosis: 2010. Cell Death Dis. 2010;1:e99. doi: 10.1038/cddis.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri KF, Jacotot E, Blanco J, et al. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases. J Exp Med. 2000;192:1081–92. doi: 10.1084/jem.192.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrhol-Riise AM, Stent G, Rosok BI, Voltersvik P, Olofsson J, Asjo B. The Fas/FasL system and T cell apoptosis in HIV-1-infected lymphoid tissue during highly active antiretroviral therapy. Clin Immunol. 2001;101:169–79. doi: 10.1006/clim.2001.5101. [DOI] [PubMed] [Google Scholar]
- Silvestris F, Cafforio P, Frassanito MA, Tucci M, Romito A, Nagata S, Dammacco F. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS. 1996;10:131–41. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–51. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Shudo E, Ortiz AM, et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 2010;6:e1000747. doi: 10.1371/journal.ppat.1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortara L, Letourneur F, Gras-Masse H, Venet A, Guillet JG, Bourgault-Villada I. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J Virol. 1998;72:1403–10. doi: 10.1128/jvi.72.2.1403-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon DF, Townsend AR, Elvin JG, Rizza CR, Gallwey J, McMichael AJ. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–7. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology. 2011;133:190–6. doi: 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiraz A, Garber OG, Harari S, Hassin D, Berke G. Switch from perforin-expressing to perforin-deficient CD8+ T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo. Immunology. 2009;128:69–82. doi: 10.1111/j.1365-2567.2009.03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin Y, Eilam Y, Hassin D, Fixler R. The effect of cytotoxic lymphocytes on contraction, action potential and calcium handling in cultured myocardial cells. Adv Exp Med Biol. 1995;382:229–38. doi: 10.1007/978-1-4615-1893-8_23. [DOI] [PubMed] [Google Scholar]
- Hassin D, Fixler R, Shimoni Y, Rubinstein E, Raz S, Gotsman MS, Hasin Y. Physiological changes induced in cardiac myocytes by cytotoxic T lymphocytes. Am J Physiol. 1987;252(1 Pt 1):C10–6. doi: 10.1152/ajpcell.1987.252.1.C10. [DOI] [PubMed] [Google Scholar]
- Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Hauptman SP, Lischner HW, Sachs M, Pomerantz RJ. Detection of human immunodeficiency virus type 1 provirus in mononuclear cells by in situ polymerase chain reaction. N Engl J Med. 1992;326:1385–91. doi: 10.1056/NEJM199205213262103. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–8. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BK, Till M, Otto P, Goolsby C, Furtado MR, McBride LJ, Wolinsky SM. Detection of HIV-1 DNA and messenger RNA in individual cells by PCR-driven in situ hybridization and flow cytometry. Science. 1993;260:976–9. doi: 10.1126/science.8493534. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV: shock and kill. Nature. 2012;487:439–40. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffenbauer YS, Kalma Y, Trubniykov E, Gal-Garber O, Weisz L, Halamish A, Sister M, Berke G. A cell chip for sequential imaging of individual non-adherent live cells reveals transients and oscillations. Lab Chip. 2009;9:2965–72. doi: 10.1039/b904778f. [DOI] [PubMed] [Google Scholar]
- Berke G, Gabison D, Feldman M. The frequency of effector cells in populations containing cytotoxic T lymphocytes. Eur J Immunol. 1975;5:813–8. [Google Scholar]
- Berke G. Killer Lymphocytes. 1st edn. Berlin: Springer; 2007. [Google Scholar]
- Bagasra O. Protocols for the in situ PCR-amplification and detection of mRNA and DNA sequences. Nat Protoc. 2007;2:2782–95. doi: 10.1038/nprot.2007.395. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Illustrates conjugation of CD4–CD8 T cells, eventually leading to apoptosis of CD4 T cells from the PBMC of an acute HIV-infected patient.
Video S2 and S3. Illustrate similar events in two chronic HIV-infected patients. In video S3 the CD4 T cell can be seen to have undergone apoptosis even after the CD8 T cell had detached from it.