INTRODUCTION
In February 2010, the National Institute for Occupational Safety and Health (NIOSH) received a confidential employee request for a Health Hazard Evaluation (HHE) at a veterinary teaching hospital that provides routine care and oncology services to large and small animals. Canines and felines constitute the majority of the oncology department’s patients. Veterinary hospital employees were concerned about adverse health effects from the use of chemotherapy drugs in the oncology department.
In 2004, NIOSH released an Alert regarding occupational exposures to antineoplastic and other hazardous drugs that are used in health care settings. Hazardous drugs were defined in the NIOSH Alert as having “specific health effects (such as skin rashes, cancer, and reproductive effects) and high toxicity at low doses.”(1) The NIOSH Alert also described best practices for handling, storage, disposal, and decontamination procedures and was updated in 2010 and 2012 to include an expanded list of hazardous drugs.(2,3) Also in 2010, NIOSH released a Workplace Solutions document to increase awareness of occupational safety and health issues in veterinary health care workers who work with antineoplastic and hazardous drugs.(4)
PROCESS DESCRIPTION
Chemotherapy drugs were received at the veterinary hospital pharmacy as powders, liquids, or premixed solutions. Pharmacy personnel dispensed oral drugs on a per patient basis for owner administration at home. Drugs that were administered at the veterinary hospital were transported to the oncology department’s chemotherapy drug preparation room where they were refrigerated and stored until needed. The drugs were removed and prepared in a Class II biological safety cabinet (BSC) in the chemotherapy drug preparation room. Air exhausted from the BSC passed through a high-efficiency particulate air (HEPA) filter and was recirculated back into the room (Figure 1).
FIGURE 1.
Veterinary technician wearing personal protective equipment while preparing chemotherapy drugs in a Class II biosafety cabinet.
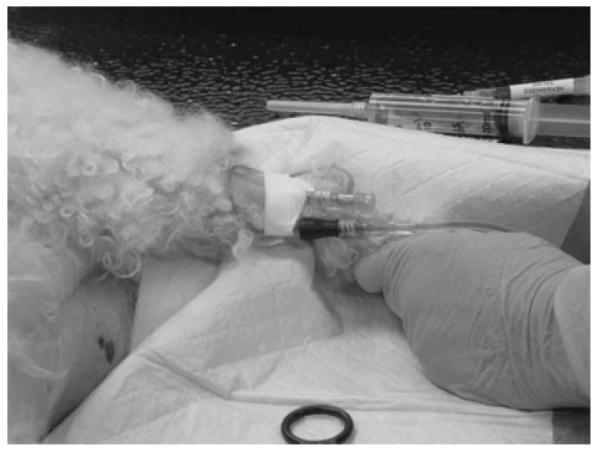
Animals typically receive several diagnostic procedures before receiving chemotherapy drugs to ensure that they are healthy enough to receive the treatment. Animals were kenneled in the drug administration area until cleared for treatment. Once cleared, animals received the chemotherapy drugs in a procedure that required at least two employees to restrain the animals for intravenous (IV) catheter placement and drug administration. Animals were placed on the treatment table and prepared by shaving and disinfecting the area where the IV catheter was placed. The administering technician then injected the chemotherapy drug. Figure 2 illustrates a chemotherapy drug injection process.
FIGURE 2.
Intravenous administration procedure for doxorubicin.
After treatment, animals were removed from the table and kenneled in the administration area or in cages or runs in adjacent areas (Figure 3). Employees then decontaminated the treatment table and surrounding floor with a bleach solution. The IV tubing, bag, and other potentially contaminated items were placed in chemotherapy-approved disposal containers, and the area was wiped with a 10% bleach solution and allowed to dry.
FIGURE 3.
A chemotherapy drug-treated canine in a labeled kennel. The label warns employees of potential chemotherapy drug cross contamination and identifies the chemotherapy drug used.
After receiving chemotherapy, animals occasionally may be taken to radiology/ultrasound, the nursing care unit, or the critical care unit for further diagnostics, medical care, or observation if needed. Animals also were walked indoors or outside the veterinary hospital. After completing treatment, animals were discharged to their owners with instructions on how to safely handle the animal’s urine, feces, and vomit that could be contaminated with chemotherapy drugs. The owners were also told how to safely administer chemotherapy drugs and clean up accidents and were informed about personal protective equipment (PPE) that should be worn when cleaning up after the animals.
ASSESSMENT
In 2010, we collected surface wipe and air samples for cyclophosphamide, ifosfamide, and doxorubicin, chemotherapy drugs commonly used at this veterinary hospital. Surface wipe samples were collected in the chemotherapy drug preparation room and administration area. Surface samples were collected in areas thought to have the greatest potential for chemotherapy drug contamination. Offices, employee break rooms, and public reception areas where no PPE was used were also sampled to learn whether chemotherapy drugs were inadvertently spread beyond treatment areas.
General area air samples were collected in the chemotherapy drug preparation room and administration area. A background area air sample was collected in a clerical area. Because cyclophosphamide, ifosfamide, and doxorubicin are unlikely to volatilize due to low vapor pressure, we used an airflow sampling rate of 15 L/min to increase our ability to detect low airborne concentrations. We also visually examined the BSC, reviewed its certification records, and asked employees and managers about their work practices when using the BSC.
We held confidential interviews with 13 randomly selected employees who worked directly with chemotherapy drugs, including veterinarians and licensed veterinary and pharmacy technicians. We asked about their work history, health concerns, and medical history. We asked for their personal assessment of safety policies and procedures, knowledge about recommended disposal methods for chemotherapy drugs and supplies, and satisfaction with the veterinary hospital’s health and safety program. We also asked about the PPE used when they handled chemotherapy drugs, and their perceptions about communication with their supervisor about safety issues related to chemotherapy drug handling and administration.
We talked informally with employees and observed work practices in other areas of the veterinary hospital, including radiology, critical care and nursing care units, other patient wards, the laundry, and groundskeeping. We assessed employees’ knowledge about handling animals that had received chemotherapy drugs, disposal of chemotherapy drugs, and cleaning procedures.
RESULTS
As shown in Table I, most of the surface sample results (40 of 44) were below the limit of detection (LOD) of 5 ng per sample for cyclophosphamide and 2 ng per sample for ifosfamide. The highest result, 240 ng/100 cm2 for cyclophosphamide, was collected beneath the BSC grate, and all detectable levels of cyclophosphamide and ifosfamide were from samples collected in and around the BSC in the chemotherapy drug preparation room. This suggests the potential for chemotherapy drug exposures to employees compounding or mixing in the BSC. No doxorubicin was detected (LOD = 7 ng per sample) in any of the surface wipe samples. However, the recovery of doxorubicin from these samples may have been poor because the samples were frozen prior to analysis.
TABLE I.
Chemotherapy Drugs in Surface Wipe Samples Collected on September 13–15, 2010
| Results, ng/100 cm2 |
|||
|---|---|---|---|
| Location | Sample Description | Cyclophosphamide | Ifosfamide |
| Pharmacy | Receiving table | ND | ND |
| Plastic bin for transporting chemotherapy drugs | ND | ND | |
| Desktop near oral chemotherapy pill cabinet | ND | ND | |
| Countertop adjacent to small refrigerator and BSC | ND | ND | |
| Top of chemotherapy waste disposal bin | ND | ND | |
| Drug Preparation Room (Room A) |
Countertop adjacent to small refrigerator and BSC | ND | ND |
| BSC working surface | 77 | ND | |
| BSC airfoil | ND | ND | |
| BSC top portion of cabinet (estimated area) | ND | ND | |
| BSC underneath the grate | 240 | 37 | |
| Floor directly in front of BSC | (5) | 29 | |
| Administration Area (Room B) |
Chemotherapy drug administration table (stainless steel area) | ND | ND |
| Tool cart with various supplies and instruments | ND | ND | |
| Exam table on the soft padding | ND | ND | |
| Exam table on the soft padding after doxorubicin administration | (11) | ND | |
| Floor near technician after doxorubicin administration | ND | ND | |
| Floor between examination tables | ND | ND | |
| Chemotherapy waste disposal lid after doxorubicin | ND | ND | |
| Cubicle | Telephone in cubicle near hallway door (estimated area) | ND | ND |
| Reception Area | Discharge medical records bin | ND | ND |
| Countertop in area behind reception desk | ND | ND | |
| Reception area floor | ND | ND | |
| Reception desk near computer | ND | ND | |
| Women’s Locker Room | Changing area floor | ND | ND |
| Floor near door | ND | ND | |
| Radiology Room | Room 4 on exam table under X-ray | ND | ND |
| Floor next to exam table | ND | ND | |
| Sandbag used to position animals | ND | ND | |
| Laundry | Floor in front of washer | ND | ND |
| Washer loading door (estimated area) | ND | ND | |
| Oncology Hallway | Floor directly outside pharmacy mixing room D157 | ND | ND |
| Floor directly outside the chemotherapy administration room | ND | ND | |
| Floor directly outside the technician offices | ND | ND | |
| Floor inside technician office area | ND | ND | |
| Floor entering technician office from Administration | ND | ND | |
| Floor at corner of hallway away from Reception area | ND | ND | |
| Floor near door to Room D165 | ND | ND | |
| Animal Run Area | Floor near drain in the run area | ND | ND |
| Technician Office | On table by door to Administration | ND | ND |
| Keyboard on the middle desk | ND | ND | |
| Conference Room | Conference room table | ND | ND |
| Ward 1 | Floor next to animal treated with vincristine | ND | ND |
| Holding Area | Floor near chemotherapy treated animal | ND | ND |
| Ultrasound | Floor near drain | ND | ND |
| Small Animal | Reception area floor near front door | ND | ND |
| LOD | 5 | 2 | |
| LOQ | 17 | 7.3 | |
Notes: ND=not detected (result was below the LOD). Sample results in parentheses were between the LOD and the LOQ, meaning that they havemore uncertainty associated with them.
Cyclophosphamide and ifosfamide were not detected (minimum detectable concentration was 0.07 ng/m3, for a 7000-L air sample) in general area air samples collected from the chemotherapy drug preparation room, the administration area, and an office behind admissions. Doxorubicin was not detected (minimum detectable concentration was 0.1 ng/m3 for a 7000-L air sample), but its recovery may have been poor because the samples were frozen prior to analysis.
Employee Interviews
The median age of the 13 employees we interviewed was 34 years (range: 27 to 55 years). The median number of years employees had worked with chemotherapy drugs, either at this veterinary hospital or in other workplaces, was 4.5 years, with a range of less than 1 to 30 years. Most employees denied any health symptoms while handling or working around chemotherapy drugs. Three employees reported headache, and one reported occasional nausea and facial flushing. One employee reported abnormal menstruation that began after starting work with chemotherapy drugs. No employees reported hair loss at the time of the interviews, although this problem had been reported at the time of the original HHE request.
When asked how satisfied they were with their work area’s health and safety program, 10 of 13 employees reported “not satisfied,” while three employees reported being “somewhat satisfied” or “satisfied.” When asked if written policies were available at their work area regarding PPE use, 11 of 13 reported no written policies were available. Regarding self-reported PPE use, 70% reported “always” wearing double gloves when administering chemotherapy drugs, and 60% reported “always” wearing disposable gowns. Several employees reported concerns about being required to re-use disposable PPE items such as gowns because of cost concerns. Most employees demonstrated a good working knowledge of the proper procedures for the disposal of chemotherapy drugs and administration supplies.
Review of the Biological Safety Cabinet and Other Workplace Observations
The Class II BSC was certified annually as recommended by the Centers for Disease Control and Prevention.(5) According to veterinary hospital records, the BSC met the recommended exhaust flow rate of 100 linear ft/min with the sash open to the typical operating height. The BSC was equipped with a HEPA filter and recirculated 100% of the exhausted air back into the chemotherapy drug preparation room.
Some veterinary hospital employees voluntarily wore elastomeric half-mask respirators equipped with organic vapor cartridges when they prepared and/or administered chemotherapy drugs. We noted that when chemotherapy drug-treated animals were taken to other areas of the hospital or returned home they were no longer visually identifiable as having recently received chemotherapy drugs. A treated animal can spread the parent drug and metabolites of the chemotherapy drugs through biological fluids or wastes such as urine, feces, and vomit.(6)
DISCUSSION
We found cyclophosphamide and ifosfamide in a few surface wipe samples, mainly in and around the BSC in the chemotherapy drug preparation room. Although we did not detect doxorubicin, it is important to note that the samples were frozen for approximately 9 months while an analytical method was developed. NIOSH has studied the stability of cyclophosphamide and ifosfamide on surface wipe samples, and no recovery degradation was observed (personal communication, G. Burr and J. Pretty, NIOSH). However, NIOSH chemists have observed in laboratory experiments that doxorubicin degraded after being frozen (personal communication; G. Burr and J. Pretty, NIOSH). Therefore, we cannot exclude the possibility that doxorubicin may have been present when the samples were collected.
One limitation to this evaluation is that we collected surface wipe samples over 3 days, which may not be representative of typical exposures. Levels of chemotherapy drugs on surfaces may vary over time depending on patient load, quantities of drugs, and whether proper work practices are followed. Another limitation is that the surface wipe samples were analyzed only for cyclophosphamide, ifosfamide, and doxorubicin, although the veterinary hospital uses other hazardous drugs.(2) Because the possibility remains that other hazardous drugs may be present or that exposures could be greater at other times, we consider it prudent to control potential chemotherapy drug exposures to levels as low as reasonably achievable.
The absence of cyclophosphamide, ifosfamide, or doxorubicin in air samples is not unexpected considering these drugs are not volatile at room temperature. However, because doxorubicin can degrade when frozen we cannot exclude the possibility that it may have been present when the air samples were collected (personal communication, G. Burr and J. Pretty, NIOSH). In this evaluation we learned that mustargen, a chemotherapy drug that is more volatile than cyclophosphamide, ifosfamide, or doxorubicin, was prepared in the BSC. Sampling and analytical methods do not exist for mustargen on work surfaces or in the air.
We learned that the veterinary hospital was transitioning to preparing mustargen in a chemical fume hood (which did not recirculate exhaust air) instead of the BSC. However, chemotherapy drugs should not be prepared in a chemical fume hood because the sterility of the drug(s) may be compromised. Since a HEPA filter in a BSC does not capture and remove drug vapors, the potential for exposure exists if some or all of the exhausted air is recirculated inside the facility.
Three employees reported acute symptoms that they associated with their work. We are unable to determine if the symptoms of headache, nausea, facial flushing, and abnormal menstruation were related to work; however, these symptoms have been associated with occupational exposure to chemotherapy drugs in other studies.(7,8) Most employees reported dissatisfaction with their work area’s health and safety program, including the lack of written policies on PPE use. Although most interviewed employees reported proper PPE use when administering chemotherapy drugs, appropriate PPE should be worn at all times during drug administration.
CONCLUSION
Cyclophosphamide and ifosfamide, but not doxorubicin, were detected on some surface wipe samples, primarily in and around the BSC. We did not detect these chemotherapy drugs in the air. Because doxorubicin degrades after being frozen (personal communication, G. Burr and J. Pretty, NIOSH), we cannot exclude the possibility that this drug may have been present when the surface or air samples were collected. More volatile chemotherapy drugs such as mustargen have the potential to enter the work area if they are prepared in the BSC because some of the exhausted air is recirculated. We could not determine if the acute health symptoms reported by employees were work related. Recommendations are provided to limit chemotherapy drug exposure, address employee concerns about their workplace health and safety program, and maintain consistent work practices and PPE use.
RECOMMENDATIONS
On the basis of our findings we recommend the actions listed below to create a more healthful workplace. We encouraged the veterinary hospital to use a labor-management health and safety committee or working group to discuss the recommendations in this report and develop an action plan. Those involved in the work can best set priorities and assess the feasibility of our recommendations for the specific situation at the veterinary hospital.
Exhaust 100% of the HEPA-filtered air from the BSC to the outdoors.(1)
Do not prepare volatile chemotherapy drugs such as mustargen in the veterinary hospital BSC if any of the exhaust air is recirculated.
Use dedicated cleaning supplies for the chemotherapy drug preparation room and administration area. If possible, store these supplies in the same area where they are used.
Improve communication with critical care and nursing care units, caretaking, and laundry employees about standard operating procedures concerning vomit, urine, and feces from animals that have been given chemotherapy drugs.
Post warning signs outside of the veterinary hospital’s comparative oncology building regarding the potential for chemotherapy drug-contaminated animal waste. Staff should use this area of the building for all chemotherapy drug-treated animals.
Identify animals that have received chemotherapy with brightly colored disposable collars or bands to alert staff that vomit, urine, and feces may contain chemotherapy drugs and that these potentially contaminated areas should be cleaned according to standard operating procedures.
Create an interdisciplinary group consisting of managers, technicians, interns, residents, and university health and safety department representatives to address safety and health of personnel who may come in contact with chemotherapy drugs. This committee should meet routinely, communicate with staff, and work cooperatively with the teaching hospital safety committee.
Encourage participation in the voluntary, university-administered surveillance program for employees who work with chemotherapy drugs. Additional information on a medical surveillance program is provided in the references.(9,10)
Involve employees in selecting training topics and in developing training materials.
Limit access to the chemotherapy preparation and administration rooms to essential employees only. Use self-closing doors to facilitate keeping doors closed.
Follow the OSHA respiratory protection standard [29 CFR 1910.134] regarding voluntary use of respirators, including providing Appendix D of the OSHA respiratory protection standard [29 CFR 1910.134] to employees.
Instruct employees to wear double chemotherapy protective gloves and a protective gown when decontaminating the BSC and when administering chemotherapy drugs.(11) Because of the risk for latex sensitivity, non-latex chemotherapy gloves are recommended. Manufacturer recommendations concerning the use of disposable PPE should be followed.
ACKNOWLEDGMENTS
The authors would like to thank Gregory Burr, Donald Booher, Karl Feldmann, Stefanie Evans, and Ellen Galloway (NIOSH) for their contributions to this project. Analytical support was provided by Jack Pretty (NIOSH) and Bureau Veritas North America. The full HHE report can be accessed at http://www.cdc.gov/niosh/hhe/.
The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of any company or product does not constitute endorsement by NIOSH.
REFERENCES
- 1.National Institute for Occupational Safety and Health (NIOSH) NIOSH Alert: Preventing Occupational Exposure to Antineoplastic and Other Hazardous Drugs in Health Care Settings. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH; Cincinnati, Ohio: 2004. DHHS (NIOSH) Pub. No. 2004-165. [Google Scholar]
- 2.National Institute for Occupational Safety and Health (NIOSH) NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2010. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH; Cincinnati, Ohio: 2010. DHHS (NIOSH) Pub. No. 2010-167. [Google Scholar]
- 3.National Institute for Occupational Safety and Health (NIOSH) NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2012. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH; Cincinnati, Ohio: 2012. DHHS (NIOSH) Pub. No. 2012-150. [Google Scholar]
- 4.National Institute for Occupational Safety and Health (NIOSH) Safe Handling of Hazardous Drugs for Veterinary Healthcare Workers. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, NIOSH; Cincinnati, Ohio: 2010. DHHS (NIOSH) Pub. No. 2010-150. [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) [accessed July 2012];Appendix A—Primary Containment for Biohazards: Selection, Installation and Use of Biological Safety Cabinets. Available at http://www.cdc.gov/biosafety/publications/bmbl5/BMBL5appendixA.pdf.
- 6.Pellicaan CH, Teske E. Risks of using cytostatic drugs in veterinary medical practice. Tijdschr Diergeneeskd. 1999;124(7):210–215. [PubMed] [Google Scholar]
- 7.Shortridge K, Lemasters G, Valanis B, Hertzberg V. Menstrual cycles in nurses handling antineoplastic drugs. Cancer Nurs. 1995;18(6):439–444. [PubMed] [Google Scholar]
- 8.Connor T, McDiarmid M. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J. Clin. 2006;56(6):354–365. doi: 10.3322/canjclin.56.6.354. [DOI] [PubMed] [Google Scholar]
- 9.Occupational Safety and Health Administration (OSHA) [accessed July 2012];OSHA Technical Manual, Section VI, Chapter 2: Controlling Occupational Exposures to Hazardous Drugs. Available at http://www.osha.gov/dts/osta/otm/otmvi/otmvihtml.
- 10.National Institute for Occupational Safety and Health (NIOSH) Medical Surveillance for Health Care Workers Who Work with Hazardous Drugs. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH; Cincinnati, Ohio: 2007. DHHS (NIOSH) Pub. No. 2007-117. [Google Scholar]
- 11.National Institute for Occupational Safety and Health (NIOSH) Personal Protective Equipment for Health Care Workers Who Work with Hazardous Drugs. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, NIOSH; Cincinnati, Ohio: 2009. DHHS (NIOSH) Pub. No. 2009-106. [Google Scholar]