Abstract
The objective of this analysis was to evaluate mortality among a cohort of 24,865 capacitor-manufacturing workers exposed to polychlorinated biphenyls (PCBs) at plants in Indiana, Massachusetts, and New York and followed for mortality through 2008. Cumulative PCB exposure was estimated using plant-specific job-exposure matrices. External comparisons to US and state-specific populations used standardized mortality ratios, adjusted for gender, race, age and calendar year. Among long-term workers employed 3 months or longer, within-cohort comparisons used standardized rate ratios and multivariable Poisson regression modeling. Through 2008, more than one million person-years at risk and 8749 deaths were accrued. Among long-term employees, all-cause and all-cancer mortality were not elevated; of the a priori outcomes assessed only melanoma mortality was elevated. Mortality was elevated for some outcomes of a priori interest among subgroups of long-term workers: all cancer, intestinal cancer and amyotrophic lateral sclerosis (women); melanoma (men); melanoma and brain and nervous system cancer (Indiana plant); and melanoma and multiple myeloma (New York plant). Standardized rates of stomach and uterine cancer and multiple myeloma mortality increased with estimated cumulative PCB exposure. Poisson regression modeling showed significant associations with estimated cumulative PCB exposure for prostate and stomach cancer mortality. For other outcomes of a priori interest – rectal, liver, ovarian, breast, and thyroid cancer, non-Hodgkin lymphoma, Alzheimer disease, and Parkinson disease – neither elevated mortality nor positive associations with PCB exposure were observed. Associations between estimated cumulative PCB exposure and stomach, uterine, and prostate cancer and myeloma mortality confirmed our previous positive findings.
Keywords: Polychlorinated biphenyls, Cohort study, Occupational exposure, Cancer, Exposure assessment
Introduction
From 1929 to 1977, 209 polychlorinated biphenyl (PCB) congeners were produced commercially in the United States. Mixtures of PCBs were used widely in industry (Robertson and Ludewig, 2011; Robertson and Ruder, 2010) and persist in humans and in the environment (Hopf et al., 2009a). Increasing concern about potential health and environmental risks led to a 1977 ban on PCB production and distribution in the United States and a 2004 ban on PCB production in 151 signatory countries (United Nations Environment Programme, 2004). In 2013 the International Agency for Research on Cancer (IARC) classified PCBs as definite human carcinogens (Lauby-Secretan et al., 2013).
Thirty-five years after their use was banned in the United States PCBs are still a potential occupational and environmental exposure for those who repair, maintain, or remove capacitors and transformers containing PCBs and for the general public, as well as for the former workers whose decades of internal exposure to PCBs continue (Beyer and Biziuk, 2009). The National Institute for Occupational Safety and Health (NIOSH) has assembled a cohort of workers who manufactured capacitors using PCBs as a dielectricfluid at plants in Indiana (1957–1977) (Ruder et al., 2006), and Massachusetts (1939–1976) and New York (1946–1977) (Prince et al., 2006). Dielectric fluid formulations (i.e., different commercial products containing some of the 209 PCB congeners) varied across time and across the plants (see Table 1).
Table 1.
Differences among the three capacitor manufacturing plants in the NIOSH Capacitor Cohort.
| Determinant | Indiana | New York | Massachusetts | |
|---|---|---|---|---|
| Chemicalsa | Aroclor 1254 use | – | 1946–1955b | 1939–1958c |
| Aroclor 1242 use | ~1958–1971 | 1955–1971 | 1958–1976 | |
| Aroclor 1016 use | 1971–1977 | 1971–1977 | 1976–~1977 | |
| Other Aroclors | Yes – (detected by EPA around the plant where used oil was dumped): Aroclors 1248, 1254, and 1260 | Yes – occasional use of Aroclor 1221 (50% mono-Cl + 35% di-Cl + 5% tri and tetra Cl) 1971–1975 | No | |
| Dielectric fluids other than PCBs | No | Yes after 1977: Bis(2-ethylhexyl) phthalate (DEHP), castor oil, and mineral oil | Yes – castor oil and mineral oils used in large capacitors | |
| Degreasing solvent | TCE | TCE, detergent from 1977 | TCE, degreasing station often leaked | |
| Process description | Method of filling capacitors with PCBs | Manual | Manual 1946–1960; some automation after 1960 | Manual |
| Flood filling temperatures >100 °C | ||||
| Process changes over time | Yes, but timing and nature of change unknown | Few changes | Very few changes made from 1947 to 1977 | |
| Plant-specific information | Years of operation using Aroclors | 1958–1977 | 1946–1977 | 1939–1977 |
| Ventilation | Leakage of oil mist from impregnation chambers because the oil seal was broken Local exhaust ventilation provided but often not used Insufficient duct size in chamber filling area Degreaser leaked TCE, open tanks Exhaust vapors re-entered work area Painting booths not |
General dilution ventilation installed in 1965; upgrade in 1976 reduced exposures 75% from 1976 to 1977 | No local ventilation for oven opening – PCBs were exhausted from the impregnation ovens by opening the doors | |
| Personal protective | Respirators provided but no training or change out schedule – mostly not used by the workers | Not documented | Not documented | |
| Plant lay-out | 1968 expansion of plant with new non-PCB production areas Very small area of the plant dedicated to manufacturing with PCBs | Two different facilities Frequent changes Large spills at both facilities frequent due to the set-up of the manufacturing process | Impregnation on 1st and 2nd floors Capacitors were moved from department to department and from floor to floor PCB areas not physically separated |
|
| Manufacture of items other than PCB-filled capacitors | Yes – manufactured shunt capacitors, series capacitors, lighting arresters, reclosers and breakers, switches, potential devices, and line traps | No | Yes – produced mica and tubular capacitors | |
| Exposure category values used in job-exposure matrix | Hopf et al. (2009b) | Hopf et al. (2013) | Hopf et al. (2010) | |
| High | 230 | 750 | 770 | |
| Medium | 140 | 300 | 550 | |
| Low | 50 | 50 | 180 | |
| Baseline | 5 | 10 | 50 |
Abbreviations: PCB, polychlorinated biphenyl; TCE, trichloroethylene.
When plants changed from one Aroclor product to another, the substitution process was gradual over several months during which more than one product would be inuse.
Different periods of use of various Aroclors have been reported: Aroclor 1254 from 1946 to 1954 and Aroclor 1242 from 1954 to 1971 (Taylor et al., 1991); Aroclor 1016from 1971 to 1976 (Jones et al., 1978; Taylor et al., 1991); occasional use of Aroclor 1221 (Jones et al., 1978).
Date of switch from Aroclor 1254 to Aroclor 1242 is uncertain.
Previous reports utilized different requirements for eligibility (one day vs. 90 days) and considered the Indiana plant separately from the Massachusetts and New York plants for most causes of death (Brown, 1987; Brown and Jones, 1981; Prince et al., 2006; Ruder et al., 2006; Sinks et al., 1992), but jointly for breast cancer incidence (Silver et al, 2009) and neurodegenerative disease mortality (Steenland et al., 2006).
The rationale for the present study was that by combining the three largest US capacitor manufacturing cohorts and incorporating ten additional years of mortality experience, previous findings and hypothesized relationships could be re-evaluated with greater statistical power due to more workers, longer follow-up, and the integration of the three cohort exposure assessments. The current study extends vital status follow-up an additional ten years through 2008 and includes all workers from the three plants with one day or more of employment during the period when PCBs were in use, but focuses on workers employed 3 months or more. Job-exposure matrixes developed for the three plants were used to estimate cumulative PCB exposure for each worker. The primary purpose was to investigate further the relationship between estimated cumulative PCB exposure and mortality outcomes, particularly among long-term workers employed for 3 months or longer. Outcomes of a priori interest included causes of death with increased risks originally observed in the subcohorts followed through 1998, including hormone-related cancers (i.e., prostate, breast, uterine, and ovarian), multiple myeloma, melanoma, brain and nervous system cancer, stomach cancer, intestinal cancer, and neurodegenerative diseases. Other a priori hypotheses were that PCB exposure would affect all-cause mortality, all-cancer mortality, and, specifically, rectal cancer, biliary passages, liver, and gall bladder cancer, and non-Hodgkin lymphoma, outcomes for which other studies indicated increased risks (Brown, 1987; Brown and Jones, 1981; Rothman et al., 1997). More recent results have motivated the addition of thyroid cancer to the a priori list (Mallin et al., 2004; Yard et al., 2011). Including all the workers in one analysis and adding ten years of vital status follow-up increases the power of the study.
Materials and methods
Cohort
The cohort included all workers employed at least one day at any of the plants. Records were maintained for 26,588 workers; however, those not employed during the years PCBs were used or employed less than one day were excluded from the final PCB cohort of 25,062 workers. Demographic data sources included work histories and plant records. Records for the New York and Massachusetts workers were originally microfilmed in 1977, although only the 2588 workers considered highly exposed were included in the first analyses (Brown, 1987; Brown and Jones, 1981).The cohort was expanded to include all exposed workers at the New York and Massachusetts plants using these microfilmed records, which were verified against Internal Revenue Service (IRS) Employer’s Quarterly Earnings Reports for the New York plant (Prince et al., 2006). Records for the Indiana workers were microfilmed and data-entered in 1989 (Sinks et al., 1992).
Exposure assessment
Exposure data sources included company and NIOSH surveys of the plants and plant records. The exposure assessment used previously created plant-specific semi-quantitative job-exposure matrices (JEMs) that are described in detail in Hopf et al. (2009b, 2010, 2013) and briefly here. All jobs were categorized based on PCB exposure intensity and frequency, and qualitatively ranked for inhalation and dermal exposure. For inhalation exposure intensity, air concentration data permitted assignment of exposure units (ppm), but the lack of historical dermal exposure measurements resulted in a unitless measure of dermal exposure intensity. For each job category the product of intensity and frequency (fraction of day exposed) was calculated. Inhalation and dermal JEMs were modified for earlier and later eras (the former with estimated 20% higher exposure). Since dermal exposures account for a significant proportion of total PCB exposure (Fishbein, 1982), a combination JEM averaging inhalation and dermal (1:1) scores was used to estimate PCB exposure for each job by era. Detailed work histories available for each worker were used to estimate cumulative PCB exposure, based on plant, department, job title, and the era-specific combination JEM, and expressed in unit-days or unit-years of exposure (but the “unit” was undefined).
Vital status follow-up
Vital status was originally determined through 1982 for the New York and Massachusetts cohorts using records from the Social Security Administration (SSA) Death Master File, state motor vehicle registration and vital statistics offices, the US Postal Service, and, after 1979, the National Death Index (NDI) (National Center for Health Statistics, 2007) (Brown, 1987; Brown and Jones, 1981). Vital status was determined through 1984 for the Indiana cohort using records from the SSA and the IRS (Sinks et al., 1992). For previous analyses (Brown, 1987; Brown and Jones, 1981; Sinks et al., 1992), death certificates had been obtained for pre-1979 deaths and causes of death coded by a trained nosologist. Subsequent vital status updates for all three subcohorts used the NDI (Prince et al., 2006; Ruder et al., 2006).
For the present update, cohort member data were submitted to the NDI for determination of vital status through 2008, and NDI-Plus causes of death were obtained. Any worker lost to follow-up before 1979 was classified ‘vital status unknown’ and considered alive until the date last observed (usually the date last employed). All causes of death (NDI and death certificate-based) were coded to the revision of the International Classification of Diseases (ICD) in effect at the time of death, and mapped to specific cause of death categories (described below). This study (HSRB 08-DSHEFS-02) has been approved by the NIOSH Human Subjects Review Board. As a records study, it was exempted from informed consent requirements.
Statistical analysis
Mortality was evaluated using the NIOSH life table analysis system (Schubauer-Berigan et al, 2011). Analyses used US mortality rates for 92 (1940–2007) or 119 (1960–2007) cause of death categories (Robinson et al, 2006) and three neurodegenerative causes of death: Alzheimer disease, amyotrophic lateral sclerosis (ALS), and Parkinson disease (Steenland et al, 2006). Race- and gender-specific person years at risk (PYAR) were accumulated for each eligible worker across five-year age and calendar year intervals, beginning on the qualified date of first exposure, or the rate file begin date (January 1, 1940 or January 1, 1960), whichever was later, and ending with the date of death, the date last known alive, or the study end date (December 31,2008), whichever was earliest. Cohort members with valid social security numbers known to be alive after January 1,1979, when the NDI began, and not identified as deceased were assumed to be alive on December 31, 2008, as the sensitivity of the NDI is over 95% when social security numbers are available (Cowper et al, 2002). For each cause of death, PYAR were multiplied by the gender-race-age-calendar period referent rates and summed to yield an expected number of deaths. The standardized mortality ratio (SMR) is the ratio of observed to expected deaths. The statistical significance of the SMR was determined by a two-tailed test based on the Poisson distribution and a 95% confidence interval (CI) was calculated based on exact (10 or fewer deaths) or approximate (more than 10 deaths) methods.
Plant-specific analyses used state mortality rates (1960–2007) for the 119 cause of death categories. State rates account for local conditions that can influence disease rates (e.g., the gradient of disease with latitude seen for some cancers (Foster et al., 2008)). A multiple-cause-of-death analysis considering all causes listed on the death certificates investigated possible excesses in nonmalignant diseases using US multiple cause referent rates (1960–2007) (Steenland et al., 1992). Because of the large number of short-term workers, and for comparability to previous updates, we conducted separate analyses of short-term (employment duration less than 3 months) and long-term (employment duration of 3 months or longer) workers.
Within-cohort comparisons
The distribution of estimated cumulative PCB exposure was highly right-skewed (range 10 to 9.6 × 106 unit-days; median 3.2 × 104 unit-days); cutpoints at 40,000, 150,000, and 600,000 unit-days defined quartiles with approximately equal numbers of deaths among long-term workers. Among long-term workers, standardized rates were calculated for each quartile using the sum of all PYAR for each gender-race-age-calendar period stratum as the stratum weight. Directly standardized rate ratios (SRRs) were calculated for each exposure quartile relative to the lowest quartile and 95% CIs were calculated based on a Taylor series approximation of the variance (Rothman and Greenland, 1998). A test for a linear trend was performed based on a weighted regression of the standardized rates (Rothman et al., 2008). To account for potential latency, exposure lag periods of 10 and 20 years were evaluated for cancer outcomes. Analyses that considered cumulative exposure excluded 515 workers with periods of unknown exposure who were included in the overall results.
Relations between estimated cumulative PCB exposure and mortality were examined using Poisson regression modeling (Frome, 1983) available in the GENMOD procedure in SAS (version 9.2, SAS Institute Inc., Cary, NC). Regression models were performed among long-term workers for prostate, uterine, ovarian, and stomach cancers and multiple myeloma because a previous analysis of the Massachusetts and New York subcohorts found an exposure-response effect for these outcomes (Prince et al, 2006). Effect modification was evaluated for gender, race, and plant using the likelihood ratio test for interaction and confounding by gender, race, age, birth year, and plant, was evaluated using a change-in-estimate criterion of 10%. In these models, estimated cumulative exposure was treated as either a categorical variable (5 categories based on quintiles of the exposure distribution among cases) or a continuous variable. Several exposure transformations (identity, natural logarithm, and square root) were evaluated and the best-fitting model (based on Akaike’s information criterion (AIC) (Akaike, 1979)) was reported. Exposure lag periods of 0, 5,10,15, and 20 years were evaluated and results of the best-fitting lag period (also based on AIC) were reported. Collinearity among model predictors, evaluated for best fitting models using methods for generalized linear models, was not observed (Lesaffre and Marx, 1993).
In the Poisson regression models, age and birth year were centered at the median age at death and birth year among cases, so the model intercept estimates the logarithm of the background rate for an unexposed worker at the median age at death and birth year. For some outcomes, variability in the estimated intercepts was observed with some models estimating implausibly low background rates. For this reason, all model forms were re-evaluated using constrained models in which the intercept was specified to fix the background rate across models.
In supplemental analyses, best fitting models were re-evaluated excluding workers with any time in a job category with potential for trichloroethylene (TCE) exposure (time in “setup and/or operate wet machines” or “setup and/or operate dry machines” at Indiana; in “degreaser” at Massachusetts; or in “degreasers and painters” at New York). Analyses were conducted excluding these solvent-exposed workers because they did not comprise a large enough group to be analyzed separately. Two-sided p < 0.05 was considered statistically significant.
Results
After exclusions (n = 197) for missing data, the final cohort of 24,865 workers contributed 1,019,128 PYAR (Table 2). Through 2008, 8749 cohort members (35%) had died.
Table 2.
Cohort demographics and vital status as of December 31, 2008, NIOSH Capacitor Cohort.
| Characteristic | Entire cohort | Plant |
||
|---|---|---|---|---|
| Indiana (1957–1977) | Massachusetts (1938–1976) |
New York (1946–1977) |
||
| Total workers | 25,062 | 3601 | 12,616 | 8845 |
| Excluded from analysisa | 197 | 21 | 58 | 118 |
| Total analyzed | 24,865 | 3580 | 12,558 | 8727 |
| Vital statusb | ||||
| Dead | 8749 (35%) | 1021 (29%) | 4489 (36%) | 3239 (37%) |
| Alive | 15,356 (62%) | 2479 (69%) | 7591 (60%) | 5286 (61%) |
| Lost to follow-up | 760 (3%) | 80 (2%) | 478 (4%) | 202 (2%) |
| Race, gender | ||||
| Caucasian females | 6719 (27%) | 555 (16%) | 3832 (31%) | 2332 (27%) |
| Females of other races | 275 (1%) | 19 (<1%) | 220 (2%) | 36 (<1%) |
| Race-unknown femalesc | 6083 (24%) | 282 (8%) | 4377 (35%) | 1424 (16%) |
| Caucasian males | 2171 (9%) | 517 (14%) | 787 (6%) | 867 (10%) |
| Males of other races | 42 (<1%) | 11 (<1%) | 25 (<1%) | 6 (<1%) |
| Race-unknown malesc | 9575 (39%) | 2196 (61%) | 3317 (26%) | 4062 (47%) |
| Age at first employment (years) | ||||
| Median (interquartile range) | 22.4 (18.7–29.7) | 24.2 (20.9–31.4) | 19.7 (17.4–27.5) | 24.4 (21.0–31.1) |
| Mean ± standard deviation | 25.6 ± 9.1 | 27.0 ± 8.2 | 24.1 ± 9.7 | 27.1±8.3 |
| Duration of employment (years) | ||||
| Median (interquartile range) | 0.77 (0.18–4.0) | 1.26 (0.29–5.6) | 0.45 (0.11–2.5) | 1.20 (0.32–5.8) |
| Mean ± standard deviation | 3.75 ± 6.33 | 3.93 ± 5.25 | 3.12 ± 6.18 | 4.57 ± 6.83 |
| Estimated cumulative exposure (unit-days)d | ||||
| Unknown | 515 | 22 | 476 | 17 |
| Lowest quartile (0–<10,000) | 7423 (30%) | 1444 (40%) | 3593 (29%) | 2386 (27%) |
| Second quartile (10,000–<60,000) | 7243 (29%) | 1116 (31%) | 3657 (29%) | 2470 (28%) |
| Third quartile (60,000–<400,000) | 6091 (24%) | 878 (25%) | 2850 (23%) | 2363 (27%) |
| Highest quartile (≥400,000) | 3593 (14%) | 120 (3%) | 1982 (16%) | 1491 (17%) |
| Person-years at risk | ||||
| Sum | 1,019,128 | 138,219 | 528,572 | 352,337 |
Missing employment dates (n = 124), missing date of birth (n = 72), missing date of death (n = 1).
For the analysis, workers lost to follow-up were considered alive until the date lost to follow-up.
Race/ethnicity was not available for the majority of cohort members. For the analysis, race was assumed to be Caucasian, based on racial demographics in the geographic areas.
Estimated cumulative exposure could not be calculated for 515 workers with periods of unknown exposure level. Cut-points based on quartiles of estimated cumulative exposure for all decedents.
In the overall cohort, all-cause mortality was similar to the US population (8749 deaths, SMR 0.97, 95% CI 0.95–1.00), but all-cancer mortality was elevated (2602 cancer deaths, SMR 1.05, 95% CI 1.01–1.09). These results, based on the 1940–2008 time period, were similar to results based on the 1960–2008 time period (presented in Table 3). As only 61 deaths occurred before 1960 and 119 category US and state rates are only available from 1960, we present here only results from 1960 on (8688 deaths). For ease of comparison with previous results we present all categories for which 3 or more deaths occurred.
Table 3.
Standardized mortality ratios overall and by duration of employment, NIOSH Capacitor Cohort, 1960–2008.a
| Underlying cause of deathb | Entire cohort (n = 24,865) |
Short-term workersc (n = 7647) |
Long-term workersc (n = 17,218) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OBS | SMRd | 95% CI | OBS | SMR | 95% CI | OBS | SMR | 95% CI | |
| All causes | 8688 | 0.99 | 0.97–1.01 | 2255 | 1.17 | 1.12–1.22 | 6433 | 0.94 | 0.91–0.96 |
| All cancers | 2593 | 1.06 | 1.02–1.10 | 686 | 1.20 | 1.11–1.30 | 1907 | 1.01 | 0.97–1.06 |
| MN of buccal cavity and pharynx | 45 | 1.12 | 0.82–1.50 | 17 | 1.89 | 1.10–3.03 | 28 | 0.90 | 0.60–1.29 |
| MN of tongue | 12 | 1.25 | 0.65–2.18 | 3 | 1.38 | 0.28–4.02 | 9 | 1.21 | 0.55–2.30 |
| MN of other parts of buccal cavity | 15 | 1.36 | 0.76–2.24 | 4 | 1.67 | 0.46–4.28 | 11 | 1.27 | 0.64–2.28 |
| MN of pharynx | 18 | 0.94 | 0.56–1.48 | 10 | 2.31 | 1.11–4.25 | 8 | 0.54 | 0.23–1.06 |
| MN of digestive organs and peritoneum | 603 | 1.09 | 1.00–1.18 | 151 | 1.23 | 1.04–1.44 | 452 | 1.05 | 0.95–1.15 |
| MN of esophagus | 60 | 1.21 | 0.92–1.55 | 10 | 0.90 | 0.43–1.66 | 50 | 1.29 | 0.96–1.71 |
| MN of stomach | 58 | 1.04 | 0.79–1.35 | 12 | 1.01 | 0.52–1.77 | 46 | 1.05 | 0.77–1.40 |
| MN of intestine except rectum | 231 | 1.11 | 0.97–1.27 | 56 | 1.23 | 0.93–1.60 | 175 | 1.08 | 0.92–1.25 |
| MN of rectum | 50 | 1.21 | 0.90–1.59 | 9 | 0.98 | 0.45–1.85 | 41 | 1.27 | 0.91–1.73 |
| MN of biliary passages, liver and gall bladder | 63 | 0.98 | 0.76–1.26 | 19 | 1.29 | 0.78–2.01 | 44 | 0.89 | 0.65–1.20 |
| MN of pancreas | 136 | 1.08 | 0.90–1.28 | 42 | 1.47 | 1.06–1.98 | 94 | 0.96 | 0.78–1.18 |
| MN of peritoneum and other and unspecified of digestive organs | 5 | 0.53 | 0.17–1.24 | 3 | 1.40 | 0.29–4.10 | 2 | 0.27 | 0.03–0.99 |
| MN of respiratory system | 797 | 1.08 | 1.00–1.15 | 230 | 1.35 | 1.19–1.54 | 567 | 0.99 | 0.91–1.08 |
| MN of larynx | 24 | 1.32 | 0.85–1.97 | 8 | 2.04 | 0.88–4.02 | 16 | 1.13 | 0.64–1.83 |
| MN of trachea, bronchus, and lung | 766 | 1.07 | 0.99–1.15 | 221 | 1.34 | 1.17–1.53 | 545 | 0.99 | 0.91–1.07 |
| MN other parts of respiratory system | 7 | 1.52 | 0.61–3.14 | 1 | 0.91 | 0.02–5.09 | 6 | 1.71 | 0.63–3.72 |
| MN of breast | 239 | 1.01 | 0.89–1.15 | 74 | 1.15 | 0.90–1.44 | 165 | 0.96 | 0.82–1.12 |
| MN of female genital organs | 143 | 1.00 | 0.84–1.18 | 39 | 1.03 | 0.73–1.41 | 104 | 0.99 | 0.81–1.20 |
| MN of cervix uteri | 31 | 1.12 | 0.76–1.60 | 10 | 1.29 | 0.62–2.37 | 21 | 1.06 | 0.66–1.62 |
| MN of other and unspecified parts of uterus | 34 | 1.07 | 0.74–1.50 | 6 | 0.75 | 0.28–1.63 | 28 | 1.18 | 0.78–1.70 |
| MN of ovary, fallopian tube, and broad ligament | 69 | 0.88 | 0.68–1.11 | 20 | 0.96 | 0.59–1.48 | 49 | 0.85 | 0.63–1.13 |
| MN of other female genital organs | 9 | 1.75 | 0.80–3.32 | 3 | 2.38 | 0.49–6.95 | 6 | 1.54 | 0.57–3.36 |
| MN of male genital organs | 87 | 0.96 | 0.77–1.18 | 9 | 0.57 | 0.26–1.09 | 78 | 1.04 | 0.82–1.30 |
| MN of prostate | 87 | 0.99 | 0.80–1.23 | 9 | 0.61 | 0.28–1.16 | 78 | 1.07 | 0.85–1.34 |
| MN of urinary organs | 109 | 1.08 | 0.89–1.31 | 31 | 1.43 | 0.97–2.02 | 78 | 0.99 | 0.78–1.24 |
| MN of kidney | 56 | 1.03 | 0.78–1.34 | 14 | 1.12 | 0.61–1.88 | 42 | 1.01 | 0.73–1.36 |
| MN of bladder and other urinary organs | 53 | 1.15 | 0.86–1.50 | 17 | 1.84 | 1.07–2.95 | 36 | 0.97 | 0.68–1.35 |
| MN of other and unspecified sites | 335 | 1.06 | 0.95–1.18 | 80 | 1.06 | 0.84–1.32 | 255 | 1.06 | 0.94–1.20 |
| MN of bone | 6 | 1.12 | 0.41–2.44 | 3 | 2.25 | 0.46–6.59 | 3 | 0.75 | 0.15–2.18 |
| Melanoma | 47 | 1.20 | 0.88–1.60 | 6 | 0.60 | 0.22–1.30 | 41 | 1.41 | 1.01–1.91 |
| Other MN of skin | 9 | 0.93 | 0.42–1.76 | 4 | 1.87 | 0.51–4.78 | 5 | 0.66 | 0.21–1.54 |
| Mesothelioma | 7 | 1.53 | 0.61–3.15 | 1 | 0.94 | 0.02–5.26 | 6 | 1.70 | 0.62–3.71 |
| MN of connective tissue | 14 | 0.94 | 0.51–1.57 | 3 | 0.78 | 0.16–2.27 | 11 | 0.99 | 0.50–1.77 |
| MN of brain and other parts of nervous system | 67 | 1.01 | 0.78–1.28 | 14 | 0.83 | 0.45–1.39 | 53 | 1.07 | 0.80–1.40 |
| MN of thyroid gland | 3 | 0.52 | 0.11–1.53 | 0 | 0.00 | 0.00–2.76 | 3 | 0.68 | 0.14–2.00 |
| MN of other and unspecified sites | 181 | 1.07 | 0.92–1.24 | 49 | 1.27 | 0.94–1.68 | 132 | 1.02 | 0.85–1.21 |
| Neoplasms of lymphatic and hematopoietic tissue | 235 | 1.01 | 0.88–1.15 | 55 | 1.02 | 0.77–1.33 | 180 | 1.01 | 0.86–1.16 |
| Hodgkin disease | 9 | 0.76 | 0.35–1.45 | 4 | 1.28 | 0.35–3.27 | 5 | 0.58 | 0.19–1.35 |
| Non-Hodgkin lymphoma | 93 | 0.99 | 0.80–1.21 | 24 | 1.11 | 0.71–1.65 | 69 | 0.95 | 0.74–1.20 |
| Multiple myeloma | 45 | 1.12 | 0.82–1.50 | 4 | 0.45 | 0.12–1.14 | 41 | 1.31 | 0.94–1.78 |
| Leukemia and aleukemia | 88 | 1.02 | 0.82–1.25 | 23 | 1.15 | 0.73–1.73 | 65 | 0.98 | 0.75–1.24 |
| Benign and unspecified neoplasms | 30 | 0.90 | 0.61–1.29 | 9 | 1.19 | 0.54–2.25 | 21 | 0.82 | 0.51–1.26 |
| Tuberculosis and HIV-related disease | 47 | 1.23 | 0.91–1.64 | 32 | 2.80 | 1.91–3.95 | 15 | 0.56 | 0.32–0.93 |
| Respiratory tuberculosis | 3 | 0.47 | 0.10–1.36 | 1 | 0.81 | 0.02–4.50 | 2 | 0.38 | 0.05–1.39 |
| HIV-related | 44 | 1.45 | 1.06–1.95 | 31 | 3.13 | 2.13–4.44 | 13 | 0.64 | 0.34–1.09 |
| Diseases of the blood and blood forming organs | 32 | 0.89 | 0.61–1.25 | 3 | 0.38 | 0.08–1.12 | 29 | 1.02 | 0.69–1.47 |
| Diabetes mellitus | 206 | 0.91 | 0.79–1.04 | 54 | 1.05 | 0.79–1.36 | 152 | 0.87 | 0.74–1.02 |
| Mental, psychoneurotic, and personality disorders | 113 | 0.84 | 0.69–1.01 | 39 | 1.40 | 0.99–1.91 | 74 | 0.69 | 0.54–0.87 |
| Alcoholism | 19 | 0.60 | 0.36–0.94 | 10 | 1.20 | 0.58–2.21 | 9 | 0.39 | 0.18–0.74 |
| Other mental disorders | 94 | 0.91 | 0.74–1.12 | 29 | 1.48 | 0.99–2.12 | 65 | 0.78 | 0.60–0.99 |
| Dementia and Alzheimer diseasee | 116 | 1.04 | 0.86–1.24 | 32 | 1.57 | 1.08–2.22 | 84 | 0.92 | 0.73–1.14 |
| Disorders of the nervous system and sense organs | 254 | 1.09 | 0.96–1.24 | 60 | 1.22 | 0.93–1.57 | 194 | 1.06 | 0.92–1.22 |
| Multiple sclerosis | 14 | 0.91 | 0.50–1.53 | 3 | 0.68 | 0.14–1.98 | 11 | 1.01 | 0.50–1.81 |
| Other diseases of the nervous system and sense organs | 240 | 1.11 | 0.97–1.26 | 57 | 1.27 | 0.96–1.65 | 183 | 1.07 | 0.92–1.23 |
| Amyotrophic lateral sclerosise | 31 | 1.34 | 0.91–1.90 | 11 | 1.95 | 0.97–3.50 | 20 | 1.14 | 0.70–1.76 |
| Parkinson diseasee | 45 | 1.14 | 0.83–1.52 | 3 | 0.42 | 0.09–1.22 | 42 | 1.30 | 0.94–1.76 |
| Diseases of the heart | 2520 | 0.96 | 0.92–1.00 | 593 | 1.14 | 1.05–1.23 | 1927 | 0.92 | 0.87–0.96 |
| Rheumatic heart disease, including fever | 28 | 0.71 | 0.47–1.03 | 6 | 0.71 | 0.26–1.55 | 22 | 0.71 | 0.45–1.08 |
| Hypertension with heart disease | 60 | 0.71 | 0.54–0.92 | 19 | 1.07 | 0.65–1.68 | 41 | 0.62 | 0.44–0.84 |
| Ischemic heart disease | 1984 | 0.96 | 0.92–1.01 | 454 | 1.12 | 1.02–1.23 | 1530 | 0.92 | 0.88–0.97 |
| Chronic disease of endocardium | 44 | 0.87 | 0.63–1.17 | 7 | 0.69 | 0.28–1.42 | 37 | 0.91 | 0.64–1.26 |
| Cardiomyopathy | 86 | 1.08 | 0.86–1.33 | 27 | 1.50 | 0.99–2.18 | 59 | 0.96 | 0.73–1.23 |
| Conduction disorder | 148 | 1.08 | 0.91–1.27 | 42 | 1.46 | 1.05–1.98 | 106 | 0.98 | 0.80–1.19 |
| Other diseases of the heart | 170 | 0.98 | 0.84–1.14 | 38 | 1.12 | 0.79–1.54 | 132 | 0.95 | 0.79–1.12 |
| Other diseases of the circulatory system | 734 | 0.96 | 0.89–1.03 | 136 | 0.89 | 0.74–1.05 | 598 | 0.98 | 0.90–1.06 |
| Cerebrovascular disease | 478 | 0.94 | 0.85–1.02 | 89 | 0.88 | 0.71–1.09 | 389 | 0.95 | 0.86–1.05 |
| Hypertension without heart disease | 43 | 0.96 | 0.69–1.29 | 3 | 0.32 | 0.07–0.95 | 40 | 1.12 | 0.80–1.53 |
| Diseases of the arteries, veins and lymphatic vessel | 213 | 1.02 | 0.88–1.16 | 44 | 1.02 | 0.74–1.37 | 169 | 1.02 | 0.87–1.18 |
| Diseases of the respiratory system | 725 | 0.98 | 0.91–1.06 | 196 | 1.29 | 1.11–1.48 | 529 | 0.91 | 0.83–0.99 |
| Influenza | 3 | 0.59 | 0.12–1.73 | 0 | 0.00 | 0.00–3.67 | 3 | 0.74 | 0.15–2.15 |
| Pneumonia (except newborn) | 213 | 1.07 | 0.93–1.23 | 51 | 1.32 | 0.98–1.74 | 162 | 1.01 | 0.86–1.18 |
| Chronic obstructive pulmonary disease | 400 | 1.00 | 0.91–1.10 | 118 | 1.40 | 1.16–1.68 | 282 | 0.90 | 0.79–1.01 |
| Asthma | 16 | 0.97 | 0.55–1.57 | 6 | 1.47 | 0.54–3.21 | 10 | 0.80 | 0.38–1.47 |
| Other respiratory diseases | 89 | 0.81 | 0.65–1.00 | 21 | 0.90 | 0.56–1.38 | 68 | 0.78 | 0.61–0.99 |
| Diseases of the digestive system | 320 | 0.85 | 0.76–0.95 | 96 | 1.11 | 0.90–1.36 | 224 | 0.78 | 0.68–0.89 |
| Diseases of the skin and subcutaneous tissue | 8 | 0.75 | 0.32–1.48 | 0 | 0.00 | 0.00–1.58 | 8 | 0.96 | 0.41–1.89 |
| Diseases of the musculoskeletal system and connective system | 33 | 0.85 | 0.58–1.19 | 10 | 1.09 | 0.52–2.00 | 23 | 0.78 | 0.49–1.16 |
| Diseases of the genito-urinary system | 136 | 0.89 | 0.75–1.05 | 34 | 1.08 | 0.74–1.50 | 102 | 0.84 | 0.69–1.02 |
| Chronic and unspecified nephritis and renal failure and other renal sclerosis | 90 | 1.12 | 0.90–1.38 | 25 | 1.46 | 0.95–2.16 | 65 | 1.03 | 0.80–1.32 |
| Symptoms and ill-defined conditions | 45 | 0.52 | 0.38–0.70 | 16 | 0.79 | 0.45–1.29 | 29 | 0.44 | 0.29–0.63 |
| Transportation injuries | 167 | 0.78 | 0.66–0.91 | 60 | 0.94 | 0.72–1.21 | 107 | 0.71 | 0.58–0.86 |
| Falls | 39 | 0.70 | 0.50–0.96 | 2 | 0.17 | 0.02–0.61 | 37 | 0.85 | 0.60–1.17 |
| Other injury | 120 | 0.79 | 0.66–0.95 | 49 | 1.19 | 0.88–1.57 | 71 | 0.65 | 0.50–0.81 |
| Intentional self-harm | 110 | 0.73 | 0.60–0.88 | 39 | 0.92 | 0.66–1.26 | 71 | 0.66 | 0.51–0.83 |
| Assault and homicide | 23 | 0.44 | 0.28–0.67 | 13 | 0.82 | 0.44–1.40 | 10 | 0.28 | 0.13–0.51 |
| Other causes | 207 | 0.91 | 0.79–1.04 | 64 | 1.17 | 0.90–1.49 | 143 | 0.82 | 0.69–0.97 |
| Unknown cause | 226 | 64 | 162 | ||||||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OBS, observed deaths; MN, malignant neoplasm; SMR, standardized mortality ratio.
All results exclude workers who died (n = 61) or were lost to follow-up (n = 324) prior to the rate file begin date (1 January 1960).
International Classification of Disease (ICD) codes were mapped to underlying cause of death categories as described by Robinson et al. (2006) and tabulated on the NIOSH website (http://www.cdc.gov/niosh/ltas/rates.html). All major categories of causes of death are presented. Some minor categories of causes of death are not presented because there were two or fewer deaths in the category or the category was not of a priori interest and the SMR was not in statistically significant excess.
Short-term workers worked less than 3 months; long-term workers worked 3 months or longer.
The SMR is the ratio of observed to expected (based on US underlying cause of death rates, 1960–2007) deaths; 95% CI was estimated based on the Poisson distribution.
Neurodegenerative outcomes based on US underlying cause of death rates, 1960–2007. Dementia and Alzheimer disease (excluding arteriosclerotic dementia) includes ICD codes 304–305 (revision 7), 290.0–290.1 (revision 8), 290.0–290.3 and 331.0 (revision 9), and G30 (revision 10); amyotrophic lateral sclerosis includes ICD codes 306 (revision 7), 348.0 (revision 8), 335.2 (revision 9), and F01 (revision 10); and Parkinson disease includes ICD codes 350 (revision 7), 342 (revision 8), 332 (revision 9), and G20–G21 (revision 10).
Compared to the US population, mortality was not significantly elevated in the overall cohort for any individual outcome of a priori interest, although statistically significant increases were observed for all cancers combined, all cancers of the digestive organs and peritoneum combined, all respiratory cancers combined, and HIV-related disease (Table 3). Furthermore, significant deficits were observed among all workers for alcoholism, heart diseases, digestive system diseases, symptoms and ill-defined conditions, injuries, suicide and homicide. Similar results were observed (results not shown) when all causes listed on the death certificate were considered.
The median duration of employment in the cohort was less than one year and short-term workers (less than 3 months of employment) comprised nearly one-third of the cohort. Among these short-term workers, all-cause and all-cancer mortality were elevated with excesses for pharyngeal, pancreatic, lung, and bladder cancers, HIV-related disease, dementia and Alzheimer disease, heart disease, and chronic obstructive pulmonary disease (Table 3). Among long-term workers there was a significant increase in melanoma mortality (41 deaths, SMR 1.41, 95% CI 1.01–1.91); elevations were not observed for other outcomes of a priori interest. Mortality among long-term workers was significantly reduced for all causes combined, tuberculosis and HIV-related disease, mental, psychoneurotic, and personality disorders, and respiratory diseases, in addition to those categories with significant decreases in the cohort overall. Additional analyses focused exclusively on the long-term workers.
Table 4 shows mortality for a priori outcomes among long-term workers stratified by plant based on state rates. Mortality was significantly elevated among long-term workers for two outcomes of a priori interest previously observed at the Indiana plant: melanoma (SMR 2.58, 95% CI 1.38–4.42) and cancers of the brain and other parts of the nervous system (SMR 2.06,95% CI 1.20–3.29); two outcomes of a priori interest previously observed at the New York plant: melanoma (SMR 1.71, 95% CI 1.04–2.64) and multiple myeloma (SMR 1.74, 95% CI 1.08–2.66); but for no outcomes at the Massachusetts plant. All-cause and all-cancer mortality were significantly reduced among long-term workers at the Indiana plant, but not among long-term workers at the Massachusetts and New York plants.
Table 4.
Standardized mortality ratios for outcomes of a priori interest among long-term workers (3 months or longer) by plant, NIOSH Capacitor Cohort, 1960–2008.a
| Underlying cause of deathb | Indiana plant (n = 2794) |
Massachusetts plant (n = 7499) |
New York plant (n = 6925) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OBS | SMRc | 95% CI | OBS | SMR | 95% CI | OBS | SMR | 95% CI | |
| All causes | 800 | 0.79 | 0.73–0.84 | 2998 | 1.02 | 0.98–1.05 | 2635 | 0.92 | 0.89–0.96 |
| All cancers | 246 | 0.83 | 0.73–0.94 | 860 | 0.99 | 0.92–1.05 | 801 | 1.02 | 0.95–1.09 |
| MN of stomach | 4 | 0.73 | 0.20–1.87 | 28 | 1.26 | 0.83–1.81 | 14 | 0.59 | 0.32–0.99 |
| MN of intestine except rectum | 21 | 0.85 | 0.53–1.30 | 80 | 1.01 | 0.80–1.25 | 74 | 0.98 | 0.77–1.23 |
| MN of rectum | 2 | 0.43 | 0.05–1.55 | 15 | 0.86 | 0.48–1.41 | 24 | 1.52 | 0.97–2.26 |
| MN of biliary passages, liver and gall bladder | 5 | 0.70 | 0.23–1.63 | 22 | 1.05 | 0.66–1.59 | 17 | 0.77 | 0.45–1.24 |
| MN of breast | 8 | 0.64 | 0.28–1.26 | 99 | 0.91 | 0.74–1.11 | 58 | 0.86 | 0.65–1.11 |
| MN of other and unspecified parts of uterus | 0 | 0 | 0–2.11 | 18 | 1.22 | 0.72–1.93 | 10 | 1.09 | 0.52–2.01 |
| MN of ovary, fallopian tube, and broad ligament | 1 | 0.24 | 0.01–1.34 | 34 | 0.99 | 0.69–1.39 | 14 | 0.65 | 0.36–1.09 |
| MN of prostate | 9 | 0.62 | 0.28–1.18 | 25 | 1.08 | 0.70–1.59 | 44 | 1.25 | 0.91–1.68 |
| Melanoma | 13 | 2.58 | 1.38–4.42 | 8 | 0.63 | 0.27–1.24 | 20 | 1.71 | 1.04–2.64 |
| MN of brain and other parts of nervous system | 17 | 2.06 | 1.20–3.29 | 18 | 0.93 | 0.55–1.47 | 18 | 0.98 | 0.58–1.55 |
| MN of thyroid | 1 | 1.71 | 0.04–9.52 | 1 | 0.49 | 0.01–2.72 | 1 | 0.49 | 0.01–2.74 |
| Non-Hodgkin lymphoma | 11 | 0.94 | 0.47–1.67 | 39 | 1.23 | 0.87–1.68 | 19 | 0.60 | 0.36–0.94 |
| Multiple myeloma | 2 | 0.40 | 0.05–1.46 | 18 | 1.32 | 0.78–2.09 | 21 | 1.74 | 1.08–2.66 |
| Dementia and Alzheimer diseased | 2 | 0.24 | 0.03–0.85 | 46 | 0.99 | 0.73–1.32 | 36 | 0.98 | 0.69–1.36 |
| Amyotrophic lateral sclerosisd | 2 | 0.72 | 0.09–2.59 | 8 | 1.07 | 0.46–2.11 | 10 | 1.37 | 0.66–2.53 |
| Parkinson diseased | 5 | 1.14 | 0.37–2.67 | 16 | 1.17 | 0.67–1.89 | 21 | 1.48 | 0.91–2.26 |
Abbreviations: CI, confidence interval; OBS, observed deaths; MN, malignant neoplasm; SMR, standardized mortality ratio.
All results are for long-term workers (employed for 3 months or longer) and exclude workers who died (n = 58) or were lost to follow-up (n = 155) prior to the rate file begin date (1 January 1960).
For cause of death outcomes of a priori interest. Complete results available from the first author.
The SMR is the ratio of observed to expected deaths (based on state-specific underlying cause of death rates, 1960–2007); 95% CI was estimated based on the Poisson distribution.
Neurodegenerative outcomes based on US underlying cause of death rates, 1960–2007.
Table 5 shows mortality for a priori outcomes among long-term workers stratified by sex. Compared to the US population, mortality was significantly elevated for long-term female workers for all cancers (SMR 1.06, 95% CI 1.00–1.13), intestinal cancer (SMR 1.23, 95% CI 1.00–1.49), and ALS (SMR 1.90, 95% CI 1.09–3.09). Among long-term male workers, all-cause mortality was significantly reduced (SMR 0.91, 95% CI 0.88–0.94) but melanoma mortality was significantly elevated (SMR 1.59,95% CI 1.06–2.28). In both sexes, multiple myeloma mortality was elevated, but neither elevation was statistically significant. While long-term male and female workers had comparable mean and median cumulative exposures in the Massachusetts subcohort, long-term female workers in the Indiana and New York subcohorts had lower mean and median cumulative exposures compared to male workers (results not shown).
Table 5.
Standardized mortality ratios for outcomes of a priori interest among long-term workers (3 months or longer) by sex, NIOSH Capacitor Cohort, 1960–2008.a
| Underlying cause of deathb | Women (n = 8642) |
Men (n = 8576) |
||||
|---|---|---|---|---|---|---|
| OBS | SMRc | 95% CI | OBS | SMR | 95% CI | |
| All causes | 3155 | 0.97 | 0.94–1.01 | 3278 | 0.91 | 0.88–0.94 |
| All cancers | 983 | 1.06 | 1.00–1.13 | 924 | 0.97 | 0.90–1.03 |
| MN of stomach | 13 | 0.75 | 0.40–1.28 | 33 | 1.25 | 0.86–1.75 |
| MN of intestine except rectum | 102 | 1.23 | 1.00–1.49 | 73 | 0.92 | 0.72–1.16 |
| MN of rectum | 22 | 1.52 | 0.95–2.30 | 19 | 1.07 | 0.65–1.67 |
| MN of biliary passages, liver and gall bladder | 25 | 1.12 | 0.73–1.65 | 19 | 0.70 | 0.42–1.10 |
| MN of breast | 163 | 0.96 | 0.82–1.12 | 2 | 1.68 | 0.20–6.07 |
| MN of other and unspecified parts of uterus | 28 | 1.18 | 0.78–1.70 | |||
| MN of ovary, fallopian tube, and broad ligaments | 49 | 0.85 | 0.63–1.13 | |||
| MN of prostate | 78 | 1.07 | 0.85–1.34 | |||
| Melanoma | 12 | 1.11 | 0.57–1.94 | 29 | 1.59 | 1.06–2.28 |
| MN of brain and other parts of nervous system | 19 | 0.85 | 0.51–1.33 | 34 | 1.25 | 0.87–1.75 |
| MN of thyroid | 2 | 0.79 | 0.10–2.84 | 1 | 0.54 | 0.01–3.04 |
| Non-Hodgkin lymphoma | 37 | 1.07 | 0.75–1.48 | 32 | 0.84 | 0.58–1.19 |
| Multiple myeloma | 20 | 1.30 | 0.80–2.01 | 21 | 1.32 | 0.82–2.01 |
| Dementia and Alzheimer diseased | 62 | 0.96 | 0.73–1.23 | 22 | 0.82 | 0.51–1.24 |
| Amyotrophic lateral sclerosisd | 16 | 1.90 | 1.09–3.09 | 4 | 0.44 | 0.12–1.12 |
| Parkinson diseased | 16 | 1.12 | 0.64–1.81 | 26 | 1.45 | 0.94–2.12 |
Abbreviations: CI, confidence interval; OBS, observed deaths; MN, malignant neoplasm; SMR, standardized mortality ratio.
All results are for long-term workers (employed for 3 months or longer) and exclude workers who died (n = 58) or were lost to follow-up (n = 155) prior to the rate file begin date (1 January 1960).
For cause of death outcomes of a priori interest. Complete results available from the first author.
The SMR is the ratio of observed to expected (using United States underlying cause of death rates, 1960–2007) deaths; 95% CI was estimated based on the Poisson distribution.
Neurodegenerative outcomes based on US underlying cause of death rates, 1960–2007.
Among long-term workers, directly standardized mortality rates increased with quartiles of estimated cumulative PCB exposure for stomach and uterine cancer and multiple myeloma; but not for other a priori outcomes evaluated (Table 6). Prostate cancer mortality was elevated in the highest quartile compared to the lowest quartile (SRR 2.11, 95% CI 1.08–4.13). Similar results were observed for cancer outcomes when a 10- or 20-year lag period was applied (results not shown).
Table 6.
Standardized rate ratio analyses among long-term workers (3 months or longer), NIOSH Capacitor Cohort, 1960–2008.
| Underlying cause of deatha | Estimated cumulative PCB exposure (unit-days) |
P valuec | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–<40,000 |
40,000–<150,000 |
150,000–<600,000 |
≥600,000 |
||||||||||
| OBS | SRRb | 95% CI | OBS | SRR | 95% CI | OBS | SRR | 95% CI | OBS | SRR | 95% CI | ||
| All causes | 1600 | 1 | [ref] | 1467 | 1.08 | 1.00–1.17 | 1531 | 1.19 | 1.10–1.28 | 1547 | 1.07 | 0.98–1.17 | 0.60 |
| All cancers | 511 | 1 | [ref] | 462 | 1.14 | 1.00–1.29 | 417 | 1.10 | 0.96–1.25 | 427 | 1.17 | 0.98–1.39 | 0.24 |
| MN of stomach | 4 | 1 | [ref] | 6 | 2.11 | 0.58–7.67 | 15 | 5.14 | 1.66–15.9 | 17 | 8.37 | 2.52–27.8 | <0.001 |
| MN of intestine except rectum | 41 | 1 | [ref] | 48 | 1.42 | 0.92–2.19 | 35 | 1.08 | 0.68–1.72 | 43 | 0.93 | 0.60–1.45 | 0.36 |
| MN of rectum | 10 | 1 | [ref] | 9 | 1.11 | 0.43–2.84 | 6 | 0.76 | 0.26–2.18 | 14 | 1.56 | 0.60–4.02 | 0.50 |
| MN of biliary passages, liver and gall bladder | 11 | 1 | [ref] | 9 | 0.98 | 0.39–2.48 | 6 | 0.76 | 0.27–2.17 | 15 | 1.53 | 0.66–3.58 | 0.27 |
| MN of breast | 41 | 1 | [ref] | 43 | 1.24 | 0.80–1.94 | 29 | 0.87 | 0.53–1.41 | 39 | 0.90 | 0.57–1.42 | 0.31 |
| MN of other and unspecified parts of uterus | 6 | 1 | [ref] | 5 | 1.06 | 0.31–3.60 | 6 | 1.29 | 0.40–4.19 | 9 | 1.35 | 0.46–3.98 | <0.001 |
| MN of ovary, fallopian tube, and broad ligament | 6 | 1 | [ref] | 14 | 3.22 | 1.19–8.69 | 9 | 1.81 | 0.62–5.26 | 12 | 2.31 | 0.83–6.42 | 0.40 |
| MN of prostate | 17 | 1 | [ref] | 21 | 1.73 | 0.90–3.32 | 14 | 1.28 | 0.62–2.64 | 25 | 2.11 | 1.08–4.13 | 0.14 |
| Melanoma | 21 | 1 | [ref] | 5 | 0.34 | 0.13–0.92 | 10 | 0.78 | 0.37–1.68 | 3 | 0.20 | 0.06–0.70 | 0.21 |
| MN of brain and other parts of nervous system | 22 | 1 | [ref] | 9 | 0.53 | 0.24–1.16 | 13 | 0.82 | 0.41–1.64 | 7 | 0.58 | 0.21–1.56 | 0.67 |
| Non-Hodgkin lymphoma | 17 | 1 | [ref] | 23 | 1.84 | 0.95–3.55 | 14 | 1.23 | 0.58–2.65 | 12 | 1.03 | 0.44–2.45 | 0.78 |
| Multiple myeloma | 9 | 1 | [ref] | 9 | 1.02 | 0.39–2.65 | 9 | 1.12 | 0.43–2.92 | 12 | 1.31 | 0.52–3.32 | <0.001 |
| Dementia and Alzheimer disease | 20 | 1 | [ref] | 10 | 0.54 | 0.25–1.17 | 16 | 0.77 | 0.39–1.51 | 35 | 1.29 | 0.73–2.29 | 0.13 |
| Amyotrophic lateral sclerosis | 6 | 1 | [ref] | 2 | 0.38 | 0.08–1.93 | 5 | 0.95 | 0.28–3.18 | 7 | 1.44 | 0.41–5.03 | 0.22 |
| Parkinson disease | 9 | 1 | [ref] | 12 | 1.68 | 0.68–4.11 | 7 | 0.89 | 0.32–2.47 | 11 | 1.19 | 0.47–2.98 | 0.97 |
Abbreviations: CI, confidence interval; MN, malignant neoplasm; OBS, observed deaths; SRR, standardized rate ratio.
For cause of death outcomes of interest a priori (thyroid cancer was omitted due to small numbers).
SRR, comparing mortality rates in higher quartiles with rate in the lowest quartile, obtained by direct standardization. Quartiles based on cumulative PCB exposure among decedents with 90 days or more of employment.
P value for test of linear trend in the standardized rates.
Poisson regression models are summarized in Table 7. For the outcomes evaluated (prostate, uterine, ovarian, and stomach cancers and multiple myeloma), the model with the best-fitting lag period was reported although model fit did not vary much across lag periods (results not shown); plant was neither an effect modifier nor a confounder; age and birth year were positive confounders; and all but two cases were among Caucasians so confounding from race was not evaluated. Prostate cancer mortality was significantly associated with estimated 20-year lagged (and log transformed) cumulative PCB exposure among long-term male workers (adjusted rate ratio (RR) 2.22 at 1000 unit-years, 95% CI 1.06–4.80); however, the RR was slightly reduced in the constrained intercept model (RR 1.73 at 1000 unit-years, 95% CI 1.08–2.74). Furthermore, prostate cancer mortality was significantly elevated in the highest exposure quintile relative to the lowest quintile.
Table 7.
Poisson regression models among long-term workers (3 months or longer) for selected underlying cause of death outcomes, NIOSH Capacitor Cohort.
| Cause of death | Rate ratio (95% confidence interval)a |
||||
|---|---|---|---|---|---|
| Continuous modelsb |
Categorical modelsc |
||||
| Unconstrainedd | Constrainedd | Estimated cumulative PCB exposure (unit-years) |
Unconstrainedd | Constrainedd | |
| Prostate cancer | 2.22 (1.06–4.80) | 1.73 (1.08–2.74) | 0 to <77 | 1 [reference] | 1 [reference] |
| 77 to <240 | 1.21 (0.58–2.49) | 1.20 (0.61–2.22) | |||
| 240 to <990 | 1.27 (0.63–2.58) | 1.27 (0.67–2.27) | |||
| 990 to <3200 | 1.52 (0.73–3.14) | 1.51 (0.78–2.75) | |||
| ≥3200 | 2.30 (1.14–4.67) | 2.28 (1.24–4.00) | |||
| Uterine cancer | 2.88 (0.62–15.2) | 1.70 (0.71–3.95) | 0 to <110 | 1 [reference] | 1 [reference] |
| 110 to <380 | 1.05 (0.30–3.51) | 1.08 (0.34–2.93) | |||
| 380 to <880 | 1.68 (0.48–5.62) | 1.72 (0.55–4.59) | |||
| 880 to <2500 | 1.28 (0.36–4.44) | 1.32 (0.41–3.59) | |||
| ≥2500 | 1.30 (0.35–4.67) | 1.34 (0.40–3.80) | |||
| Ovarian cancer | 1.07 (0.96–1.15) | 1.08 (0.98–1.16) | 0 to <120 | 1 [reference] | 1 [reference] |
| 120 to <250 | 3.29 (1.26–9.08) | 3.47 (1.46–7.89) | |||
| 250 to <870 | 1.52 (0.54–4.36) | 1.61 (0.64–3.75) | |||
| 870 to <3200 | 1.49 (0.52–4.32) | 1.57 (0.61–3.74) | |||
| ≥3200 | 2.57 (0.89–7.60) | 2.71 (1.03–6.58) | |||
| Stomach cancer | 4.79 (1.83–13.3) | 1.99 (1.05–3.71) | 0 to <110 | 1 [reference] | 1 [reference] |
| 110 to <650 | 1.48 (0.54–4.03) | 0.95 (0.39–2.09) | |||
| 650 to <1000 | 7.07 (2.66–19.0) | 4.43 (1.91–9.41) | |||
| 1000 to <2100 | 5.13 (1.98–13.6) | 3.20 (1.43–6.67) | |||
| ≥2100 | 3.04 (1.11–8.42) | 1.88 (0.80–4.07) | |||
| Multiple myeloma | 1.09 (0.99–1.17) | 1.09 (0.99–1.17) | 0 to <54 | 1 [reference] | 1 [reference] |
| 54 to <170 | 1.19 (0.42–3.45) | 1.28 (0.49–3.11) | |||
| 170 to <980 | 0.78 (0.28–2.26) | 0.84 (0.33–2.02) | |||
| 980 to <3300 | 1.27 (0.46–3.66) | 1.37 (0.54–3.27) | |||
| ≥3300 | 1.46 (0.48–4.47) | 1.59 (0.57–3.96) | |||
Rate ratios are adjusted for age and birth year; rate ratios for stomach cancer and multiple myeloma are additionally adjusted for gender. All models incorporate the best-fitting (based on model AIC) exposure lag period: 20 years for prostate, 10 years for uterine, 5 years for ovarian, 15 years for stomach, and 10 years for multiple myeloma.
For continuous model, the rate ratio is at 1000 unit-years of lagged exposure and based on the regression model with the best-fitting (based on model AIC) exposure transformation: natural logarithm (prostate, uterine, and stomach cancers) and identity (ovarian cancer and multiple myeloma).
For categorical models, cutpoints were selected based on quintiles of the lagged exposure distribution among cases.
The unexposed baseline rate (or low exposed for the categorical model) was either unconstrained or constrained to be 180 deaths per 100,000 for prostate cancer, 17 deaths per 100,000 for uterine cancer, 31 deaths per 100,000 for ovarian cancer, 30 deaths per 100,000 for stomach cancer, and 19 deaths per 100,000 for multiple myeloma, respectively.
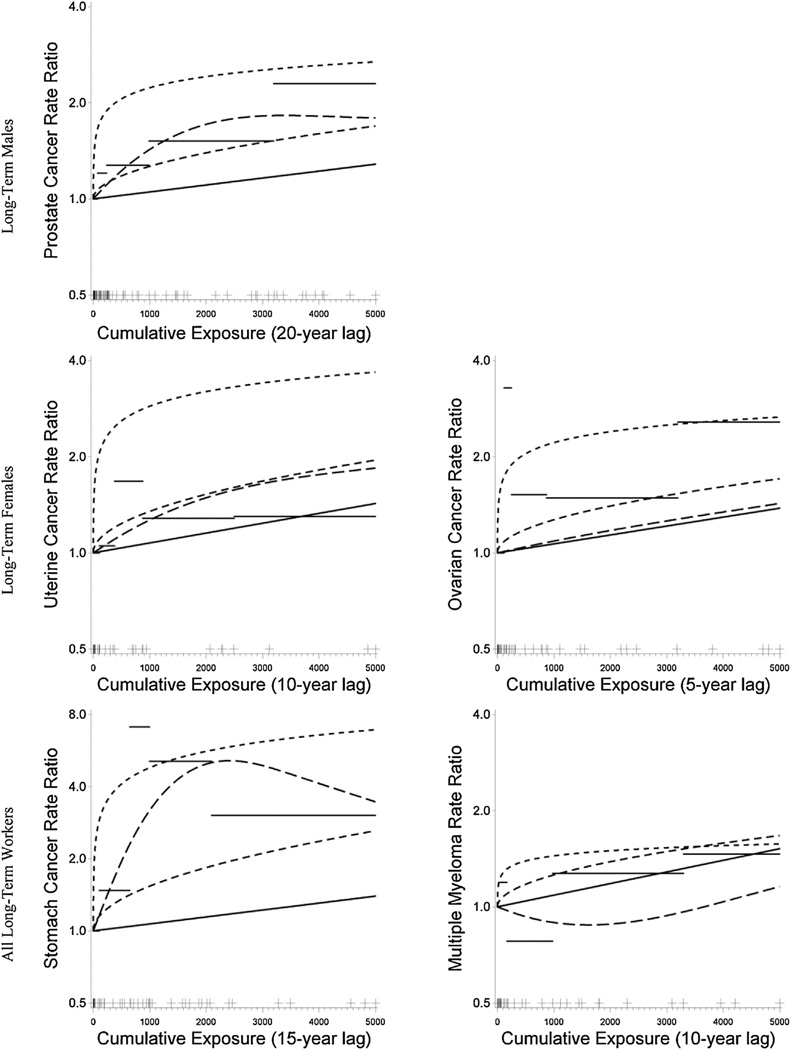
Shorter lag periods provided the best fit for Poisson regression models of uterine (10-year lag period) and ovarian (5-year lag period) cancer mortality among long-term female workers; however, no significant associations were observed with estimated cumulative PCB exposure in any Poisson model evaluated for uterine or ovarian cancer. Stomach cancer mortality among long-term workers was significantly associated with estimated 15-year lagged (and log transformed) cumulative PCB exposure (RR 4.79 at 1000 unit-years, 95% CI 1.83–13.3); however, the rate ratio was diminished, but remained statistically significant in the constrained intercept model. A 10-year exposure lag period was best fitting for Poisson models of multiple myeloma mortality; however, multiple myeloma mortality was not significantly associated with estimated cumulative PCB exposure in any model evaluated. Estimated rate ratios are graphically displayed in Fig. 1 along with other model forms evaluated.
Fig. 1.
Poisson regression models with lagged cumulative exposure (unit-years) among long-term workers. Rate ratios are adjusted for age and year of birth for prostate, uterine, and ovarian cancer, and for gender, age, and year of birth for stomach cancer and multiple myeloma. Cumulative exposure for the cases, indicated by the cross-hatches on the horizontal axis, was truncated at 5000 unit-years for 7 prostate, 3 uterine, 5 ovarian, 3 stomach, and 3 multiple myeloma cases. Horizontal lines indicate categorical treatment of cumulative exposure; curves indicate identity (solid), natural log (short-dash), square root (medium-dash), and restricted cubic spline (long-dash) transformations.
Similar results were obtained for best-fitting models for prostate, uterine, and ovarian cancer and multiple myeloma (data not shown) when workers with any time in jobs with potential TCE exposure were excluded; however, for stomach cancer, the RR associated with a 1000 unit-year increase (based on the natural log transformation) decreased to 3.49 (95% CI 1.21–10.8) when workers potentially exposed to TCE were excluded.
Discussion
This update of our capacitor-manufacturing worker study, combining three subcohorts previously analyzed separately, adding ten years of vital status data, and focusing on long-term workers, confirmed and strengthened previous findings. Mortality was elevated for some outcomes of a priori interest among subgroups of long-term workers: all cancer, intestinal cancer and amyotrophic lateral sclerosis (women); melanoma (men); melanoma and brain and nervous system cancer (Indiana plant); and melanoma and multiple myeloma (New York plant). Internal comparisons showed increasing rates of stomach and uterine cancer and multiple myeloma mortality with increasing estimated cumulative PCB exposure. Poisson regression modeling showed significant associations with estimated cumulative PCB exposure for prostate and stomach cancer mortality.
For other outcomes of a priori interest – rectal, liver, ovarian, breast, and thyroid cancer, non-Hodgkin lymphoma, Alzheimer disease, and Parkinson disease – neither elevated mortality nor positive associations with PCB exposure were observed. Studies of mortality in cohorts occupationally exposed to PCBs have presented inconsistent findings. Nine cohorts of electrical capacitor and transformer manufacturers have been studied to date (see supplemental table to Ruder et al., 2006). In some cases SMRs were elevated for one sex but not the other. Excess deaths from particular cancers or other diseases have been reported, but there has not been consistency from cohort to cohort, or even within cohort across studies. These inconsistencies might be related to differences in the exposure assessments, to differing procedures at different plants and in different eras, to differing properties and modes of action of the PCB congeners, and to these manufacturing facilities having used varying commercial mixtures of PCB congeners (AccuStandard Inc., 2012).
In our study, the excess melanoma mortality among Indiana and New York workers, but lack of excess among Massachusetts workers, could be due to lifestyle factors, differences in the PCB mixtures used, occupational exposures other than to PCBs, or interplant differences in work practices (see Table 1 comparing the three plants). The original study of the Indiana plant noted that PCB-exposed workers were at greater risk of skin effects, including chloracne and hyperpigmentation (Sinks et al., 1992). Several recent papers support an association of melanoma and PCB exposure: Behrens and colleagues observed an increased risk of uveal melanoma among men with occupational exposure to transformer or capacitor oils (odds ratio 2.74, 95% CI 1.07–7.02) (Behrens et al., 2010); Gallagher et al. saw a dose-response effect for melanoma risk and plasma levels of PCBs (p for trend <0.001), comparing melanoma patients and controls (Gallagher et al., 2011); and Loomis and colleagues reported increased melanoma risk among electrical utility workers exposed to PCBs (Loomis et al., 1997). A review (which included the original study of the Indiana cohort (Sinks et al., 1992)) of non-solar occupational risk factors for melanoma found increased risks for workers in the electric and electronic industry (Fortes and de Vries, 2008). In 2013 an International Agency for Research on Cancer Working Group concluded that there was sufficient evidence for human carcinogenity of PCBs, based on the analyses of melanoma and PCB exposure (Lauby-Secretan et al., 2013). The deficit of melanoma in the Massachusetts subcohort might be related to its ethnic composition; an estimated 30% are of Portuguese or Cape Verdean ethnicity with darker skin pigmentation, which is a protective factor for melanoma (Fortes and de Vries, 2008).
Breast, ovarian, uterine and prostate cancer are among those cancers associated with hormonal factors, and endocrine disruptors such as polychlorinated biphenyls (National Research Council, 2000) may modify risk. For each of these categories of disease there are additional demographic risk factors which can affect the results, such as shift work, family history, ethnicity, parity and reproductive history (Poole et al, 2011; Silver et al, 2009). In this analysis, which did not include demographic risk factors, we did not see increased risk of breast cancer. However, our nested case-control study of breast cancer, which adjusted for those risk factors, did find increased risk among women of races other than Caucasian (Silver et al., 2009). Compared to the lowest exposure quartile, ovarian cancer mortality was elevated in categories of higher estimated cumulative PCB exposure, but there was no trend of increasing risk with increasing exposure, while we observed increasing standardized rates of uterine cancer mortality across quartiles of estimated cumulative PCB exposure (Table 6). There are recognized reproductive and hormonal risk factors for these cancers but few strong occupational associations (Slack et al., 2012).
The significantly increased risk of prostate cancer in the highest quartile of estimated cumulative PCB exposure (Table 6) and in the Poisson regression analysis (Table 7) support the association of PCB exposure and increased prostate cancer mortality observed in other cohorts (Charles et al., 2003; Pesatori et al., 2013). Our previous analyses showed this association among the more highly exposed Massachusetts and New York workers (Prince et al., 2006), but not the Indiana workers (Ruder et al., 2006).
Stomach cancer mortality was not elevated in the cohort although we observed significant trends with increasing estimated cumulative PCB exposure in SRR and Poisson regression analyses. Poisson regression models of stomach cancer, however, were influenced by widely varying background rates. When the model intercept was constrained to avoid implausibly low background rates, both of these rate ratios were diminished, but remained statistically significant. Park et al. (2012) similarly observed implausibly low model intercepts in some Poisson regression models of the lung cancer SMR in a cohort of workers exposed to cadmium and arsenic; to account for this, they additionally reported rate ratios adjusted to a common unexposed SMR of 0.8. In our study, when workers potentially exposed to TCE were excluded from the analysis, the stomach cancer rate ratio was attenuated (but remained statistically significant), indicating that some of the increased risk was due to confounding by TCE exposure. TCE exposure was associated with stomach cancer in one cohort study of TCE-exposed workers (Anttila et al., 1995) but not in others (Blair et al., 1998; Boice et al., 2006). Our Poisson models would have been more robust if the numbers of deaths had been larger; this limitation could be alleviated for outcomes with long survival using a cancer incidence study.
There were some major differences between male and female workers in our cohort. Generally the men exhibited the “healthy worker effect”, while the women did not (Table 5). It has been noted that working and general populations of women differ in risk factors (Bond et al., 1987) and female and male employees in the same industry may have different tasks even if they have the same job title (Stellman, 1999). The observed mortality differences between male and female workers may be due to differences in work assignments, differences in physiology, and differences in how their bodies reacted to PCBs (Eng et al, 2011; Messing and Mager Stellman, 2006; Silvaggio and Mattison, 1994). Since various PCB congeners have estrogenic, anti-estrogenic, androgenic or anti-androgenic properties (Diamanti-Kandarakis et al., 2009), it follows that female-male hormonal differences might be associated with the activity of PCBs in the body (Meeker and Hauser, 2010; Salehi et al, 2008).
Our a priori causes of interest, based on several case-control studies, included non-Hodgkin lympohoma (Engel et al, 2007; Rothman et al., 1997). However, we did not find an increase in NHL mortality nor an association between NHL and estimated cumulative exposure. The findings of our cohort mortality study differed from those of the case–control studies of NHL incidence. This discrepancy may be due to differences in the study populations and the outcome variable (death vs. diagnosis).
We did not find excesses for other a priori outcomes (rectal cancer, biliary passages, liver, and gall bladder cancer, or thyroid cancer). The cancer incidence study we are conducting on this combined cohort may provide some additional insight, particularly for cancers with high survival rates such as thyroid cancer (National Cancer Institute, 2011).
A strength of this combined cohort mortality update study is the detailed exposure assessment. The data available to construct the job-exposure matrices included individual work histories, detailed job descriptions for hourly jobs, and limited sets of exposure measurements collected at the plants (Hopf et al., 2009b, 2010, 2013). However, as in most records-based retrospective occupational cohort studies, we had no information on risk factors explored in case-control studies, such as family history or genetic susceptibility; lifestyle choices, such as smoking or sun exposure, that could affect mortality; or on previous or subsequent employment.
This last limitation is significant because a majority of cohort members worked a year or less at one of the plants (Table 2). Increased mortality among short-term workers has been reported, particularly for causes of death associated with disorders that might affect employment or with an unhealthy lifestyle. Those who are only briefly in a workplace might leave because of sensitization to work materials, and possibly the beginning of work-related chronic conditions (Langseth and Kjaerheim, 2006), or shorter durations of employment might be associated with more pre-employment hospitalizations for alcohol use, accidents, and the effects of violence (Kolstad and Olsen, 1999). We have no lifestyle or hospitalization information for our cohort. However, our analyses showed different mortality profiles for short-term and long-term workers, as have been found in other studies (Boffetta et al., 1998; Kolstad and Olsen, 1999). Significant excess mortality from all cancers, digestive and respiratory cancers, and HIV-related diseases were seen among the third of the cohort that worked fewer than 3 months. Among long-term workers only the excess of melanoma deaths was statistically significant. These differences could be due to lifestyle differences as well as differences in occupational exposure or physiological reactions to that exposure.
In conclusion, we found evidence of associations between employment in capacitor manufacturing and increased total cancer and intestinal cancer mortality among female long-term workers and excess melanoma mortality for male long-term workers. The associations in our life table analyses convey moderate increased risk. We did not observe increased risk for several a priori causes of death for which case-control studies observed increased risk. The internal comparisons show increasing risk with estimated cumulative exposure for uterine, prostate and stomach cancer, and multiple myeloma.
Thirty-five years after PCB use was banned, the former workers in our cohort continue to be exposed internally to PCBs. Occupational and environmental exposure also continues for those who repair, maintain, or remove capacitors and transformers containing PCBs and for the general public, emphasizing the continued importance of understanding health risks associated with PCB exposures.
Acknowledgments
Thanks to reviewers, for their valuable comments. Thanks to Christine M Gersic and Patricia A Laber for assistance in data preparation.
This study was entirely funded by National Institute for Occupational Safety and Health base operating funds.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Conflict of interest statement
The authors (all current or former NIOSH employees) state they have no conflict of interest or competing financial interest.
References
- AccuStandard Inc. Similar But Different - Reference Standards of Aroclor Mixtures (by GC Analysis) 2012 [Google Scholar]
- Akaike H. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika. 1979;66(2):237–242. [Google Scholar]
- Anttila A, Pukkala E, Sallmen M, Hernberg S, Hemminki K. Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. J. Occup. Environ. Med. 1995;37(7):797–806. doi: 10.1097/00043764-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Behrens T, Kaerlev L, Cree I, Lutz JM, Afonso N, Eriksson M, Guenel P, Merletti F, Morales-Suarez-Varela M, Stengrevics A, et al. Hormonal exposures and the risk of uveal melanoma. Cancer Causes Control. 2010;21(10):1625–1634. doi: 10.1007/s10552-010-9591-9. [DOI] [PubMed] [Google Scholar]
- Beyer A, Biziuk M. Environmental fate and global distribution of polychlorinated biphenyls. Rev. Environ. Contam. Toxicol. 2009;201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Blair A, Hartge P, Stewart PA, McAdams M, Lubin J. Mortality and cancer incidence of aircraft maintenance workers exposed to trichloroethylene and other organic solvents and chemicals: extended follow up. Occup. Environ. Med. 1998;55(3):161–171. doi: 10.1136/oem.55.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Sali D, Kolstad H, Coggon D, Olsen J, Andersen A, Spence A, Pesatori AC, Lynge E, Frentzel-Beyme R, et al. Mortality of short-term workers in two international cohorts. J. Occup. Environ. Med. 1998;40(12):1120–1126. doi: 10.1097/00043764-199812000-00012. [DOI] [PubMed] [Google Scholar]
- Boice JD, Jr, Marano DE, Cohen SS, Mumma MT, Blot WJ, Brill AB, Fryzek JP, Henderson BE, McLaughlin JK. Mortality among Rocketdyne workers who tested rocket engines, 1948–1999. J. Occup. Environ. Med. 2006;48(10):1070–1092. doi: 10.1097/01.jom.0000240661.33413.b5. [DOI] [PubMed] [Google Scholar]
- Bond GG, McLaren EA, Cartmill JB, Wymer KT, Lipps TE, Cook RR. Mortality among female employees of a chemical company. Am. J. Ind. Med. 1987;12(5):563–578. doi: 10.1002/ajim.4700120510. [DOI] [PubMed] [Google Scholar]
- Brown DP. Mortality of workers exposed to polychlorinated biphenyls—an update. Arch. Environ. Health. 1987;42(6):333–339. doi: 10.1080/00039896.1987.9934355. [DOI] [PubMed] [Google Scholar]
- Brown DP, Jones M. Mortality and industrial hygiene study of workers exposed to polychlorinated biphenyls. Arch. Environ. Health. 1981;36(3):120–129. doi: 10.1080/00039896.1981.10667615. [DOI] [PubMed] [Google Scholar]
- Charles LE, Loomis D, Shy CM, Newman B, Millikan R, Nylander-French LA, Couper D. Electromagnetic fields, polychlorinated biphenyls, and prostate cancer mortality in electric utility workers. Am. J. Epidemiol. 2003;157(8):683–691. doi: 10.1093/aje/kwg044. [DOI] [PubMed] [Google Scholar]
- Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann. Epidemiol. 2002;12(7):462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng A, t Mannetje A, McLean D, Ellison-Loschmann L, Cheng S, Pearce N. Gender differences in occupational exposure patterns. Occup. Environ. Med. 2011;68(12):888–894. doi: 10.1136/oem.2010.064097. [DOI] [PubMed] [Google Scholar]
- Engel LS, Laden F, Andersen A, Strickland PT, Blair A, Needham LL, Barr DB, Wolff MS, Helzlsouer K, Hunter DJ, et al. Polychlorinated biphenyl levels in peripheral blood and non-Hodgkin’s lymphoma: a report from three cohorts. Cancer Res. 2007;67(11):5545–5552. doi: 10.1158/0008-5472.CAN-06-3906. [DOI] [PubMed] [Google Scholar]
- Fishbein L. An overview of the structural features of some mutagenic and teratogenic pesticides. In: Chambers JE, Yarbrough JD, editors. Effects of Chronic Exposures to Pesticides on Animal Systems. New York: Raven Press; 1982. pp. 177–209. [Google Scholar]
- Fortes C, de Vries E. Nonsolar occupational risk factors for cutaneous melanoma. Int. J. Dermatol. 2008;47(4):319–328. doi: 10.1111/j.1365-4632.2008.03653.x. [DOI] [PubMed] [Google Scholar]
- Foster SL, Stewart SL, Trivers KF. Geographic distribution of prostate cancer incidence in the United States; ESRI Health GIS Conference; Washington, DC. 2008. pp. 1–25. http://proceedings.esri.com/library/userconf/health08/docs/monday/geo_dist.pdf. [Google Scholar]
- Frome EL. The analysis of rates using Poisson regression models. Biometrics. 1983;39(3):665–674. [PubMed] [Google Scholar]
- Gallagher RP, Macarthur AC, Lee TK, Weber JP, Leblanc A, Mark Elwood J, Borugian M, Abanto Z, Spinelli JJ. Plasma levels of polychlorinated biphenyls and risk of cutaneous malignant melanoma: a preliminary study. Int. J. Cancer. 2011;128(8):1872–1880. doi: 10.1002/ijc.25503. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Succop P. Background levels of polychlorinated biphenyls in the U.S. population. Sci. Total Environ. 2009a;407:6106–6119. doi: 10.1016/j.scitotenv.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Waters MA. Historical reconstruction of polychlorinated biphenyl (PCB) exposures for workers in a capacitor manufacturing plant. Environ. Sci. Pollut. Res. Int. 2013 doi: 10.1007/s11356-013-1590-4. http://dx.doi.org/10.1007/s11356-013-1590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf NB, Waters MA, Ruder AM. Cumulative exposure estimates for polychlorinated biphenyls using a job-exposure matrix. Chemosphere. 2009b;76(2):185–193. doi: 10.1016/j.chemosphere.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Hopf NB, Waters MA, Ruder AM, Prince MM. Development of a retrospective job exposure matrix for PCB exposed workers in capacitor manufacturing. J. Occup. Health. 2010;52(4):199–208. doi: 10.1539/joh.l9151. [DOI] [PubMed] [Google Scholar]
- Jones M, Phillips R, Sandusky T, Becker J, Fogarty T, Blade L, Marlow D. Plant 1, Hudson Falls, NY, Fort Edward, NY NIOSH, Cincinnati, Ohio. Report nr. 1978;95:12.1–12.22. [Google Scholar]
- Kolstad HA, Olsen J. Why do short term workers have high mortality? Am. J. Epidemiol. 1999;149(4):347–352. doi: 10.1093/oxfordjournals.aje.a009819. [DOI] [PubMed] [Google Scholar]
- Langseth H, Kjaerheim K. Mortality from non-malignant diseases in a cohort of female pulp and paper workers in Norway. Occup. Environ. Med. 2006;63(11):741–745. doi: 10.1136/oem.2005.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, et al. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013 doi: 10.1016/S1470-2045(13)70104-9. http://dx.doi.org/10.1016/S1470-2045(13)70104-9(0) [DOI] [PubMed] [Google Scholar]
- Lesaffre E, Marx BD. Collinearity in generalized linear regression. Commun. Stat. Theory Methods. 1993;22(7):1933–1952. [Google Scholar]
- Loomis D, Browning SR, Schenck AP, Gregory E, Savitz DA. Cancer mortality among electric utility workers exposed to polychlorinated biphenyls. Occup. Environ. Med. 1997;54(10):720–728. doi: 10.1136/oem.54.10.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallin K, McCann K, D’Aloisio A, Freels S, Piorkowski J, Dimos J, Persky V. Cohort mortality study of capacitor manufacturing workers, 1944–2000. J. Occup. Environ. Med. 2004;46(6):565–576. doi: 10.1097/01.jom.0000128156.24767.12. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst. Biol. Reprod. Med. 2010;56(2):122–131. doi: 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- Messing K, Mager Stellman J. Sex, gender and women’s occupational health: the importance of considering mechanism. Environ. Res. 2006;101(2):149–162. doi: 10.1016/j.envres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, et al., editors. National Cancer Institute. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- National Center for Health Statistics. National Death Index. 2007 http://www.cdc.gov/nchs/ndi.htm.
- National Research Council. Hormonally Active Agents in the Environment. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Park RM, Stayner LT, Petersen MR, Finley-Couch M, Hornung R, Rice C. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup. Environ. Med. 2012;69(5):303–309. doi: 10.1136/oemed-2011-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesatori AC, Grillo P, Consonni D, Caironi M, Sampietro G, Olivari L, Ghisleni S, Bertazzi PA. Update of the mortality study of workers exposed to polychlorinated biphenyls (PCBs) in two Italian capacitor manufacturing plants. Med. Lav. 2013;104(2):107–114. [PubMed] [Google Scholar]
- Poole EM, Schernhammer ES, Tworoger SS. Rotating night shift work and risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2011;20(5):934–938. doi: 10.1158/1055-9965.EPI-11-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MM, Ruder AM, Hein MJ, Waters MA, Whelan EA, Nilsen N, Ward EM, Schnorr TM, Laber PA, Davis-King KE. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Environ. Health Perspect. 2006;114(10):1508–1514. doi: 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Ludewig G. Polychlorinated biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst. Reinhalt. Luft. 2011;71(1–2):25–32. [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Ruder AM. Polychlorinated biphenyls. In: Straif K, Cogliano V, editors. Identification of Research Needs to Resolve the Carcinogenicity of High-Priority IARC Carcinogens. Lyon, France: International Agency for Research on Cancer; 2010. pp. 166–182. [Google Scholar]
- Robinson CF, Schnorr TM, Cassinelli RT, II, Calvert GM, Steenland NK, Gersic CM, Schubauer-Berigan MK. Tenth revision U.S. mortality rates for use with the NIOSH Life Table Analysis System. J. Occup. Environ. Med. 2006;48(7):662–667. doi: 10.1097/01.jom.0000229968.74906.8f. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott; 1998. [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia, PA: Lippincott; 2008. [Google Scholar]
- Rothman N, Cantor KP, Blair A, Bush D, Brock JW, Helzlsouer K, Zahm SH, Needham LL, Pearson GR, Hoover RN, et al. A nested case-control study of non-Hodgkin lymphoma and serum organochlorine residues. Lancet. 1997;350(9073):240–244. doi: 10.1016/S0140-6736(97)02088-6. [DOI] [PubMed] [Google Scholar]
- Ruder AM, Hein MJ, Nilsen N, Waters MA, Laber P, Davis-King K, Prince MM, Whelan E. Mortality among workers exposed to polychlorinated biphenyls (PCBs) in an electrical capacitor manufacturing plant in Indiana: an update. Environ. Health Perspect. 2006;114(1):18–23. doi: 10.1289/ehp.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi F, Turner MC, Phillips KP, Wigle DT, Krewski D, Aronson KJ. Review of the etiology of breast cancer with special attention to organochlorines as potential endocrine disruptors. J. Toxicol. Environ. Health B Crit. Rev. 2008;11(3–4):276–300. doi: 10.1080/10937400701875923. [DOI] [PubMed] [Google Scholar]
- Schubauer-Berigan MK, Hein MJ, Raudabaugh WR, Ruder AM, Silver SR, Spaeth S, Steenland K, Petersen MR, Waters KM. Update of the NIOSH Life Table Analysis System: a person-years analysis program for the Windows computing environment. Am. J. Ind. Med. 2011;54(12):915–924. doi: 10.1002/ajim.20999. [DOI] [PubMed] [Google Scholar]
- Silvaggio T, Mattison DR. Setting occupational health standards: toxicokinetic differences among and between men and women. J. Occup. Med. 1994;36(8):849–854. [PubMed] [Google Scholar]
- Silver SR, Whelan EA, Deddens JA, Steenland NK, Hopf NB, Waters MA, Ruder AM, Prince MM, Yong LC, Hein MJ, et al. Occupational exposure to polychlorinated biphenyls and risk of breast cancer. Environ. Health Perspect. 2009;117(2):276–282. doi: 10.1289/ehp.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinks T, Steele G, Smith AB, Watkins K, Shults RA. Mortality among workers exposed to polychlorinated biphenyls. Am. J. Epidemiol. 1992;136(4):389–398. doi: 10.1093/oxfordjournals.aje.a116511. [DOI] [PubMed] [Google Scholar]
- Slack R, Young C, Rushton L. Occupational cancer in Britain. Female cancers: breast, cervix and ovary. Br. J. Cancer. 2012;107(Suppl. 1):S27–S32. doi: 10.1038/bjc.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RT, II, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17(1):8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Steenland K, Nowlin S, Ryan B, Adams S. Use of multiple-cause mortality data in epidemiologic analyses: US rate and proportion files developed by the National Institute for Occupational Safety and Health and the National Cancer Institute. Am. J. Epidemiol. 1992;136(7):855–862. doi: 10.1093/aje/136.7.855. [DOI] [PubMed] [Google Scholar]
- Stellman JM. Women workers: the social construction of a special population. Occup. Med. 1999;14(3):559–580. [PubMed] [Google Scholar]
- Taylor PR, Reilly AA, Stelma JM, Lawrence CE. Estimating serum polychlorinated biphenyl levels in highly exposed workers: an empirical model. J. Toxicol. Environ. Health. 1991;34(4):413–422. doi: 10.1080/15287399109531579. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme. Consultation Meeting on PCB Management and Disposal under the Stockholm Convention on Persistent Organic Pollutants. Switzerland: Geneva; 2004. pp. 1–70. [Google Scholar]
- Yard EE, Terrell ML, Hunt DR, Cameron LL, Small CM, McGeehin MA, Marcus M. Incidence of thyroid disease following exposure to polybrominated biphenyls and polychlorinated biphenyls, Michigan, 1974–2006. Chemosphere. 2011;84(7):863–868. doi: 10.1016/j.chemosphere.2011.06.020. [DOI] [PubMed] [Google Scholar]