Abstract
Objective
Conflicting results have been reported for adeno- and adenosquamous carcinomas of the cervix with respect to their response to therapy and prognosis. The current study sought to evaluate impact of adeno- and adenosquamous histology in the randomized trials of primary cisplatin-based chemoradiation for locally advanced cervical cancer.
Methods
Patients with adeno- and adenosquamous cervical carcinomas were retrospectively studied and compared to squamous cell carcinomas in GOG trials of chemoradiation.
Results
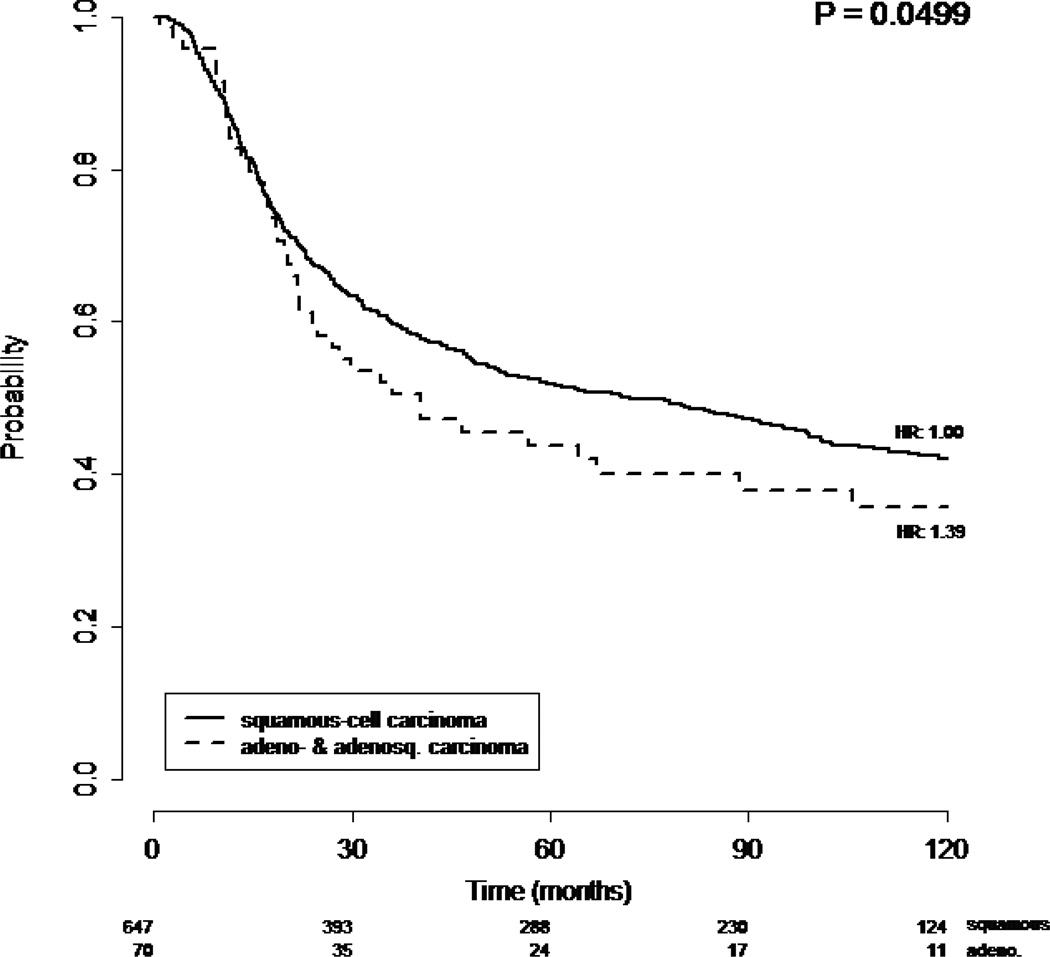
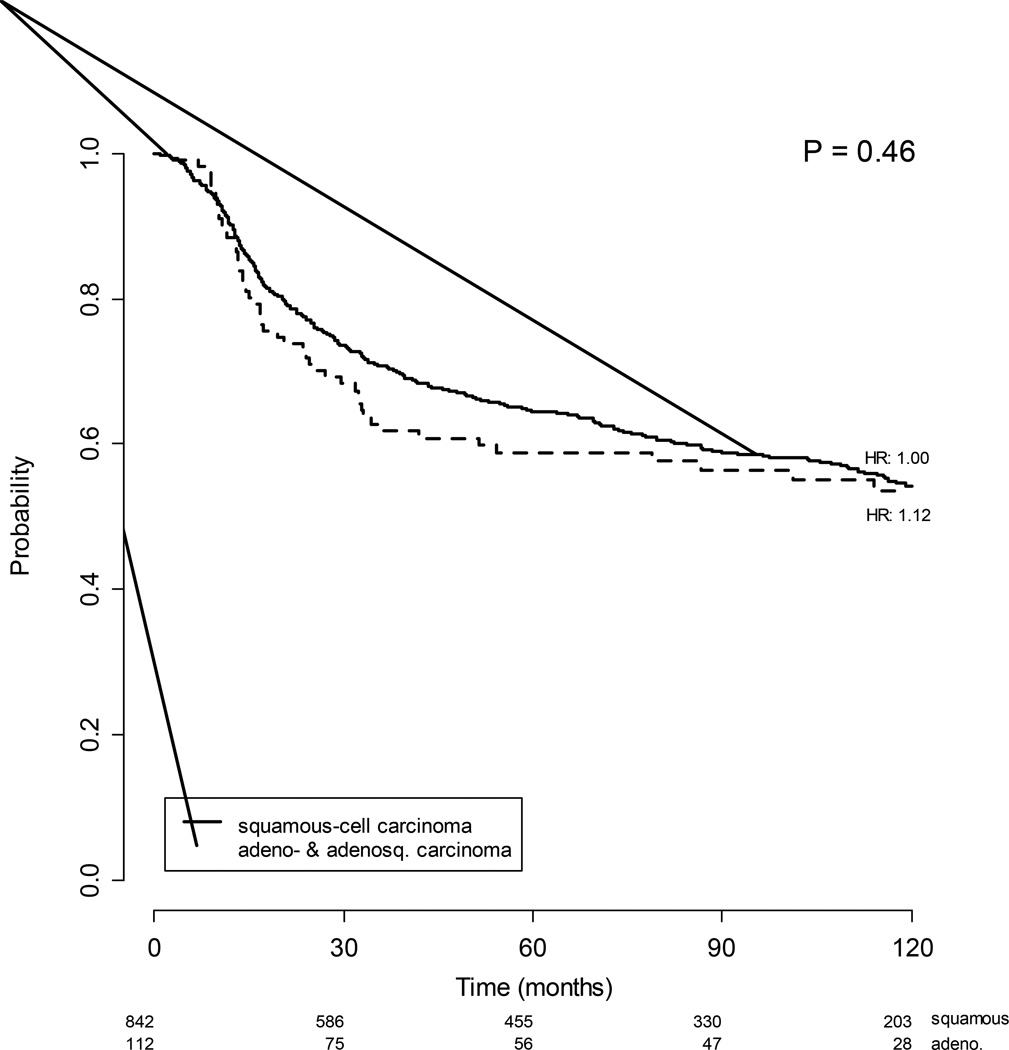
Among 1671 enrolled in clinical trials of chemoradiation, 182 adeno- and adenosquamous carcinomas were identified (10.9%). A higher percentage of adeno- and adenosquamous carcinomas were stage IB2 (27.5% versus 20.0%) and fewer had stage IIIB (21.4% versus 28.6%). The mean tumor size was larger for squamous than adeno- and adenosquamous. Adeno- and adenosquamous carcinomas were more often poorly differentiated (46.2% versus 26.8%). When treated with radiation therapy alone, the 70 patients with adeno- and adenosquamous carcinoma of the cervix showed a statistically poorer overall survival (p=0.0499) compared to the 647 patients with squamous cell carcinoma of the cervix. However, when treated with radiation therapy with concurrent cisplatin-based chemotherapy, the 112 patients with adeno- and adenosquamous carcinomas had a similar overall survival (p=0.459) compared the 842 patients with squamous cell carcinoma. Adverse effects to treatment were similar across histologies.
Conclusion
Adeno- and adenosquamous carcinomas of the cervix are associated with worse overall survival when treated with radiation alone but with similar progression-free and overall survival compared to squamous cell carcinomas of the cervix when treated with cisplatin based chemoradiation.
INTRODUCTION
Although adeno- and adenosquamous cancers of the cervix comprise a minority of cervical cancers, their relative and absolute frequency has increased over the last 4 decades despite the wider application of cervical cancer screening [1,2]. A recent large SEER (Surveillance, Epidemiology, and End Results) database study from 1988–2005 found a 1–2 % increase in the incidence of adenocarcinoma and adenosquamous carcinomas with each 6 year increment. Collectively, adenocarcinoma and adenosquamous cancers totaled 21.3%, 22.9% and 24.1%, for the years 1988–1993, 1994–1999 and 2000–2005, respectively [2]. This study also reported that patients with adenocarcinoma have a poorer overall survival than similarly staged squamous cell cancer patients [2]. Patients with advanced stage adenocarcinoma or adenosquamous carcinoma were 21% more likely to die from their disease than advanced stage squamous cell cancer patients. In contrast to the SEER data, a recent large single institution study (N=423) by Katanyoo et al suggests that, when treated according to a standard treatment protocol, adenocarcinoma and adenosquamous carcinomas have a similar outcome to squamous cell carcinoma [3].
In 1999, the NCI released a clinical announcement in strongly urging the use of cisplatin-based chemoradiation for cervical cancer patients requiring radiation for their treatment [4]. However, the role of primary chemoradiation for locally advanced adenocarcinoma and adenosquamous cancers of the cervix has not been established by level 1evidence. Although adeno- and adenosquamous carcinomas were included in randomized trials of cisplatin-based chemoradiation, there were too few patients with adenocarcinoma and adenosquamous histology to allow a subset analysis. Only Peters et al evaluated the role of tumor histology in their randomized trial of post-radical hysterectomy adjuvant chemoradiation for women with positive nodes, parametria or margins [5]. This demonstrated that patients with adenocarcinoma had an apparent poorer 5 year progression-free survival (40% vs 65%) when treated with radiation alone but similar outcome with chemoradiation (80% vs 77%). However, this did not reach statistical significance due to the small number of patients with adenocarcinoma.
Based on their relatively small size, prior retrospective studies of adenocarcinoma and adenosquamous cervical cancers have not established a clear role for cisplatin-based chemoradiation. In the Katanyoo et al study, concurrent platinum-based chemotherapy did not significantly improve overall survival, although only 37.6% of patients received chemoradiation [3]. The impact of concurrent chemotherapy during radiation has been addressed in other retrospective studies with no apparent improvement although the authors have included disclaimers about the regimens employed [6].
Therefore, the current GOG ancillary data study was undertaken to evaluate impact of histology in the prospective randomized trials of primary cisplatin-based chemoradiation for locally advanced cervical cancer.
METHODS
We retrospectively analyzed GOG trials numbered 85, 120, 123, 165 and 191 [7–11]. Patients provided written informed consent consistent with federal, state and local institutional requirements. These trials have been reported previously and included patients with stage IB2: GOG Trials 123, 191 and stages IIA: GOG Trial 191 and stage IIB-IVA: GOG Trials 85,120, 165, and 191. In GOG trials 85 and 120 patients underwent surgical staging to exclude para-aortic nodal metastasis and pelvic nodal dissection was optional. While in GOG trials 123, 165 and 191 surgical staging was optional and performed on 7.5%, 18% and 23.7% respectively. Tumor size measured clinically within 0.5 cm was obtained before treatment. All patients were treated with a combination of external radiation and brachytherapy per protocol guidelines. The duration of external radiation for GOG trials 85, 120 and 123 required external radiation treatment to be given over 10 weeks, while GOG trials 165 and 191 required external radiation treatment to be given over 8 weeks. All patients’ tumors underwent central pathologic review for confirmation of histology and tumor grade. Due to the small sample size, we wanted to combine patients with adenocarcinoma and adenosquamous carcinoma based on the fact that in previous studies these two entities had similar patterns of failure, progression-free and overall survival [12]. To justify this combination we performed an analysis comparing adenosquamous carcinoma with adenocarcinoma. With adenosquamous as the referent, adenocarcinoma patients in the PFS model had a HR of 0.93 (95% CI, 0.59–1.48, P=0.769). In the OS model, adenocarcinoma patients had a HR of 0.84 (95% CI, 0.52–1.36, P=0.484). In both models the histology variables were not significant. Therefore, patients with subgroups of adenocarcinoma and adenosquamous carcinoma were combined and compared to patients with squamous cell carcinoma.
Categorical variables were compared between the histology groups by the Pearson chisquare test [13], and continuous variables by the Wilcoxon–Mann–Whitney test [14]. Overall survival was estimated using the Kaplan–Meier method [15]. The Cox proportional hazards model was used to evaluate independent prognostic factors and to estimate their covariateadjusted effects on progression-free and overall survival [16]. Continuous variables exhibiting skewed distribution (i.e. tumor size) were included in the survival model after log transformation, and the nonlinearity of the effect of continuous variables was assessed using restricted cubic splines. All statistical tests were two-tailed with the significance level set at α=0.0499. Statistical analyses were performed using the R programming language and environment [17].
RESULTS
One thousand six hundred and seventy-one patients treated on the GOG studies were analyzed of which 89.1% (1489) were squamous, and 10.9% (182) had adenocarcinoma or adenosquamous cancers (6% had adenosquamous and 4.8% had adenocarcinoma). The demographics of squamous and adeno- and adenosquamous carcinomas of the cervix are compared in Table 1. Adeno- and adenosquamous carcinomas were more often stage IB2, 27.5% versus 20.0%, and fewer had stage IIIB, 21.4% versus 28.6%, though these differences were not statistically significant (p=0.102). The mean tumor size was larger for squamous (6.5 cm) than adeno- and adenosquamous carcinomas (5.9 cm; t-test for difference in means, p<0.001). Adeno- and adenosquamous carcinomas were more often poorly differentiated, 46.2% versus 26.9%.
Table 1.
Patient Characteristics by Tumor Histology
| N | squamous-cell carcinoma N = 1489 |
adeno- & adenosq. carcinoma N = 182 |
Test Statistic | |
|---|---|---|---|---|
| Age (years) | 1671 | 46.5 (38.8–55.9) | 46.0 (54.6–39.4) | P = 0.561 |
| Race/Ethnicity | 1671 | P = 0.0772 | ||
| White | 59.9% (892) | 64.8% (118) | ||
| Black | 24.3% (362) | 15.9% (29) | ||
| Hispanic, other | 12.2% (182) | 15.4% (28) | ||
| Asian | 3.6% (53) | 3.8% (7) | ||
| Performance status | 1671 | P = 0.4592 | ||
| normal, asymptomatic | 70.9% (1055) | 75.3% (137) | ||
| symptomatic, ambulatory | 25.4% (378) | 21.4% (39) | ||
| symptomatic, in bed | 3.8% (56) | 3.3% (6) | ||
| Tumor size (cm) | 1659 | 6.0 (5.0–8.0) | 6.0 (5.0–7.0) | P < 0.0011 |
| Tumor size (cm) | 1659 | P = 0.0052 | ||
| < 5.0 | 15.8% (234) | 22.5% (41) | ||
| 5.0–6.0 | 18.8% (278) | 24.7% (45) | ||
| 6.0–7.5 | 38.9% (575) | 35.2% (64) | ||
| ≥ 7.5 | 26.4% (390) | 17.6% (32) | ||
| FIGO stage | 1671 | P = 0.1022 | ||
| IB2 | 20.0% (298) | 27.5% (50) | ||
| IIB | 47.1% (702) | 47.3% (86) | ||
| IIIA | 1.3% (20) | 1.6% (3) | ||
| IIIB | 28.6% (426) | 21.4% (39) | ||
| IVA | 2.9% (43) | 2.2% (4) | ||
| Tumor grade (differentiation) | 1671 | P < 0.0012 | ||
| well | 5.5% (82) | 14.3% (26) | ||
| moderate | 65.3% (972) | 37.9% (69) | ||
| poor | 26.9% (400) | 46.2% (84) | ||
| not graded | 2.4% (35) | 1.6% (3) | ||
| Hydronephrosis | 1671 | P = 0.62 | ||
| none | 88.9% (1324) | 91.2% (166) | ||
| unilateral | 8.8% (131) | 6.6% (12) | ||
| bilateral | 2.3% (34) | 2.2% (4) | ||
| Parametrial involvement | 1671 | P = 0.1082 | ||
| none | 21.7% (323) | 28.6% (52) | ||
| unilateral | 46.3% (689) | 42.9% (78) | ||
| bilateral | 32.0% (477) | 28.6% (52) | ||
| Pelvic nodes | 1671 | P = 0.0332 | ||
| positive | 10.3% (154) | 15.9% (29) | ||
| negative | 62.8% (935) | 63.2% (115) | ||
| unknown | 26.9% (400) | 20.9% (38) |
b (a–c) represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
N is the number of non–missing values.
Numbers after percents are frequencies.
Tests used:
Wilcoxon test;
Pearson test
The treatment regimens by tumor histology are represented in Table 2. Cisplatin-based chemoradiation was utilized in 56.5% of squamous cell carcinoma patients compared to 61.5% of adenocarcinoma and adenosquamous cervical carcinoma patients. Adjusted rates of morbidity for adeno- and adenosquamous - vs. squamous histology are presented in table 3. Although most toxicities were equivalent between the 2 histologies, neurologic, auditory and visual toxicities were more common in squamous cell carcinoma patients, while pulmonary toxicities were more common among adenocarcinoma and adenosquamous carcinoma patients.
Table 2.
Treatment Regimen by Tumor Histology
| N | squamous-cell carcinoma N = 1489 |
Adeno/adenosquamous carcinoma N = 182 |
|
|---|---|---|---|
| Treatment | 1671 | ||
| RT | 11.6% (173) | 14.3% (26) | |
| RT+5FU | 8.9% (133) | 12.1% (22) | |
| RT+HU | 22.9% (341) | 12.1% (22) | |
| RT+CDDP | 32.6% (485) | 37.4% (68) | |
| RT+CDDP+5FU | 10.4% (155) | 11.5% (21) | |
| RT+CDDP+5FU+HU | 10.2% (152) | 10.4% (19) | |
| RT+CDDP+rHuEPO | 3.4% (50) | 2.2% (4) |
RT = Radiation therapy
5FU = 5-fluorouracil
HU = hydroxyurea
CDDP = cis-diamminedichloroplatinum
rHuEPO = recombinant human erythropoietin
N is the number of non–missing values.
Numbers after percents are frequencies.
Table 3.
Adjusted Rates of Morbidity for Adeno- vs. Squamous Histology
| Variable | Odds ratio* | 95% CI |
|---|---|---|
| Toxicity | ||
| WBC | 0.85 | 0.60–1.18 |
| peripheral neuropathy | 0.87 | 0.13–3.35 |
| neurologic, auditory, visual | 0.32 | 0.08–0.89 |
| other hematologic | 0.74 | 0.52–1.06 |
| skin | 1.00 | 0.56–1.68 |
| genitourinary | 0.98 | 0.57–1.61 |
| lymphatic | 2.15 | 0.29–10.24 |
| gastrointestinal, hepatic | 0.91 | 0.65–1.26 |
| constitutional, fever | 0.82 | 0.43–1.44 |
| pulmonary | 3.05 | 1.04–7.91 |
| allergy, immunological | 0.99 | 0.05–5.78 |
| cardiovascular | 1.52 | 0.56–3.54 |
| other | 0.84 | 0.47–1.43 |
Adjusted for age, race, performance status, tumor size, stage, grade, and treatment.
Multivariate Cox modeling was used to analyze prognostic factors for progression-free and overall survival for all patients (Table 4), controlling for treatment by stratification; and for patients treated without concurrent cisplatin (i.e. radiation alone, radiation with 5- fluorouracil, and radiation with hydroxyurea) and for patients treated with concurrent cisplatin (i.e. cisplatin alone; cisplatin with 5- fluorouracil; cisplatin, 5- fluorouracil and hydroxyurea; and cisplatin with recombinant human erythropoietin) respectively. Overall survival was poorer for adeno- and adenosquamous carcinomas compared to squamous carcinomas when analyzed together. African-American race is associated with a significantly poorer overall survival (p=0.010) and Asian race is associated with a significantly improved overall survival (p=0.013). However, African-Americans did equally as poorly with squamous and non-squamous histologies. However, the effect of race was only present for patients receiving radiation without cisplatin and was not present among cisplatin-treated patients. Performance status 0 versus 1 and 0 versus 2 significantly affected overall survival p=0.019 and 0.014, respectively. For both squamous and adeno- and adenosquamous carcinomas there were statistically poorer overall survival rates for patients with stage IIB and greater stages as compared with stage IB2. Similarly, when analyzed by tumor size, poorer overall survival was noted for each increment in tumor size for both squamous and adeno- and adenosquamous carcinomas. Based on the Cox model, each 10% increase in tumor size was associated with a 3% increase in risk of disease progression and a 2% decrease in overall survival.
Table 4.
Progression-free and Overall survival Analysis of Patients with Squamous or Adeno- and Adenosquamous Carcinoma
| N | Nevent | Adj. HR (PFS) | P† | Nevent | Adj. HR (OS) | P† | |
|---|---|---|---|---|---|---|---|
| Histology | |||||||
| squamous-cell carcinoma | 1489 | 777 | referent | — | 718 | referent | — |
| adeno- & adenosq. carcinoma | 182 | 97 | 1.21 (0.98–1.51) | 0.083 | 92 | 1.25 (1.00–1.56) | 0.0499 |
| Age (years) | 1671 | 874 | 1.00 (0.99–1.01) | 0.883 | 810 | 1.00 (1.00–1.01) | 0.299 |
| Race/Ethnicity | |||||||
| White | 1010 | 530 | referent | — | 495 | referent | — |
| Black | 391 | 227 | 1.21 (1.03–1.42) | 0.018 | 215 | 1.24 (1.05–1.46) | 0.010 |
| Hispanic, other | 210 | 92 | 0.91 (0.73–1.14) | 0.419 | 82 | 0.87 (0.69–1.11) | 0.265 |
| Asian | 60 | 25 | 0.76 (0.50–1.15) | 0.196 | 18 | 0.54 (0.33–0.88) | 0.013 |
| Performance status | |||||||
| normal, asymptomatic | 1192 | 580 | referent | — | 526 | referent | — |
| symptomatic, ambulatory | 417 | 251 | 1.16 (0.99–1.36) | 0.061 | 242 | 1.22 (1.03–1.43) | 0.019 |
| symptomatic, in bed | 62 | 43 | 1.39 (1.01–1.93) | 0.046 | 42 | 1.51 (1.09–2.11) | 0.014 |
| log Tumor size (cm) | 1659 | 874 | 1.32 (1.07–1.62) | 0.009 | 810 | 1.28 (1.04–1.58) | 0.021 |
| FIGO stage | |||||||
| IB | 348 | 133 | referent | — | 113 | referent | — |
| IIB | 788 | 385 | 1.39 (1.06–1.84) | 0.018 | 357 | 1.51 (1.12–2.03) | 0.006 |
| IIIA | 23 | 17 | 3.47 (1.96–6.14) | < 0.001 | 17 | 4.61 (2.58–8.24) | < 0.001 |
| IIIB | 465 | 302 | 2.13 (1.59–2.85) | < 0.001 | 289 | 2.37 (1.74–3.24) | < 0.001 |
| IVA | 47 | 37 | 3.46 (2.25–5.31) | < 0.001 | 34 | 3.27 (2.08–5.15) | < 0.001 |
| Tumor grade (differentiation) | |||||||
| good | 108 | 51 | referent | — | 50 | referent | — |
| moderate | 1079 | 552 | 1.11 (0.83–1.49) | 0.481 | 507 | 1.04 (0.78–1.40) | 0.773 |
| poor | 484 | 271 | 1.44 (1.06–1.95) | 0.018 | 253 | 1.35 (0.99–1.84) | 0.055 |
b (a–c) represent the lower 95% confidence limit a, the value b, and the upper 95% confidence limit c.
Progression-free survival (PFS) and overall survival (OS) are given in months; hazard ratios (HR) are unitless.
Tests used:
Wald test
When treated with radiation therapy without concurrent cisplatin, the 70 patients with adeno- and adenosquamous carcinomas of the cervix showed a statistically slightly poorer overall survival (p=0.0499) compared to the 647 patients with squamous cell carcinoma of the cervix. (Figure 1) However, when treated with concurrent cisplatin-based chemotherapy during radiation therapy the 112 patients with adeno- and adenosquamous carcinomas had a similar progression-free (p=0.315) and overall survival (p=0.459) compared the 842 patients with squamous cell carcinoma (Figure 2). Adverse effects to treatment were similar among squamous and non-squamous patients with the exception of pulmonary toxicity seen in 1.1% of squamous cell patients and 3.3% of adeno- and adenosquamous carcinoma patients (and neurologic, auditory and visual symptoms which are slightly more common in the squamous cell carcinoma patients 5.4% versus 1.6%.
Figure 1.
Overall survival for patients with squamous and adeno- and adenosquamous carcinomas treated with radiation alone
Figure 2.
Overall survival for patients with squamous and adeno- and adenosquamous carcinomas treated with radiation and concurrent cisplatin chemotherapy
DISCUSSION
This is the first GOG study to retrospectively evaluate locally advanced stage adeno- and adenosquamous carcinomas of the cervix treated with radiation with or without concurrent chemotherapy. Compared to squamous carcinomas, adenocarcinoma and adenosquamous carcinomas were more commonly stage IB2 27% versus 20%. This is not completely unexpected, since in contrast to squamous cell cervical carcinoma, adenocarcinoma and adenosquamous carcinomas arise within the endocervical canal. As they grow, they distend the cervical stroma creating a barrel shaped cervix, which does to have definitive parametrial invasion and precludes classification as stage IIB. There was statistically poorer overall survival for patients with stage IIB and greater compared with stage IB2. The confidence intervals of stage IIB to IVA overlapped. Since large tumor size can often be reached with expansile endocervical tumors, we chose to analyze the effect of clinical tumor size on overall survival, which was poorer with increasing tumor size. However, the adverse effect of increasing tumor size was equal among squamous and non-squamous histologies.
Despite smaller tumor size, which should confer a better prognosis, patients with adenocarcinoma and adenosquamous carcinoma had a poorer overall survival when treated with radiation alone. There are inherent limitations to tumor measurement clinically, however, a previous GOG study demonstrated clinical tumor measurement was closely associated with progression-free and overall survival [18]. Clinical tumor measurement may be more significant in adeno- and adenosquamous carcinomas which occur higher in the genital tract. MRI was not available in the earlier years of these studies and is an option although not currently mandated in our most recent cervical cancer trials of chemoradiation.
The most important finding of our study is the poorer outcome noted for locally advanced stage adenocarcinoma and adenosquamous cancers treated with radiation without cisplatin when compared to squamous cancers and nullification of this difference with the use of concurrent cisplatin-based chemoradiation. This provides the strongest support to date for the use of concurrent cisplatin-based chemoradiation for locally advanced non-squamous cervical cancer. Recently, Wright et al characterized the molecular profile of 40 adenocarcinomas and 40 squamous of the cervix [19]. They found significant differences in KRAS mutations 17.5% vs 0% and non-significant differences in EGFR mutations 0% vs 7.5%, PIK3CA mutations 25% vs 37.5%, for adenocarcinoma and squamous carcinoma, respectively. Adjusted analysis demonstrated tumors with PIK3CA mutations were associated with a shorter overall survival, HR=9.1. They proposed this may account for the differences in outcome and targeting distinct subsets of cervical cancer patients may improve outcome. However, since PIK3CA mutations were more common in squamous cancers, they should have the poorer overall survival. The current study finds a poorer outcome for adenocarcinoma and adenosquamous cancers compared to squamous carcinomas treated with radiation alone but not when treated by cisplatin-based chemoradiation. Toxicity of chemoradiation was very similar between the two histologies. Other strategies to incorporate chemotherapy into the primary radiation treatment of cervical cancer include adjuvant and neoadjuvant. Phase II and III studies that have used concurrent and post-radiation chemotherapy have demonstrated improvement in overall survival [5,12,20]. In these studies, it is difficult to know the relative benefit of concurrent vs adjuvant chemotherapy. The impact of post-radiation adjuvant chemotherapy following concurrent chemotherapy for locally advanced cervical cancer is unknown but is currently being studied in the Outback trial in which patients are randomized to adjuvant chemotherapy with carboplatin and paclitaxel for 4 cycles or observation following weekly cisplatin during radiation.[21] The impact of concurrent and adjuvant cisplatin-based chemoradiation on adenocarcinoma and adenosquamous cervical carcinomas was noted in the study of post-radical hysterectomy by Peters et al [5]. This trial administered two doses of cisplatin and 5-fluorouracil chemotherapy during radiation therapy and two courses following radiation. An improvement was noted for patients who received the post-radiation chemotherapy but a subset analysis by histology was not performed.
The use of both neoadjuvant and adjuvant chemotherapy with concurrent chemoradiation in adenocarcinomas was recently reported by Tang et al [22]. In their trial, 880 patients with adenocarcinoma of the cervix were randomized to concurrent chemoradiation or concurrent chemoradiation preceded by one cycle of neoadjuvant and two cycles of adjuvant chemotherapy with cisplatin and paclitaxel. Patients who received chemoradiation with adjuvant chemotherapy showed a significantly longer disease-free (P<.05), cumulative overall survival (P<.05) and long-term local tumor control (P<.05). They also had decreased rates of both local and distant failure (P<.05).
In summary, this analysis demonstrates that both adenocarcinoma/adenosquamous and squamous cell cancer appear to respond well to chemoradiation with no differences detected in this large retrospective analysis of prospectively collected data. The role of other strategies of chemoradiation awaits ongoing and future trials.
Research Highlights.
We retrospectively analyzed histology in locally advanced cancers of the cervix treated in GOG randomized trials of chemoradiation.
Adenocarcinoma or adenosquamous (non-squamous) carcinoma had a poorer survival compared to squamous carcinoma.
Non-squamous patients had poorer survivals when treated with radiation without concurrent cisplatin but similar survival when treated with concurrent cisplatin.
ACKNOWLEDGEMENTS
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Medical Center, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group, P.C., University of California at Los Angeles, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical Center, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, SUNY Downstate Medical Center, Eastern Virginia Medical School, Johns Hopkins Cancer Center, State University of New York at Stony Brook, Eastern Pennsylvania Gynecology/Oncology Center, P.C., Southwest Oncology Group, Cooper Hospital/University Medical Center and Columbus Cancer Council, University of Oklahoma, Wayne State University, Ellis Fischel Cancer Center, Tampa Bay/H. Lee Moffitt Cancer Center, New York Hospital/Cornell Medical Center, University of Kentucky, Case Western Reserve University, Stanford University Medical Center, Tacoma General Hospital, University of Washington/Puget Sound Oncology Consortium, Cleveland Clinic Foundation, Fox Chase Cancer Center, Women's Cancer Center, University of Massachusetts Medical Center, University of Chicago, University of Minnesota Medical School, Emory University Clinic, Community Clinical Oncology Program, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, National Cancer Institute of Cancer, MD Anderson CCOP, Thomas Jefferson University Hospital, University of Virginia, University of Texas-Galveston and Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors wish to disclose that there are no conflicts of interest.
REFERENCES
- 1.Smith HO, Tiffany MF, Qualls CR, Key CR. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi: 10.1006/gyno.2000.5826. [DOI] [PubMed] [Google Scholar]
- 2.Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125:287–291. doi: 10.1016/j.ygyno.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Katanyoo K, Sanguanrungsirikul S, Manusirivithaya S. Comparison of treatment outcomes between squamous cell carcinoma and adenocarcinoma in locally advanced cervical cancer. Gynecol Oncol. 2012;125:292–296. doi: 10.1016/j.ygyno.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 4.NCI Clinical Announcement. U.S. Dept. of Health and Human Services, Public Health Service. National Institutes of Health; 1999. Feb, [Google Scholar]
- 5.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 6.Huang YT, Wang CC, Tsai CS, Lai CH, Chang TC, Chou HH, et al. Long-term outcome and prognostic factors for adenocarcinoma/adenosquamous carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:429–436. doi: 10.1016/j.ijrobp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC, Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 8.Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 9.Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL, 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 10.Lanciano R, Calkins A, Bundy BN, Parham G, Lucci JA, 3rd, Moore DH, et al. Randomized comparison of weekly cisplatin or protracted venous infusion of fluorouracil in combination with pelvic radiation in advanced cervix cancer: a gynecologic oncology group study. J Clin Oncol. 2005;23:8289–8295. doi: 10.1200/JCO.2004.00.0497. [DOI] [PubMed] [Google Scholar]
- 11.Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JL-Y, Cheng JC-H, Kuo S-H, Chen C-A, Lin M-C, Huang C-Y. Outcome analysis of cervical adenosquamous carcinoma compared with adenocarcinoma. Acta Obstet Gynecol Scand. 2012;91(10):1158–1166. doi: 10.1111/j.1600-0412.2012.01420.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philosophical Magazine Series 5. 1900;50:157–175. [Google Scholar]
- 14.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. URL http://www.R-project.org/, ISBN 3-900051-07-0. [Google Scholar]
- 18.Stehman FB, Bundy B, DiSaia PJ, Keys HM, Larson JE, Fowler WC. Carcinoma of the cervix treated with radiation therapy: A multi-variate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–2785. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: Genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119:3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duenas-Gonzalez A, Zarba JJ, Patel F, Alcedo JC, Beslija S, Casanova L, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol. 2011;29:1678–1685. doi: 10.1200/JCO.2009.25.9663. [DOI] [PubMed] [Google Scholar]
- 21. [2/25/2014]; clinicaltrials.gov, NCT01414608.
- 22.Tang J, Tang Y, Yang J, Huang S. Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma. Gynecol Oncol. 2012;125:297–302. doi: 10.1016/j.ygyno.2012.01.033. [DOI] [PubMed] [Google Scholar]