Abstract
The protein CD46 protects cells from complement attack by regulating cleavage of C3b and C3d. CD46 also regulates the adaptive immune response by controlling T-cell activation and differentiation. Co-engagement of the T-cell receptor and CD46 notably drives T-cell differentiation by switching production of interferon-γ to secretion of anti-inflammatory interleukin-10. This regulatory pathway is altered in several chronic inflammatory diseases, highlighting its key role for immune homeostasis. The manipulation of the CD46 pathway may therefore provide a powerful means to regulate immune responses. Herein, we investigated the effect of recombinant proteins derived from the fibre knob of the adenovirus serotype 35 (Ad35) that uses CD46 as its entry receptor, on human T-cell activation. We compared the effects of Ad35K++, engineered to exhibit enhanced affinity to CD46, and of Ad35K−, mutated in the binding site for CD46. Ad35K++ profoundly affects T-cell activation by decreasing the levels of CD46 at the surface of primary T cells, and impairing T-cell co-activation, shown by decreased CD25 expression, reduced proliferation and lower secretion of interleukin-10 and interferon-γ. In contrast, Ad35K− acts a potent co-activator of T cells, enhancing T-cell proliferation and cytokine production. These data show that recombinant Ad35 proteins are potent modulators of human T-cell activation, and support their further development as potential drugs targeting T-cell responses.
Keywords: adenovirus 35, agonist, antagonist, CD46, human T cells
Introduction
The complement regulator CD46 is a type I transmembrane protein that protects cells from autolysis by its regulation of the activating complement components C3b and C4b.1–3 The CD46 ectodomain consists of four short-consensus repeat domains (SCR1–4), which are followed by a region rich in serine-threonine-proline (STP region), a transmembrane domain and one of two short cytoplasmic tails produced by alternative splicing.4,5 Besides binding to complement components, CD46 also acts as a cellular receptor for many pathogens, and has been dubbed a ‘pathogen’s magnet’.6 Pathogens using CD46 as their receptor include several serotypes of adenovirus such as Ad35,7 the vaccinal strain of measles virus, herpes virus 6 and some strains of bacteria such as Neisseria gonorrhoeae and Neisseria meningitides, as well as group A streptococcus. Moreover, CD46 is key in the regulation of the adaptive immune response by controlling T-cell activation, differentiation and polarity.8–12 Co-ligation of CD46 and the T-cell receptor provides a strong co-stimulatory signal to T cells,8,11 and expression of either the CD46 Cyt1 or the Cyt2 isoform in transgenic mice exerted antagonist effects in vivo on T-cell-mediated inflammation, which was correlated with different effects in vitro on cytokine production and T-cell proliferation.10 CD46 expression at the surface of human T cells is tightly regulated, being shed by matrix metalloproteases (MMP) upon ligation, and CD46 cleavage is important for its functions.13,14 In the presence of interleukin-2 (IL-2), CD46 drives differentiation of human CD4+ T cells towards a regulatory T-cell type I (Tr1), characterized by increased production of IL-10 and reduced secretion of interferon-γ (IFN-γ).9,15 This regulatory pathway is altered in T cells from patients with multiple sclerosis, rheumatoid arthritis and asthma, as IL-10 production upon CD46 co-stimulation is impaired.15–20 The dysregulation of this pathway in several chronic inflammatory diseases highlights its key role in regulating the homeostasis of the immune response, and therefore makes it an attractive pathway to modulate in these pathologies. Moreover, most cancer cells up-regulate CD46 to avoid complement lysis, and inhibition of CD46 on cancer cells may be a promising strategy to enhance antibody-dependent cytotoxicity of therapeutic monoclonal antibodies.21–24
Ad35K++ is a small recombinant protein derived from the fibre knob of the adenovirus serotype 35 that has been modified to bind to CD46 with picomolar affinity.25 Previous studies have shown that this CD46 antagonist could transiently remove CD46 from the surface of several cancer cell lines, and as a consequence render them more sensitive to antibody-dependent complement lysis. For example, Ad35K++ promotes killing of several lymphoma cell lines by rituximab, a humanized IgG1 targeting CD20.25 Moreover, in vivo administration of Ad35K++ to non-human primates, that ubiquitously express CD46, appears to be safe and well tolerated.26 Therefore, using recombinant proteins targeting CD46 may be of potential use in clinical trials. Considering the key role of the CD46 pathway in controlling human T-cell activation, we investigated herein the effect of Ad35K++ on T-cell responses. We also assessed the effects of Ad35K−, which has a point mutation (Arg279) that affects binding to CD46.25 Our data show that Ad35K++ strongly affected CD46 expression at the surface of primary T cells, and importantly that it was able to significantly impair T-cell activation. T cells co-activated in presence of Ad35K++ had normal induction of CD69, an early marker of T-cell activation, but failed to express high levels of CD25, which was correlated with decreased proliferation and reduced cytokine production. In contrast, Ad35K− surprisingly led to enhanced T-cell activation, with increased proliferation and cytokine production. These data emphasize the potency of recombinant adenoviral proteins in modulating the CD46 pathway, and provide the rationale to further investigate their effects on the immune response to maximize their therapeutic potential.
Methods
Cell purification and activation
Peripheral blood mononuclear cells were isolated by Ficoll–Hypaque density gradient centrifugation (GE Healthcare, Uppsala, Sweden), from venous blood from healthy donors obtained after informed consent. Naive CD4+ T cells were negatively isolated using magnetic beads (STEMCELL technologies, Grenoble, France, purification > 95%), and cultured in RPMI-1640 with 10% fetal calf serum at 0·5 × 106 per well in 48-well plates pre-coated with α-CD3 (OKT3, 5 μg/ml) or α-CD3/α-CD46 (OKT3, 5 μg/ml, MC120.6, 10 μg/ml) (MC120.6 was kindly provided by Dr Chantal Rabourdin-Combe, France, and recognized the SCR1 domain of CD46). Activated T cells also received recombinant human IL-2 (Life Technologies, Paisley, UK; 10 U/ml) to induce a Tr1-like phenotype.9 Ad35K++ and Ad35K− were added to the wells at the start of the culture at the concentrations indicated. In some experiments, GM6001, a broad MMP inhibitor, was also added to the culture (SigmaAldrich, St Louis, MO; 10 μm).14
Ad35K++ and Ad35K−
Recombinant, synthetic proteins derived from the fibre knob adenovirus Ad35 serotype. Both Ad35K++ and Ad35K− were selected using a display library as previously described,27,28 with the Ad35K++ version having greatly increased affinity for the CD46 target. The coding sequences were cloned into a prokaryotic expression vector and gene expression was induced by the addition of Isopropyl β-D-1-thiogalactopyranoside to the Escherichia coli culture medium. Cells were lysed and the proteins were purified using a combination of nickel affinity chromatography and ion exchange chromatography. The final proteins had high purity by SDS–PAGE and did not contain appreciable amounts of endotoxin that could affect cellular readouts.
Cytokine detection
Cell culture supernatants from the 48-well plates (as described in the cell activation section) were collected after 5 days of stimulation, and both IL-10 and IFN-γ secretion was determined by ELISA specific for human IL-10 (BD Pharmingen, San Diego, CA) and IFN-γ (Endogen, Rockford, IL).
Flow cytometry
The expression level of CD46, CD69 and CD25 was assessed by flow cytometry, by incubating the cells with the antibodies at 4° for 20 min in FACS buffer (PBS containing 1% fetal calf serum). We used different antibodies against CD46 as described in the Results section: anti-CD46-FITC (clone MEM-258 recognizing the SCR4 domain – BioLegend, London, UK), anti-CD46-phycoerythrin (clone 344519; R&D Systems, Minneapolis, MN) or the MCI20.6 clone recognizing the SCR1 domain followed by anti-IgG1-FITC antibodies. For activation experiments, we used the following antibodies: anti-CD46-phycoerythrin, anti-CD69-FITC (BioLegend) and anti-CD25-allophycocyanin (BioLegend). Samples were run with a FACSCalibur and data were analysed using FlowJo. Relative expression to staining with the control was calculated by calculating the ΔMFI [mean fluorescence intensity (MFI) obtained with antibody – MFI obtained with isotype control]. Staining for intracellular expression of CD46 was performed in FACS buffer containing 0·1% saponin (SigmaAldrich) for 30 min at room temperature, after fixation of the cells. Proliferation was determined by pre-labelling purified T cells with eFluor 670 cell proliferation stain (eBioscience, Hatfield, UK) before activation following the manufacturer’s instructions, and assessing remaining fluorescence after 4 days.
Statistics
The groups were analysed using Graphpad Prism software. Flow cytometry data were analysed using the Wilcoxon test, when assessing paired samples. ELISA data are the average of duplicate wells, and the average obtained for the different donors was analysed using the Wilcoxon test. All P-values are two-tailed and with a 95% confidence interval *P < 0·05; **P < 0·01; and ***P < 0·001.
Results
Effect of Ad35K++ and Ad35K− on CD46 expression on Jurkat cells
We first examined the effect of incubating Jurkat cells, a T leukaemia cell line, with Ad35K++ and Ad35K− on CD46 surface expression. Jurkat cells were cultured in the presence or absence of a fixed concentration of both proteins (20 μg/ml) for various periods of time, and CD46 expression was then assessed by flow cytometry using an anti-CD46-FITC antibody. Addition of Ad35K++ for 1 hr led to a total lack of detection of CD46, and the lack of CD46 expression was still observed after 48 hr of culture (Fig1a). A shorter kinetic experiment showed that a total lack of detection of CD46 was also observed after a 5-min incubation period (not shown). Incubation with Ad35K− led to a slight decrease in the level of CD46 detected after 24 and 48 hr, suggesting a residual binding to CD46. We next incubated Jurkat cells with various concentrations of Ad35K++, ranging from 1 pg/ml to 20 μg/ml, for 3 hr. A dose-dependent decrease in CD46 detection was observed. We observed a total effect with 10 ng/ml while there was no more effect on CD46 expression at 1 pg/ml, with a partial effect observed at 100 pg/ml (Fig1b). These data suggested that Ad35K++ binding to CD46 could either mask the epitope in CD46 that is recognized by the antibody used for detection, or induce its internalization or cleavage.
Figure 1.
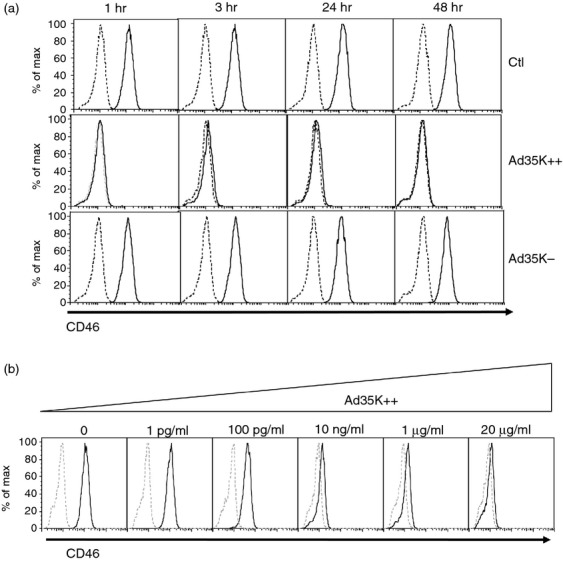
Ad35K++ affects the detection of CD46 expression on Jurkat cells. (a) Jurkat cells were incubated with Ad35K++ or Ad35K− (20 μg/ml) for various lengths of time, as indicated. CD46 surface expression was then monitored by flow cytometry using an anti-CD46-FITC antibody. (b) Jurkat cells were incubated for 3 hr with various concentrations of Ad35K++ as indicated and CD46 surface expression was monitored by flow cytometry using the anti-CD46-FITC antibody. Dotted lines represent isotype controls.
To determine whether Ad35K++ binding to CD46 was masking CD46 antibody binding sites, we performed some competition experiments using different anti-CD46 antibodies recognizing distinct epitopes. We assessed the anti-CD46-FITC antibody used above, which recognizes the SCR4 domain; an anti-CD46-phycoerythrin antibody whose binding site has not been identified but that did not compete with the FITC antibody (Fig2a); and the MCI20.6 monoclonal antibody, which recognizes the SCR1 domain. Jurkat cells were first incubated with Ad35K++ for 20 min on ice, washed and then stained with these different anti-CD46 antibodies. Again, the use of the anti-CD46-FITC antibody led to a total lack of detection of CD46, suggesting that binding of Ad35K++ to CD46 on Jurkat cells was masking the epitope of CD46 recognized by this clone (Fig2b). The use of the anti-CD46-phycoerythrin antibody only led to a partial decrease, suggesting a partial blockage. Importantly, there was no competition between the MCI20.6 clone and Ad35K++, indicating that MCI20.6 could be used to activate cells in the presence of Ad35K++.
Figure 2.
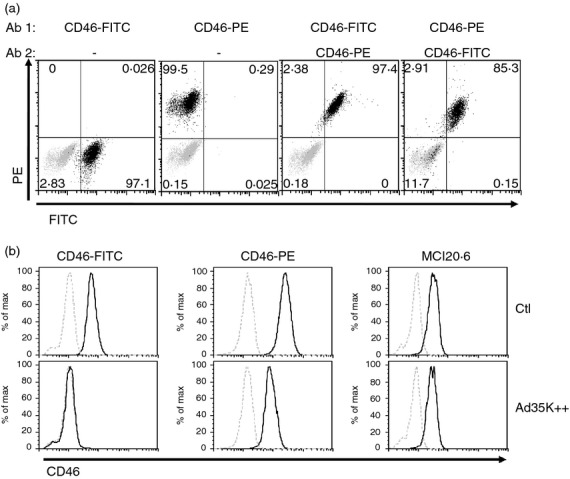
Competition experiments between Ad35K++ and anti-CD46 antibodies. (a) Jurkat cells were stained with anti-CD46-FITC or anti-CD46-phycoerythrin (PE), or with both antibodies added in the different orders, as indicated, and CD46 expression was assessed by flow cytometry. These two clones do not compete with each other. (b) Jurkat cells were first incubated with Ad35K++ (500 ng/nl) for 20 min, washed and then stained with the anti-CD46-FITC, anti-CD46-PE, or indirectly labelled with the MCI20.6 clone followed by anti-mIgG1-FITC. CD46 expression was assessed by flow cytometry. Dotted lines represent isotype controls.
Ad35K++ and Ad35K− affect the phenotype of primary human T cells
We next determined the effect of Ad35K++ and Ad35K− on the expression of CD46 on primary T cells. Purified naive CD4+ T cells were cultured with Ad35K++ or Ad35K− (500 ng/ml), and expression of CD46 was assessed after 24 hr using the MCI20.6 antibody that does not compete with Ad35K++. As binding of Ad35K++ to CD46 in several lymphoma cell lines has been shown to induce CD46 internalization,25 we also assessed intracellular CD46 expression after cell permeabilization. Incubation of T cells with Ad35K++ for 24 hr resulted in a reduced surface expression of CD46 on these cells (Fig.3a,b) while Ad35K− had a lesser effect. Incubation with Ad35K++ had no significant effect on intracellular CD46 expression. We also assessed whether down-regulation of CD46 expression by Ad35K++ could be affected by lowering the temperature to 4°. T cells were incubated with Ad35K++ or Ad35K− (500 ng/ml) at 4° or 37°, and CD46 expression was assessed by flow cytometry. Down-regulation of CD46 expression was only observed at 37° (Fig.3c). These data suggest that internalization and/or MMP-dependent shedding of CD46 was blocked.14 We next investigated whether MMP inhibition could modulate the effect of Ad35K++ on CD46 expression in T cells. Naive CD4+ T cells were cultured in the presence or absence of Ad35K++ (500 ng/ml) and/or GM6001, a broad MMP inhibitor, and CD46 expression was monitored by flow cytometry using the MCI20.6 antibody (Fig.3d). Inhibition of MMP increased the levels of surface CD46 in Ad35K++-treated T cells. These data suggest that ligation of Ad35K++ to CD46 on primary T cells led to, at least, a partial shedding of CD46.
Figure 3.
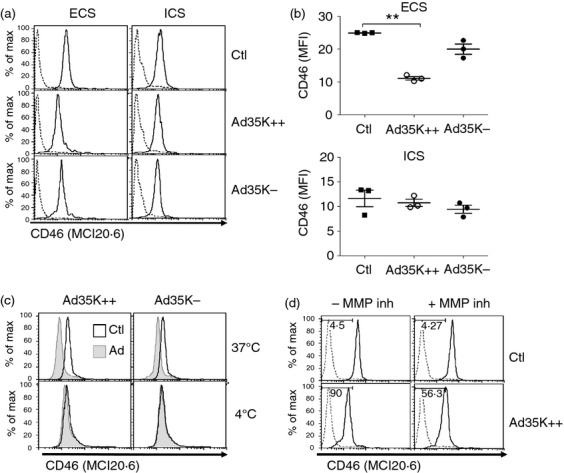
Ad35K++ affects expression of surface CD46 on primary naive CD4+ T cells. (a) Naive T cells were cultured with Ad35K++ or Ad35K− (500 ng/ml) for 24 hr, and CD46 expression was determined at the cell surface or after intracellular staining using the MCI20.6 clone that does not compete with Ad35K++. Dotted lines represent isotype controls. (b) Data obtained with three different donors. (c) Naive T cells were cultured with Ad35K++ or Ad35K− (500 ng/ml) for 24 hr either at 4° or 37°, and CD46 expression was then determined using the MCI20.6 monoclonal antibody. Representative of two experiments. (d) Naive T cells were cultured in the presence of Ad35K++ (500 ng/ml) and with or without addition of GM6001, a broad matrix metalloprotease (MMP) inhibitor. Forty-eight hours later, CD46 surface expression was then analysed by flow cytometry using the MCI20.6 antibody. **P = 0·0017.
We next investigated whether activation of primary T cells was affected in the presence of Ad35K++ or Ad35K−. Purified human naive CD4+ T cells were left unstimulated or were activated with pre-immobilized anti-CD3 or anti-CD3/anti-CD46 (using the MCI20.6 antibody that does not compete with Ad35K++), as previously described,9,17 in the presence or absence of Ad35K++ or Ad35K− (500 ng/ml). CD46 surface expression, as well as expression of the activation markers CD69 and CD25, was examined by flow cytometry after 2 and 5 days (Fig4). As expected, CD3/CD46 co-stimulation of control cells induced a strong down-regulation of CD46 surface expression, and this was correlated with an increased expression of CD69 and CD25, at both day 2 and day 5.14 In the presence of Ad35K++, a marked decrease in CD46 surface expression was observed in all conditions of activation, an effect that was still observed after 5 days of culture. The presence of Ad35K++ did not affect the induction of CD69 expression in T cells activated with either anti-CD3 or anti-CD3/CD46. In contrast, the presence of Ad35K++ significantly impaired CD25 induction in CD3/CD46-co-stimulated T cells. As previously observed, a slight decrease in surface CD46 expression was noted with Ad35K−. Surprisingly, Ad35K− significantly enhanced CD69 induction on CD3-activated T cells compared with control cells, and a significant effect, albeit more moderate, was also observed for CD25 expression. These data suggest that while Ad35K++ blocks late CD46-mediated T-cell co-stimulation, the residual binding of Ad35K− to CD46 is sufficient to co-stimulate T cells activated by CD3.
Figure 4.
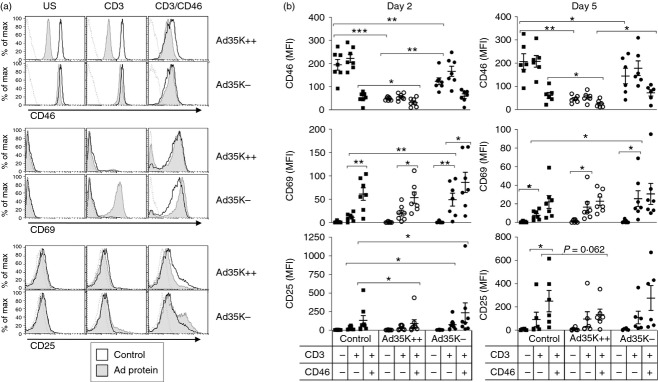
Ad35K++ impairs induction of CD25 while Ad35K− promotes co-stimulation. Naive CD4+ T cells were left unstimulated (US) or were activated by immobilized anti-CD3 or anti-CD3/anti-CD46 for 2 or 5 days in the presence or absence of Ad35K++ or Ad35K− (500 ng/ml). Expression of CD46 (using the anti-CD46-phycoerythrin clone), CD69 and CD25 was assessed. Dotted lines represent isotype controls. (a) Raw data obtained for one donor at day 2, and (b) data obtained for the different donors analysed at day 2 and day 5 (n = 6). *P < 0·05; **P < 0·01; and ***P < 0·001.
Ad35K++ and Ad35K− modulate primary human T-cell responses
In order to determine whether these changes in phenotype would affect T-cell functions, we next examined T-cell responses. Naive T cells were stained with eFluro670 before activation with immobilized anti-CD3 or anti-CD3/anti-CD46, in the presence of Ad35K++ or Ad35K−, and cell proliferation was determined after 4 days (Fig5a). As expected, control cells proliferated upon CD3 activation and the proliferation was further increased upon CD3/CD46 co-stimulation.8 Cells incubated in the presence of Ad35K++ proliferated in response to CD3 ligation but failed to show increased proliferation upon CD3/CD46 co-stimulation. In contrast, cells cultured in the presence of Ad35K− showed increased proliferation in response to CD3 ligation compared with control cells, but no co-stimulation was observed following CD3/CD46 co-ligation.
Figure 5.
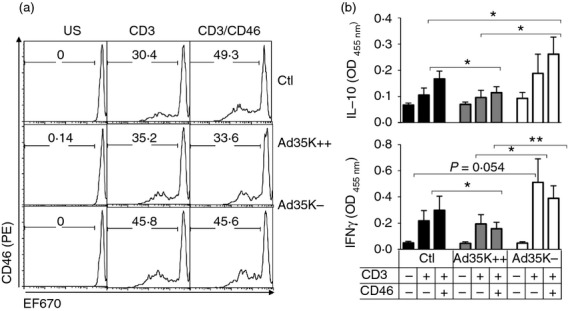
Ad35K++ inhibits T-cell co-stimulation while Ad35K− increases activation. (a) Purified naive CD4+ T cells were pre-labelled with eFluor 670 before activation by immobilized anti-CD3 or anti-CD3/anti-CD46 in the presence or absence of Ad35K++ or Ad35K−. Four days later, proliferation was monitored by flow cytometry. (b) The production of interleukin-10 (IL-10) and interferon-γ (IFN-γ) in the culture supernatants was assessed by ELISA (n = 6). *P < 0·05; **P < 0·01; and ***P < 0·001.
Next, we determined cytokine production of cells activated in the presence of Ad35K++ and Ad35K−. As CD46 co-stimulation controls production of IFN-γ and IL-10,15 we assessed the levels of these two cytokines in the culture supernatants of primary T cells activated for 5 days in the presence of Ad35K++ or Ad35K− (Fig5b). Cells co-stimulated in the presence of Ad35K++ secreted reduced amounts of cytokines compared with control cells. In contrast, T cells co-stimulated in the presence of Ad35K− secreted increased levels of IL-10 when compared with control cells or cells incubated with Ad35K++.
Discussion
In the past decade, the complement regulator CD46 has been of increasing interest to immunologists as several groups reported that its ligation on human T cells could profoundly affect T-cell functions. Although the first studies highlighted the role of CD46 ligation on T-cell activation and proliferation,8,11 further reports have demonstrated its key role in the control of inflammation by regulating not only T-cell activation but also its ability to differentiate T cells towards an anti-inflammatory phenotype.9,10,12 Strikingly, this pathway is dysregulated in a number of pathologies (multiple sclerosis, rheumatoid arthritis and asthma), which underlines the key role of CD46 in the control of inflammation.15,17–20 Moreover, the role of CD46 as a cellular receptor for multiple pathogens suggests that this is a powerful pathway that can be exploited to the pathogens’ advantage.29 Therefore, specifically targeting CD46 may have potential use in several clinical settings.
Ad35K++ is a small recombinant protein derived from the fibre knob of the adenovirus serotype 35 (Ad35) that uses CD46 as its receptor. It has been previously shown that Ad35K++ could increase the complement-dependent lysis of tumour cell lines,25 highlighting again the attractiveness of this drug to improve antibody-based cancer treatments. This suggestion is further supported by the fact that injection of Ad35K++ to monkeys, the only mammals that express CD46 in a pattern resembling humans, appears to be safe and well tolerated, and indeed in vivo administration of Ad35K++ could increase B-cell depletion by rituximab.26
Herein, we have focused on the effects of Ad35K++ and of a mutant Ad35K− on human T-cell responses. We have examined CD46 expression, and response of T cells co-stimulated by CD46 in the presence of both recombinant proteins. Our data show that Ad35K++ binds to CD46 at the surface of primary T cells, and this interaction appears to be very stable as decreased CD46 expression was still detected after 5 days of culture. Of note, intravenous administration of Ad35K++ to macaques results in a decreased expression of CD46 on peripheral blood mononuclear cells that is still observed after 2 weeks after injection.26 These data may be explained by the picomolar affinity of Ad35K++ for CD46. Previous studies have shown internalization of CD46 after binding to Ad35K++ in lymphoma cell lines.25 Our results from experiments to examine intracellular staining of CD46 in primary T cells do not completely support internalization of CD46 in primary T cells, although down-regulation of CD46 was blocked at 4°, and we cannot exclude CD46 internalization and rapid degradation. Our data also suggest that binding of Ad35K++ to CD46 leads to a partial MMP-dependent cleavage as partially blocked by addition of a broad MMP inhibitor.
The structure of the CD46 ectodomain resembles the shape of a ‘hockey stick’,30 which probably explains the data obtained with the different clones of anti-CD46 antibodies used in the competition experiments with Ad35K++. Binding of Ad35K++ to the SCR1/2 domains of CD46 may also cause changes in CD46 conformation, as it totally competes with the anti-CD46-FITC antibody that recognizes the SCR4 domain.31 Importantly, binding of Ad35K++ to CD46 does not affect recognition by the MCI20.6 antibody that also recognizes the SCR1 domain, allowing us to use this antibody to determine the effect of Ad35K recombinant proteins on CD46 co-stimulation. Our data show that early T-cell activation appears to be normal in Ad35K++-treated cells, as we could observe a strong co-stimulation effect on CD69 induction between CD3 and CD3/CD46 co-stimulated T cells. However, there was no further increase in CD25 in CD46-co-stimulated T cells in the presence of Ad35K++, especially after longer activation periods, and CD46-mediated co-stimulation was strongly impaired, as evidenced by reduced proliferation and cytokine production. Altogether, these data indicate that, although early T-cell co-activation may remain unchanged, Ad35K++ blocks late T-cell co-stimulation. Interestingly, recombinant Ad35 fibre knob proteins could inhibit CD3/CD28-mediated T-cell activation, albeit inhibition could only be observed when the Ad35 knob was immobilized,32,33 suggesting that multimerization was required to suppress activation, possibly by hindering the spatial organization of the immune synapse. Therefore, Ad35K++ is a potent protein that strongly impairs the CD46-mediated pathway, at least in vitro.
Our data show a surprising effect of Ad35K− on T-cell functions. This result was unexpected as Ad35K− was designed as a negative control, having a point mutation supposed to abrogate CD46 binding. However, incubation of primary T cells with Ad35K− led to a significant decrease in CD46 expression (see Fig4b), indicating that there is a residual binding of Ad35K− to CD46. Indeed, binding of Ad35K− to several human cell lines was also observed (data not shown). Moreover, addition of Ad35K− was able to modulate T-cell responses, providing a co-stimulatory signal to CD3-activated T cells, and leading to enhanced secretion of IL-10, suggesting its ability to promote Tr1 differentiation. Hence, in contrast to Ad35K++, Ad35K− acts as a CD46 agonist, which may be useful to boost the CD46 pathway in vivo.
In conclusion, both recombinant proteins differently modulate primary T-cell responses, at least in vitro. Therefore, caution should be exerted in targeting this pathway, as ligation of CD46 can either promote or inhibit activation, probably depending on conformational changes triggered by ligand binding and subsequent signalling cascades. Nevertheless, our data emphasize the potency of recombinant proteins to modulate the CD46-mediated pathway, which is key to control immune homeostasis. The analysis of the effects of these two recombinant proteins on other immune cell types for which ligation of CD46 has been shown to modulate their response, such as dendritic cells34 and B cells,35 will further support the development of these potential drugs into clinical trials.
Acknowledgments
We thank Dongqing Ma who performed some preliminary experiments during her Masters. JH has done the experiments, AB and DC have provided the recombinant proteins, have performed experiments (data not shown) and discussed data; AA has written the manuscript. We thank Fiona Rossi and Shonna Johnston for their help with flow cytometry. We are grateful to Ian Dransfield for the critical reading of the manuscript. AA is an MRC academic fellow and detached member of CNRS, France. JH is an MRC-PhD student. AL and DC were supported by NIH grant 2R44CA162582-02.
Glossary
- IFN-γ
interferon-γ
- IL-10
interleukin-10
- MMP
matrix metalloprotease
- SCR
short-consensus repeat
- Tr1
type 1 regulatory T cells
Disclosures
The authors declare no competing interests.
References
- Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–55. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland B, Russell S, Purcell D, Johnstone R, Thorley B, Sparrow R, McKenzie I. The molecular basis of CD46 polymorphic expression and demonstration of a protective role against lysis by xenosera. Transplant Proc. 1992;24:685–6. [PubMed] [Google Scholar]
- Russell SM, Johnstone RW, Wilton A, Sparrow RL, McKenzie IF, Purcell DF. Molecular characterization of the polymorphic expression of CD46: a cell surface molecule protecting cells from complement attack. Transplant Proc. 1992;24:211–3. [PubMed] [Google Scholar]
- Purcell DF, Russell SM, Deacon NJ, Brown MA, Hooker DJ, McKenzie IF. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics. 1991;33:335–44. doi: 10.1007/BF00216692. [DOI] [PubMed] [Google Scholar]
- Seya T, Hirano A, Matsumoto M, Nomura M, Ueda S. Human membrane cofactor protein (MCP, CD46): multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–60. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–8. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–12. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–5. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–66. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–5. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- Oliaro J, Pasam A, Waterhouse NJ, Browne KA, Ludford-Menting MJ, Trapani JA, Russell SM. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci USA. 2006;103:18685–90. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Astier AL. CD46 processing: a means of expression. Immunobiology. 2012;217:169–75. doi: 10.1016/j.imbio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Choileain S, Weyand NJ, Neumann C, Thomas J, So M, Astier AL. The dynamic processing of CD46 intracellular domains provides a molecular rheostat for T cell activation. PLoS ONE. 2011;6:e16287. doi: 10.1371/journal.pone.0016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone J, Le Friec G, Vantourout P, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–71. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL. T-cell regulation by CD46 and its relevance in multiple sclerosis. Immunology. 2008;124:149–54. doi: 10.1111/j.1365-2567.2008.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–7. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Xiong Z, Hu Y, et al. Dysfunction of IL-10-producing type 1 regulatory T cells and CD4+ CD25+ regulatory T cells in a mimic model of human multiple sclerosis in Cynomolgus monkeys. Int Immunopharmacol. 2009;9:599–608. doi: 10.1016/j.intimp.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, et al. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–86. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Gao YD, Yang J, Guo W. A defect of CD4+ CD25+ regulatory T cells in inducing interleukin-10 production from CD4+ T cells under CD46 costimulation in asthma patients. J Asthma. 2010;47:367–73. doi: 10.3109/02770903.2010.481340. [DOI] [PubMed] [Google Scholar]
- Di Gaetano N, Cittera E, Nota R, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- Kolev M, Towner L, Donev R. Complement in cancer and cancer immunotherapy. Arch Immunol Ther Exp (Warsz) 2011;59:407–19. doi: 10.1007/s00005-011-0146-x. [DOI] [PubMed] [Google Scholar]
- Carter D, Lieber A. Protein engineering to target complement evasion in cancer. FEBS Lett. 2014;588:334–40. doi: 10.1016/j.febslet.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis N, Zell S, Rutz R, Li W, Giese T, Mamidi S, Schultz S, Kirschfink M. Inhibition of membrane complement inhibitor expression (CD46, CD55, CD59) by siRNA sensitizes tumor cells to complement attack in vitro. Curr Cancer Drug Targets. 2010;10:922–31. doi: 10.2174/156800910793357952. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2009;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer I, Cao H, Persson J, et al. Transient removal of CD46 is safe and increases B-cell depletion by rituximab in CD46 transgenic mice and macaques. Mol Ther. 2012;21:291–9. doi: 10.1038/mt.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Y, Li Z, et al. In vitro and in vivo properties of adenovirus vectors with increased affinity to CD46. J Virol. 2008;82:10567–79. doi: 10.1128/JVI.01308-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–8. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- Persson BD, Schmitz NB, Santiago C, Zocher G, Larvie M, Scheu U, Casasnovas JM, Stehle T. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog. 2010;6:e1001122. doi: 10.1371/journal.ppat.1001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischli C, Verhaagh S, Havenga M, Sirena D, Schaffner W, Cattaneo R, Greber UF, Hemmi S. The distal short consensus repeats 1 and 2 of the membrane cofactor protein CD46 and their distance from the cell membrane determine productive entry of species B adenovirus serotype 35. J Virol. 2005;79:10013–22. doi: 10.1128/JVI.79.15.10013-10022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WC, Gujer C, McInerney G, Gall JG, Petrovas C, Karlsson Hedestam GB, Koup RA, Lore K. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc Natl Acad Sci USA. 2011;108:7499–504. doi: 10.1073/pnas.1017146108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WC, Berenson RJ, Karlsson Hedestam GB, Lieber A, Koup RA, Lore K. Attenuation of CD4+ T-cell function by human adenovirus type 35 is mediated by the knob protein. J Gen Virol. 2012;93:1339–44. doi: 10.1099/vir.0.039222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–74. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- Jabara HH, Angelini F, Brodeur SR, Geha RS. Ligation of CD46 to CD40 inhibits CD40 signaling in B cells. Int Immunol. 2011;23:215–21. doi: 10.1093/intimm/dxq474. [DOI] [PMC free article] [PubMed] [Google Scholar]


