Abstract
Hepatitis B virus (HBV) infection causes liver diseases and hepatocellular carcinoma. Immunotolerance in HBV-infected patients is one of the factors that incur failure of HBV clearance and persistent HBV amplification. However, the mechanisms underlying immunotolerance after HBV infection are yet to be thoroughly understood. Using a novel HBV mouse model, we found for the first time that epidermal growth factor receptor (EGFR) is up-regulated on intrahepatic regulatory T (Treg) cells in HBV-infected mouse livers. The EGFR-positive Treg cells are more immunosuppressive than EGFR-negative Treg cells, demonstrated by higher expression of immunosuppressive cytokines and robust inhibition of CD8+ T-cell proliferation in vitro. Furthermore, EGFR-positive Treg cells potently restrain CD8+ T-cell-mediated anti-viral activity, leading to higher HBV burden in hepatocytes. Amphiregulin, a cytokine of the EGF family, is significantly up-regulated in HBV-infected livers, but the cellular sources of amphiregulin are still elusive. Amphiregulin promotes the immunosuppressive activity of EGFR-positive Treg cells in vitro, so as to profoundly inhibit production of anti-viral components in CD8+ T cells. Taken together, our discovery elucidated a novel mechanism contributing to immunotolerance and viral amplification after HBV infection. Our study may provide new clues for developing therapeutic strategies against HBV infection.
Keywords: amphiregulin, epidermal growth factor receptor, hepatitis B virus, regulatory T cells
Introduction
As a Hepadnaviridae family member, hepatitis B virus (HBV) is a small DNA virus with unusual features similar to retroviruses.1 HBV replicates in the hepatocytes and can integrate into the host genome. Hepatitis B virus infects more than 300 million people worldwide and is a common cause of liver disease and liver cancer.2 Infection with HBV can cause a wide spectrum of liver disease ranging from acute to chronic hepatitis, cirrhosis and even hepatocellular carcinoma. Most HBV-infected adults recover, but 5–10% are unable to clear the virus and become chronically infected individuals.3 The failure of efficient viral clearance contributes to persistent viral replication and spreading, as well as development of active disease, which can eventually progress to cirrhosis and liver cancer.
There have been accumulating studies on understanding the virology and immune response of HBV infection in the past decades. However, the cellular and molecular mechanisms by which the host fails to clear the virus and develops chronic infection remain largely elusive. Both innate and adaptive host immune responses play important roles in the successful restraint and elimination of HBV.4 Innate immunity against HBV includes the production of anti-viral cytokines such as type I interferon (IFN-α and IFN-β) and the activation of innate immune cells such as natural killer cells.5 Even though innate immune response is crucial for the outcome of HBV infection, the hallmark of successful viral eradication is a sustained adaptive immune response. Specifically, CD4+ and CD8+ T-cell responses have been shown to play a central role in the fight against HBV infection.6
Regulatory T cells (Treg cells) are a group of CD4+ T cells expressing high levels of interleukin-2 (IL-2) receptor α chain (CD25) and nuclear Foxp3. Treg cells have been considered critical in maintaining immune homeostasis, restraining autoimmune reaction and alleviating inflammatory responses.7 In HBV infection, the significance of Treg cells has been taken into account for the development and prognosis. Although Treg cells might contribute to the protection of overwhelming liver tissue damage, it has been considered that Treg cells are the negative determinant factor of hepatitis B infection prognosis.8,9 The level of Treg cells among hepatitis B patients is higher than in healthy persons, and patients with higher levels of Treg cells have more HBV gene copies than those with fewer Treg cells.9,10 Studies have demonstrated the negative role of Treg cells in immune response in hepatitis B infection, including suppressing HBV-specific helper T cells,8,11 suppressing proliferation of viral antigen-specific CD8+ T cells.11–13 However, the mechanisms underlying the suppressive activity of Treg cells in HBV infection is still unclear. The genesis, activation, migration, activity modulation and fate decision of Treg cells after HBV infection have not been fully studied. Also, it is not clear whether the intrahepatic micro-environment influences Treg cell function after they infiltrate into the infected liver.
In this study, we found that intrahepatic Treg cells up-regulated epidermal growth factor receptor (EGFR) on their surface in a mouse HBV infection model. Intrahepatic EGFR+ Treg cells possessed higher immunosuppressive activity than EGFR− Treg cells in HBV-infected mice. The level of amphiregulin, an EGF-like cytokine, was also significantly increased in the liver after HBV infection. Importantly, amphiregulin promoted suppressive activity of Treg cells to inhibit the anti-viral activity of CD8+ T cells. Taken together, our work disclosed an important mechanism by which Treg cell activity is modulated in HBV infection.
Materials and methods
Animal model
All animal experiments were conducted in compliance with institutional guidelines and Wuhan University Guidelines for the Use of Animals. All animal procedures were approved by the Wuhan University School of Medicine Animal Care and Use Committee. C57BL/6 mice were purchased from Vital River Laboratories (Beijing, China). Six- to eight-week-old male mice were used in all experiments unless otherwise specified. All mice were housed under controlled temperature and light conditions following the Institutional Animal Care guidelines. The adeno-associated virus (AAV)/HBV infection model was established following the previously published protocol.14 Briefly, control AAV and AAV/HBV were provided by Beijing FivePlus Molecular Medicine Institute (Beijing, China). Adult C57BL/6 mice were injected with recombinant virus at 1 × 1011 viral genome equivalents (diluted to 200 ml with PBS) through tail vein injection.
Isolation of intrahepatic and splenic immune cells
Intrahepatic and splenic immune cells were prepared according to previous publications with several modifications.15–17 Briefly, mice were killed by inhalation of carbon dioxide, following which the abdomen of each animal was immediately opened and the spleen was collected and put in ice-cold PBS. Then 10 ml cold PBS (pH 7·4) was injected via the right ventricle of the heart to perfuse the liver until this organ became blanched and swollen. The gallbladder was removed and the liver was removed. The spleen and liver were then minced into small pieces with surgical scissors and pressed through a 40-μm cell strainer with a 3-ml syringe plunger. The obtained preparation was suspended in RPMI-1640 medium and centrifuged at 300 g for 10 min to pellet the cells. The cell pellet was resuspended in a Tris–NH4Cl solution to lyse red blood cells. Cells were washed with PBS twice and were subject to further processing.
Flow cytometry analysis
The following anti-mouse antibodies were used for detection of immune cells: phycoerythrin-conjugated (PE) anti-CD3 (17A2), PE anti-T-cell receptor-β (TCR-β; H57-597), allophycocyanin-Cy7-conjugated (APC-Cy7) anti-CD4 (GK1.5), PE-Cy7 anti-CD25 (PC61), Alexa Fluor® 488 anti-Foxp3 (R16-715), PE-Cy7 anti-CD45 (30-F11), PE-Cy7 anti-CD8a (53-6·7), APC anti-IFN-γ (XMG1.2), and FITC anti-tumour necrosis factor (TNF-α; MP6-XT22) were purchased from BD Pharmingen (San Diego, CA). FITC anti-perforin (eBioOMAK-D) and FITC anti-granzyme B (NGZB) were purchased from eBioscience (San Diego, CA). Biotinylated epidermal growth factor (EGF) complexed with avidin-Alexa Fluor 647 was purchased from Invitrogen (Carlsbad, CA). Cells were incubated with the corresponding antibodies and complexed EGF in PBS for 15 min on ice before analysis on a BD FACSCalibur. For intracellular staining, cells were fixed and permeabilized with BD Cytofix/Perm and Perm/wash buffer according to the manufacturer’s manuals. Then cells were stained at room temperature for 20 min with PE anti-IL-10 (JESS5-16E3; eBioscience) and PE anti-transforming growth factor-β (TGF-β; TW7-16B4; BD Pharmingen), respectively, before analysis on a BD FACSCalibur. All flow cytometry data were further analysed with a CellQuest Pro software (Becton Dickinson, San Jose, CA). Cell sorting was performed on a BD FACSAria cell sorter based on cell surface marker staining.
RNA isolation, reverse transcription and quantitative PCR
Total RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany). One microgram of total RNA from each sample was reverse-transcribed into cDNA using SuperScript® III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Quantitative RT-PCR (qRT-PCR) was performed using Fast SYBR® Green Master Mix (Invitrogen) on a 7300 Real-Time PCR System (Invitrogen). Data were analysed with 7300 system software. Primer sequences for each gene are as follows: β-actin (5′-AGAGGGAAATCGTGCGTGAC-3′ and 5′-CAATAGTGATGACCTGGCCGT-3′); IL-10 (5′-GATGCCTTCAGCAGAGTGAA-3′ and 5′-GCAACCCAGGTAACCCTTAAA-3′); TGF-β1 (5′-TGACGTCACTGGAGTTGTACGG-3 and 5′-GGTTCATGTCATGGATGGTGC-3′); Perforin (5′-CTGGCAGGGACGATGACCT-3′ and 5′-GGGAACCAGACTTGGGAGC-3′); Granzyme B (5′-ATCAAGGATCAGCAGCCTGA-3′ and 5′-TGATGTCATTGGAGAATGTCT-3′); IFN-γ (5′-TGAACG CTACACACTGCATCTTGG-3′ and 5′-CGACTCCTTTTC CGC TTC CTG AG-3′); TNF-α (5′-GCCTCTTCTCATTCCTGCTTG-3′ and 5′-CTGATGAGAGGGAGGCCATT-3′). HBV (5′-CACATCAGGATTCCTAGGACC-3′ and 5′-GGTGAGTGATTGGAGGTTG-3′). PCR conditions used for all primer sets were as follows: 95° hot start for 10 min, followed by 40 amplification cycles of 95° for 30 s (denaturing), 59° for 40 s (annealing), and 72° for 40 s (extension). Relative abundance of RNA was analysed using the 2−ΔΔCt method.
Enzyme linked immunosorbent assay
The liver was cut into small pieces and homogenized manually in a homogenizer containing homogenization buffer (PBS containing 0·05% sodium azide, 0·5% Triton X-100, and a protease inhibitor cocktail, pH 7·2, 4°) and then sonicated for 10 min. Homogenates were centrifuged at 12 000 g for 10 min and the supernatant was collected for ELISA. Blood serum was 1 : 5 diluted in PBS containing 1% BSA. For cell lysate preparation, 1 × 106 white blood cells or hepatocytes were resuspended in 60 μl of homogenization buffer and stayed on ice for 30 min. Cell lysates were centrifuged at 12 000 g for 10 min and the supernatant was collected for ELISA. Amphiregulin concentration was determined with a Mouse Amphiregulin DuoSet (R&D Systems, Minneapolis, MN) according to the manufacturer’s manual. The plate was read on a TECAN Infinite 200 PRO microplate reader.
Enrichment of intrahepatic Treg cells
Intrahepatic CD4+ CD25+ T cells were enriched from isolated intrahepatic immune cells using the EasySep™ Mouse CD25 Regulatory T Cell Positive Selection Kit (StemCell Technologies, Vancouver, BC, Canada) following the manufacturer’s instructions. Foxp3 staining was conducted to confirm that 80% enriched cells were Foxp3+ Treg cells. In some experiments, enriched cells were further stained with avidin-Alexa Fluor 647-complexed EGF on ice for 15 min before being sorted on a BD FACSAria cell sorter.
In vitro cell culture
Treg-enriched cells isolated from AAV/HBV-infected mouse livers were cultured at the cell density of 1 × 106/ml in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Recombinant mouse amphiregulin (R&D Systems) was added into culture to reach the final concentration of 100 ng/ml for 16 hr.
For detecting the effects of Treg cells on CD8+ T cells, 1 × 104 intrahepatic Treg-enriched cells and 1 × 104 intrahepatic CD3+ CD4− T cells were co-cultured in 96-well V-bottom microplates at 37° in supplemented RPMI-1640 medium. After co-culture for 24 hr, cell clusters were dissociated by vigorous pipetting in PBS buffer containing 1 mm EDTA and cells were stained with PE-Cy7 anti-CD8a antibody for 15 min on ice. CD8+ cells were sorted by flow cytometry and subject to RNA extraction, reverse transcription and qPCR. For cell proliferation assay, 96-well round-bottom microplates were coated with 5 μg/ml anti-CD3 antibody (17A2, eBioscience) at 4° overnight. Splenic CD8+ T cells were labelled with 5 μm CFSE following the manufacturer’s instructions. Treg-enriched cells (1 × 105) and CFSE-labeled CD8+ T cells (1 × 105) were co-cultured in each well of pre-coated microplates for 72 hr in the presence of 2 μg/ml anti-CD28 antibody (37.51, eBioscience) and 100 U/ml recombinant mouse IL-2 (eBioscience). CFSE dilution was determined by flow cytometry.
The primary mouse hepatocyte culture was conducted following the previous method with a few modifications.18 Briefly, hepatocytes were isolated from livers of AAV/HBV-infected C57BL/6 mice by collagenase IV perfusion. Hepatocytes then were plated on collagen-coated 48-well microplates and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 1% penicillin–streptomycin, 1% HEPES, and 0·05 mm 2-mercaptoethanol. After 4-hr attachment, the medium was replaced by fresh culture medium. Before co-culture with CD8+ T cells, the medium was removed. Two hundred microlitres of CD8+ T cell–Treg cell mixture (in supplemented Dulbecco’s modified Eagle’s medium) was added to each well for culture for 24 hr. The ratio between Treg cells, CD8+ T cells and hepatocytes was 1 : 1 : 1. Then the supernatant was collected and stored on ice. The T-cell–hepatocyte mixture was digested by TrypLE-Express (Invitrogen) at room temperature for 10 min and then the mixture was stained with PE anti-TCR-β antibody on ice. T cells were removed by flow cytometry cell sorting while hepatocytes were subject to further experiments. Secreted HBV DNA in the supernatant and HBV DNA in the hepatocytes were extracted using a QIAamp DNA minikit (Qiagen) and quantified with real time-PCR as previously described.
Statistical analysis
Quantitative data were expressed as mean ± SEM from the indicated number of experiments. Student’s t-test or one-way analysis of variance was used for comparison of mean between the groups. P values < 0·05 were considered significant.
Result
Intrahepatic Treg cells are more immunosuppressive than splenic Treg cells in AAV/HBV-infected mice
To compare the immunoregulatory activity of Treg cells in the spleen and liver during HBV infection, we first measured the immunosuppressive cytokines produced by Treg cells 2 weeks after infection. In the control AAV (cAAV)-infected mice, splenic Treg cells expressed low levels of IL-10 and TGF-β1, in accordance with previous studies showing that inactivated Treg cells produce very little immunosuppressive cytokines. AAV/HBV injection significantly up-regulated mRNA levels of both cytokines in splenic Treg cells, suggesting that Treg cells are activated after HBV infection. Furthermore, compared with splenic Treg cells, intrahepatic Treg cells expressed even higher IL-10 and TGF-β1, suggesting that intrahepatic Treg cells enhanced their immunosuppressive and/or anti-inflammatory activities (Fig.1a, d, e). The proportion of Foxp3+ Treg cells and the expression level of Foxp3 were not significantly altered in either the spleen or the liver (Fig.1b, c).
Figure 1.
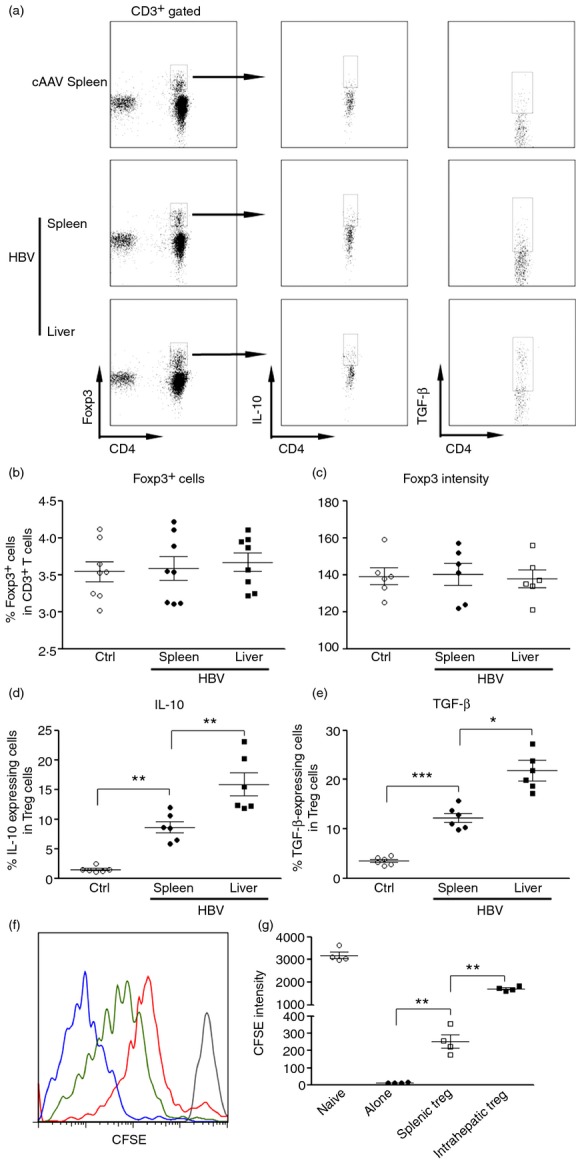
Intrahepatic regulatory T (Treg) cells are more immunosuppressive than splenic regulatory T cells. (a) Representative flow cytometry dot plots indicate expression of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) in intrahepatic Treg cells and splenic Treg cells. Splenic and intrahepatic immune cells were isolated and stained with anti-CD3, anti-CD4 and anti-Foxp3, anti-IL-10 and anti-TGF-β antibodies for flow cytometry as described in the Materials and methods. CD3+ CD4+ Foxp3+ cells were gated to show staining of IL-10 and TGF-β. Control adeno-associated virus (cAAV) spleen, spleens of cAAV-infected mice; hepatitis B virus (HBV) spleen or liver, spleens and livers of AAV/HBV-infected mice. (b) Percentage of Foxp3+ cells in total T cells. (c) Foxp3 intensity in Foxp3+ T cells. (d,e) Percentage of IL-10-expressing (d) and TGF-β-expressing (e) cells in Foxp3+ Treg cells. Ctrl, control AAV-infected mice; HBV spleen or liver, spleens and livers of AAV/HBV-infected mice. (f) Representative histogram of CFSE dilution in CD8+ T cells. Grey curve, unstimulated naive CD8+ T cells; blue curve, stimulated CD8+ T cells alone; green curve, stimulated CD8+ T cells co-cultured with splenic Treg cells; red curve, stimulated CD8+ T cells co-cultured with intrahepatic Treg cells. (g) Statistical analysis for CFSE dilution in CD8+ T cells. Each dot represents an individual mouse. *P < 0·05; **P < 0·01; ***P < 0·001.
To further determine the intrahepatic Treg cell function after HBV infection, we conducted in vitro assay to measure the Treg cell-induced immunoregulation on CD8+ T cells, because cellular immunity is critical for resisting viral challenge and CD8+ T cells have been shown to be crucial for controlling HBV replication. We tested the suppression of CD8+ T-cell proliferation by Treg cells. Normal splenic CD8+ T cells readily proliferated after agonistic antibody stimulation. Splenic and intrahepatic Treg cells significantly inhibited CFSE dilution in CD8+ T cells, while intrahepatic Treg cells induced more potent inhibition on CD8+ T-cell division than splenic Treg cells (Fig.1f, g). Taken together, our data indicated that intrahepatic Treg cells are more immunosuppressive than splenic Treg cells in HBV-infected mice.
Intrahepatic regulatory T cells up-regulated EGFR in AAV/HBV-infected mice
A recent study has demonstrated that Foxp3+ Treg cells express EGFR under inflammatory conditions. And the engagement of EGFR and an EGF-like cytokine, amphiregulin, markedly enhanced Treg cell function.19 To explore the possibility that EGFR is also involved in Treg cell function after HBV infection, we tested EGFR expression on splenic and intrahepatic Treg cells by incubating these cells with fluorochrome-conjugated EGF. We found that in cAAV-infected mice, the EGFR expression on splenic and intrhepatic CD4+Foxp3+ T cells was relatively low, ranging from only 5 to 15% EGFR+ Treg cells. In AAV/HBV-infected mice, both splenic and intrahepatic CD4+ Foxp3+ T cells increased their expression of EGFR, and the up-regulation of EGFR expression was marked in the liver, suggesting that the HBV-infected liver micro-environment might have enhanced EGFR expression on Treg cells (Fig.2a, b). The expression of EGFR on intrahepatic Treg cells was regulated in a time-dependent manner, with a peak at the second week post-inoculation, and remaining at high levels for at least 2 weeks (Fig.2c). In addition, intrahepatic CD4+ non-Treg cells and CD8+ T cells did not express EGFR after infection (Fig.2d).
Figure 2.
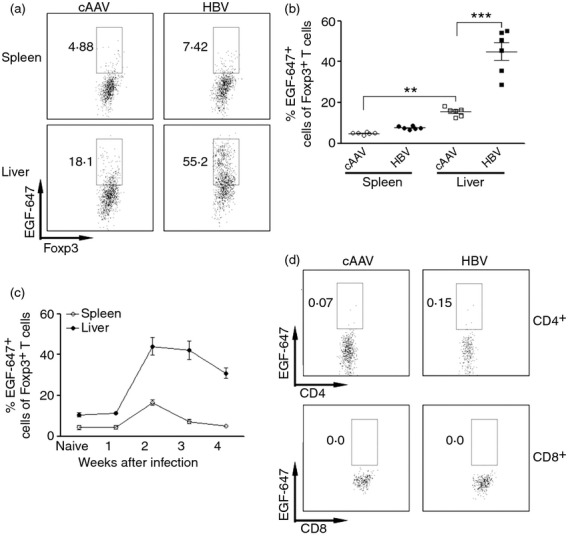
Intrahepatic regulatory T (Treg) cells up-regulate epidermal growth factor receptor (EGFR) expression after hepatitis B virus (HBV) infection. (a) Representative dot plots showing the staining of EGF-Alexa Fluro 647 on Treg cells. CD4+ Foxp3+ cells were gated as in Figure1. Only Foxp3+ cells are shown here. (b) Statistical analysis for proportion of EGF-Alexa Fluro 647+ cells in Treg cells. (c) Temporal change of EGFR expression on Treg cells after adeno-associated virus (AAV)/HBV infection; n = 4 per group. (d) Expression of EGFR on non-Treg CD4+ and CD8+ T cells 2 weeks after AAV/HBV infection. Numbers in the dot plots are the percentage of EGFR+ cells. Control AAV, control AAV-infected mice; HBV, AAV/HBV-infected mice. *P < 0·05; **P < 0·01; ***P < 0·001.
Intrahepatic EGFR+ Treg cells are more immunosuppressive than EGFR− Treg cells
To explore the role of EGFR on intrahepatic Treg cells, we separated intrahepatic Treg cells based on their EGFR expression. The expression of IL-10 and TGF-β1 was determined by flow cytometry (Fig.3a). Our data demonstrated that EGFR+ Treg cells produced more anti-inflammatory cytokines than EGFRlo/− Treg cells (Fig.3b, c), suggesting that EGFR could be crucial for modulating Treg cell functions. Moreover, EGFR+ Treg cells more strongly inhibited CD8+ T-cell proliferation than EGFRlo/− Treg cells (Fig.3d), further confirming the stronger immunosuppressive activity of intrahepatic EGFR+ Treg cells.
Figure 3.
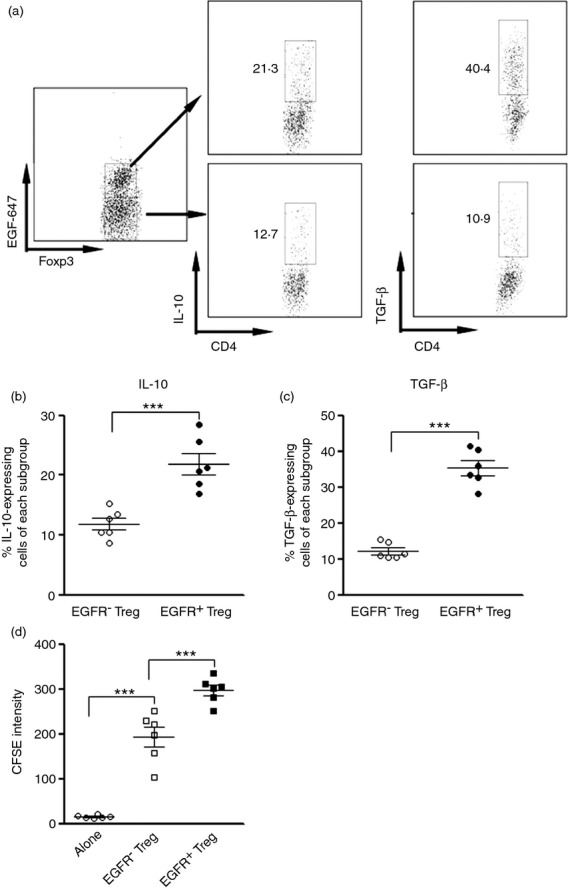
Intrahepatic epidermal growth factor receptor positive (EGFR+) regulatory T (Treg) cells are more immunorepressive than EGFR− Treg cells. (a) Representative dot plots showing expression of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) in EGFR+ Treg cells and EGFR− Treg cells. Intrahepatic CD4+ Foxp3+ cells were gated as in Figure1. Only Foxp3+ cells were shown here. Numbers in the dot plots are the percentage of EGFR+ cells. (b, c) Statistical analysis for proportion of IL-10-expressing (b) and TGF-β-expressing (c) cells in intrahepatic EGFR+ and EGFR− Treg cells, respectively. (d) CFSE dilution in stimulated CD8+ T cells co-cultured with EGFR+ and EGFR− Treg cells, respectively. Alone, CD8+ T cells cultured alone; EGFR− Treg, CD8+ T cells co-cultured with EGFR− Treg cells; EGFR+ Treg, CD8+ T cells co-cultured with EGFR+ Treg cells. Each dot represents an individual mouse. ***P < 0·001.
The association of EGFR expression with Treg cell immunoregulatory activity let us assume that EGFR+ Treg cells are also more active in restraining the anti-viral response of CD8+ T cells. Hence, we co-cultured intrahepatic CD3+ CD4− T cells with intrahepatic CD4+ CD25+ EGFR+ or CD4+ CD25+ EGFRlo/− Treg-enriched cells isolated from AAV/HBV-infected mice for 24 hr. Production of cytolytic mediators and anti-viral cytokines in CD8+ T cells were determined by qRT-PCR and flow cytometry. We found that both EGFR+ and EGFRlo/− Treg cells strongly decreased transcripts of IFN-γ, TNF-α, perforin and granzyme B, in comparison with CD8+ T cells cultured alone. Furthermore, compared with EGFRlo/− Treg cells, EGFR+ Treg cells more significantly inhibited production of IFN-γ, TNF-α, perforin and granzyme B, suggesting that EGFR+ Treg cells could more efficiently suppress CD8+ T-cell activity, which might subsequently influence intracellular viral replication (Fig.4a–e).
Figure 4.

Intrahepatic epidermal growth factor receptor positive (EGFR+) regulatory T (Treg) cells more potently inhibit CD8+ T-cell-mediated anti-viral effects. (a–d) Levels of mRNA of interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), perforin and granzyme B in intrahepatic CD8+ T cells co-cultured with EGFR+ or EGFR− Treg cells. Alone, CD8+ T cells cultured alone; EGFR− Treg, CD8+ T cells co-cultured with EGFR− Treg cells; EGFR+ Treg, CD8+ T cells co-cultured with EGFR+ Treg cells. Each dot represents Treg cells from an individual mouse. (e) Representative FACS dot plots for the expression of IFN-γ, TNF-α, perforin and granzyme B in intrahepatic CD8+ T cells co-cultured with EGFR+ or EGFR− Treg cells. Alone, CD8+ T cells cultured alone; EGFR− Treg, CD8+ T cells co-cultured with EGFR− Treg cells; EGFR+ Treg, CD8+ T cells co-cultured with EGFR+ Treg cells. Number in each plot is the frequency (mean ± SEM) of T cells expressing corresponding proteins. (f, g) Levels of cytoplasmic and secreted hepatitis B virus DNA after co-culture of hepatocytes, CD8+ T cells and Treg cells. Each dot represents a single well of culture. This result is a combination of three independent experiments. E− Treg, EGFR− Treg cells; E+ Treg, EGFR+ Treg cells. *P < 0·05; **P < 0·01; ***P < 0·001.
To further test our hypothesis, we isolated intrahepatic CD8+ T cells and hepatocytes 2 weeks after AAV/HBV infection, and co-cultured them in the presence of EGFR+ or EGFRlo/− Treg cells for 24 hr. The ratio between Treg cells, CD8+ T cells and hepatocytes was 1 : 1 : 1. The HBV DNA both in the supernatant and in hepatocytes was quantified to monitor the viral replication. Our data indicated that in the presence of EGFR+ Treg cells, the cytoplasmic viral DNA amount was significantly up-regulated in comparison with the EGFRlo/− Treg cell-containing group, suggesting that EGFR+ Treg cells strongly inhibited CD8+ T-cell-mediated down-regulation of HBV replication. And the increased viral replication was not the result of the direct effect of Treg cells towards hepatocytes, because Treg cells were not able to elicit a similar effect without CD8+ T cells (Fig.4f). However, there was a decrease of supernatant HBV DNA level in the presence of EGFR+ or EGFRlo/− Treg cells (Fig.4g). This decrease could be due to inhibited cytolytic activity of CD8+ T cells on hepatocytes.
Amphiregulin production is up-regulated in AAV/HBV-infected liver
One of the EGFR ligands, amphiregulin, has been shown to promote Treg cell function in inflammation sites. To clarify whether amphiregulin is also responsible for the enhanced immunoregulatory activity of intrahepatic Treg cells, we detected its abundance in the liver and blood using ELISA. In normal mice, the baseline expression of amphiregulin in either blood or liver homogenate was almost below the detection threshold of the ELISA kit, but AAV/HBV infection significantly increased amphiregulin protein in the liver. In the blood, however, amphiregulin level was only moderately increased, not as profoundly as in the liver (Fig.5a). This result suggests that the source of amphiregulin might be in the liver rather than in the blood. The up-regulation of amphiregulin was time dependent, reaching the peak at the second week after infection and gradually returning to normal level within 4 weeks (Fig.5a). Interestingly, the up-regulation of intrahepatic amphiregulin level coincided with an increment of intrahepatic EGFR+ Treg cells during AAV/HBV infection. The qRT-PCR data revealed that amphiregulin mRNA level was increased in both hepatocytes and intrahepatic white blood cells, but not in circulating white blood cells 2 weeks after infection (Fig.5b). Moreover, the increase of amphiregulin mRNA in hepatocytes was more robust than in infiltrating white blood cells, suggesting that hepatocytes would be the main source of amphiregulin in infected livers. ELISA for cell lysate confirmed higher amphiregulin expression in intrahepatic white blood cells and hepatocytes (Fig.5c).
Figure 5.
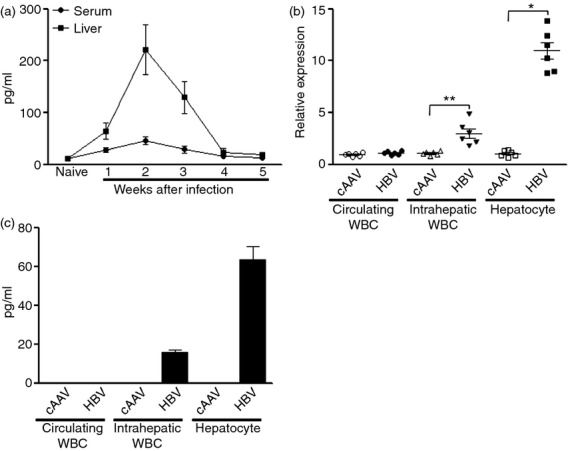
Amphiregulin expression is up-regulated in adeno-associated virus (AAV)/hepatitis B virus (HBV)-infected livers. (a) Temporal change of amphiregulin expression in the serum and liver homogenate after AAV/HBV infection; n = 5 mice per group. (b) Amphiregulin mRNA level in different cell types. WBC, white blood cells. Each dot represents an individual mouse. (c) Amphiregulin concentration in cell lysates detected by ELISA. Cells were lysed as mentioned in the Materials and Methods. The supernatants of cell lysate were used for ELISA; n = 6 mice per group.*P < 0·05; **P < 0·01; ***P < 0·001.
Amphiregulin promoted immunosuppressive activity of EGFR+ Treg cells
To ascertain the role of amphiregulin in modulating intrahepatic Treg cell function, we sorted intrahepatic CD4+ CD25+ Treg cells and cultured them with IL-2 in the presence or absence of amphiregulin. Production of IL-10 and TGF-β was detected with flow cytometry and qRT-PCR. Amphiregulin treatment significantly increased both protein levels and mRNA levels of these two anti-inflammatory cytokines (Fig.6a–d), suggesting that amphiregulin promoted Treg activity. Amphiregulin treatment did not significantly alter Treg cell apoptosis, because the proportion of Annexin-V+ cells was comparable between treated and untreated groups (Fig.6e).
Figure 6.
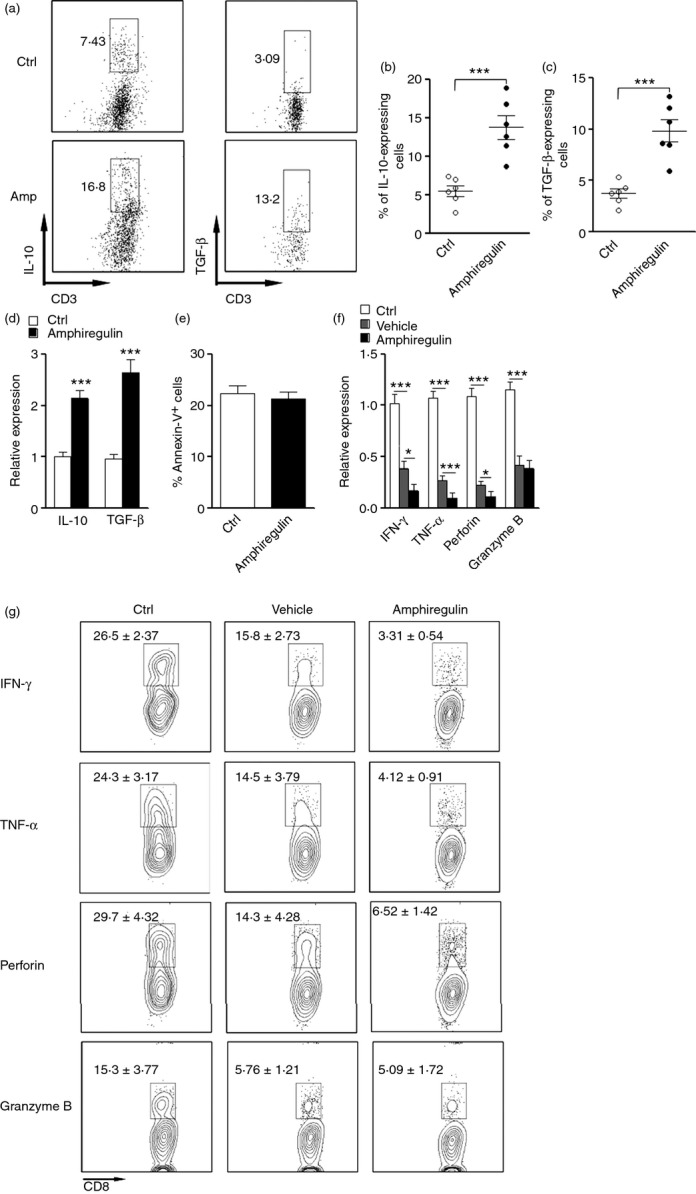
Amphiregulin enhances the immunosuppressive activity of epidermal growth factor receptor positive (EGFR+) regulatory T (Treg) cells to inhibit CD8+ T-cell-mediated anti-viral function. (a) Representative dot plots showing interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) staining in EGFR+ Treg cells. FACS sorted intrahepatic CD4+ CD25+ Treg cells were culture in vitro in the presence or absence of 100 ng/ml Amphiregulin for 24 hr. Surface staining for CD3 and intracellular staining for IL-10 and TGF-β were then conducted. CD3+ cells were gated. Numbers in the dot plots are the percentage of IL-10-expressing or TGF-β-expressing cells. Ctrl, without amphiregulin; Amp, with amphiregulin. (b, c) Statistical analysis for proportion of IL-10-expressing (b) and TGF-β-expressing (c) cells in cultured Treg cells. Each dot represents an individual mouse. (d) mRNA levels of IL-10 and TGF-β in cultured Treg cells; n = 6 per group. (e) Percentage of Annexin V+ cells in Treg cells after culture; n = 6 per group. Ctrl, Treg cells cultured without amphiregulin; Amphiregulin, Treg cells cultured with amphiregulin. (f) mRNA levels of anti-viral components in CD8+ T cells after co-culture with intrahepatic CD4+ CD25+ Treg cells. Ctrl, CD8+ T cells culture without Treg cells; Vehicle, CD8+ T cells co-cultured with Treg cells but without amphiregulin; Amphiregulin, CD8+ T cells co-cultured with Treg cells with amphiregulin. (g) Representative FACS quadrant plots for the expression of interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), perforin and granzyme B in CD8+ T cells co-cultured with Treg cells. Number in each plot is the frequency (mean ± SEM) of T cells expressing corresponding proteins; n = 5 per group. *P < 0·05; **P < 0·01; ***P < 0·001.
To check the impact of amphiregulin on Treg cell-induced inhibition of CD8+ T cells, intrahepatic Treg cells were co-cultured with intrahepatic CD3+ CD4− T cells isolated from AAV/HBV-infected mice in the presence or absence of amphiregulin. After 24 hr of co-culture, CD8+ T cells were sorted as mentioned in the Materials and methods. CD8+ T cells were subject to qRT-PCR and flow cytometry to determine the expression of IFN-γ, TNF-α, perforin and granzyme B. We found that when co-cultured with amphiregulin and Treg cells, CD8+ T cells expressed significantly less IFN-γ, TNF-α and perforin, but not granzyme B, in comparison with CD8+ T cells co-cultured without amphiregulin (Fig.6f, g). Hence, amphiregulin indeed enhanced Treg cell-induced inhibition of CD8+ T-cell anti-viral function.
Discussion
Hepatitis B virus is a small DNA virus with unusual features similar to retroviruses.1 Successful HBV replication in host cells is associated with insufficient immune response to neutralize and eradicate the virus. Both humoral and cellular immune responses are mounted after infection. It has been shown that humoral responses such as virus-specific neutralizing antibodies exist.6 However, their function does not significantly contribute to viral clearance during acute HBV infection. In contrast, several studies have shown that CD4+ T-cell-mediated and CD8+ T-cell-mediated immune responses decide the outcome of HBV infection.6,20 Hence, spontaneous viral clearance of HBV infection is characterized by vigorous and sustained virus-specific CD4+ and CD8+ T-cell responses during the acute phase of infection. However, T-cell-mediated immunity is a double-edged sword, because it is also critical for liver injury and the establishment of liver diseases during viral infections. CD4+ T cells are important for helping B cells to develop into antibody-secreting plasma cells,21 and they also possess cytotoxic function.22 Moreover, CD4+ T cells seem to be responsible for the induction and maintenance of successful CD8+ T-cell responses.23,24 CD8+ T cells control HBV replication in both cytotoxic and non-cytotoxic manners, through releasing cytotoxic mediators and anti-viral cytokines, respectively.25 A strong correlation between vigorous, virus-specific CD8+ T-cell responses and HBV clearance has been demonstrated in several studies,26–28 suggesting that these cells are critical for HBV progression. Failure of the adaptive immune response in HBV infection is believed to contribute to sustained viral replication. However, the main factors driving failure of T-cell responses in hepatitis are not completely elucidated, but studies suggest that T-cell dysfunction and viral escape contribute to the failure.6
Within CD4+ T cells, a subpopulation expressing Foxp3 has been identified as Treg cells, which restrain autoimmunity and excess inflammation.29 Treg cells are armed with several weapons to fight against autoimmune diseases and inflammatory disorders, including IL-10, TGF-β, recently identified IL-35, CD39, CD73, etc.30 Regulatory CD4+ T cells have been suggested to contribute to the protection from overwhelming liver tissue damage, but also to the failure of CD8+ T cells in HBV infection.31,32 Interleukin-10 and TGF-β have been shown to generate a unique cytokine environment, mainly inducing tolerance of liver-infiltrating lymphocytes. It has been shown that patients with chronic HBV infection have elevated frequencies of regulatory CD4+ T cells in the blood that inhibit the proliferation of HBV-specific CD8+ T cells.10,33,34 Chronically HBV-infected patients with high viral loads have higher frequencies of regulatory CD4+ T cells in the liver. A previous study has demonstrated that in hepatitis C virus-infected patients, Treg cells are able to suppress hepatitis C virus-specific CD8+ T-cell proliferation and IFN-γ secretion,13 which could also be the case for HBV infection. Taken together, these studies suggest that Treg cells play a role in the inhibition of HBV-specific T-cell function. However, the mechanisms by which Treg cell function is regulated are still elusive.
In this study we characterized the up-regulated expression of EGFR on intrahepatic Treg cells in HBV infection in a mouse model. It has been known that EGFR exists on the cell surface and transduces intrinsic intracellular signal pathways to induce DNA synthesis and cell proliferation upon ligand engagement.35 The relationship between EGFR and cancer has been elucidated in previous research.36,37 However, its role in regulating T-cell functions has not been addressed. Actually, little information about the expression of EGFR on T cells has been generated until recently, suggesting that under most conditions EGFR might not be expressed on T cells. The only literature addressing this issue is published recently, stating that EGFR is expressed on Treg cells under inflammatory conditions.19 Because HBV infection elicits robust inflammation in the liver, we hypothesized that Treg cells infiltrating the infected liver might also express EGFR to adaptive themselves to the hepatitis. Indeed, we found a higher expression level of EGFR on intrahepatic Treg cells. The expression level of EGFR on intrahepatic cells is higher than on splenic Treg cells, suggesting that although Treg activation in the spleen might induce EGFR expression, the most potent factor driving EGFR expression is in the infected liver. However, the identity of the factor is still unknown. Early studies have propose several candidate factors that increase EGFR expression in different cell types, including androgen,38,39 TNF-α,40 sphingosine 1-phosphate41 and TGF-β.42 Further studies are needed to identify the soluble and/or cellular components that induce EGFR expression in Treg cells.
Interestingly, the higher EGFR level is associated with enhanced immunosuppressive activity of intrahepatic Treg cells. This relationship posed the possibility that EGFR with its ligand could modulate Treg cell function in HBV-infected livers, so as to influence the anti-HBV immune reaction. EGFR can be bound with ligands such as EGF, TGF-α and amphiregulin. Inspired by the previous study, we found that amphiregulin is at least partially responsible for the promoted Treg cell function in the liver. Amphiregulin, also known as AREG, belongs to the EGF family. Synthesized as a transmembrane protein, amphiregulin’s extracellular domain is proteolytically processed to release the mature protein. It has been regarded as an autocrine growth factor and a mitogen for astrocytes, Schwann cells, fibroblasts and epithelial cells.43 Amphiregulin also inhibits the growth of certain carcinoma cell lines.43 Recent study indicates that amphiregulin also plays a role in regulating Treg cell function.19 Our data demonstrated elevated expression of amphiregulin in AAV/HBV-infected mouse livers. Furthermore, exogenous amphiregulin enhanced immunosuppressive activity of Treg cells in vitro. Our discovery poses the possibility of neutralizing amphiregulin in the liver as a therapeutic approach for HBV infection, if future studies can confirm the presence and similar activity of amphiregulin in the livers of HBV-infected patients. However, we cannot exclude the possibility that other EGFR ligands including EGF and TGF-α could also potentially affect Treg cell function. Level of EGF could be increased during HBV infection,44 and TGF-α expression in HBV-infected livers has been confirmed previously.45,46 To our knowledge, there is no literature showing the effects of EGF and TGF-α on Treg cells. In one of our pilot studies EGF was incapable of promoting Treg cell function in vitro. Further studies are required to test the effects of EGF and TGF-α on Treg cells, especially EGFR+ Treg cells.
However, the source of amphiregulin after HBV infection is still unknown. We found that the level of amphiregulin in the liver was higher than in the blood, suggesting that HBV-infected liver could be its source. The cellular source of amphiregulin is still elusive. Hepatocytes could be a candidate, because amphiregulin gene expression is not detected in healthy liver but is up-regulated in hepatocytes during chronic human and rat liver injury.47 Amphiregulin expression is induced in isolated hepatocytes by IL-1β and prostaglandin E2, but not by hepatocyte growth factor, IL-6 or TNF-α.47 Other potential cellular sources of amphiregulin include mast cells,19 dendritic cells,48 monocytes49 and neutrophils.50 It is possible that HBV-infected hepatocytes up-regulate amphiregulin expression as an injury-induced response, followed by infiltrated immune cells releasing amphiregulin to modulate the inflammatory response in the liver. Ongoing research in our laboratory is focusing on this issue and the amphiregulin-producing cells will be disclosed.
Taken together, our current study is the first to link the amphiregulin–EGFR interaction to the Treg cell function in HBV-infected livers. Our research might provide novel clues for how the anti-viral immune response is regulated in the liver, and also might inspire new therapeutic strategies for HBV infection.
Acknowledgments
This study was supported by a grant from the Chinese Foundation for hepatitis prevention and control (Tianqing liver disease research fund No. CFHPC20132133).
Glossary
- HBV
hepatitis B virus
- EGFR
epidermal growth factor receptor
- Treg
regulatory T
Disclosures
The authors have no financial or commercial conflicts of interest.
References
- Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13–21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–40. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- Merican I, Guan R, Amarapuka D, et al. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356–61. doi: 10.1046/j.1440-1746.2000.0150121356.x. [DOI] [PubMed] [Google Scholar]
- Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–49. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. doi: 10.1186/1743-422X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia Schmidt HEB, Robert T. T-cell responses in hepatitis B and C virus infection: similarities and differences. Emerg Microbes Infect. 2013;2:e15. doi: 10.1038/emi.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A, Saxena R. Regulatory T cells: a review. Natl Med J India. 2012;25:341–51. [PubMed] [Google Scholar]
- Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, Kusters JG, Janssen HL. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–8. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- Aalaei-Andabili SH, Alavian SM. Regulatory T cells are the most important determinant factor of hepatitis B infection prognosis: a systematic review and meta-analysis. Vaccine. 2012;30:5595–602. doi: 10.1016/j.vaccine.2012.06.063. [DOI] [PubMed] [Google Scholar]
- Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008;123:57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect Dis. 2007;7:804–13. doi: 10.1016/S1473-3099(07)70289-X. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, TrehanPati N, Patra S, Kottilil S, Pande C, Trivedi SS, Sarin SK. Increased regulatory T cells and impaired functions of circulating CD8 T lymphocytes is associated with viral persistence in hepatitis B virus-positive newborns. J Viral Hepat. 2013;20:582–91. doi: 10.1111/jvh.12078. [DOI] [PubMed] [Google Scholar]
- Franzese O, Kennedy PT, Gehring AJ, Gotto J, Williams R, Maini MK, Bertoletti A. Modulation of the CD8+ T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J Virol. 2005;79:3322–8. doi: 10.1128/JVI.79.6.3322-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Liu L, Zhu D, Peng H, Su L, Fu YX, Zhang L. A mouse model for HBV immunotolerance and immunotherapy. Cell Mol Immunol. 2014;11:71–8. doi: 10.1038/cmi.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom KG, Qazi MR, Matos JB, Nelson BD, DePierre JW, Abedi-Valugerdi M. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol. 2009;155:320–9. doi: 10.1111/j.1365-2249.2008.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. Isolation of mouse intrahepatic lymphocytes. Curr Protoc Immunol. 2001;3:Unit–3. doi: 10.1002/0471142735.im0321s22. [DOI] [PubMed] [Google Scholar]
- Wang J, Holmes TH, de Guevara LL, Cheung R, Wright TL, He XS, Greenberg HB. Phenotypic and functional status of intrahepatic T cells in chronic hepatitis C. J Infect Dis. 2006;194:1068–77. doi: 10.1086/507681. [DOI] [PubMed] [Google Scholar]
- Bongfen SE, Torgler R, Romero JF, Renia L, Corradin G. Plasmodium berghei-infected primary hepatocytes process and present the circumsporozoite protein to specific CD8+ T cells in vitro. J Immunol. 2007;178:7054–63. doi: 10.4049/jimmunol.178.11.7054. [DOI] [PubMed] [Google Scholar]
- Zaiss DM, van Loosdregt J, Gorlani A, et al. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–84. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana D, Menachery J, Chawla Y, Duseja A, Dhiman R, Arora S. HBV specific T-cell responses in hepatitis B. Trop Gastroenterol. 2011;32:273–8. [PubMed] [Google Scholar]
- Tsai LM, Yu D. Follicular helper T-cell memory: establishing new frontiers during antibody response. Immunol Cell Biol. 2014;92:57–63. doi: 10.1038/icb.2013.68. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol. 2011;2011:954602. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun. 2007;75:3556–60. doi: 10.1128/IAI.00086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8+ T cell control of hepatitis B virus replication: direct comparison between cytolytic and noncytolytic functions. J Immunol. 2010;184:287–95. doi: 10.4049/jimmunol.0902761. [DOI] [PubMed] [Google Scholar]
- Riedl P, Bertoletti A, Lopes R, Lemonnier F, Reimann J, Schirmbeck R. Distinct, cross-reactive epitope specificities of CD8 T cell responses are induced by natural hepatitis B surface antigen variants of different hepatitis B virus genotypes. J Immunol. 2006;176:4003–11. doi: 10.4049/jimmunol.176.7.4003. [DOI] [PubMed] [Google Scholar]
- Das A, Hoare M, Davies N, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205:2111–24. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang W, Wang S, Meng G, Zhang M, Ni B, Wu Y, Wang L. An immunodominant HLA-A*1101-restricted CD8+ T-cell response targeting hepatitis B surface antigen in chronic hepatitis B patients. J Gen Virol. 2013;94:2717–23. doi: 10.1099/vir.0.052167-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13:461–7. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatrakchi N, Koziel M. Regulatory T cells and viral liver disease. J Viral Hepat. 2009;16:223–9. doi: 10.1111/j.1365-2893.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Stross L, Gunther J, Gasteiger G, et al. Foxp3+ regulatory T cells protect the liver from immune damage and compromise virus control during acute experimental hepatitis B virus infection in mice. Hepatology. 2012;56:873–83. doi: 10.1002/hep.25765. [DOI] [PubMed] [Google Scholar]
- Miroux C, Vausselin T, Delhem N. Regulatory T cells in HBV and HCV liver diseases: implication of regulatory T lymphocytes in the control of immune response. Expert Opin Biol Ther. 2010;10:1563–72. doi: 10.1517/14712598.2010.529125. [DOI] [PubMed] [Google Scholar]
- Barboza L, Salmen S, Goncalves L, et al. Antigen-induced regulatory T cells in HBV chronically infected patients. Virology. 2007;368:41–9. doi: 10.1016/j.virol.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131:1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meel R, Oliveira S, Altintas I, et al. Inhibition of tumor growth by targeted anti-EGFR/IGF-1R nanobullets depends on efficient blocking of cell survival pathways. Mol Pharm. 2013;10:3717–27. doi: 10.1021/mp400212v. [DOI] [PubMed] [Google Scholar]
- Pignon JC, Koopmansch B, Nolens G, Delacroix L, Waltregny D, Winkler R. Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res. 2009;69:2941–9. doi: 10.1158/0008-5472.CAN-08-3760. [DOI] [PubMed] [Google Scholar]
- Mukherjee B, Mayer D. Dihydrotestosterone interacts with EGFR/MAPK signalling and modulates EGFR levels in androgen receptor-positive LNCaP prostate cancer cells. Int J Oncol. 2008;33:623–9. [PubMed] [Google Scholar]
- Yoo J, Rodriguez Perez CE, Nie W, Edwards RA, Sinnett-Smith J, Rozengurt E. TNF-α induces upregulation of EGFR expression and signaling in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2012;302:G805–14. doi: 10.1152/ajpgi.00522.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Sun CC, Wu CB, Wu CY, Tung WH, Wang HH, Yang CM. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-κB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J Cell Biochem. 2008;103:1732–46. doi: 10.1002/jcb.21563. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118–22. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A. The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta. 2011;1816:119–31. doi: 10.1016/j.bbcan.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Barreiros AP, Sprinzl M, Rosset S, et al. EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes. Int J Cancer. 2009;124:120–9. doi: 10.1002/ijc.23921. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Axiotis CA, Di Bisceglie AM, Tabor E. Transforming growth factor-α in human hepatocellular carcinoma and coexpression with hepatitis B surface antigen in adjacent liver. Cancer. 1992;70:1049–56. doi: 10.1002/1097-0142(19920901)70:5<1049::aid-cncr2820700507>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Morimitsu Y, Kleiner DE, Jr, Conjeevaram HS, Hsia CC, Di Bisceglie AM, Tabor E. Expression of transforming growth factor α in the liver before and after interferon α therapy for chronic hepatitis B. Hepatology. 1995;22:1021–6. doi: 10.1016/0270-9139(95)90604-5. [DOI] [PubMed] [Google Scholar]
- Berasain C, Garcia-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, Avila MA. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005;128:424–32. doi: 10.1053/j.gastro.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Bles N, Di Pietrantonio L, Boeynaems JM, Communi D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood. 2010;116:3219–26. doi: 10.1182/blood-2010-01-265611. [DOI] [PubMed] [Google Scholar]
- Mograbi B, Rochet N, Imbert V, Bourget I, Bocciardi R, Emiliozzi C, Rossi B. Human monocytes express amphiregulin and heregulin growth factors upon activation. Eur Cytokine Netw. 1997;8:73–81. [PubMed] [Google Scholar]
- Adib-Conquy M, Pedron T, Petit-Bertron AF, Tabary O, Corvol H, Jacquot J, Clement A, Cavaillon JM. Neutrophils in cystic fibrosis display a distinct gene expression pattern. Mol Med. 2008;14:36–44. doi: 10.2119/2007-00081.Adib-Conquy. [DOI] [PMC free article] [PubMed] [Google Scholar]


