Abstract
Research Significance
Toxicological evidence suggests the potential for a wide range of health effects from exposure to carbon nanotubes (CNTs) and carbon nanofibers (CNFs). To date, there has been much focus on the use of direct-reading instruments (DRIs) to assess multiple airborne exposure metrics for potential exposures to CNTs and CNFs due to their ease of use and ability to provide instantaneous results. Still, uncertainty exists in the usefulness and interpretation of the data. To address this gap, air-monitoring was conducted at six sites identified as CNT and CNF manufacturers or users and results were compared with filter-based metrics.
Methods
Particle number, respirable mass, and active surface area concentrations were monitored with a condensation particle counter, a photometer, and a diffusion charger, respectively. The instruments were placed on a mobile cart and used as area monitors in parallel with filter-based elemental carbon (EC) and electron microscopy samples. Repeat samples were collected on consecutive days, when possible, during the same processes. All instruments in this study are portable and routinely used for industrial hygiene sampling.
Results
Differences were not observed among the various sampled processes compared with concurrent indoor or outdoor background samples while examining the different DRI exposure metrics. Such data were also inconsistent with results for filter-based samples collected concurrently at the same sites [Dahm MM, Evans DE, Schubauer-Berigan MK et al. (2012) Occupational exposure assessment in CNT and nanofiber primary and secondary manufacturers. Ann Occup Hyg; 56: 542–56]. Significant variability was seen between these processes as well as the indoor and outdoor backgrounds. However, no clear pattern emerged linking the DRI results to the EC or the microscopy data (CNT and CNF structure counts).
Conclusions
Overall, no consistent trends were seen among similar processes at the various sites. The DRI instruments employed were limited in their usefulness in assessing and quantifying potential exposures at the sampled sites but were helpful for hypothesis generation, control technology evaluations, and other air quality issues. The DRIs employed are nonspecific, aerosol monitors, and, therefore, subject to interferences. As such, it is necessary to collect samples for analysis by more selective, time-integrated, laboratory-based methods to confirm and quantify exposures.
Keywords: carbon nanofibers, carbon nanotubes, exposure assessment, nanomaterials, nanoparticles
INTRODUCTION
Carbon nanotube (CNT) and carbon nanofiber (CNF) production and incorporation into consumer and commercial materials are steadily rising. These materials have moved beyond small, laboratory-scale syntheses to large-scale production and manufacturing methods (Invernizzi, 2011). Applications for CNTs and CNFs include advanced composite materials, batteries, fuel cells, and electronics—such as memory devices and flat panel displays. The global production capacity for multiwalled carbon nanotubes (MWCNTs) has increased from 390 ton in 2008 to nearly 3400 ton in 2010 (Innovative Research and Products Inc., 2011). As this market continues to expand, the potential for worker exposure, especially those involved in the manual handling, transferring, or conveyance of these materials will also increase.
Despite the rapid rise in production and commercialization of CNT/CNF materials and concerns about their possible health hazards, little is known about the potential adverse effects from workplace exposures. Studies suggest that exposures to CNTs/CNFs may result in pulmonary inflammation, granulomas, oxidative stress, and fibrosis (Lam et al., 2006; Mercer et al., 2008; Shvedova et al., 2008; Kisin et al., 2010). Possible extrapulmonary effects under investigation include cardiovascular inflammation (Duffin et al., 2007), immunological effects (Kunzmann et al., 2011), systemic exposure (Riviere et al., 2009; Simeonova et al., 2009), genotoxicity (Sargent et al., 2009), and penetration of the blood–brain barrier (Mercer et al., 2009).
Currently, there is a lack of epidemiologic evidence linking exposure to CNTs/CNFs to human health effects. However, in the wake of the results of animal studies, there has been great emphasis on following the precautionary principal to limit exposure levels as low as possibly achievable (Schulte et al., 2008). Therefore, several international agencies, groups, and authors have made recommendations for characterizing emissions/exposures to various nanomaterials (Brouwer et al., 2009, 2012; Methner et al., 2010a; British Standards Institute, 2010; Institute of Energy and Environmental Technology et al., 2011; Ramachandran et al., 2011). For the most part, these sampling approaches have focused on application of various direct-reading instruments (DRIs), with filter-based chemical analyses or microscopy used as confirmatory samples. Similar DRI methodologies have been used to conduct a majority of the published exposure assessment studies for CNTs/CNFs (Bello et al., 2008, 2009, 2010; Han et al., 2008; Tsai et al., 2009; Evans et al., 2010; Lee et al., 2010; Methner et al., 2010b; Cena and Peters, 2011), as well as for other nanomaterials (Demou et al., 2008, 2009; Peters et al., 2009; Plitzko 2009).
In addition to the benefits of on-site measurement, a major reason for this focus on DRIs has been the fact that the current toxicological and epidemiological research has not determined the most relevant dose metric(s) to monitor exposures to CNTs/CNFs. It has been proposed that at least particle number, surface area, and mass be simultaneously monitored when attempting to assess potential nanoparticle/nanomaterial exposures (Maynard and Kuempel, 2005; Oberdörster et al., 2005; Heitbrink et al., 2009; Ramachandran et al., 2011).
In an effort to understand the interrelationship between these metrics and provide insights into respiratory deposition and transport through the workplace, particle size distributions are desirable (Heitbrink et al., 2009). Current methods and equipment most capable to size particles in real time are relatively large and expensive, and they can only practically be used as area monitors, such as a scanning mobility particle sizer (SMPS) or electrical low-pressure impactor (ELPI). The SMPS or ELPI can provide valuable information on the nature and sources of many types of aerosols in the workplace, in ways that filter-based samples cannot, but they are not specific to any particular particle type. The portable, empirical instruments more commonly used by industrial hygienists provide more limited data, but at lower cost, and offer greater mobility. One major advantage for all DRIs is their ability to provide nearly instantaneous results, allowing investigators to make sampling/exposure mitigation decisions in the field. DRIs can be useful in determining how exposures may be occurring in the workplace, a crucial prerequisite to controlling them (Evans et al., 2010).
A combination of portable DRIs and filter-based sampling was employed to assess exposures at primary (producers) and secondary (downstream users) CNT/CNF manufacturers. All instruments used at these site visits have been routinely employed for industrial hygiene sampling. This manuscript reports the DRI results, while a previous article reports the filter-based sample results co-collected at the same facilities (Dahm et al., 2012). The primary objective of this study was to identify, characterize, and differentiate exposure points by job task at various CNT/CNF primary and secondary manufacturers within the USA using various metrics (particle number, respirable mass, and active surface area concentrations). Secondary goals for this study were to examine data analysis methods for the DRIs in addition to determining the utility of the data.
METHODS
Plant and process descriptions
A total of six site visits were conducted between May and September 2010 at primary and secondary manufacturers of CNTs/CNFs. Of the six companies, three were primary manufacturers of MWCNTs, double-walled CNTs (DWCNTs), or single-walled CNTs (SWCNTs) (coded as Sites A–C). The remaining three sites were secondary manufacturers of MWCNTs or CNFs (coded as Sites D–F). Facility and process descriptions are briefly discussed below, while specific uses of engineering controls and personal protective equipment are discussed in detail elsewhere (Dahm et al., 2012).
Site descriptions
Site A was a primary manufacturing facility specializing in the production of MWCNTs and DWCNTs. Two processes were primarily sampled: the production of MWCNTs and subsequent harvesting of two batches of MWCNTs and DWCNTs. During a typical day, two batches of MWCNTs were produced and harvested. The production process for MWCNTs was enclosed and process gases exhausted out of the building. No forms of exposure controls were used during harvesting.
Site B was a primary SWCNT manufacturer. SWCNT production was run once per day and yielded ~5 g of material. After production, the raw material was harvested and weighed in a chemical fume hood. The entire production process was under vacuum with the product being harvested within a baghouse. The reactor was cleaned using a HEPA vacuum cleaner in combination with wet wiping. Replicate samples were completed on two separate days for the same processes.
Site C was a primary and secondary manufacturer of MWCNTs. Multiple days of sampling were conducted, which focused on the production of MWCNTs within a reactor, under vacuum, and the harvesting of two batches of MWCNTs in a custom-made glove box. Further sampling focused on the sonication of a 1-l solution of MWCNTs housed within an unventilated enclosure and the sieving of 5 g of MWCNTs in a chemical fume hood concurrent to spray coating of copper plates with an MWCNT aqueous solution.
Site D was a secondary manufacturer of MWCNTs for the development of semiconductor devices. MWCNT in powder and aqueous form was handled within a cleanroom. Two processes were sampled: sonication and the weighing of 100 g of powdered MWCNTs. A single employee oversaw the sonication of a 5-l MWCNT aqueous solution while the sonicator was housed within a HEPA-ventilated chemical fume hood. Another employee weighed 100 g of dry powdered MWCNTs inside a HEPA-ventilated glove box.
Site E was a secondary manufacturer of MWCNTs, which mixed MWCNTs with different resin formulations. Sampling was focused on several processes which included the weighing and extrusion of 1 kg of MWCNTs mixed with a polyvinyl chloride-based resin, the weighing of 15 g of MWCNTs and use of a batch mixer, and the milling of CNT composite materials. Powdered MWCNTs were weighed and added to the extruder, while another employee weighed and mixed 15 g of MWCNTs with various resins. A HEPA vacuum was used intermittently for clean-up. The final milling operation was conducted using an automated milling machine with exhaust ventilation.
Site F was a secondary manufacturer of MWCNTs and CNFs. The processes sampled at Site F included weighing, mixing, and sonicating of several grams of MWCNTs and CNFs within chemical fume hoods (hoods were not always in operation during handling of nanomaterials) and on an open table, as well as the transferring of ~1 kg of CNFs within a custom unventilated glove box. All sampled processes were conducted within a cleanroom.
Direct-reading instruments
Three DRIs were employed: a condensation particle counter (CPC), photometer, and diffusion charger (DC). All DRIs were placed on carts for enhanced mobility and used as area samplers. The DRIs were arranged with all sampling inlets placed ~14 cm apart from each other in order to sample the same air space. These area samples were generally located as close as possible to the potential emission source, but the location depended on the process. Area filter-based samples for the mass concentration of elemental carbon (EC) and transmission electron microscopy (TEM) were collected in parallel with the DRIs for comparison. Sampling and analysis methods for the filter-based samples are discussed in a related article. CNT/CNF microscopy images showed few single-fiber CNTs/CNFs; a majority of the materials observed consisted of agglomerated nanomaterials estimated to range from several nanometers to 10 μm (Dahm et al., 2012).
Direct-reading measurements of particle number concentration were performed with a CPC (CPC 3007; TSI Inc., Shoreview, MN, USA). The CPC measures the total particle number concentration (TNC) in the size range from 10 to 1000 nm and has an inlet flow rate of 0.7 l min−1. Data are expressed as total number of particles per cubic centimeter (P cm−3), with an upper linear limit of 100 000 P cm−3. Real-time respirable mass estimates were obtained using a photometer (DustTrak Models 8533, 8520; TSI Inc.). The operating range of the DustTrak 8520 (used for the first two sites) is 0.001–100 mg m−3 and the instrument utilized a 10-mm nylon Dorr-Oliver cyclone as a (particle size) pre-classifier. The cyclone provides a 50% penetration diameter at 4 μm operating at a flow rate of 1.7 l min−1. The operating range for the DustTrak DRX 8533 (used for remaining sites) is 0.001–150 mg m−3 and the respirable size fraction is determined optically and has an inlet flow rate of 3 l min−1. Active surface area measurements were provided by a diffusion charging-based instrument (DC 2000 CE; EcoChem Analytics, Murrieta, CA, USA). Units are expressed as μm2 cm−3, and the instrument has an operating range of 0–2000 μm2 cm−3 and an inlet flow rate of 2 l min−1.
All instruments were factory calibrated prior to sampling. On-board data-logging capabilities were utilized for all DRI and the shortest logging intervals were selected for process and indoor background samples. All instruments were time synchronized each day at the commencement of sampling.
Background samples
Outdoor and indoor background measurements were collected at each site when feasible. These samples were used as a comparison to the process samples due to the potential for interference from incidental particle sources. Anthropogenic sources of nanoscale particulate (i.e. ultrafine aerosol) can result from combustion or hot processes such as vehicle exhaust, industrial dryers, compressors, and vacuum cleaners which can contribute to the total particle concentration measured by the CPC (Vincent and Clement, 2000; Shi et al., 2001; Heitbrink and Collingwood, 2005; Lam et al., 2006; Heitbrink et al., 2007; Szymczak et al., 2007; Demou et al., 2008; Evans et al., 2008, 2010; Peters et al., 2009). To account for this, background samples were collected using at least a CPC and at certain sites, when available, a photometer. Due to the small size of the background aerosol, it was expected to affect the number concentrations to a greater extent than mass.
Generally, background measurements were collected throughout the full day of sampling, concurrent to the sampled processes. Indoor background samples were collected before work operations began, in-between processes, and after completion when possible. Locations for indoor and outdoor background monitoring were selected based on professional judgment, once knowledge of the facility and surroundings was gained.
Data analysis
The distributions of all DRI observations were examined graphically via probability plots and histograms and were found to be log-normal; therefore, a log transformation was applied. Before log-transforming, all 0 values for the CPC and DC data were replaced by one half of the lowest nonzero value, which always resulted in the value 0.5 (only occurring within cleanrooms at Sites D and F for the CPC and all sites, except Site E, for the DC). For measurements collected every second, values were averaged over every minute; the final analysis was performed on these observations. Entire processes were analyzed as a whole, and for select sites each process was also broken up into smaller subtasks consisting of 25- to 30-min periods for further analysis.
As anticipated for DRI data, all observations were highly autocorrelated. For whole processes and subtask periods with corresponding outdoor background measurements, the analysis was performed on the difference between the process and concurrent outdoor background observations. Since these differences were also autocorrelated, PROC AUTOREG in SAS was used with order (p) = 2 to model the differences using a second-order autoregressive [AR(2)] covariance structure.
If the whole processes and subtask periods did not contain corresponding outdoor background measurements, the average of the log-transformed process measurements was compared with the average for the entire outdoor background time period during the same process or subtask. For whole processes or subtask periods with no collected outdoor background measurements, measurements were compared with the average of the log-transformed outdoor background measurements for the entire day. For processes for which there were no outdoor background measurements collected on that day, measurements were compared with the average of the log-transformed indoor background measurements. PROC AUTOREG with p = 2 was used to estimate the mean and standard error for both the whole process or subtask measurements and the outdoor or indoor background measurements. The ratio of the difference between the mean estimates and the square root of the sum of the squares of the standard errors was then computed. Since both sample sizes were fairly large, the P-value using the Z-distribution was calculated based on this ratio. In order to adjust for performing multiple hypothesis tests, a Bonferroni adjustment was used (adjusted P-value = number of comparisons × usual P-value) for each site, day, and instrument.
PROC AUTOREG in SAS was used with p = 2 to model all autocorrelated data. p = 2 was chosen for consistency, since many series were not adequately modeled using AR(1), while very few required AR(p) with p > 2. In addition, all estimates for p = 2 and p > 2 were similar. For a random sample with autocorrelated errors, AUTOREG estimates the mean and standard error. However, it does not directly estimate the standard deviation. For an auto-correlated time series, the usual standard error estimate underestimates the true standard error.
The statistical correlations between background-corrected DRIs and in parallel filter-based samples for the mass concentration of EC and CNT structure counts were examined using PROC CORR in SAS, version 9.1 (Cary, NC, USA). Statistical significance was evaluated using the Spearman rank-order correlation coefficient (due to the extreme skewness of the DRI data) and a cut-point for P of 0.05. Filter-based samples, which may encompass several processes, were compared with the time-weighted averages of the corresponding processes collected by the DRIs.
RESULTS
In total, 37 processes/activities were assessed using the DRIs at six facilities. Results are presented in Table 1 (primary manufacturers, A–C) and Table 2 (secondary manufacturers, D–F) stratified by site, process (including the averaged outdoor and indoor background samples), and instrument, which are further stratified by number of minutes sampled (n), geometric mean (GM), geometric standard deviation (GSD), maximum value, and the adjusted P-values.
Table 1.
Summary statistics of primary manufacturers by process and instrument.
| Summary statistic by instrument | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Sample characteristics | CPC | DustTrak | DC | ||||||||||||||
|
| |||||||||||||||||
| Particle concentration | Mass concentration | Surface area | |||||||||||||||
|
| |||||||||||||||||
| particles cm−3 | mg m−3 | μm2 cm−3 | |||||||||||||||
|
| |||||||||||||||||
| Site | Material, quantity handled |
Process | n | GM | GSD | Max | Adjusted P-valuea |
n | GM | GSD | Max | Adjusted P-valuea |
n | GM | GSD | Max | Adjusted P-valuea |
| A | MWCNT, DWCNT 40–50 g day−1, powder form(15 nm diameter; 100 μm length) | Outdoor backgroundb | 510 | 2.9E+03 | 1.54 | 3.07E+04 | — | 513 | 0.035 | 1.35 | 0.330 | — | — | — | — | — | — |
| MWCNT production-A | 132 | 1.01E+04 | 1.55 | 2.69E+04 | 0.000 | 131 | 0.026 | 1.15 | 0.053 | 0.000 | 182 | 8.8 | 2.00 | 45 | — | ||
| MWCNT production-B | 52 | 2.1E+03 | 1.14 | 2.8E+03 | 0.000c | 52 | 0.019 | 1.07 | 0.022 | 0.000c | — | — | — | — | — | ||
| Harvesting DWCNTs | 6 | 3.18E+04 | 1.04 | 3.32E+04 | 0.000 | 6 | 0.032 | 1.04 | 0.035 | 0.008 | 6 | 33.5 | 1.28 | 47 | — | ||
| Harvesting MWCNTs | 45 | 3.18E+04 | 1.04 | 3.44E+04 | 0.000 | 45 | 0.032 | 1.07 | 0.037 | 0.001 | 45 | 24.7 | 1.39 | 42 | — | ||
| Harvesting MWCNTs (second batch) | 17 | 8.7E+03 | 1.02 | 8.9E+03 | 0.000 | 17 | 0.036 | 1.04 | 0.040 | 0.000 | 17 | 38.3 | 1.18 | 59 | — | ||
| B | SWCNT 5–15 g day−1, powder form(1.1 nm diameter; 2 μm length) | Outdoor backgroundb | 283 | 5.8E+03 | 1.35 | 4.02E+04 | — | 289 | 0.037 | 1.14 | 0.083 | — | — | — | — | — | — |
| SWCNT production | 30 | 1.12E+04 | 1.87 | 4.36E+04 | 0.315 | 41 | 0.023 | 1.19 | 0.046 | 0.000 | 41 | 23.0 | 1.65 | 64 | — | ||
| harvesting SWCNTs | 8 | 5.9E+03 | 1.09 | 6.9E+03 | 0.142 | 8 | 0.017 | 1.03 | 0.018 | 0.000 | 8 | 15.6 | 1.37 | 26 | — | ||
| Reactor clean-out | 20 | 1.43E+04 | 2.43 | 7.69E+04 | 0.275 | 17 | 0.022 | 1.04 | 0.026 | 0.000 | 17 | 21.4 | 3.03 | 69 | — | ||
| Outdoor background (Day 2) | 113 | 8.9E+03 | 1.55 | 5.35E+04 | — | 299 | 0.046 | 1.40 | 0.077 | — | — | — | — | — | — | ||
| Indoor background (Day 2) | 38 | 8.5E+03 | 1.35 | 1.72E+04 | 1.00d | 38 | 0.033 | 1.09 | 0.043 | 0.434d | 38 | 7.4 | 2.01 | 24 | — | ||
| SWCNT production-A (Day 2) | 113 | 9.0E+03 | 1.46 | 2.92E+04 | 1.000 | 159 | 0.040 | 1.27 | 0.067 | 0.431 | 161 | 13.8 | 1.73 | 43 | 0.001e | ||
| SWCNT production-B (Day 2) | 48 | 6.0E+03 | 1.28 | 1.17E+04 | 0.181c | — | — | — | — | — | — | — | — | — | — | ||
| Harvesting SWCNTs (Day 2) | 19 | 1.01E+04 | 1.69 | 2.17E+04 | 1.00d | 19 | 0.041 | 1.07 | 0.046 | 0.008 | 19 | 14.6 | 1.47 | 28 | 0.000e | ||
| Reactor clean-out (Day 2) | 25 | 6.6E+03 | 1.46 | 1.23E+04 | 1.00d | 25 | 0.016 | 1.12 | 0.028 | 0.000 | 25 | 13.5 | 1.79 | 72 | 0.004e | ||
| C | MWCNT 50–100 g day−1, powder and aqueous forms(15 nm diameter; 15 μm length) | Outdoor Backgroundb | 210 | 7.9E+03 | 1.43 | 2.58E+04 | — | — | — | — | — | — | — | — | — | — | — |
| Indoor Background | 22 | 1.23E+04 | 1.10 | 1.45E+04 | 0.000d | 26 | 0.038 | 1.02 | 0.041 | — | 25 | 61.8 | 1.11 | 72 | — | ||
| Harvesting MWCNTs | 20 | 1.21E+04 | 1.05 | 1.40E+04 | 0.000d | 20 | 0.037 | 1.02 | 0.047 | 0.113e | 20 | 55.2 | 1.27 | 86 | 0.197e | ||
| MWCNT Production-A | 204 | 1.53E+04 | 2.79 | 9.79E+04 | 0.161 | 319 | 0.044 | 1.08 | 0.050 | 0.000e | 319 | 63.4 | 1.53 | 159 | 1.000e | ||
| MWCNT Production-B | 91 | 1.13E+04 | 2.33 | 9.51E+04 | 0.000c | — | — | — | — | — | — | — | — | — | — | ||
| Harvesting second batch of MWCNTs | 23 | 4.4E+03 | 1.03 | 4.6E+03 | 0.000d | 20 | 0.032 | 1.04 | 0.035 | 0.000e | 20 | 44.3 | 1.63 | 104 | 0.232e | ||
| Outdoor background (Day 2)b | 240 | 5.5E+03 | 1.57 | 3.33E+04 | — | — | — | — | — | — | — | — | — | — | — | ||
| Indoor background (Day 2) | 26 | 3.2E+03 | 1.04 | 3.4E+03 | 0.00d | 7 | 0.011 | 1.02 | 0.012 | — | — | — | — | — | — | ||
| Sonication-A (Day 2) | 71 | 7.4E+03 | 1.30 | 1.44E+04 | 0.136 | 94 | 0.011 | 1.02 | 0.012 | 0.049e | 85 | 31.9 | 1.75 | 93 | — | ||
| Sonication-B (Day 2) | 23 | 3.5E+03 | 1.07 | 4.0E+03 | 0.041c | — | — | — | — | — | — | — | — | — | — | ||
| Spray coating and sieving-A (Day 2) | 150 | 3.21E+04 | 1.43 | 4.75E+04 | 0.000 | 166 | 0.012 | 1.08 | 0.017 | 0.002e | 165 | 30.4 | 1.61 | 66 | — | ||
| Spray coating and sieving-B (Day 2) | 16 | 4.26E+04 | 1.05 | 4.73E+04 | 0.000c | — | — | — | — | — | — | — | — | — | — | ||
Note: All P-values were adjusted using a Bonferroni adjustment for the number of subtasks in a given day.
Default P-values were computed using a paired analysis compared with either indoor or outdoor background; all statistical tests accounted for autocorrelation of data using a second-order autoregressive model.
Outdoor background used for paired analysis compared with process measurements.
No completely concurrent background sample collected, compared with averaged background measurements during concurrently sampled process.
Compared with entire day outdoor background since no concurrent outdoor background collected during entire process.
Compared with indoor background since no outdoor background samples were collected.
Table 2.
Summary statistics of secondary manufacturers by process and instrument.
| Summary statistic by instrument | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| Sample characteristics | CPC | DustTrak | DC | ||||||||||||||
|
| |||||||||||||||||
| Particle concentration | Mass concentration | Surface area | |||||||||||||||
|
| |||||||||||||||||
| particles cm−3 | mg m−3 | μm2 cm−3 | |||||||||||||||
|
| |||||||||||||||||
| Site | Material, quantity handled |
Process | n | GM | GSD | Max | Adjusted P-valuea |
n | GM | GSD | Max | Adjusted P-valuea |
n | GM | GSD | Max | Adjusted P-valuea |
| D | MWCNT 100 g day−1, powder and aqueous forms(2.5 nm diameter; 1 μm length) | Weighing MWCNTs | 25 | 5.1E+02 | 2.30 | 1.45E+04 | — | — | — | — | — | — | 28 | 16.1 | 3.93 | 741 | — |
| Sonication of MWCNTs | 157 | 1.0E+01 | 1.80 | 9.6E+01 | — | — | — | — | — | — | 158 | 30.8 | 1.71 | 120 | — | ||
| E | MWCNT 14.75 g to 1 kg day−1, powder form(12 nm diameter; 5 μm length) | Indoor background | 17 | 1.53E+04 | 1.42 | 2.91E+04 | — | 18 | 0.029 | 1.12 | 0.034 | — | 15 | 140.0 | 1.46 | 246 | — |
| Extrusion of MWCNTs | 115 | 1.60E+04 | 1.81 | 1.56E+05 | 1.000b | 114 | 0.107 | 2.58 | 3.468 | 0.000b | 108 | 148.3 | 2.37 | 2501 | 0.960b | ||
| Batch mixing of MWCNTs | 21 | 9.4E+03 | 1.30 | 2.00E+04 | 0.048b | 21 | 0.033 | 2.45 | 0.284 | 0.838b | — | — | — | — | — | ||
| Milling MWCNT composite | 22 | 6.8E+03 | 1.87 | 2.02E+04 | 0.005b | 22 | 0.016 | 1.04 | 0.018 | 0.000b | — | — | — | — | — | ||
| F | MWCNT/CNF 2 g to 1 kg day−1, powder form(140 nm diameter; 100 μm length) | Weighing and mixing | 122 | 3.5E+01 | 2.15 | 5.3E+02 | — | 122 | 0.006 | 1.06 | 0.010 | — | 122 | 9.3 | 2.00 | 247 | — |
| Transferring CNFs | 37 | 0.5E+01 | 1.56 | 6.1E+01 | — | 37 | 0.006 | 1.25 | 0.037 | — | 37 | 13.9 | 1.61 | 96 | — | ||
| Mixing and sonicating | 140 | 1.2E+01 | 2.72 | 1.0E+02 | — | 140 | 0.006 | 1.03 | 0.012 | — | 140 | 27.4 | 1.87 | 90 | — | ||
Note: All P-values were adjusted using a Bonferroni adjustment for the number of subtasks in a given day.
All statistical tests accounted for autocorrelation of data.
Compared with indoor background since no outdoor background samples were collected; P-values were computed using a paired analysis of indoor versus outdoor background.
Tables 1 and 2 provide summary statistics for the entire duration of the process, while Table 3 provides summary statistics for subtasks at Site A. The averaging times were reduced for the subtasks to determine if there were intermittent peak exposures during the process, as a whole, which could impact the variance of exposures.
Table 3.
Summary statistics for Site A divided into subtasks by instrument.
| Summary statistics by instrument | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| CPC | DustTrak | |||||||||||
|
| ||||||||||||
| Particle concentration | Mass concentration | |||||||||||
|
| ||||||||||||
| particles cm−3 | mg m−3 | |||||||||||
|
| ||||||||||||
| Process | Subtask | n | Subtask GM | Subtask GSD |
Background GM |
Background GSD |
Adjusted P-valuea |
Subtask GM |
Subtask GSD |
Background GM |
Background GSD |
Adjusted P-valuea |
| MWCNT production | A-1 | 26 | 1.9E+03 | 1.04 | 2.9E+03 | 1.55 | 0.002b | 0.018 | 1.03 | 0.045 | 1.03 | 0.000b |
| A-2 | 26 | 2.3E+03 | 1.11 | 2.9E+03 | 1.55 | 0.025b | 0.020 | 1.06 | 0.045 | 1.03 | 0.000b | |
| A-3 | 28 | 5.5E+03 | 1.46 | 3.4E+03 | 1.13 | 0.000 | 0.022 | 1.05 | 0.044 | 1.04 | 0.000 | |
| A-4 | 28 | 8.9E+03 | 1.13 | 2.5E+03 | 1.08 | 0.000 | 0.024 | 1.05 | 0.045 | 1.01 | 0.000 | |
| A-5 | 28 | 1.10E+04 | 1.06 | 2.6E+03 | 1.12 | 0.000 | 0.027 | 1.04 | 0.046 | 1.02 | 0.000 | |
| A-6 | 28 | 1.27E+04 | 1.15 | 3.1E+03 | 1.19 | 0.000 | 0.029 | 1.17 | 0.045 | 1.02 | 0.000 | |
| A-7 | 20 | 1.85E+04 | 1.23 | 3.3E+03 | 1.09 | 0.000 | 0.030 | 1.05 | 0.044 | 1.02 | 0.000 | |
| Harvesting MWCNTs | B-1 | 23 | 3.13E+04 | 1.04 | 5.3E+03 | 1.13 | 0.000 | 0.030 | 1.05 | 0.046 | 1.17 | 0.000 |
| B-2 | 22 | 3.23E+04 | 1.04 | 4.2E+03 | 1.24 | 0.000 | 0.034 | 1.04 | 0.040 | 1.08 | 0.000 | |
| Harvesting DWCNTs | C-1 | 6 | 3.18E+04 | 1.04 | 1.8E+03 | 1.05 | 0.000 | 0.032 | 1.04 | 0.025 | 1.02 | 0.018 |
| Harvesting MWCNTs (second batch) | D-1 | 17 | 8.7E+03 | 1.02 | 2.4E+03 | 1.15 | 0.000 | 0.036 | 1.04 | 0.029 | 1.02 | 0.000 |
Note: All P-values were adjusted using a Bonferroni adjustment for the number of subtasks in a given day.
Default P-values were computed using a paired analysis of indoor versus outdoor background; all statistical tests accounted for autocorrelation of data.
No concurrent background sample collected, compared with averaged process measurements during entire process; Outdoor background used for paired analysis compared with process measurements.
Number concentration
Outdoor background GM concentrations collected by the CPC at primary manufacturers (Sites A–C) ranged from 2.9 × 103 to 8.9 × 103 particles cm−3, while indoor background samples collected at Site C, on consecutive days, varied from 3.2 × 103 to 1.23 × 104 particles cm−3. TNC GMs at primary manufacturers collected during specific processes fluctuated from site to site as well as day to day (2.1 × 103 to 4.26 × 104 particles cm−3). TNC GMs during these processes ranged from 0.55 to 17 times higher than their concurrent process background GMs as opposed to using the full day GM displayed in Table 1. A majority of the highest TNC processes seen above background were found at Site A (3–17 times higher). All P-values associated with the processes were significantly different compared with background (P-value <0.05). This indicated a probable indoor nanoparticle source but did not reflect CNT exposure. In this case, the elevation in TNC was due to an inoperable exhaust system for reaction byproducts (Discussion section). No changes in significance were seen when processes were divided into subtasks at Site A, seen in Table 3.
Other notably high TNC GMs observed at primary manufacturers included the reactor clean-out process on day 1 at Site B (1.43 × 104 particles cm−3, 2.8 times higher than background) and spray coating and sieving (A and B) processes sampled at Site C (3.21 × 104 particles cm−3, 5.5 times higher than background; 4.26 × 104 particles cm−3, 7.3 times higher than background).
Outdoor background samples were not collected at secondary manufacturers due to the use of cleanrooms (Sites D and F) or a small single enclosed lab (Site E), where only an indoor pretask sample was taken (1.53 × 104 particles cm−3). TNC GMs within the cleanroom atmospheres ranged from 5 to 512 particles cm−3, while the non-cleanroom secondary manufacturing site (Site E) TNC GMs ranged from 6.8 × 103 to 1.60 × 104 particles cm−3, across a wide range of tasks. The highest TNC GMs measured at Site D were seen during the weighing of 100 g of MWCNTs inside of a HEPA-ventilated glove box (512 particles cm−3). However, this elevation was unlikely due to the MWCNTs. Site E saw its highest TNC during the extrusion of MWCNTs with a polyvinyl chloride-based resin (1.60 × 104 particles cm−3). A HEPA vacuum was used intermittently, and most likely contributed to the elevation in TNC. These were very similar to the collected pretask, indoor background samples (GM ratio of 0.96; P-value 1.000). However, one of the highest filter-based samples for the mass concentration of EC was also collected at Site E from a personal breathing zone sample during this same process (7.86 μg m−3, 0.242 CNT structures cm−3; Dahm et al., 2012). The area filter-based samples, collected next to the DRIs, were not nearly as high (1.01 μg m−3, 0.008 CNT structures cm−3).
Site F saw its highest TNC GMs during various weighing and mixing operations of 2–100 g of MWCNTs (35 particles cm−3). Similar to Site E, one of the highest filter-based results for the mass concentration of EC was collected for a personal breathing zone sample during this process (7.54 μg m−3, 0.065 CNT structures cm−3). The concurrent area sample did not show evidence of exposure (nondetectable mass concentration, 0.003 CNT structures cm−3).
Overall, no consistent trends were seen between similar processes among all sites using the TNC GMs (Fig. 1a). A majority of the grouped processes were fairly similar to the collected outdoor background samples. There was very little to no correlation between the CPC and the area filter-based samples for the mass concentration of EC and CNT structure counts collected in parallel with the CPC, seen in Fig. 2a, 2b (r = 0.13, P-value 0.64 compared with the mass concentration of EC and r = 0.23, P-value 0.41 for the CNT structure counts).
Fig. 1.
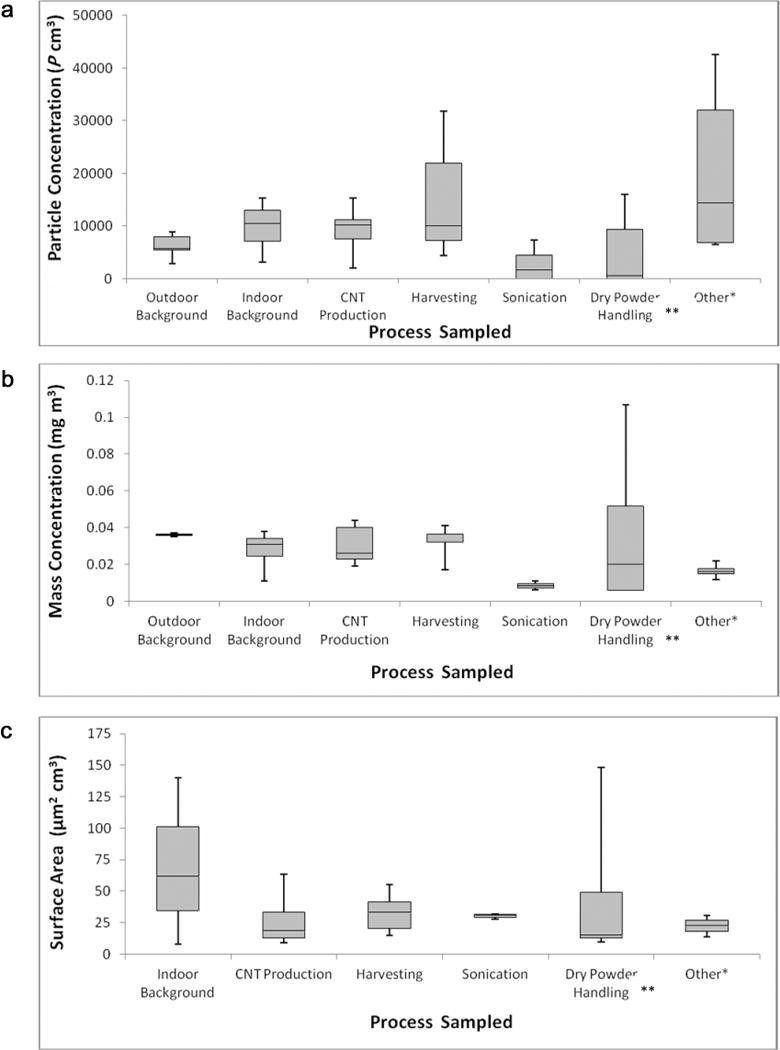
DRI GM results by process and instrument. Samples were not background corrected (source sample background). Box plot represents range (minimum to maximum), 25th percentile, median, and 75th percentile. (a) TNC plotted by process sampled, (b) respirable mass concentration plotted by process sampled, and (c) active surface area plotted by process sampled. **Tasks include weighing and mixing; extrusion; transferring CNFs, batch mixer use; and weighing. *Tasks include spray coating and sieving; reactor clean-out; and milling CNT composite.
Fig. 2.
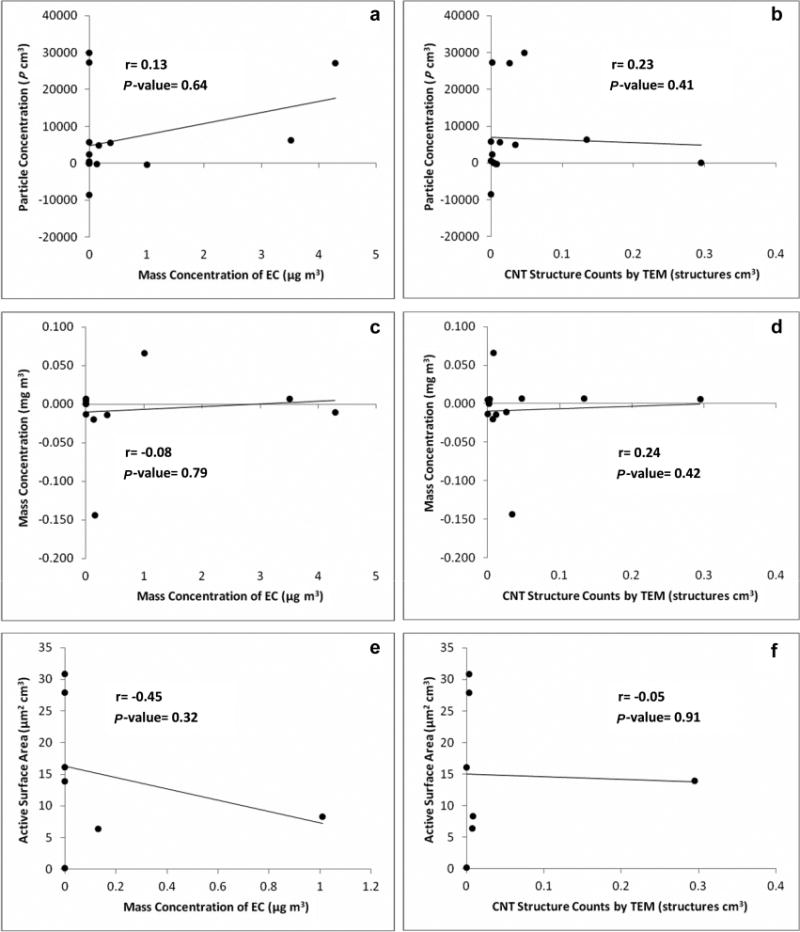
Correlation graphs of the background-corrected DRI results (process/task − background) compared with filter-based results for TEM and background-corrected EC. (a) CPC particle concentration results compared with area EC mass concentrations. (b) CPC particle concentration results compared with area TEM structure count concentrations. (c) DustTrak mass concentration results compared with area EC mass concentrations. (d) DustTrak mass concentration results compared with area TEM structure count concentrations. (e) DC results compared with area EC mass concentrations. (f) DC results compared with area TEM structure count concentrations. Filter-based samples, which may encompass several processes, were compared with the time-weighted averages of the corresponding processes collected by the DRIs. All EC mass concentration samples were background corrected (process/task − background), and nondetected samples subtracted by background were treated as zero.
Mass concentration
For the most part, the respirable mass concentration samples measured by the photometer were comparable to the indoor or outdoor background samples (Fig. 1b). Similar to the CPC, there was no correlation seen between the area filter-based samples and the photometer (Fig. 2c, 2d; r = −0.08, P-value 0.79 for mass of EC; r = −0.24, P-value 0.42 for CNT structure counts). However, most P-values did show processes to be significantly different than background (P-value <0.05) at both primary and secondary manufacturers. However, most were lower than background. Indoor and outdoor background respirable mass concentration samples at both primary and secondary manufacturers ranged from 0.011 to 0.046 mg m−3, while specific processes ranged from 0.011 to 0.107 mg m−3 (Tables 1 and 2). GM concentrations ranged from 0.5 to 4 times higher than background samples although a majority of the processes sampled had ratios of 1. For the most part, the photometer lacked the sensitivity to reflect changes for most emissions of CNTs/CNFs during the sampled process, due to either the small quantities of CNTs/CNFs, the distance from the source, or the use of exposure controls in the facilities. However, small, but transient elevations were observed with the time-series data on occasions. Furthermore, no differences in significance were seen when individual processes were divided into shorter subtasks (Table 3).
Surface area
Indoor background active surface area measurements were 7.4 and 61.8 μm2 cm−3 at primary manufacturers (Table 1). The remaining surface area GM measurements collected during specific tasks at primary manufacturers ranged from 8.8 to 63.4 μm2 cm−3. The sole surface area GM indoor background value at a secondary manufacturer was 140 μm2 cm−3. The remaining surface area GMs collected at secondary manufacturers during specific tasks ranged from 9.3 to 148.3 μm2 cm−3. The highest surface area observations at both primary and secondary manufacturers were statistically similar to the collected indoor background sample (63.4 μm2 cm−3, P-value 1.0; 148.3 μm2 cm−3, P-value 0.96) and GM surface area values ranged from 0.7 to 1.9 times higher than indoor background GMs. Fig. 1c displays the collected DC measurements by process and indicates results consistent with the previous observations from the CPC and photometer; no clear exposure patterns were seen. Compared with the filter-based samples, negative correlations were seen (Fig. 2e, 2f; r = −0.45, P-value 0.32 for mass of EC; r = −0.05, P-value 0.91 for CNT structure counts).
DISCUSSION
Number concentration
Overall, no consistent trends were seen among similar processes at the various sites or between the various DRIs (Fig. 1a). There was also little correlation seen among the side-by-side filter-based samples compared with the background corrected DRI data (Fig. 2a, 2b). TNCs varied widely depending on the site, process, and time of day. Of all the instruments, the CPC was most often affected by other particle sources which included hot processes such as CNT production, electric HEPA-filtered vacuum cleaners, anthropogenic outdoor sources, and indoor sources found in manufacturing environments, making interpretation of the data difficult. Similar findings were reported in the Evans et al. (2010) study, where the TNCs measured by the CPC were dominated by other nanoparticle sources, not the material of interest.
An example of this can be seen in Fig. 3, which displays the time-series plot for all instruments at Site A. An elevation in TNC occurred during the initiation of MWCNT production at 6:50 (processes I and II; Fig. 3), while the other DRIs did not show a similar response. It was later determined that the elevation in TNC was due to an inoperable exhaust system for the MWCNT and other production processes. Thus, the DRIs were useful in this instance in alerting the authors and the facility staff to faulty process controls, which resulted in reactor ultrafine emissions (not MWCNTs) into the workplace. Time-integrated samples cannot provide this time resolved information in the timely manner required. The TNC elevations above background during these sampled processes later in the day were due to an R&D reactor in operation (the same inoperable exhaust system was used). Area filter-based samples collected during the same period of MWCNT production found little mass or visual evidence of MWCNT exposure (0.49 μg m−3, 0.034 CNT structures cm−3). Another example of potential misclassification of exposure occurred from elevations at Site B during the reactor clean-out process. The elevations during the clean-out process were likely due to the operation of a HEPA vacuum that was used intermittently, and most likely contributed to the TNC [also observed by Maynard et al. (2004)]. The filter-based, area samples associated with these tasks found little visual evidence or mass-based evidence of exposure (1.13 μg m−3, 0.012 CNT structures cm−3).
Fig. 3.
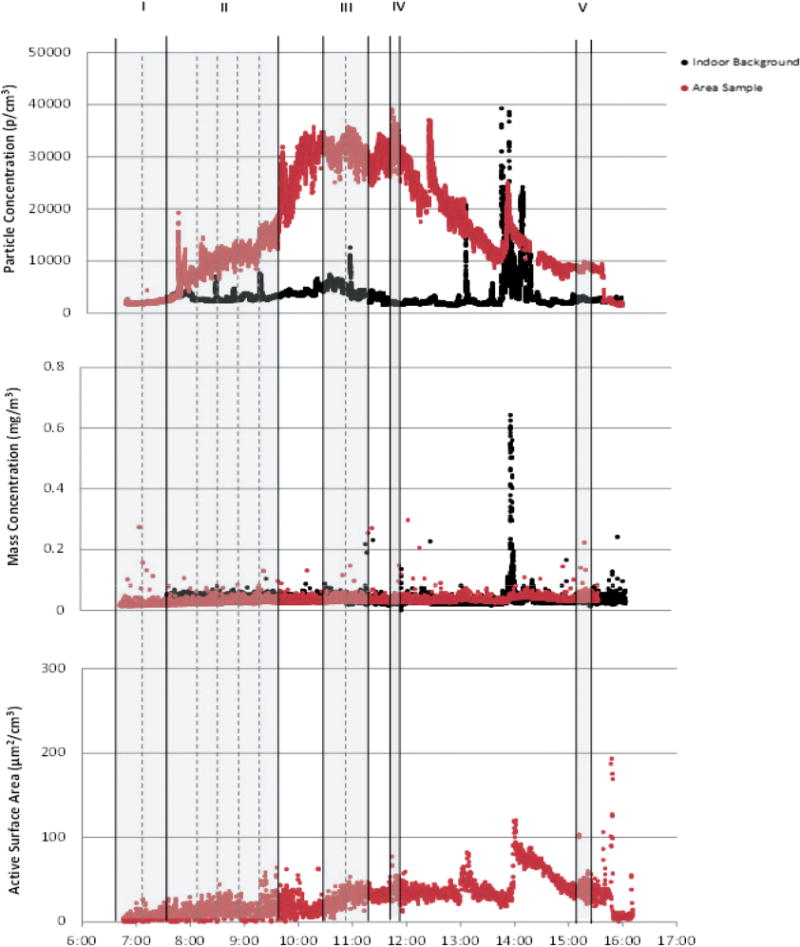
Time series of particle number, respirable mass, and active surface area during the five highlighted processes: (I) MWCNT Production-B, (II) MWCNT Production-A, (III) Harvesting MWCNTs, (IV) Harvesting DWCNTs, and (V) Harvesting MWCNTs (second batch). Solid black lines denote processes (Table 1), while dotted lines denote subtasks (Table 3). Red data consist of at-source samples of processes. Black data are corresponding outdoor background sample.
Mass concentration
In general, and at all sites, the respirable mass concentration data collected with the photometer do not reflect changes in emissions of CNTs/CNFs during the sampled process, most likely due to inadequate sensitivity. Similar results were seen with a photometer during solid core drilling on various CNT composites (Bello et al., 2010). However, in a previous study by Evans et al. (2010), conducted at a CNF primary producer, the respirable mass concentration measured by a comparable photometer was found to be the most useful monitoring metric. This can likely be attributed to the large quantity of CNFs produced and handled, as well as the general lack of exposure controls within the facility.
Surface area
Active surface area measurements by the DC appeared to closely follow TNC, more so than respirable mass, as previously observed by Heitbrink et al. (2009) and Evans et al. (2010). Due to the relatively high and transient-background ultrafine particle concentrations, it was often difficult to differentiate background ultrafine aerosol from any CNT/CNF contribution, if any, limiting the usefulness of the data. However, from an indoor air quality and mixed exposure perspective, these measurements may provide some useful information and also highlight other issues within the workplace (Evans et al., 2010; Birch, 2011).
Data analysis
The time-series analysis methods used to interpret the data were complicated, time consuming, and for the most part outside the typical statistical methods used for industrial hygiene. It may be difficult to develop a standardized protocol to analyze all data sets with consistency since there can be varying levels of autocorrelation. It was also problematic to determine the best averaging time to further analyze the data, due to the range of exposure variances seen from task to task. However, no differences in significance were seen in this study when processes were further subdivided into subtasks. The assistance of a statistician may be needed to help develop an analysis plan, on a case-by-case basis.
However, time-series plots of the data, combined with concurrent background samples collected at carefully selected locations, and detailed worker observations can provide useful insight on possible breaches of engineering controls or on specific employee actions which may increase the risk of exposure. Nevertheless, caution has to be used when interpreting the data due to the potential for transient changes in particle background, simultaneous emissions of ultrafine particles, or the potential influence from other particle sources (Evans et al., 2010).
Data utility
It has been mentioned previously that the pooling of data requires consistent methodologies of assessing exposures which should consist of at least particle number concentration, surface area, and mass (Maynard and Kuempel, 2005; Brouwer et al., 2009; Ramachandran et al., 2011). Ideally, the data collected with similar instruments could be useful for various applications, including risk assessment, standards development and epidemiologic studies (Woskie et al., 2010). While it is still unclear which exposure metric is the most relevant to human health, it is obvious that the choice of the exposure metric will affect the classification of workers into exposure groups for future epidemiologic studies. DRIs may not be the most appropriate tools for collecting quantitative exposure assessment data for epidemiologic studies because of the inability to assign gradient levels of exposure, but they may be used as a nonspecific, integrative measure of ultrafine or other mixed particle exposure.
Less-sophisticated, empirical instruments were used throughout this study, compared with the instruments used in more refined laboratory studies, but all data provided by nonselective instruments for particle measurement should be interpreted cautiously. For field application, interpretation is further confounded by uncontrolled aerosol sources. Taken alone, the DRI data from this study and others may not be useful for future risk assessments and epidemiologic research on engineered nanomaterials because of the lack of specificity and inability to assign gradient levels of exposure based on the processes sampled. However, in parallel work using filter-based samples for the mass of EC and electron microscopy, we found positive correlations between the mass and microscopy metrics and saw common trends between processes. Currently, it is unclear whether a metric other than mass is a more appropriate metric to predict human health effects due to CNT/CNF exposure.
At present, there are no readily available instruments that provide reliable and cost effective online or off-line data on the previously suggested, alternative metrics, which ultimately limits their usefulness. To date, filter-based, chemical analyses and TEM structure count methods, although burdensome, appear to be the most reliable, selective, and feasible way to conduct quantitative exposure assessments of workers exposed to CNTs/CNFs (Birch et al., 2011; Dahm et al., 2012).
Limitations
As previously emphasized, the DRIs employed are nonspecific measurement tools that cannot distinguish CNT/CNF particles from other particle types. In order to account for other sources, preprocess indoor and concurrent outdoor background samples were collected, based on knowledge of the facility and various work-related actions being performed in or around the facility. However, with simultaneous operations occurring at various locations within complex workplaces, at times, it may be difficult to select appropriate positions for indoor or outdoor background samples, which could greatly affect the determination of the contribution of CNTs/CNFs to the measured aerosol exposures.
Another notable limitation is that many instruments rely on a particle’s optical properties or refractive index as the basis for operation. Further, the accuracy in response to particles or fibers of irregular shape may vary (Gebhart, 1991; Umhauer and Bottlinger, 1991; Szymanski et al., 2009). The varying and highly irregular shapes of CNT/CNF particles, many of which are complex aggregates, may result in erroneous measurements. Calibration procedures using CNT/CNF reference materials, rather than spherical reference materials, should be adopted. However, the CNT/CNF materials may differ widely in both their propensity for dispersal and their airborne properties (Evans et al., in press); so, ideally a calibration is required for each material encountered.
Moreover, from a practical standpoint, even if DRIs were reliable indicators of CNTs/CNFs, most DRI instruments can only be practically used as static area samplers, which can greatly limit their utility for workplace monitoring. It has been previously noted that area samples for CNTs/CNFs can be substantially lower than personal breathing zone samples (Birch et al., 2011; Dahm et al., 2012). This is highlighted at Sites E and F where the personal exposures, measured with the filter-based samples, were much higher than the corresponding area filter-based samples, even though they were only 1 or 2 m away from the source. There has been a recent emergence of small, portable, battery-operated instruments that use electrical diffusion charging principles to provide total and size-selective number concentrations. In a recent study comparing these instruments, a CPC was found to provide the best accuracy for number concentration determination. All instruments reacted quite differently to the test aerosols and none were challenged with high-aspect ratio nanomaterials such as CNTs/CNFs (Asbach et al., 2012).
CONCLUSIONS
The DRIs used in this study (CPC, photometer, and DC) are nonselective assessment tools, perhaps best used to determine how and where exposures may be occurring, rather than to quantify exposures (Evans et al., 2010). The statistical treatment of the time-series data in this study did not identify meaningful trends between any of the DRI’s sampling at similar processes at various sites, or between the side-by-side filter-based samples for the mass concentration of EC- or TEM-based CNT structure counts. The differentiation of CNT/CNF exposure points by job tasks at various CNT/CNF sites was not determined through the use of the DRIs described here, but through the time-integrated approaches described in a previous article (Dahm et al., 2012). Data from these instruments can be misinterpreted without an understanding of other particle sources within workplaces. Though simple industrial hygiene approaches with a primary focus on DRIs have been previously recommended (Methner et al., 2010a), erroneous conclusions can potentially be drawn when these simple tools are used to assess complex workplace exposures and processes (Evans et al., 2010; Birch, 2011; Birch et al., 2011). A more comprehensive, multimetric approach is preferable for conducting workplace studies with CNTs/CNFs, particularly when a mixed exposure is suspected, and/or for resolving other indoor air quality issues. However, for industrial hygiene applications and control evaluations, using filter-based (time integrated) samples in combination with good professional industrial hygiene judgment may be more effective in terms of cost, time, and data quality. Given the uncertainties in the data provided by DRIs, the CPC, photometer, and DC are unreliable tools for quantifying exposure to CNTs/CNFs.
Acknowledgments
The authors would like to thank Catherine Beaucham, Greg Kinnes for their assistance in collecting field data; Donnie Booher, Karl Feldmann, and Kevin L. Dunn for their technical assistance. The authors would also like to thank Steve Wurzelbacher, Ken Martinez, and Cherie Estill for their review of the manuscript.
FUNDING
NIOSH Nanotechnology Research Center supplemental funding.
Footnotes
Disclaimer—The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the NIOSH. Mention of any company name or product does not constitute endorsement by NIOSH.
References
- Asbach C, Kaminski H, von Barany D, et al. Comparability of portable nanoparticle exposure monitors. Ann Occup Hyg. 2012;56:606–21. doi: 10.1093/annhyg/mes033. [DOI] [PubMed] [Google Scholar]
- Bello D, Hart JA, Ahn K, et al. Particle exposure levels during CVD growth and subsequent handling of vertically aligned carbon nanotube films. Carbon. 2008;46:974–81. [Google Scholar]
- Bello D, Wardle BL, Yamamoto N, et al. Exposure to nanoscale particles and fibers during machining of hybrid advanced composites containing carbon nanotubes. J Nanopart Res. 2009;11:231–49. [Google Scholar]
- Bello D, Wardle BL, Zhang J, et al. Characterization of exposures to nanoscale particles and fibers during solid core drilling of hybrid carbon nanotube advanced composites. Int J Occup Environ Health. 2010;16:434–50. doi: 10.1179/107735210799159996. [DOI] [PubMed] [Google Scholar]
- Birch ME. Exposure and emissions monitoring during carbon nanofiber production–Part II: polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2011;55:1037–47. doi: 10.1093/annhyg/mer070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch ME, Ku BK, Evans DE, et al. Exposure and emissions monitoring during carbon nanofiber production–Part I: elemental carbon and iron-soot aerosols. Ann Occup Hyg. 2011;55:1016–36. doi: 10.1093/annhyg/mer073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer DH, Berges M, Virji M, et al. Harmonization of measurement strategies for exposure to manufactured nano-objects; report of a workshop. Ann Occup Hyg. 2012;56:1–9. doi: 10.1093/annhyg/mer099. [DOI] [PubMed] [Google Scholar]
- Brouwer DH, van Duuren-Stuurman B, Berges M, et al. From workplace air monitoring results towards estimates of exposure: development of a strategy to assess exposure to manufactured nano-objects. J Nanopart Res. 2009;11:1867–81. [Google Scholar]
- British Standards Institute. Nanotechnologies—Part 3: guide to assessing airborne exposure in occupational settings relevant to nanomaterials. London, UK: British Standards Institution; 2010. (BSI PD 6699-3:2010) [Google Scholar]
- Cena LG, Peters TM. Characterization and control of airborne particles emitted during production of epoxy/carbon nanotube nanocomposites. J Occup Environ Hyg. 2011;8:86–92. doi: 10.1080/15459624.2011.545943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, et al. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg. 2012;56:542–56. doi: 10.1093/annhyg/mer110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demou E, Peter P, Hellweg S. Exposure to manufactured nanostructured particles in an industrial pilot plant. Ann Occup Hyg. 2008;52:695–706. doi: 10.1093/annhyg/men058. [DOI] [PubMed] [Google Scholar]
- Demou E, Stark WJ, Hellweg S. Particle emission and exposure during nanoparticle synthesis in research laboratories. Ann Occup Hyg. 2009;53:829–38. doi: 10.1093/annhyg/mep061. [DOI] [PubMed] [Google Scholar]
- Duffin R, Mills NL, Donaldson K. Nanoparticles-a thoracic toxicology perspective. Yonsei Med J. 2007;48:561–72. doi: 10.3349/ymj.2007.48.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Heitbrink WA, Slavin TJ, et al. Ultrafine and respirable particles in an automotive grey iron foundry. Ann Occup Hyg. 2008;52:9–21. doi: 10.1093/annhyg/mem056. [DOI] [PubMed] [Google Scholar]
- Evans DE, Ku BK, Birch ME, et al. Aerosol monitoring during carbon nanofiber production: mobile direct-reading sampling. Ann Occup Hyg. 2010;54:514–31. doi: 10.1093/annhyg/meq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Turkevich LA, Roettgers CT, et al. Dustiness of fine and nanoscale powders. Ann Occup Hyg. doi: 10.1093/annhyg/mes060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart J. Response of single-particle optical counters to particles of irregular shape. Part Part Syst Charact. 1991;8:40–7. [Google Scholar]
- Han JH, Lee EJ, Lee JH, et al. Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol. 2008;20:741–9. doi: 10.1080/08958370801942238. [DOI] [PubMed] [Google Scholar]
- Heitbrink WA, Collingwood S. Aerosol generation by blower motors as a bias in assessing aerosol penetration into cabin filtration systems. J Occup Environ Hyg. 2005;2:45–53. doi: 10.1080/15459620590903020. [DOI] [PubMed] [Google Scholar]
- Heitbrink WA, Evans DE, Ku BK, et al. Relationships among particle number, surface area, and respirable mass concentrations in automotive engine manufacturing. J Occup Environ Hyg. 2009;6:19–31. doi: 10.1080/15459620802530096. [DOI] [PubMed] [Google Scholar]
- Heitbrink WA, Evans DE, Peters TM, et al. Characterization and mapping of very fine particles in an engine machining and assembly facility. J Occup Environ Hyg. 2007;4:341–51. doi: 10.1080/15459620701290081. [DOI] [PubMed] [Google Scholar]
- Innovative Research and Products Inc. (iRAP) Productions and application of carbon nanotubes, carbon nanofibers, fullerenes, graphene and nano-diamonds: a global technology survey and market analysis. 2011 Available at http://www.innoresearch.net/report_summary.aspx?id=77&pg=531&rcd=ET-113&pd=2/1/2011. Accessed 10 October 2012.
- Institute of Energy and Environmental Technology, Federal Institute for Occupational Safety and Health (BAuA), German Social Accident Insurance Institution (BG RCI) et al. Tiered approach to an exposure measurement and assessment of nanoscale aerosols released from engineered nanomaterials in workplace operations. 2011 Available at https://www.vci.de/Downloads/Tiered-Approach.pdf. Accessed 10 October 2012.
- Invernizzi N. Nanotechnology between the lab and the shop floor: what are the effects on labor? J Nanopart Res. 2011;13:2249–68. [Google Scholar]
- Kisin ER, Murray AR, Sargent L, et al. Pulmonary response, oxidative stress and genotoxicity induced by carbon nanofibers. Toxicologist. 2010;114:169. [Google Scholar]
- Kunzmann A, Andersson B, Thurnherr T, et al. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2011;1810:361–73. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, et al. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee SB, Bae GN, et al. Exposure assessment of carbon nanotube manufacturing workplaces. Inhal Toxicol. 2010;22:369–81. doi: 10.3109/08958370903367359. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, et al. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Maynard A, Kuempel E. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7:587–614. [Google Scholar]
- Mercer RR, Scabilloni J, Wang L, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Wang L, et al. Use of labeled single walled carbon nanotubes to study translocation from the lungs. Toxicologist. 2009;108(Suppl. 1):A2192. [Google Scholar]
- Methner MM, Hodson L, Geraci C. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials–Part A. J Occup Environ Hyg. 2010a;7:127–32. doi: 10.1080/15459620903476355. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, Dames A, et al. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials–Part B: Results from 12 field studies. J Occup Environ Hyg. 2010b;7:163–76. doi: 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters TM, Elzey S, Johnson R, et al. Airborne monitoring to distinguish engineered nanomaterials from incidental particles for environmental health and safety. J Occup Environ Hyg. 2009;6:73–81. doi: 10.1080/15459620802590058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitzko S. Workplace exposure to engineered nanoparticles. Inhal Toxicol. 2009;21(Suppl. 1):25–9. doi: 10.1080/08958370902962317. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Ostraat M, Evans DE, et al. A strategy for assessing workplace exposures to nanomaterials. J Occup Environ Hyg. 2011;8:673–85. doi: 10.1080/15459624.2011.623223. [DOI] [PubMed] [Google Scholar]
- Riviere JE. Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:26–34. doi: 10.1002/wnan.24. [DOI] [PubMed] [Google Scholar]
- Sargent LM, Shvedova AA, Hubbs AF, et al. Induction of aneuploidy by single-walled carbon nanotubes. Environ Mol Mutagen. 2009;50:708–17. doi: 10.1002/em.20529. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Trout D, Zumwalde RD, et al. Options for occupational health surveillance of workers potentially exposed to engineered nanoparticles: state of the science. J Occup Environ Med. 2008;50:517–26. doi: 10.1097/JOM.0b013e31816515f7. [DOI] [PubMed] [Google Scholar]
- Shi JP, Evans DE, Khan AA, et al. Sources and concentrations of nanoparticles in the urban atmosphere. Atmos Environ. 2001;35:1193–202. [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–65. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Erdely A. Engineered nanoparticle respiratory exposure and potential risks for cardiovascular toxicity: predictive tests and biomarkers. Inhal Toxicol. 2009;21(Suppl. 1):68–73. doi: 10.1080/08958370902942566. [DOI] [PubMed] [Google Scholar]
- Szymczak W, Menzel N, Keck L. Emission of ultrafine copper particles by universal motors controlled by phase angle modulation. J Aerosol Sci. 2007;38:520–31. [Google Scholar]
- Szymanski W, Nagy A, Czitrovszky A. Optical particle spectrometry – problems and prospects. J Quant Spectrosc Radiat Trans. 2009;110:918–29. [Google Scholar]
- Tsai SJ, Hofmann M, Hallock M, et al. Characterization and evaluation of nanoparticle release during the synthesis of single-walled and multiwalled carbon nanotubes by chemical vapor deposition. Environ Sci Technol. 2009;43:6017–23. doi: 10.1021/es900486y. [DOI] [PubMed] [Google Scholar]
- Umhauer H, Bottlinger M. Effect of particle shape and structure on the results of single-particle light-scattering size analysis. Appl Opt. 1991;30:4980–6. doi: 10.1364/AO.30.004980. [DOI] [PubMed] [Google Scholar]
- Vincent JH, Clement CF. Ultrafine particles in workplace atmospheres. Phil Trans R Soc Lond. 2000;358:2673–82. [Google Scholar]
- Woskie SR, Bello D, Virji MA, et al. Understanding workplace processes and factors that influence exposures to engineered nanomaterials. Int J Occup Environ Health. 2010;16:365–77. doi: 10.1179/107735210799159950. [DOI] [PubMed] [Google Scholar]


