Abstract
Generalized convulsive status epilepticus (GCSE) is one of the most common neurologic emergencies and can be associated with significant morbidity and mortality if not treated promptly and aggressively. Management of GCSE is staged and generally involves the use of life support measures, identification and management of underlying causes, and rapid initiation of anticonvulsants. The purpose of this article is to review and evaluate published reports regarding the treatment of impending, established, refractory, and super-refractory GCSE in pediatric patients.
INDEX TERMS: anticonvulsants, pediatrics, status epilepticus
INTRODUCTION
Generalized convulsive status epilepticus (GCSE) is a common neurologic emergency that represents 1% to 2% of all emergency department visits.1 Status epilepticus (SE) is traditionally defined as any seizure lasting longer than 30 minutes, whether or not consciousness is impaired or seizures recur without an intervening period of consciousness.2 An operational definition that allows for prompt treatment is generally considered to be seizures lasting at least 5 minutes. In reality, the average seizure is less than 2 minutes, and only 40% of seizures lasting 10 to 29 minutes cease without treatment.3,4 The definition of GCSE has evolved to include not only the number and duration of seizures but also the number and types of drugs.5–7 GCSE can be divided into 4 stages: impending, established, refractory, and super-refractory (Table 1).
Table 1.
Stages of Generalized Convulsive Status Epilepticus from Onset of the Seizure
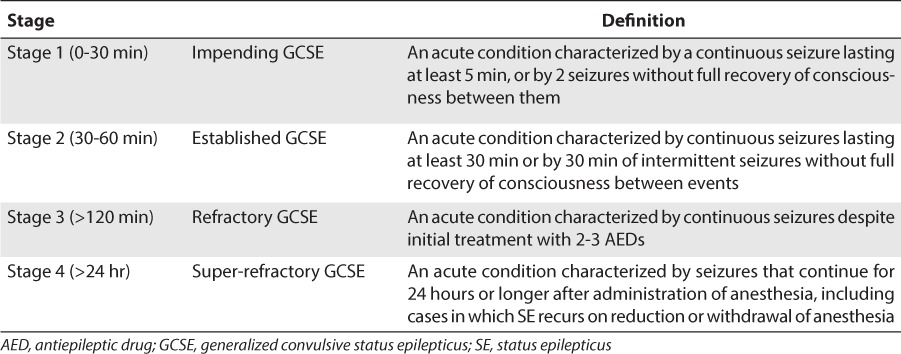
Because SE becomes increasingly resistant to anticonvulsant drugs over time, it can be associated with significant morbidity and mortality if not treated promptly and aggressively.8 Regardless of patient age, the estimated mortality rate due to GCSE in the United States ranges between 22,000 and 42,000 individuals per year,9 with estimated mortality rates as high as 16% in children.10 This article reviews and evaluates the body of research regarding the treatment of impending, established, refractory, and super-refractory GCSE in pediatric patients.
BASIC SCIENCE OF STATUS EPILEPTICUS
After a single generalized tonic-clonic seizure, there is often a postictal state lasting several minutes, during which the seizure threshold is massively elevated. This change restores homeostasis and prevents runaway excitation. During GCSE, those mechanisms fail and seizures occur in succession or even become self-sustaining. How this occurs is unknown, but recent advances suggest potential explanations. Gamma-aminobutyric acid class A (GABAA)-mediated inhibition becomes less effective, whereas glutamate's excitatory actions are enhanced.11 This has implications for sequencing anticonvulsant medication during GCSE and for understanding how GCSE progresses to refractory GCSE.
Repeated seizures produce complex patho-physiological and biochemical changes in the brain. Initially, there is release of neurotransmitters and opening and closing of voltage-gated ion channels. Within minutes, receptor trafficking, primarily of the GABA and glutamate receptors, is responsible for a key adaptation that effects medication efficacy. During repetitive seizures, GABA receptors are internalized (moving from the synaptic membrane to the cytoplasm, where they are functionally inactive), and the γ2 and β2–3 subunits decrease in number on the synaptic membrane.12–15 The γ2 subunit is associated with sensitivity to benzodiazepines, so their loss on the synaptic surface would result in a time-dependent pharmacoresistance to benzodiazepines. These changes may in part explain the failure of GABA inhibition and the development of pharmacoresistance to benzodiazepines. The potency of benzodiazepines may decrease 20-fold over 30 minutes of SE.12,13
While this maladaptive alteration is occurring, excitatory synapses show changes in the opposite direction from those of GABA synapses. N-methyl-d-aspartate (NMDA) receptor subunits are recruited to the synaptic membrane where they form additional receptors that are proconvulsant.12,14,16 Other changes in synaptic activity may increase glutamate release. A vicious cycle ensues, whereby loss of inhibition sustains seizure activity, and surface accumulation of NMDA receptors further upsets a breaking mechanism of ongoing seizure activity.
These receptor changes have important implications for treatment, as impending SE (which may be self-limiting) progresses to established and refractory SE. Seizures generate a transient but severe loss of synaptic inhibitory receptors and peptides, and an increase in synaptic excitatory receptors and peptides. This gives a possible explanation for the tendency of seizures to become self-sustaining and the time-dependent development of pharmacoresistance to GABA-ergic drugs.17 This has clinical implications: 1) prehospital treatment for GCSE should be routine as this has the potential of preventing seizure-induced receptor trafficking and pharmacoresistance; 2) in order to avoid time-dependent complications, pharmacoresistance, and brain damage, rapid and vigorous treatment should be administered for GCSE; and 3) increasing seizure activity causes a progressive loss of synaptic GABA receptors; hence, as initial treatment of GCSE, a benzodiazepine should be combined with another drug acting at a different site.18–20
REVIEW OF MANAGEMENT
Standard of Care
The management of GCSE is staged and generally involves the use of life support measures, identification, and management of underlying causes and rapid initiation of anticonvulsant medications (Table 2). Although there have been some randomized controlled trials comparing therapeutic options, our understanding of the management of impending and established GCSE is driven primarily by consensus protocols or guidelines.2,21–26 Unfortunately, there are no evidence-based studies or commonly accepted consensus protocols for the management of refractory and super-refractory GCSE; hence, optimal treatment for these stages of GSCE remains unclear.
Table 2.
Management of Generalized Convulsive Status Epilepticus
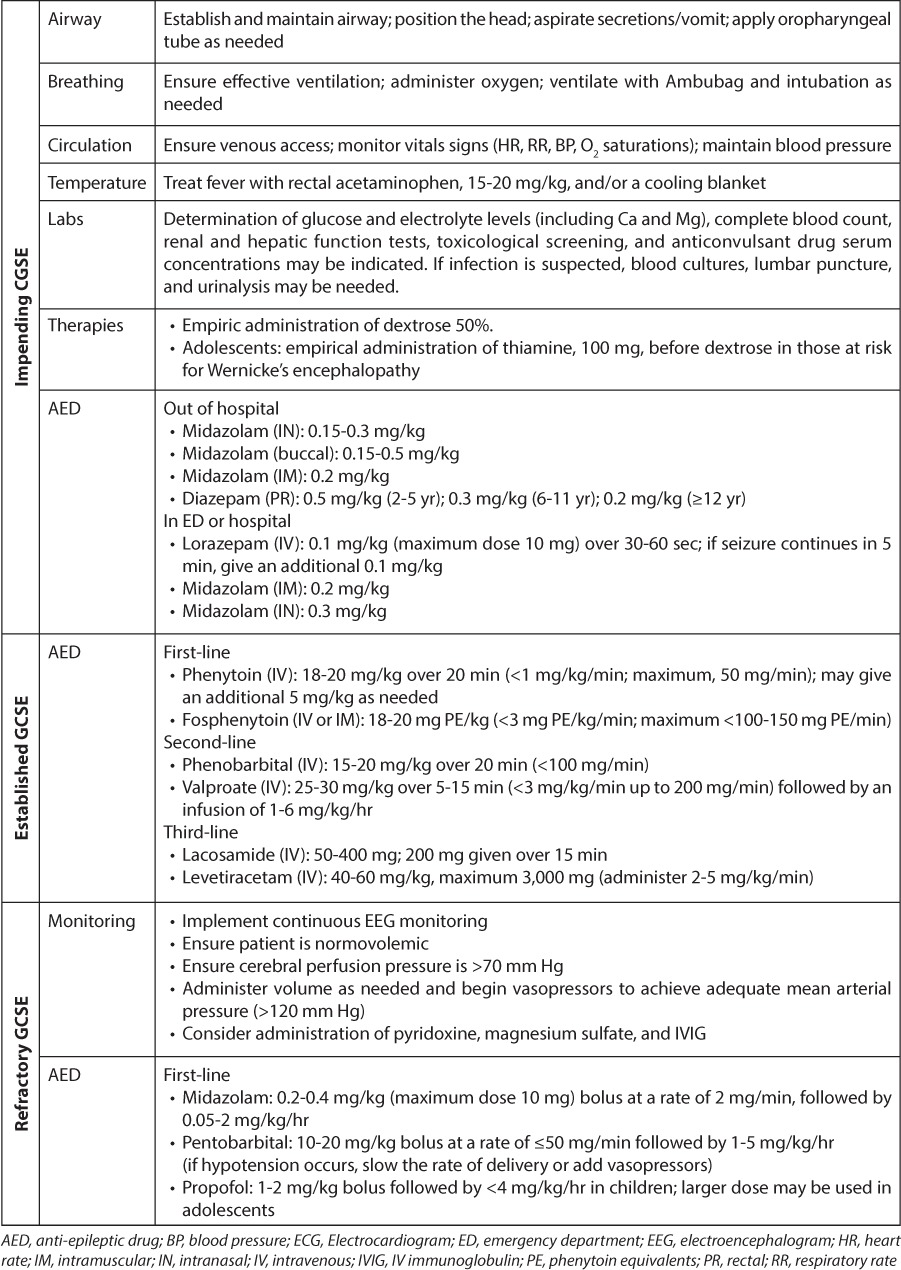
The initial management of GCSE includes securing an airway and monitoring vital signs, especially blood pressure. Arterial blood pressure should remain above 120 mm Hg as cerebral blood flow is dependent upon systemic pressure; pressures of <90 mm Hg must be avoided as a decrease in oxygen and glucose may result in neurologic injury. The patient's temperature should be monitored and fever treated aggressively. Febrile GCSE is most common in pediatric patients, and normalization of body temperature helps minimize neurologic morbidity. Intravenous (IV) access should be established. When IV access is not possible, intraosseous administration may be considered.
Laboratory studies of serum glucose and electrolyte levels (including calcium and magnesium), complete blood count, and renal and hepatic function tests should be performed. Anticonvulsant serum concentration should be obtained as needed, and a urine drug screen should be performed if there is suspicion of ingestion. If infection is suspected, blood cultures, lumbar puncture, and urinalysis may be needed. Antibiotic administration does not need to wait until after the lumbar puncture if the patient is medically unstable.
Patients should be given dextrose 50% as empirical therapy in the event they are hypoglycemic. Adolescents, or those at risk for Wernicke's encephalopathy, should be given 100 mg of thiamine before dextrose is administered. Patients with refractory GCSE also should undergo arterial blood gas and continuous electroencephalogram (EEG) monitoring.
Impending GCSE
Impending GCSE is characterized by continuous generalized seizures lasting at least 5 minutes or 2 seizures without full recovery of consciousness between events (Table 1). Impending GCSE may terminate spontaneously or may respond to a benzodiazepine (BDZ). Benzodiazepines are the preferred therapy for this stage, but the specific chemical entity used will depend on formulation availability and route of administration.
The anticonvulsant effect of BDZs is related to GABA inhibition that occurs as a direct result of binding to the GABAA receptor (Figure). This increases the frequency of channel opening to produce a surge in chloride conductance, which causes the cell to become less responsive to excitatory inputs. This effect occurs at serum concentrations achieved by doses used in clinical practice. Animal data suggest that the pharmacologic effect of a benzodiazepine is altered by prolonged seizures; hence, efficacy is changed when therapy is delayed. The mechanisms for these functional changes involve alterations in the cellular expression of GABAA receptors, which undergo a rapid internalization during SE. Changes in excitatory neurotransmission may also underlie the alterations that make prolonged GCSE less responsive to benzodiazepines. A direct effect on voltage-gated sodium channels is also suspected.
Figure.
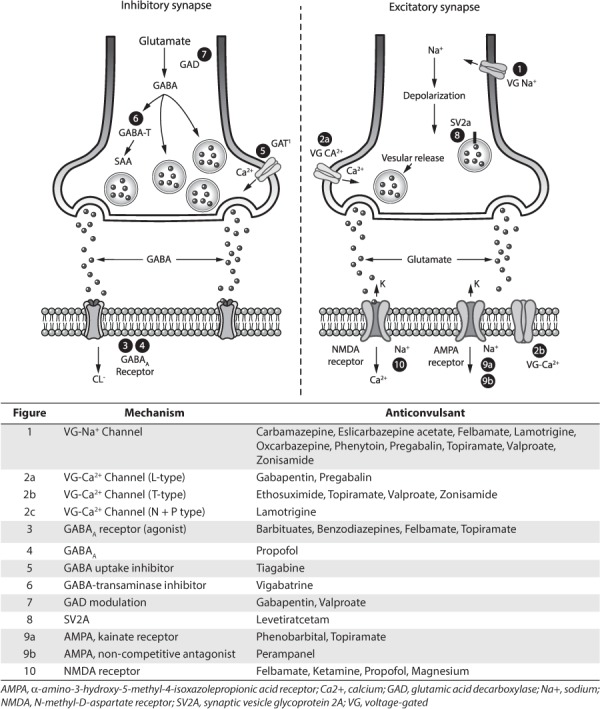
Sites of action of antiepileptic drugs used in the treatment of status epilepticus.
Historically, the discussion of management has centered on which BDZ to use as first-line therapy in patients in the emergency department, diazepam (DZP) or lorazepam (LZP).21,23,24,27–30 Although some consensus guidelines suggest either agent is acceptable,31 most recommend IV LZP as the initial treatment of choice.2,21,23–25,27–29 More recent studies have investigated the use of intranasal32–35 or buccal36 BDZs in the emergency department (Table 2).
Research has begun to focus on novel methods of BDZ administration in the outpatient setting. The Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) was a double-blind randomized trial that compared intramuscular (IM) midazolam (MDL) to IV LZP.37,38 Investigators found that IM MDL, given by paramedics using an autoinjector, was more likely to terminate seizures prior to arrival at the emergency department than IV LZP. The authors also noted that patients who received IM MDL required fewer other rescue therapies and were unlikely to require hospitalization or intensive care unit admission. As expected, time to administration of the first dose of BDZ was shorter with IM administration (1.2 vs. 4.8 minutes, respectively), but cessation of seizure activity after administration was quicker with IV administration (1.6 vs. 3.3 minutes, respectively). The authors concluded that IM MDL is the best option for treatment of impending SE in the emergency department setting.
Diazepam given IM using an autoinjector was also investigated in a 2-part phase III trial.39,40 The first part was a randomized, double-blind, parallel group, placebo-controlled multicenter study (n=234) that found a longer time to next seizure or rescue in the DZP autoinjector group compared to the placebo autoinjector group (hazard ratio: 0.55; 95% confidence interval: 0.34–0.88; p=0.012).39 The second part was an open-label continuation study (n=129; 1380 treatments) that assessed the long-term safety and effectiveness of DZP autoinjector for acute repetitive seizures.40 That study found that 77.6% of cases either had no seizure or did not require rescue treatment in the 12 hours after administration of DZP by autoinjector. The most common treatment-emergent adverse events were pain, hemorrhage, and bruising at the injection site; however, none of the events was considered severe or clinically significant.
Recent studies have focused on aborting impending SE through transmucosally delivered BDZs when IV and/or IM administration may be difficult or impossible (e.g., home setting, extended care, paramedic attendance). Benzodiazepines may be given via rectal (PR),34,35,41–45 intranasal (IN),32–35,44–57 and buccal36,41,42,52,58–62 routes (Table 3).
Table 3.
Use of Intranasal Benzodiazepines for Seizure Cessation in Pediatric Patients
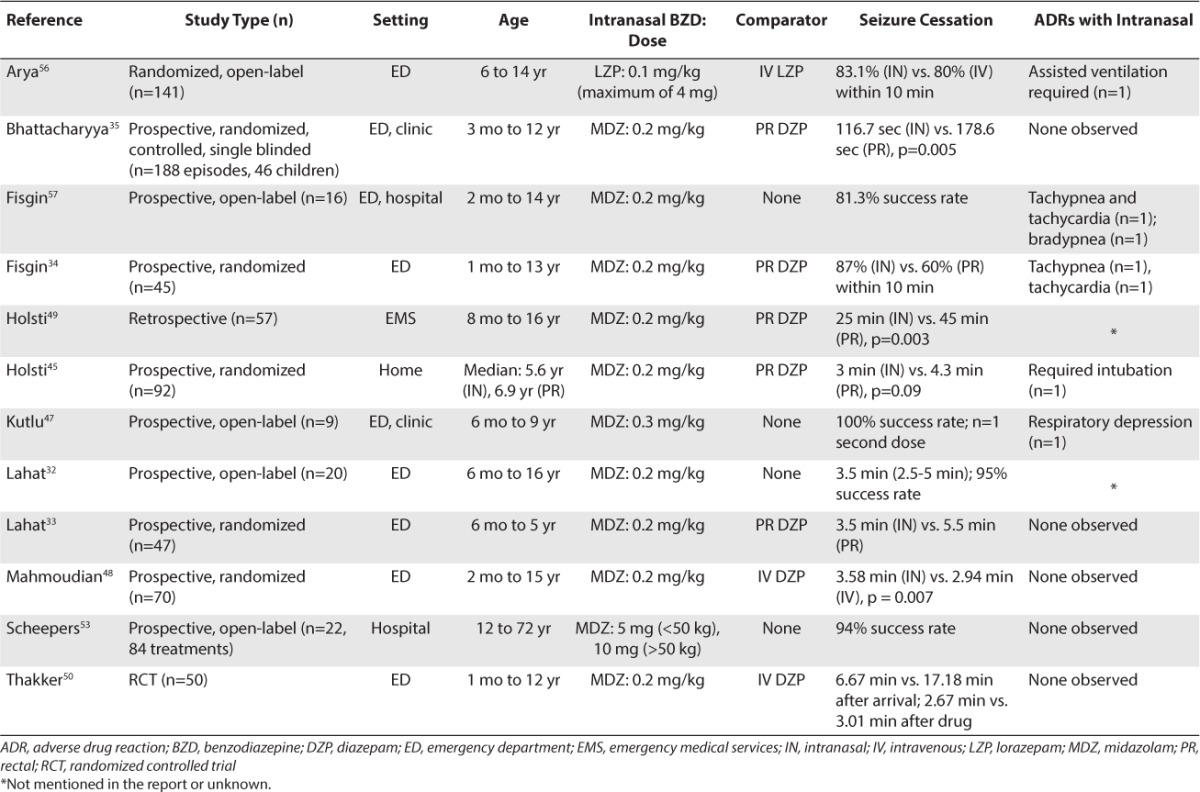
Rectal absorption of DZP is rapid but varies significantly (50%–100%) due to first-pass metabolism.63 Although a kit for rectal administration of DZP is commercially available (Diastat; Valeant Pharmaceuticals, Bridgeville, NJ), medication errors have been reported due to failure to properly dial and lock the prescribed dose. Compared to PR BDZ administration, IN administration results in higher serum concentrations, faster onset of action, and more effective seizure control.34,35,49 All studies have concluded that IN MDL is more effective than PR DZP and that it is convenient, easier to use, and as safe as PR DZP. Buccal and sublingual routes also bypass gastric and hepatic first-pass metabolism, but bioavailability can be incomplete as the drug is often swallowed. Successful administration via this route in a patient with GCSE is also unlikely due to muscular contractions of the jaw and clenching of teeth and/or patient secretions that can be associated with seizures.
The IN route is being used in virtually all settings; however, use in the home setting is complicated by the lack of a commercially available kit for IN use and difficulties finding a pharmacy willing to dispense a schedule 4 control substance injectable product for a non-parenteral route. MZL and LZP readily cross the nasal mucosa and blood–brain barrier to produce a rapid rise in both serum and cerebrospinal fluid concentrations.55,64 In fact, sera concentrations are comparable to those noted following IV injection.55,65 Rey et al54 evaluated the pharmacokinetics of IN and IV MDL in 12 children (1–5 years old) given 0.2 mg/kg and found that the half-life was similar (2.2 hours IN vs. 2.4 hours IV). The apparent plasma clearance and volume of distribution after IN administration were approximately 2 times greater than that observed after IV delivery. Intranasal LZP has also been investigated. The lag time for IN was less than that noted for buccal administration (0.6 ± 1.6 minutes versus 2.6 ± 2.7 minutes, respectively).55 The time to maximum concentration was also faster for IN administration (105 ± 75 minutes) than buccal delivery (161 ± 47 minutes).
The efficacy of IN MDL has been demonstrated in the pediatric population.32–35,44–50,53 Clinical trials have compared the safety and efficacy of IN MDL to those of IV DZP33,48,50 and PR DZP.34,35,45,49 Several studies have also evaluated buccal administration of MDL versus that of PR DZP.41,59,60,66,67 All studies preferred IN or buccal MDL for use in the community,49,62,67 emergency medical system/paramedics,43,45 and emergency department settings.32,34–36,50,59,61 When medication is given IN, one should consider using a mucosal atomizing device.
Historically, IV LZP has been the agent of choice in impending GCSE in the emergency department or hospital setting; however, studies have attempted to address the role of IN BDZs (Table 3).32–35,45,47–50,53,56,57,62 Lahat et al32 prospectively compared IN MDL (0.2 mg/kg) to IV DZP (0.3mg/kg) in 47 children (6 months–5 years of age) whose seizures lasted 10 minutes or longer. The 2 routes of administration were equally effective, stopping seizures in 88% (IN) and 92% (IV) of patients. The mean time from hospital arrival to starting treatment was shorter in the IN group (3.5 ± 1.8 minutes vs. 5.5 ± 2.0 minutes, respectively), allowing for faster seizure cessation with IN MDL (6.1 vs. 8 minutes, respectively).
A similar study by Mahmoudian and Zadeh48 compared the efficacy of IN MDL (0.2 mg/kg) to that of IV DZP (0.2 mg/kg) in 70 patients (2 months to 15 years of age) seen in the emergency department for seizures. Both of the methods were equally effective, and no adverse effects occurred. The mean time to seizure control following medication administration was 3.58 ± 1.68 minutes (IN MDL) and 2.94 ± 2.62 minutes (IV DZP).
Thakker and Shanbag50 conducted a randomized, controlled study in a pediatric emergency department of a tertiary general hospital, which compared the safety and efficacy of IN MDL (0.2 mg/kg) to that of IV DZP (0.3 mg/kg) in 50 children (1 month to 12 years of age) with acute seizures lasting at least 10 minutes. Overall, 75% of seizures were controlled with IN MDL compared to 65.2% with IV DZP. The mean time between arrival at hospital and anticonvulsant administration was significantly shorter with IN MDL (3.37 ± 2.46 minutes vs. 14.13 ± 3.39 minutes, respectively). Once the medication was given, the mean time to control of seizures was shorter with IV DZP (2.67 ± 2.31 minutes) than with IN MDL (3.01 ± 2.79 minutes). No significant side effects were observed in either group.
Arya et al56 conducted a randomized, open-label trial comparing the efficacy and adverse effects of IN LZP with that of IV LZP in 141 children (6–14 years of age) who presented consecutively to their hospital with acute seizures. After children were stabilized in the emergency department, they were randomized to receive either IN (n=71) or IV (n=70) LZP (0.1 mg/kg, maximum 4 mg). The primary outcome was clinical seizure remission within 10 minutes of drug administration, which occurred in 83.1% (IN) and 80% (IV) of patients. All studies comparing IN with IV BDZ have reported similar efficacy, but remission was accomplished within a shorter time using IN administration. Although most practitioners will continue to consider IV LZP the agent of choice in the emergency department, it is very likely that an IN BDZ will become the standard of practice due to ease and quick administration.
Because the dose must be administered using a 100- to 200-μL spray or solution, the IN route can only be used for drugs that are highly concentrated and have good aqueous solubility.68,69 Although most reports have used a variety of delivery systems (e.g., drops, sprays, and atomization devices), the lack of standardization in delivery has not impacted efficacy and tolerability outcomes. Effective delivery is best achieved by distributing the drug as a mist rather than as larger droplets that may aggregate and run out of the nose or down the back of the throat, rendering it ineffective. Placing half the dose into each nostril can double the surface area available for absorption. Upper airway infections, extent of nasal mucosa irritation, and differences in the amount of spray that is swallowed may all impact absorption. However, variability in the amount absorbed after nasal administration should be comparable to that after oral administration.
Established GCSE
Established GCSE occurs when a BDZ fails to terminate seizures (Table 1). This is characterized by at least 30 minutes of continuous seizures or 30 minutes of intermittent seizures without full recovery of consciousness between seizure events. Management generally involves administration of phenytoin (PHT), fosphenytoin (fPHT), phenobarbital (PB), or valproate (VPA). Recently, levetiracetam and lacosamide also have been used, especially during times of medication shortages that have rendered traditional drugs unavailable. Most guidelines recommend giving either PHT/fPHT2,21–23,25,26,31 or PB2,24,25 after a full dose of BDZs has failed (Table 2), but there is little level 1 evidence to support that practice.
Phenytoin
For over 75 years, numerous studies have attempted to elucidate the mechanism of action of phenytoin. Studies have noted that it influences ion conductance, Na/K-ATPase activity, post-tetanic potentiation, calcium systems, and neurotransmitter release (Figure). The major antiseizure effect is due to inhibition of voltage-dependent sodium channels. Phenytoin works on the intracellular part of the ion channel to decrease the influx of sodium into neurons and thereby decreases excitability (Figure). Activity is use- and concentration-dependent; hence, the onset of action should occur as soon as an effective concentration is achieved. At serum concentrations obtained in clinical practice, phenytoin does not modify response to pre- or postsynaptic GABA, nor does it impact NMDA or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors.
Although a hydantoin has historically been used as the first-line agent in established GCSE, there is little evidence to support the fact that it is as effective as PB. Recent studies comparing IV PHT/fPHT to other agents will be detailed in later sections. If IV access cannot be obtained and IM administration is undesirable, PHT or fPHT can be given via the interosseous (IO) route. Dosing recommendations for IO administration are derived from a prospective, controlled, randomized porcine study comparing plasma drug concentrations and pharmacodynamics of IV versus IO fPHT, 20 mg PE/kg, with those of PHT, 20 mg/kg.70 Significantly higher free and total PHT concentrations were found with IO administration of fPHT at 0 to 10 minutes (p<0.05) and 0 to 20 minutes after infusion (p<0.05). There were no significant differences for either medication beyond 20 minutes. Therefore, the authors concluded that PHT should be given IO at the standard dose of 20 mg/kg, and if fPHT is used, a slower infusion rate may be needed to avoid initial supratherapeutic concentrations.70
Phenobarbital
PB binds to inhibitory GABAA receptors at the beta subunit to increase the duration of chloride ion channel opening, thereby potentiating the effect of GABA at this receptor (Figure). At high serum concentrations, PB can activate the GABAA receptor directly to inhibit calcium-dependent release of neurotransmitters. Importantly, prolonged seizure activity does not alter the effectiveness of PB at the GABAA receptor as it can inhibit this receptor in the absence of GABA. Phenobarbital also inhibits glutamate-activated sodium and calcium channels at the AMPA receptor to reduce the release of glutamate. This mechanism requires higher concentrations than those needed to produce an effect on GABA. PB has no affect on voltage-gated sodium channels.
Only 2 randomized, controlled trials71,72 have attempted to determine whether PHT or PB should be used in established GCSE; however, only 1 of those studies71 included pediatric patients. Shaner et al71 prospectively compared PB to DZP plus PHT in 36 children (18 in each group) with established GCSE. In the first group, PB was given IV at a dose of 20 to 30 mg/kg. Patients in the second group received IV doses of DZP as small as 2 mg and as large as 20 mg plus a PHT loading dose (range: 6–21 mg/kg) that was based on the patient's admission serum PHT concentration. The authors concluded that PB was rapidly effective and comparable in safety to DZP plus PHT.
Although it is impossible to determine whether DZP was responsible for efficacy in the DZP/PHT group, the study by Treiman et al72 would suggest that might be the case. That study is the largest randomized, controlled trial to date, in which 384 adult patients with established GCSE received either IV LZP (0.1 mg/kg), PB (15 mg/kg), DZP (0.15 mg/kg) plus PHT (18 mg/kg), or PHT alone (18 mg/kg). LZP successfully stopped seizures in 64.9% of patients compared to 58.2%, 55.8%, and 43.6% of patients given PB, DZP/PHT, or PHT alone, respectively. The trial findings noted that DZP/PHT was more effective than PHT as monotherapy. When patients with established and refractory GCSE were combined in a post hoc analysis, the differences between PB and PHT as monotherapies approached significance, with PB being superior. This is an important finding in light of the low dose of PB that was used in the trial.
Recently Yasiry et al73 reported the effectiveness of 4 anticonvulsants (i.e., levetiracetam, PB, PHT, and VPA) in the treatment of BDZ-resistant convulsive SE, using meta-analysis of 27 published studies. Evidence did not support the first-line use of PHT. Efficacy data for PB (remission: 73.6% [95% confidence interval [CI]: 58.3–84.8%]), VPA (remission: 75.7% [95% CI: 63.7–84.8%]), and levetiracetam (68.5% [95% CI: 56.2–78.7%]) supported their use as first-line therapy in BDZ-resistant SE.
Valproic Acid
Although the precise mechanism of action is not known, several mechanisms have been suggested. VPA may potentiate postsynaptic GABA, inhibit GABA-transaminase and succinic acid decarboxylase, and/or increase glutamic acid decarboxylase. It may also block voltage-gated sodium channels to limit depolarization-induced, sustained repetitive firing and may block low-threshold T-type calcium channels (Figure).
Many consensus guidelines also recommend the use of VPA as an alternative second-line treatment.21,24,25,31 Reports in adults74–79 and pediatric patients78–83 with SE unresponsive to first-line agents have generally noted successful termination of seizures following IV VPA (Table 4).
Table 4.
Use of Valproate in Pediatric Patients With Status Epilepticus Unresponsive to Benzodiazepines
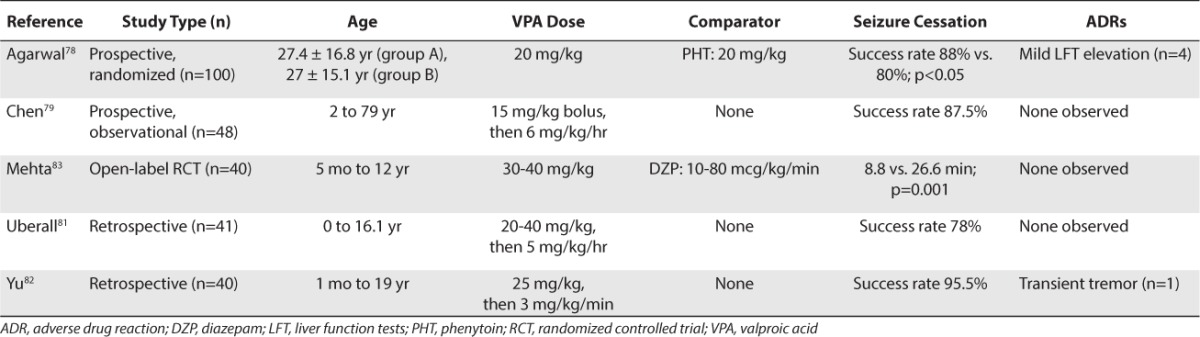
To date, there have been 3 retrospective reports of IV VPA in pediatric patients.80–82 Hovinga et al80 successfully used IV VPA in 2 pediatric patients with GCSE who failed to respond to BDZ, PB, PHT, and pentobarbital (PTB). Both of the patients were successfully treated with a loading dose of 20 mg/kg followed by a maintenance infusion of 4 to 6 mg/kg/hr. Uberall et al81 evaluated IV VPA (loading dose of 20–40 mg/kg followed by 5 mg/kg/hr) in 41 children who did not respond to DZP, PB, or PHT therapy. VPA stopped clinical and bioelectric seizures in 78% of all types of SE but was effective in 90% of patients with GCSE. Yu et al82 conducted a retrospective review of the use of IV VPA in 40 pediatric patients (1 month to 19 years of age) with either multiple types of SE (n=18) or acute repetitive seizures (n=22). Patients who were naïve to VPA received a loading dose of 25 mg/kg with an average infusion rate of 2.8 mg/kg/hr. Seizures stopped within 20 minutes in 100% of patients with SE and in 95% of those with acute repetitive seizures.
Two prospective evaluations of IV VPA for seizure control in pediatric patients also have been published.79,83 Chen et al79 evaluated VPA in 48 adult and pediatric patients with convulsive SE for whom IV DZP and IM PB failed. Five of the patients given VPA were <5 years of age, and the youngest was 2-years-old. Patients received a loading dose of 30 mg/kg that was followed by 6 mg/kg/hr. Seizures stopped in 87.5% of patients within 1 hour of administration. Mehta et al83 randomized 40 children to receive either IV VPA or continuous infusion DZP. There were no significant differences in efficacy (80% VPA vs. 85% DZP); however, there were differences in the median time to control seizures (5 minutes with VPA vs. 17 minutes with DZP).
Several studies have compared IV VPA to PHT.78,84–86 Misra et al84 compared IV VPA, 30 mg/kg (n=35) to PHT, 18 mg/kg (n=33) as the first-line agent in a randomized, prospective, head-to-head open study. Benzodiazepines were not given, and most of the patients were adults. Valproate was significantly more effective (66%) than PHT (42%). As a second choice in patients unresponsive to the first agent (i.e., cross-over), VPA was also more effective than PHT (79% vs. 25%, respectively). Tiamkao et al85 also retrospectively evaluated VPA (n=37) and PHT (n=17) as first-line agents in patients older than 15 years of age. All but 1 patient received 10 mg of IV DZP. The mean IV loading dose of PHT (743 ± 116 mg) was followed by 300 mg/day, and the mean IV loading dose of VPA (1000 ± 239.14 mg) was followed by 1200 mg/day. There were no significant differences between the PHT and VPA groups in all outcome variables. Chitsaz et al86 also compared VPA (20 mg/kg followed by 1 mg/kg/hr) with PHT (20 mg/kg with supplemental 10 mg/kg if needed, followed by 4.5 mg/kg/day) in a predominantly adult population for whom IV DZP had failed. There were no significant differences in response within the first 12 hours (73.3% vs. 60%, respectively; p=0.06). In a randomized study, Agarwal et al78 compared IV PHT (n=50) with IV VPA (n=50) in patients with SE refractory to BDZs and found no differences in efficacy (88% vs. 84%, respectively). In patients younger than 18 years of age, seizures stopped in 20 of 22 patients (91%) given VPA and 12 of 16 patients (75%) given PHT. The authors concluded that there were no differences in seizure recurrence at 12 hours regardless of age.
Only one study has compared VPA (n=30) with PB (n=30).87 VPA was given via rapid IV loading, which was defined as 5 to 6 mg/kg/min over 5 to 10 minutes. VPA, in doses of 20 mg/kg, successfully terminated GCSE within 20 minutes in 90% of children compared to 77% of children given IV PB, 20 mg/kg (p=0.189). Clinically significant adverse effects occurred in 74% of the PB group and 24% of patients in the VPA group (p<0.001).
Brigo et al88 used 5 randomized, controlled trials to perform a meta-analysis comparing the efficacy and safety of VPA, PB, and PHT in GCSE.88 They reported that VPA and PHT have similar efficacy as VPA and PB; however, VPA had a better safety profile than either PHT or PB. Because many of the studies were underpowered, there was insufficient evidence to support a change in clinical practice. That group also conducted a second meta-analysis comparing IV VPA with PB.89 Again, both agents had comparable efficacy, but VPA had a better safety profile.
Levetiracetam
Historically, levetiracetam was used only in cases of super-refractory SE. In recent years, it has been used earlier due to medication shortages that have made conventional drugs unavailable. Levetiracetam has no effect on voltage-gated Na channels or GABAergic transmission and no affinity for either GABAergic or glutamatergic receptors (Figure). The drug has recently been noted to bind to a presynaptic vesicle glycoprotein (sv2A) that has been proposed to act as a transporter for presynaptic P/Q type voltage-dependent calcium ([Ca2+]c). This is considered an important second messenger system in neurons, which might contribute to its unique mechanism of action. Levetiracetam has also been noted to reduce neurotransmitter release by inhibiting presynaptic L-type calcium channels.
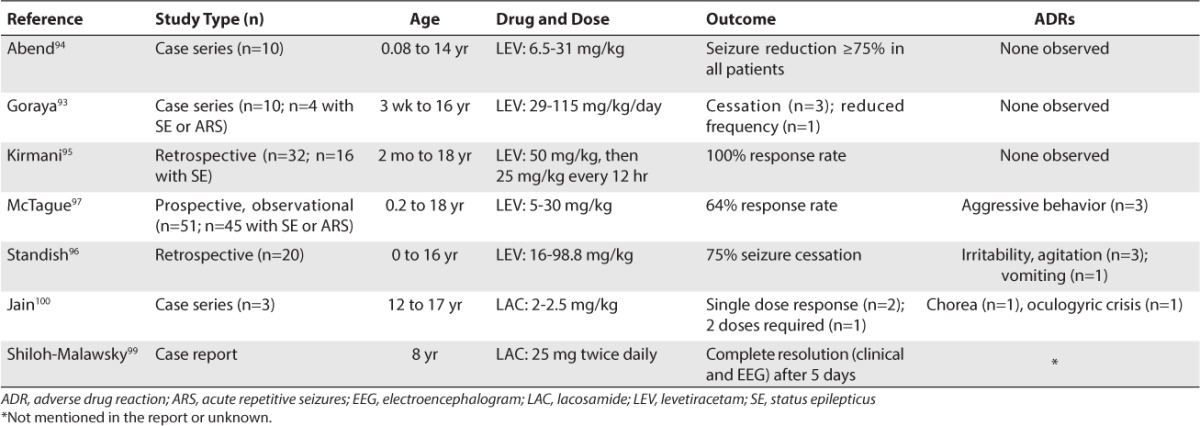
Levetiracetam has been considered a potentially useful agent for SE due to its few adverse effects, limited drug interactions, and ease of IV administration compared to other IV anticonvulsants. Early investigations of levetiracetam for SE used the oral formulation and found it to be effective for various types of SE and well tolerated by patients.90–92 There are 5 published case series of the use of IV levetiracetam for SE in the pediatric population (Table 5).93–97 One retrospective analysis included 4 children who received IV levetiracetam for acute repetitive seizures or SE in doses as large as 115 mg/kg/day and as small as 29 mg/kg/day.93 Three of 4 patients had seizure termination; the fourth patient had a reported decrease in seizure frequency. Another retrospective case series noted that 8 of 10 critically ill pediatric patients with SE or acute repetitive seizures responded and that the other 2 children exhibited a reduction of seizures (levetiracetam IV loading dose ranged from 6.5 to 31 mg/kg).94 Kirmani et al95 reported a retrospective study of 32 patients treated with an IV loading dose of levetiracetam (25 of 32 patients received 50 mg/kg; range: 25–70 mg/kg), and 16 of those patients received treatment for SE. All patients responded both clinically and according to EEG monitoring. The rate of seizure cessation using IV levetiracetam for patients with SE was determined in a case series by Standish et al.96 The seizure cessation rate was 75% (15 of 20 patients) with a mean dose of 37.5 mg/kg (range: 16–98.8 mg/kg). Three patients experienced adverse events of behavioral side effects, 1 of whom was treated with pyridoxine. An observational study of 45 patients (mean 7.1 years of age) with acute repeated seizures or SE were initially treated with 5 to 30 mg/kg levetiracetam IV.97 A total of 29 patients responded to the treatment, and 3 patients displayed aggressive behavior as a side effect of the medication. These case series suggest that IV levetiracetam seems to be safe and effective for the termination of SE when first-line agents fail to control the seizure. When levetiracetam is used, the patient should be given a loading dose of 40 to 60 mg/kg (maximum 3000 mg) at a rate of 2 to 5 mg/kg/min.
Table 5.
Newer Agents Used in Pediatric Cases of Established Status Epilepticus
Lacosamide
Like levetiracetam, lacosamide also has been used in pediatric patients for the treatment of SE (Table 5). A review paper published in 2013 found 19 publications, including 10 case reports and 9 case series, reporting IV lacosamide for the treatment of SE.98 The review cited 1 pediatric case report99 and 1 pediatric case series.100
The case report99 involved an 8-year-old boy with new onset seizures that progressed to refractory SE, which was treated successfully with enteral lacosamide, 25 mg twice daily. This success came after attempting the following treatments over a 10-week period: combinations of PHT, levetiracetam, PB, topiramate, felbamate, and VPA; propofol (PRO), PTB, MDL, and ketamine to induce EEG burst suppression; ketogenic diet; high-dose corticosteroids; IV immunoglobulin (IVIG); plasmapheresis; and vitamin B6, folic acid, carnitine, and biotin.
The case series (n=3) described the successful implementation of lacosamide in pediatric patients (12–17 years of age) with refractory tonic SE.100 After 3 or more standard anticonvulsants were tried over the course of 8 to 29 hours, an IV loading dose of lacosamide (2–2.5 mg/kg) was given. Two patients responded to a single dose, and 1 patient required 2 doses of lacosamide.
Adverse effects
Generally, the hesitation to use a hydantoin or barbiturate has not been based on their effectiveness as an anticonvulsant but upon potential adverse effects. The vasodilatory and cardio-depressant effects of PB may cause profound hypotension and hypopnea. The respiratory depressant effects are compounded when used in conjunction with a BDZ. Interestingly, Treiman et al72 noted no differences in incidence of adverse effects between adults given IV PB and those given DZP plus PHT.72 Among 91 patients treated with PB, 12 patients (13.2%) experienced hypoventilation, 31 (34%) showed hypotension, and 3 (3.3%) had cardiac arrhythmias; among the 101 patients receiving PHT, 10 patients (9.9%) experienced hypoventilation, 27 (26.7%) showed hypotension, and 7 (6.9%) had cardiac arrhythmia. Shaner et al71 noted that when the serum concentration of PB exceeded 70 mg/L, the level of consciousness was almost always impaired. That said, 83% of patients with overt SE in the study by Trieman et al72 had not fully regained consciousness 12 hours after administration of any anticonvulsant; hence, consideration of sedation may not be as important in selecting an agent.
When given in large doses and by rapid infusion, VPA rarely causes cardiovascular toxicity such as hypotension or dysrhythmia.24,74,75,81,82 It has not been noted to cause respiratory depression, and it does not cause sedation.75,82 However, VPA can cause coagulopathies such as platelet dysfunction, thrombocytopenia, and hypofibrinogenemia.101,102 Acute encephalopathy after IV VPA was reported in a 45-year-old patient with non-convulsive SE.103 Although rare, pancreatitis has been noted with VPA. Both PB and PHT contain a polypropylene solvent that may cause local irritation, necrosis, hemolysis, and an infusion syndrome. Phenytoin may cause significant peripheral vascular side effects, all of which can be avoided with fPHT.
Adverse events with the use of lacosamide have been reported. Non–life-threatening adverse events of chorea (n=1) and oculogyric crisis (n=1) were reported in the aforementioned case series.100 Neurotoxicity consisting of mostly dizziness, imbalance, diplopia, and sedation has also been reported with the use of lacosamide.104 Because this adverse event has been reported only in patients taking lacosamide as a maintenance medication in conjunction with a voltage-gated sodium channel-blocking anticonvulsant, neurotoxicity would not be expected from the use of lacosamide in SE.
Established GCSE: summary of treatment options
Although a hydantoin has historically been used as the first-line agent in established GCSE, there is little evidence to support it is as effective as PB. While numerous studies have validated the effectiveness of PB, its adverse effect profile relegates it to a second-line agent in the minds of many practitioners. Clearly, VPA, levetiracetam, and lacosamide have better safety profiles than either PHT/fPHT or PB. Because most studies evaluating VPA, levetiracetam, or lacosamide were underpowered, they enable only cautious interpretation that is not accompanied by evidenced-based decisions. The established SE trial 2013 is currently being conducted among patients older than 2 years of age.105 This study will compare fPHT, levetiracetam, and VPA. Regardless of which agent is used, Olsen et al76 noted that only 5% of patients treated within 3 hours required anesthesia compared to 38% treated 3 to 24 hours and 60% treated after 24 hours. Limdi et al74 and Agarwal et al75 also noted that time to treat affected outcome.
Refractory GCSE
The definition of refractory GCSE is based on the number of anticonvulsants used. A patient is considered to have refractory SE when seizures continue despite first- and second-line treatments,5,8,21,22,72 seizure duration is greater than 1 hour,7,8,27,72 or there is a need for general anesthesia (Table 1).
The variability in definitions makes it difficult to estimate the incidence, but studies suggest that refractory GCSE occurs in 9% to 44% of patients with GCSE.106,107 Prolonged seizures cause severe neurological sequelae108,109 and/or death.110–113 Sahin et al109 studied morbidity in normal healthy individuals with acute refractory generalized seizures and found that none returned to baseline neurological status and all developed intractable epilepsy. Mortality also may be high, ranging from 16% to 77%.110–113 In a meta-analysis of 111 children with refractory GCSE, the overall mortality was 20% in symptomatic cases and 4% in idiopathic cases.111
There is a lack of consensus regarding the optimal therapeutic approach, but patients who continue to be unresponsive are treated with continuous infusion of barbiturate (i.e., PTB, thiopental), BDZ (i.e., DZP, LZP, MDL), or PRO. Thiopental has not been available for many years, and there is currently no manufacturer of this product in the United States. The effectiveness of these agents is such that Fountain and Fugate141 suggested that practitioners should skip second-line therapy to avoid major time delays and move immediately to anesthesia with IV MDL in those failing initial BDZ therapy.
Unfortunately there are no randomized, controlled trials comparing these agents. Surveys conducted in the United States115 and Europe116 almost a decade ago noted that barbiturates are the most popular agent in refractory GCSE, followed by PRO and MDL. More recent consensus protocols/guidelines suggest that thiopental or PRO,31 PTB or PRO,26 or PRO, PTB, or MDL2,21,25 are preferred. Capovilla et al2 noted that the selection of 1 agent over another should depend on the patient's general condition and the agent's benefits versus risk of adverse effects. Use of an agent is also driven by the availability of medical staff with expertise in general anesthesia.2 Claassen et al117 evaluated 28 retrospective trials that used barbiturates, MDL, or PRO in 193 adult patients with refractory GCSE. The authors concluded that PTB was more effective than PRO or MDL in preventing seizure recurrence, but it was associated with more adverse effects.
Pentobarbital
Barbiturates such as PTB, PB, and thiopental have been used for decades to induce general anesthesia in pediatric patients with refractory GCSE.113,118–124 Although PB has been used,125,126 PTB is preferred for general anesthesia because its rapid penetration into the brain results in a quicker onset of antiepileptic activity. It has a shorter half-life in serum than PB, but both agents distribute into fat causing prolonged elimination after continued use. Pentobarbital is highly effective in terminating seizures in 74% to 100% of patients with refractory GCSE.111,113,123,124
Most reports describing the use of PTB involve case reports118–120,122 or retrospective reviews.113,124 Kim et al113 reviewed the use of PTB in 23 children with refractory GCSE. Patients received a small loading dose (5 mg/kg) followed by 1 to 3 mg/kg/hr for at least 48 hours. The dose was adjusted based on clinical seizures and EEG findings. Among the 23 patients reviewed, 12 were controlled, 6 were unresponsive, and 5 relapsed after discontinuation or during tapering. The mortality rate among the relapsed and nonresponder groups combined was 90.9%, but no deaths occurred among the responder group (p<0.001).
Barberio et al124 evaluated the use of PTB in 30 pediatric patients given a mean loading dose of 5.4 ± 2.8 mg/kg and an initial infusion of 1.1 ± 0.4 mg/kg/hr (maximum: 4.8 ± 2 mg/kg/hr). Thirty-three percent of patients achieved a sustained burst suppression pattern on EEG without relapse. Another 66.7% experienced relapse, but 60% of those eventually re-achieved burst suppression. Children achieving burst suppression within 24 hours of PTB initiation and those older than 5 years of age were 1.5 times more likely to have a positive outcome.
Pentobarbital should be given as a loading dose of 10–20 mg/kg over 1 hour, followed by a maintenance infusion of 1 to 5 mg/kg/hr (Table 2). Most patients achieve burst suppression at a dose of 1 mg/kg/hr,124 but the dose should be increased every 2 minutes by 1 mg/kg/hr until the desired effect is achieved.2 Subsequent administration of an additional dose of 5 mg/kg (combined with EEG monitoring) may allow for more rapid titration of dose to the therapeutic goal.
Pentobarbital duration of therapy remains controversial, as the endpoint for success continues to be debated. After a period of control, a loss of EEG burst suppression may suggest the development of pharmacologic tolerance, which may require a dosage increase. Seizures during withdrawal are not only frustrating and time-consuming but also predict a worsened outcome. Seizures tend to occur if the infusion is abruptly discontinued; hence, tapering the dose is recommended. Most practitioners recommend tapering PTB when burst suppression has been achieved for 12 hours119,122 or 24 hours.127 However, others argue that longer periods (24–48 hours) are associated with lower rates of recurrent seizures.128–130 Still others suggest a criterion of 3 to 9 bursts/min131 or advocate dividing burst duration by suppression duration to determine the burst suppression-to-duration ratio. Patients who received prolonged therapy and those taking PB at the time of withdrawal may be less likely to relapse.128 If seizures reoccur during tapering, the last successful dose should be reinstituted. Once seizure control is regained, the medication should be tapered at a slower rate than what was previously used.
Continuous infusion of anesthetic agents usually requires mechanical ventilation and immobilization, which may lead to a multitude of complications including infections and thromboembolism. Large doses of PTB may be associated with myocardial depression and low cardiac output. Barbiturate-induced hypotension is a frequent complication but rarely requires discontinuation of therapy. When it does occur, the rate of infusion should be decreased, fluids should be increased, or a vasopressor should be given.124 Patients who do not develop hypotension requiring vasopressors have better survival rates.120 Pulmonary edema, skin edema, and ileus have also been described when the duration of PTB therapy exceeds 4 days.118
Pentobarbital may cause white blood cell dysfunction that contributes to an increased rate of nosocomial infections, especially pneumonia. Barbiturates may induce hepatic isoenzymes, which may cause drug–drug interactions. It may take days for a patient to regain full consciousness following discontinuation of PTB. In fact, Lowenstein et al119 reported that spontaneous eye opening and respirations in a 14-year-old occurred after 36 hours and 3 days, respectively.119
Propylene glycol (1,2-propanediol) is the pharmaceutical solvent used to formulate parenteral LZP, DZP, and PTB. Although large doses of propylene glycol have been associated with toxicity, the incidence is unknown. Acute kidney injury, hyperosmolality, and lactic acidosis that presents as a high-anion gap metabolic acidosis and osmolar gap has been reported.132–134 In critically ill patients receiving agents containing propylene glycol, an osmolar gap greater than 10 was reported to cause toxicity.135 Although most patients respond to discontinuation of agents containing propylene glycol accompanied by hemodynamic support, those with multiorgan failure may require fomepizole or hemodialysis.136,137
Midazolam
Midazolam is an injectable BDZ that is fast acting, rapidly penetrates the blood–brain barrier, and exerts a short duration of action. Studies suggest efficacy in 71% to 97% of patients.138–145 A meta-analysis of 111 children indicated that MDL was as effective as other coma-inducing medications.111 Important in the discussion of efficacy is the target goal for success. Most studies used termination of seizures on EEG as the endpoint for success. EEG burst suppression is rarely achieved with the recommended doses of MDL, and some suggest that it should not be the goal for success.26
Although different dosing regimens have been used, many suggest that an initial bolus dose of 0.1–0.5 mg/kg, followed by an infusion of 0.05–2 mg/kg/hr will control refractory GCSE in most children (Table 2).138–145 Doses as large as 24 mcg/kg/min have been required.140
Very rapid control of seizures has been reported, occurring in 0.3–1.1 hours.138–144 Some138,139,143 but not all144,145 of the studies noted a longer time to control seizures when smaller loading doses were used. If a patient is not controlled, the dose should be increased every 15 minutes by 1–2 mcg/kg/min (0.06–0.12 mg/kg/hr).2,140 If clinical seizures persist 5 minutes after the initial MDL bolus, then administer an additional 0.2 mg/kg while continuing the infusion. If clinical seizures continue after another 5 minutes, then administer another MDL bolus of 0.2 mg/kg and increase the infusion to 0.2 mg/kg/hr. If seizures persist at the maximum MDL infusion rates (generally, 2 mg/kg/hr) or the infusion is not tolerated, another agent should be considered.
Tachyphylaxis rapidly develops within 24 to 48 hours; hence, the dose is often increased to prevent seizure relapse.146 After initial control, seizures have been observed in 47% to 57% of patients,142,144 and generalized convulsive seizures recurred in 6% to 19%.142–144 This may require an increase in dosage or a change in therapy. Ferlisi and Shorvon147 reported seizure control was achieved with a change in dose in 12%, 7%, and 6% of those given PRO, MDL, and barbiturates, respectively. Seizures occurred even when MDL infusion rates were 1.44 mg/kg/hr. Recurrent seizures that required a change in therapy occurred most frequently with MDL (3%) compared to PRO (1.3%) and barbiturates (0%).147 Withdrawal seizures that occurred within 48 hours of discontinuation of MDL have been reported less frequently in those receiving large doses.148 If withdrawal seizures occur, the anticonvulsant should be reintroduced at the dosage that previously achieved seizure control.
The optimal length and tapering of any anesthetic treatment has not been addressed in prospective studies, and retrospective observations do not clearly favor any specific protocol. Wilkes and Tasker149 recommended a seizure-free period of 24 to 48 hours before undertaking a trial of weaning from the MDL infusion. When tapering, the rate should be decreased by 1 to 2 mcg/kg/min every 15 minutes.140
Many studies report no adverse effects with MDL.138,140,141,143 A few reports have described hypotension requiring IV fluids or vasopressor support,142,150 whereas others describe cardiovascular stability even in children receiving large doses (24 mcg/kg/min140 or 32 mcg/kg/min142). Hypotension occurred more frequently with PTB compared to MDL and PRO (77% vs. 34%).117 Endotracheal intubation and mechanical ventilation was required less frequently with MDL. Due to MDL's short half-life, waking after discontinuation should not be excessively prolonged; however, MDL does accumulate with continued use, which can result in a longer terminal half-life.151 Rivera et al138 reported that patients were completely awake within a mean of 4 hours (range, 2–8.5 hours) after the MDL infusion was discontinued. When adverse effects do occur, the patient's recovery is quick. In addition, the availability of a pharmacological antidote for BDZs, flumazenil, lends to the safety of MDL.
Propofol
Propofol is an IV alkyl-phenol general anesthetic unrelated to BDZ and barbiturates. It has a short half-life that allows for easy titration and withdrawal and rapid awakening after drug cessation. Although it does accumulate with prolonged administration, the consequences are not as significant as that seen with MDL and PTB. Like other anesthetics, there are few studies of its efficacy in refractory GCSE152–155 and little information that helps to clarify its use relative to other therapies. It is effective in quickly terminating refractory GCSE, but it is no more effective than barbiturates.154–157
Propofol increases the potency of GABA-ergic inhibitory neurotransmission (Figure). At concentrations that are clinically achieved, it reversibly but non-competitively inhibits cellular excitation at the NMDA glutamate receptor to reduce the probability of channel opening. It is also thought that propofol stimulates the production and release of nitric oxide and at high concentrations protects against the toxicity of NMDA and glutamate. It does not affect kainic acid or AMPA.
Although several studies have compared PRO to barbiturates, most studies were underpowered. Propofol induces burst suppression within 35 minutes of initiation, but maintenance of burst suppression may require frequent dosage titration.155 Once EEG burst suppression has been achieved, the dose should be reduced.
Propofol is given as a loading dose of 1–2 mg/kg that can be repeated every 3 to 5 minutes until clinical response is achieved, up to a maximum of 10 mg/kg (Table 2). This is followed by a continuous infusion (1–4 mg/kg/hr).31 Although much larger doses have been reported (10–15 mg/kg/hr),21 prolonged infusions >4 mg/kg/hr have been associated with PRO-related infusion syndrome (PRIS) in critically ill adults and pediatric patients receiving continuous infusion PRO for anesthesia or sedation.158 Signs and symptoms of PRIS include progressive metabolic acidosis, hemodynamic instability, and bradyarrhythmia that are refractory to aggressive pharmacological treatments. It may occur with or without the presence of hepatomegaly, rhabdomyolysis, or lipemia.
Vital signs, especially blood pressure for hypotension, should be carefully monitored. Continuous ECG should assess for dysrhythmias, including bradycardia, ventricular tachycardia or fibrillation, right bundle branch block, and a widened QRS complex. Although no guidelines have been proposed for laboratory monitoring in patients receiving PRO, it would seem prudent to assess serum lactic acid, serum triglycerides, serum creatinine, CK, and hepatic enzymes in anyone given the drug in doses larger than 4 mg/kg/hr and/or those receiving an infusion for longer than 48 hours.
Cardiotoxicities should be managed with aggressive pharmacological therapies and cardiac pacing. Practitioners have successfully managed PRIS with venovenous hemodiafiltration,159–161 charcoal hemofiltration,162 or extracorporeal membrane oxygenation.163–165 However, plasmapheresis does not seem beneficial.160 A retrospective case series of 41 patients with refractory GCSE noted that 10% of patients had sudden unexplained cardiorespiratory arrests (2 fatalities) and 35% had non–life-threatening features of PRIS.166 Fatalities were also reported when PRO was administered in conjunction with a ketogenic diet.167
Propofol has also been reported to be proconvulsant in some patients.168,169 It may also cause involuntary movements having a myoclonic appearance that resolve following withdrawal of PRO.170 Differentiation from seizures may be challenging even with EEG monitoring, as the events may be obscured by movement artifacts.
Ketamine
Prolonged SE results in an increase in NMDA receptors. Stimulation of these receptors by glutamate may promote seizure activity. For this reason, NMDA-receptor antagonists such as ketamine have been investigated (Figure). A summary of the findings from more than 40 preclinical studies can be found in a recently published review paper.171 Ketamine can be used to successfully treat soman-induced prolonged SE within 1 hour of intoxication, reduce neuro-inflammation, and change brain metabolism. In this review, it was also noted that seizures lasting more than 1 hour were not successfully treated with ketamine alone. 171
There are several case reports and retrospective studies describing the use of IV ketamine for refractory GCSE in pediatric patients (Table 6).172–174 A 13-year-old patient with SE refractory to PB, PHT, continuous MDL, PRO, IV VPA, IV lidocaine, and PTB coma was treated with a loading dose of 2 mcg/kg ketamine, which provided clinical and electrographic control within 90 seconds. The patient's seizures ceased for 15 minutes and were subsequently controlled by using a continuous infusion of ketamine (maximum rate: 7.5 mcg/kg/hr).172 A retrospective review found that 6 of 9 pediatric patients (16 months to 10 years of age) had both clinical and electrical resolution following a median dose of 40 mcg/kg/min.173 The only adverse events reported were increased saliva production (n=9) and mild increase in liver enzymes with concomitant PB (n=4). A multicenter, retrospective study evaluated the use of IV ketamine in 60 episodes of refractory GCSE in 46 adult and 12 pediatric patients.174 A loading dose (median 1.5 mg/kg; maximum 5 mg/kg) and a subsequent continuous infusion (median 2.75 mg/kg/hr; maximum 10 mg/kg/hr) controlled seizures in 19 episodes (32%). Adverse events included a syndrome similar to PRIS (n=1), supraventricular tachycardia (n=2), and atrial fibrillation (n=1).
Table 6.
Use of Ketamine for Refractory Status Epilepticus in Pediatric Patients
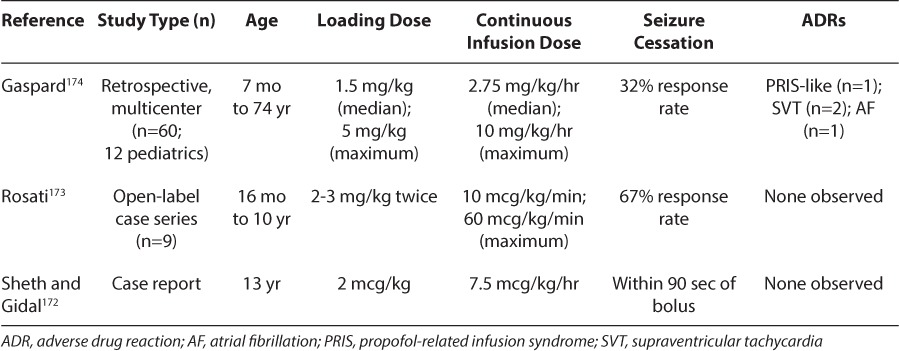
An advantage of ketamine is its ability to maintain arterial blood pressure, pulse rate, and cardiac output.175 It may cause hallucinations upon awakening, increased salivation, and increased intraocular and intracranial pressures. Although neurotoxicity has rarely been reported in infants or children, it has not been reported in any pediatric cases of SE. Finally, ketamine has been reported to be proconvulsant in some patients. In summary, ketamine is a reasonable agent to consider in refractory GCSE for which general anesthesia has failed, especially in those with cardiac instability.
Inhaled anesthetics
Isoflurane produces a dose-related reduction in cortical electrical activity, decreases cerebral metabolic rate and oxygen consumption, and does not increase cerebral blood flow. Although the mechanism of its anticonvulsant effects is not fully elucidated, it may be attributable to the potentiation of inhibitory postsynaptic GABAA receptors. Today, inhaled anesthetics are not used until other approaches fail, and only a few studies have used inhaled anesthetics (particularly isoflurane) for the treatment of refractory SE.176–178
Although concentrations required to maintain burst suppression are variable, isoflurane generally stops seizure at concentrations of 0.5% to 3%, which are not ordinarily associated with hemodynamic effects. Major limitations to the use of inhaled anesthetic involves logistical difficulties in using this in the intensive care unit, adverse effects, and the high rate of seizure recurrence after discontinuation of the therapy. Isoflurane can induce hypotension, so close hemodynamic monitoring is necessary, with administration of isotonic fluids and vasopressors as needed. Long-term use may cause atelectasis, infections, paralytic ileus, and deep venous thrombosis.177 Fugate et al178 also raised concerns about isoflurane and central nervous system toxicity, especially in thalamic and cerebellar regions.
Magnesium
Intravenous magnesium sulfate has been used primarily for seizures associated with eclampsia. Although magnesium has antiseizure properties, the mechanism by which it produces this effect is unknown. It has been shown to influence several receptors and ion channels. It is thought to work with divalent cations (e.g., Ca++) to maintain electrical stability of the neuronal membrane (Figure). That said, magnesium and calcium have opposing effects on synaptic release of neutro-transmitters. Calcium facilitates synaptic release whereas magnesium inhibits release; hence, blockage can be excitatory or inhibitory based on the nature of the synapse. Although magnesium causes voltage-dependent blockade of NMDA-induced currents, this effect tends to disappear at depolarized levels of the membrane potential. Therefore, it is not certain that magnesium blocks NMDA channels. Because it has no significant toxicity, it seems reasonable to give it in all cases of super-refractory SE, even in the presence of a normal serum magnesium value.147 One regimen suggested administering an initial loading dose and continuous infusion sufficient to increases the serum level above 7 mEq/L.179
Super-refractory GCSE
Super-refractory GCSE involves seizures that have persisted or recurred for 24 hours or more after the administration of anesthesia (Table 1). This includes exacerbations that occur when reducing the dose of an anticonvulsant or withdrawing anesthesia. To date, no randomized trials have evaluated treatment options for super-refractory GCSE; hence, the basis of information is generally derived from case reports. Other anticonvulsants (e.g., topiramate), immuno-modulatory compounds (steroids, IVIG), and non-pharmacological approaches (hypothermia, electroconvulsive treatment, vagus nerve stimulation, and the ketogenic diet) have been used in cases of protracted, super-refractory GCSE. Surgical intervention is rarely an option. There are no clear guidelines about dose or duration of therapy and often little to no evaluation of effectiveness.
Topiramate
Topiramate influences a variety of receptors and ion channels. Similar to the barbiturates and hydantoins, topiramate exhibits voltage-sensitive sodium-channel blockade. It also potentiates GABA activity at some subtype of the GABAA receptor (Figure) to significantly increase brain concentrations of GABA. This effect occurs independent of the BDZ site on the GABAA receptor; hence, topiramate may be effective in patients who display pharmacoresistance to BDZ. Topiramate also antagonizes excitatory glutamatergic transmission at the AMPA/kainite subtype of the glutamate receptor, which may be its most influential mechanism in termination of electrical discharges in patients with refractory epilepsy. Although topiramate inhibits high-voltage-activated T-type calcium channels this action does not significantly contribute to its effectiveness as an anticonvulsant.
Several reports in pediatric patients have described the successful use of rapidly titrated, nasogastrically administered topiramate (Table 7).180–184 These reports have included 1 prospective, observational study (n=14 patients) and 4 case series/reports (n=9 patients). Patients ranged from 2 months to 16 years of age, and 10 experienced new onset seizures. Doses ranged from 2 to 25 mg/kg/day. Time to response was from 2 hours to 6 days.
Table 7.
Use of Topiramate for Refractory Status Epilepticus in Pediatric Patient
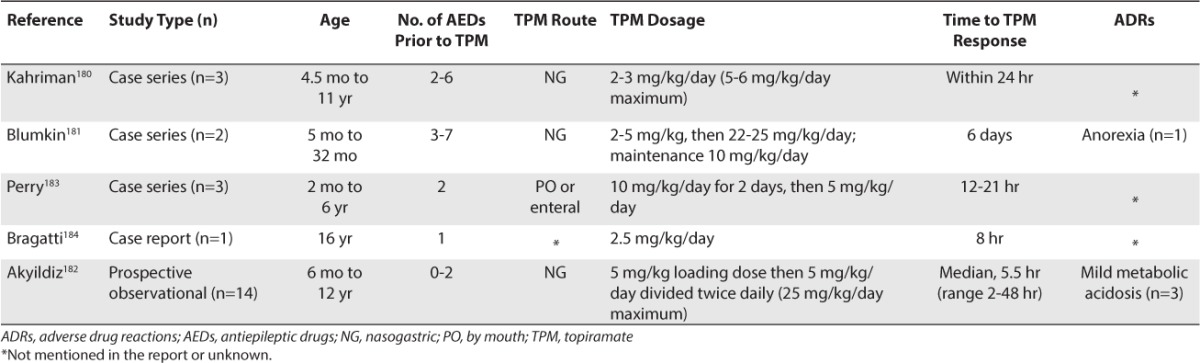
Topiramate should be implemented at full therapeutic doses (5–10 mg/kg/day) divided 3 times a day. To administer nasogastrically, the topiramate tablets should be crushed to a powder, mixed with water, and administered by syringe into the nasogastric tube. Once seizures are controlled, the topiramate dose should be tapered to a normal age/weight-appropriate maintenance dosage.
A concern with the aggressive implementation of large doses is possible hyperchloremic, non-anion gap, metabolic acidosis due to inhibition of type ll and lV carbonic anhydrase enzymes.185 Contributing factors are not fully understood, but metabolic acidosis does occur more frequently in children. Dose does not appear to be the sole determinate as it has been noted at doses as small as 50 mg/day185,186 and following overdoses.187,188 If metabolic acidosis occurs it generally responds to discontinuation of topiramate; however, it can be treated with orally administered citrates with the goal of maintaining a serum bicarbonate concentration of at least 20 mEq/L.
Immunomodulatory compounds
Immunomodulating therapies (i.e., corticosteroids, IV immune globulin, and plasmapheresis) have been given in cases of super-refractory GCSE.189,190 Their use is predicated upon recent discoveries that super-refractory GCSE may be due to antibodies directed against the voltage-gated potassium channels and the NMDA receptor. There is increasing evidence that inflammation plays an important role in epileptogenesis, especially the activation of specific inflammatory signaling pathways, such as the interleukin-1 receptor/Toll-like receptor (IL-1R/TLR) pathway.189 There is also the possibility that other undiscovered antibodies may contribute to the pathogenesis of super-refractory GCSE. Steroids may also decrease intracranial pressure, reverse blood–brain barrier opening, and reverse GABAergic inhibition, which may influence persistent seizures.
There is little evidence to support the use of steroids; however, unless there are specific contraindications, a trial of large doses of steroids should be given to all patients with an unidentified cause of super-refractory GCSE. Although prednisolone is no longer available in the United States, Shorvon and Ferlisi189 recommend prednisolone, 1 g/day IV, for 3 days, followed by 0.25 mg/kg/day four times daily.189 If there is a response, treatment is continued with long-term steroids, IVIG, and later, other immunomodulatory agents such as cyclophosphamide or rituximab.189 Neuroactive steroids (e.g., allopregnanolone) are also being explored.191
If there is no response this should be followed by 1 or 2 courses of IVIG, 0.4 g/kg/day for 5 days.189 This is especially true if inflammatory processes such as Rasmussen's encephalitis or new onset refractory SE are suspected.192,193
Although plasma exchange is rarely used, it has been tried.189 If plasmapheresis is used, it may reduce serum concentrations of an anticonvulsant; therefore, therapeutic drug monitoring is critical to the maintenance of serum concentrations as supplemental antiepileptic dosing may be required. This is especially true for those agents that are not highly protein bound.
Hypothermia
Animal studies show that controlled, moderate hypothermia reduces excitatory transmission and epileptic discharges, reduces brain edema by altering permeability of the blood–brain barrier and pro-inflammatory reactions, and has neuroprotective effects (decreases apoptosis). It also decreases cerebral metabolic rate, oxygen use, and ATP consumption.189,194
Few data have assessed the efficacy or safety of hypothermia in refractory GCSE. One case series described 3 children with refractory SE who were successfully treated with hypothermia (30°C–31°C) and barbiturate coma for 48 to 120 hours. The effects of hypothermia could not be separated from those of barbiturate.195 Corry et al196 reported a series of 4 patients who successfully gained seizure control after receiving therapeutic hypothermia (31°C–35°C) for 20 to 61 hours using endovascular cooling. Mild hypothermia (31°C–36°C) together with MDL, ketamine, or thiopental for 1 to several days has been reported to control refractory SE, but seizures can recur after rewarming. Rossetti197 recommended that only mild hypothermia (32°C–35°C) be given for 24 to 48 hours as a trial; however, it should not be used in combination with barbiturate anesthetics.
Adverse effects include acid–base and electrolyte disturbances, disseminated intravascular coagulation, coagulation disorders, thrombosis, infection, cardiac arrhythmia, bowel ischemia, and paralytic ileus.196 Cardiovascular and coagulation parameters, biochemistry and acid-base balance, and serum lactate should be monitored. Hypothermia may significantly reduce the clearance of several drugs, including anesthetics and anti-epileptics, resulting in the need for monitoring of serum concentrations. Reduced drug clearance is attributed to decreased activity of the cytochrome P450 enzyme system, reduced cardiac output, and decreased glomerular filtration rate.198,199
Electroconvulsive therapy
Electroconvulsive therapy (ECT) is listed as an indication for refractory SE by the American Psychiatric Association Task Force and has been used mostly in cases of refractory nonconvulsive SE.200 Its mechanism of action is unknown, but some evidence suggests that it enhances pre-synaptic transmission of GABA and prolongs the refractory period following a seizure.189,201 Lambrecq et al202 reviewed available information on the use of ECT in refractory SE. They noted cessation of SE in 80% of cases and complete recovery in 27% of patients. It remains unclear whether ECT or subdural electrode stimulation will terminate refractory GCSE; however, it is an option when established treatments including anesthesia have failed to abort the most severe cases of refractory GCSE.
Ketogenic diet
There have been case reports,203 case series,204,205 and retrospective reviews206,207 of the successful use of ketogenic diet in patients with refractory SE. The reports convincingly show that the diet should probably be tried in all severe cases of super-refractory SE.
Nabbout et al205 reported the successful use of a fat-to-combined protein and carbohydrate ratio of 4:1 in 9 cases of super-refractory SE caused by febrile illness.205 Efficacy occurred in 7 patients within 2 to 4 days of the onset of ketonuria, which occurred 4 to 6 days after implementing the diet. One patient experienced relapsed intractable SE and died following early disruption of the diet. Nam et al206 retrospectively reviewed the medical records of 4 children and 1 adult with refractory SE presumed to be due to viral encephalitis.206 The overall seizure frequency decreased to <50% of baseline in a median of 8 days. At 1 month, 2 patients were seizure-free, 1 patient showed seizure reduction of >90%, and the other 2 patients had >75% decrease without generalized seizures.
In a retrospective review of data from 4 centers, Thakur et al207 described successful resolution of SE in 90% of critically ill adult patients (n=10) with super-refractory SE who undertook the diet. The median duration of SE and the median number of anticonvulsants used before initiating the ketogenic diet was 21.5 days and 7 drugs, respectively. Ninety percent of patients achieved ketosis, and SE ceased in all patients achieving ketosis in a median of 3 days. Cobo et al204 reported the use of the ketogenic diet in 4 critically ill children (9 weeks to 13.5 years of age) with refractory SE. Although delayed ketosis and persistently elevated glucose measurements posed challenges in implementation, patients were weaned from continuous infusions of anesthetics without recurrence of SE. None of the patients experienced complete seizure cessation.
Wheless190 suggested that the patient be screened for metabolic disorders as a possible cause of the refractory SE before initiating the diet. Children in the intensive care unit are often receiving multiple medications, requiring consultation with the pharmacist to switch to formulations with no or minimal carbohydrate content. Close monitoring of total daily fluid, ketosis, and potential complications is essential. If metabolic acidosis develops, treatment is suggested to maintain serum bicarbonate concentrations of >18 to 20 mEq/L. PRIS was reported in a 10-year-old patient with refractory SE who received the ketogenic diet along with PRO.167 The authors speculated that the 2 agents impaired fatty-acid oxidation and recommended that they not be used simultaneously.167
Vagus nerve stimulation
Although acute vagus nerve stimulation (VNS) has been suggested as an effective therapy in patients with super-refractory GCSE, it has been used in few children208 and adults.209,210 After 2 weeks of aggressive medical treatment, placement of VNS resulted in immediate cessation of seizures in a 13-year-old patient with refractory SE.208 A recent publication describing 153 pediatric patients treated with VNS reported complete seizure remission in approximately 5%.211 However, other pediatric case series have reported a reduced seizure frequency in as many as 68% of patients.212
CONCLUSIONS
GCSE is one of the most common neurologic emergencies and can be associated with significant morbidity and mortality if not treated promptly and aggressively. Management is staged and generally involves the use of life support measures, identification, and management of underlying causes, and rapid initiation of antiepileptic medications. In a non-hospital setting, PR DZP is being replaced by IN MDL or IM LZP. Impending GCSE is best managed by IV LZP in the emergency department, but intranasal MDL is being used increasingly. Although a hydantoin has historically been used as the first-line anticonvulsant in patients unresponsive to a BDZ, there is little evidence to support that it is as effective as PB. There is a lack of consensus regarding the optimal therapeutic approach for refractory GCSE, but continuous infusion of barbiturates (PTB, thiopental), BDZs (LZP, MDL), or PRO may be used. Super-refractory GCSE has been successfully treated with other anticonvulsants (e.g., topiramate), immunomodulatory compounds (steroids, IVIG), and non-pharmacological approaches (hypothermia, electroconvulsive treatment, vagus nerve stimulation, and ketogenic diet), but there are no studies or clear guidelines to drive therapeutic decisions.
ABBREVIATIONS
- BDZ
benzodiazepine
- DZP
diazepam
- EEG
electroencephalogram
- fPHT
fosphenytoin
- GCSE
generalized convulsive status epilepticus
- IM
intramuscular
- IV
intravenous
- LZP
lorazepam
- MDL
midazolam
- NMDA
N-methyl-d-aspartate
- PB
phenobarbital
- PHT
phenytoin
- PR
rectal
- PRO
propofol
- PRIS
propofol-related infusion syndrome
- PTB
pentobarbital
- SE
status epilepticus
- VPA
valproate
Footnotes
Disclosures Dr. Wheless is a consultant and/or member of the speaker's bureau for Cyberonics, Eisai, Lundbeck, Pfizer, Questcor, Sunovion, Supernus, and Upsher-Smith and has received grants from Cyberonics, Eisai, GSK, Novartis, and Upsher-Smith. Drs. Alford and Phelps declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Huff JS, Morris DL, Kothari RU, Gibbs MA. Emergency department management of patients with seizures: a multicenter study. Acad Emerg Med. 2001;8(6):622–628. doi: 10.1111/j.1553-2712.2001.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 2.Capovilla G, Beccaria F, Beghi E et al. Treatment of convulsive status epilepticus in childhood: Recommendations of the Italian League Against Epilepsy. Epilepsia. 2013;54(suppl 7):23–34. doi: 10.1111/epi.12307. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenzo RJ, Garnett LK, Towne AR et al. Comparison of status epilepticus with prolonged seizure episodes lasting from 10 to 29 minutes. Epilepsia. 1999;40(2):164–169. doi: 10.1111/j.1528-1157.1999.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 4.Jenssen S, Gracely EJ, Sperling MR. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47(9):1499–1503. doi: 10.1111/j.1528-1167.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 5.Lowenstein DH, Bleck T, Macdonald RL. It's time to revise the definition of status epilepticus. Epilepsia. 1999;40(1):120–122. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- 6.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659–664. [PubMed] [Google Scholar]
- 7.Wasterlain CG, Chen JW. Definition and classification of status epilepticus. In: Westerlain CG, Treiman DM, editors. Status Epilepticus. Cambridge, MA: MIT Press; 2006. pp. 11–16. [Google Scholar]
- 8.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5suppl 2):13–23. [PubMed] [Google Scholar]
- 9.DeLorenzo RJ, Towne AR, Pellock JM, Ko D. Status epilepticus in children, adults, and the elderly. Epilepsia. 1992;33(suppl 4):S15–S25. doi: 10.1111/j.1528-1157.1992.tb06223.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh RK, Gaillard WD. Status epilepticus in children. Curr Neurol Neurosci Rep. 2009;9(2):137–144. doi: 10.1007/s11910-009-0022-9. [DOI] [PubMed] [Google Scholar]
- 11.Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814(1–2):179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- 12.Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand. 2007;186:7–15. [PubMed] [Google Scholar]
- 13.Naylor DE. Glutamate and gaba in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia. 2010;51(suppl 3):106–109. doi: 10.1111/j.1528-1167.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodkin HR, Joshi S, Kozhemyakin M, Kapur J. Impact of receptor changes on treatment of status epilepticus. Epilepsia. 2007;48(suppl 8):14–15. doi: 10.1111/j.1528-1167.2007.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodkin HP, Sun C, Yeh JL et al. Gaba(a) receptor internalization during seizures. Epilepsia. 2007;48(suppl 5):109–113. doi: 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(suppl 9):63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol Ther. 2001;90(1):21–34. doi: 10.1016/s0163-7258(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246–256. doi: 10.1016/S1474-4422(06)70374-X. [DOI] [PubMed] [Google Scholar]
- 19.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004;10(7):685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- 20.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4(1):18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brophy GM, Bell R, Claassen J et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 22.Treatment of convulsive status epilepticus. Recommendations of the epilepsy foundation of america's working group on status epilepticus. JAMA. 1993;270(7):854–859. [PubMed] [Google Scholar]
- 23.Appleton R, Choonara I, Martland T et al. The treatment of convulsive status epilepticus in children. The Status Epilepticus Working Party, members of the Status Epilepticus Working Party. Arch Dis Child. 2000;83(5):415–419. doi: 10.1136/adc.83.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9(4):353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
- 25.Shorvon S, Baulac M, Cross H et al. The drug treatment of status epilepticus in europe: Consensus document from a workshop at the First London Colloquium on Status Epilepticus. Epilepsia. 2008;49(7):1277–1285. doi: 10.1111/j.1528-1167.2008.01706_3.x. [DOI] [PubMed] [Google Scholar]
- 26.Meierkord H, Boon P, Engelsen B et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 27.Alldredge BK, Gelb AM, Isaacs SM et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 28.Leppik IE, Derivan AT, Homan RW et al. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA. 1983;249(11):1452–1454. [PubMed] [Google Scholar]
- 29.Cock HR, Schapira AH. A comparison of lorazepam and diazepam as initial therapy in convulsive status epilepticus. QJM. 2002;95(4):225–231. doi: 10.1093/qjmed/95.4.225. [DOI] [PubMed] [Google Scholar]
- 30.Appleton R, Macleod S, Martland T. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. 2008;(3):Cd001905. doi: 10.1002/14651858.CD001905.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Minicucci F, Muscas G, Perucca E et al. Treatment of status epilepticus in adults: guidelines of the Italian League Against Epilepsy. Epilepsia. 2006;47(suppl 5):9–15. doi: 10.1111/j.1528-1167.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 32.Lahat E, Goldman M, Barr J et al. Intranasal midazolam for childhood seizures. Lancet. 1998;352(9128):620. doi: 10.1016/S0140-6736(05)79574-X. [DOI] [PubMed] [Google Scholar]
- 33.Lahat E, Goldman M, Barr J et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: Prospective randomised study. BMJ. 2000;321(7253):83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisgin T, Gurer Y, Tezic T et al. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17(2):123–126. doi: 10.1177/088307380201700206. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya M, Kalra V, Gulati S. Intra-nasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–359. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Tonekaboni SH, Shamsabadi FM, Anvari SS et al. A comparison of buccal midazolam and intravenous diazepam for the acute treatment of seizures in children. Iran J Pediatr. 2012;22(3):303–308. [PMC free article] [PubMed] [Google Scholar]
- 37.Silbergleit R, Lowenstein D, Durkalski V, Conwit R. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(suppl 8):45–47. doi: 10.1111/j.1528-1167.2011.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silbergleit R, Durkalski V, Lowenstein D et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 16 2012;366(7):591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abou-Khalil B, Wheless J, Rogin J et al. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia. 2013;54(11):1968–1976. doi: 10.1111/epi.12373. [DOI] [PubMed] [Google Scholar]
- 40.Rogin J, Wheless J, Abou-Khalil B et al. Safety and effectiveness of long-term treatment with diazepam auto-injector administered by caregivers in an outpatient setting for the treatment of acute repetitive seizures. Epilepsia. 2014;55(9):1444–1451. doi: 10.1111/epi.12685. [DOI] [PubMed] [Google Scholar]
- 41.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: A randomised trial. Lancet. 1999;353(9153):623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 42.Camfield PR. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. J Pediatr. 1999;135(3):398–399. [PubMed] [Google Scholar]
- 43.Warden CR, Frederick C. Midazolam and diazepam for pediatric seizures in the prehospital setting. Prehosp Emerg Care. 2006;10(4):463–467. doi: 10.1080/10903120600885126. [DOI] [PubMed] [Google Scholar]
- 44.Harbord MG, Kyrkou NE, Kyrkou MR et al. Use of intranasal midazolam to treat acute seizures in paediatric community settings. J Paediatr Child Health. 2004;40(9–10):556–558. doi: 10.1111/j.1440-1754.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 45.Holsti M, Dudley N, Schunk J et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747–753. doi: 10.1001/archpediatrics.2010.130. [DOI] [PubMed] [Google Scholar]
- 46.O'Regan ME, Brown JK, Clarke M. Nasal rather than rectal benzodiazepines in the management of acute childhood seizures? Dev Med Child Neurol. 1996;38(11):1037–1045. doi: 10.1111/j.1469-8749.1996.tb15064.x. [DOI] [PubMed] [Google Scholar]
- 47.Kutlu NO, Yakinci C, Dogrul M, Durmaz Y. Intranasal midazolam for prolonged convulsive seizures. Brain Dev. 2000;22(6):359–361. doi: 10.1016/s0387-7604(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 48.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5(2):253–255. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Holsti M, Sill BL, Firth SD et al. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23(3):148–153. doi: 10.1097/PEC.0b013e3180328c92. [DOI] [PubMed] [Google Scholar]
- 50.Thakker A, Shanbag P. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260(2):470–474. doi: 10.1007/s00415-012-6659-3. [DOI] [PubMed] [Google Scholar]
- 51.McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17(6):575–582. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson M, Tambe P, Sammons H et al. Pharmacokinetics of buccal and intranasal lorazepam in healthy adult volunteers. Eur J Clin Pharmacol. 2012;68(2):155–159. doi: 10.1007/s00228-011-1109-1. [DOI] [PubMed] [Google Scholar]
- 53.Scheepers M, Scheepers B, Clarke M et al. Is intranasal midazolam an effective rescue medication in adolescents and adults with severe epilepsy? Seizure. 2000;9(6):417–422. doi: 10.1053/seiz.2000.0425. [DOI] [PubMed] [Google Scholar]
- 54.Rey E, Delaunay L, Pons G et al. Pharmacokinetics of midazolam in children: comparative study of intranasal and intravenous administration. Eur J Clin Pharmacol. 1991;41(4):355–357. doi: 10.1007/BF00314967. [DOI] [PubMed] [Google Scholar]
- 55.Wermeling DP, Miller JL, Archer SM et al. Bioavailability and pharmacokinetics of lorazepam after intranasal, intravenous, and intramuscular administration. J Clin Pharmacol. 2001;41(11):1225–1231. doi: 10.1177/00912700122012779. [DOI] [PubMed] [Google Scholar]
- 56.Arya R, Gulati S, Kabra M et al. Intranasal versus intravenous lorazepam for control of acute seizures in children: a randomized open-label study. Epilepsia. 2011;52(4):788–793. doi: 10.1111/j.1528-1167.2010.02949.x. [DOI] [PubMed] [Google Scholar]
- 57.Fisgin T, Gurer Y, Senbil N et al. Nasal midazolam effects on childhood acute seizures. J Child Neurol. 2000;15(12):833–835. doi: 10.1177/088307380001501219. [DOI] [PubMed] [Google Scholar]
- 58.Anderson M. Buccal midazolam for pediatric convulsive seizures: efficacy, safety, and patient acceptability. Patient Prefer Adherence. 2013;7:27–34. doi: 10.2147/PPA.S39233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntyre J, Robertson S, Norris E et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 60.Mpimbaza A, Ndeezi G, Staedke S et al. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 61.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain Dev. 2009;31(10):744–749. doi: 10.1016/j.braindev.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Khan A, Baheerathan A, Setty G, Hussain N. Carers' express positive views on the acceptability, efficacy and safety of buccal midazolam for paediatric status epilepticus. Acta Paediatr. 2014;103(4):e165–168. doi: 10.1111/apa.12529. [DOI] [PubMed] [Google Scholar]
- 63.Ivaturi V, Kriel R, Brundage R et al. Bio-availability of intranasal vs. rectal diazepam. Epilepsy Res. 2013;103(2–3):254–261. doi: 10.1016/j.eplepsyres.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Malinovsky JM, Lejus C, Servin F et al. Plasma concentrations of midazolam after i.V., nasal or rectal administration in children. Br J Anaesth. 1993;70(6):617–620. doi: 10.1093/bja/70.6.617. [DOI] [PubMed] [Google Scholar]
- 65.Knoester PD, Jonker DM, Van Der Hoeven RT et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol. 2002;53(5):501–507. doi: 10.1046/j.1365-2125.2002.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baysun S, Aydin OF, Atmaca E, Gurer YK. A comparison of buccal midazolam and rectal diazepam for the acute treatment of seizures. Clin Pediatr (Phila) 2005;44(9):771–776. doi: 10.1177/000992280504400904. [DOI] [PubMed] [Google Scholar]
- 67.Ashrafi MR, Khosroshahi N, Karimi P et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010;14(5):434–438. doi: 10.1016/j.ejpn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery. Adv Drug Deliv Rev. 2012;64(10):911–918. doi: 10.1016/j.addr.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Humphries LK, Eiland LS. Treatment of acute seizures: Is intranasal midazolam a viable option? J Pediatr Pharmacol Ther. 2013;18(2):79–87. doi: 10.5863/1551-6776-18.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan TM, Kissoon N, Hasan MY et al. Comparison of plasma levels and pharmacodynamics after intraosseous and intravenous administration of fosphenytoin and phenytoin in piglets. Pediatr Crit Care Med. 2000;1(1):60–64. doi: 10.1097/00130478-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38(2):202–207. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- 72.Treiman DM, Meyers PD, Walton NY et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative study group. N Engl J Med. 17. 1998;339(12):792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 73.Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure. 2014;23(3):167–174. doi: 10.1016/j.seizure.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Limdi NA, Shimpi AV, Faught E et al. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353–355. doi: 10.1212/01.WNL.0000149527.47600.5A. [DOI] [PubMed] [Google Scholar]
- 75.Peters CN, Pohlmann-Eden B. Intravenous valproate as an innovative therapy in seizure emergency situations including status epilepticus—experience in 102 adult patients. Seizure. 2005;14(3):164–169. doi: 10.1016/j.seizure.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Olsen KB, Tauboll E, Gjerstad L. Valproate is an effective, well-tolerated drug for treatment of status epilepticus/serial attacks in adults. Acta Neurol Scand Suppl. 2007;187:51–54. doi: 10.1111/j.1600-0404.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- 77.Gilad R, Izkovitz N, Dabby R et al. Treatment of status epilepticus and acute repetitive seizures with I.V. Valproic acid vs phenytoin. Acta Neurol Scand. 2008;118(5):296–300. doi: 10.1111/j.1600-0404.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 78.Agarwal P, Kumar N, Chandra R et al. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16(6):527–532. doi: 10.1016/j.seizure.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Chen L, Feng P, Wang J et al. Intravenous sodium valproate in mainland China for the treatment of diazepam refractory convulsive status epilepticus. J Clin Neurosci. 2009;16(4):524–526. doi: 10.1016/j.jocn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Hovinga CA, Chicella MF, Rose DF et al. Use of intravenous valproate in three pediatric patients with nonconvulsive or convulsive status epilepticus. Ann Pharmacother. 1999;33(5):579–584. doi: 10.1345/aph.18349. [DOI] [PubMed] [Google Scholar]
- 81.Uberall MA, Trollmann R, Wunsiedler U, Wenzel D. Intravenous valproate in pediatric epilepsy patients with refractory status epilepticus. Neurology. 13 2000;54(11):2188–2189. doi: 10.1212/wnl.54.11.2188-a. [DOI] [PubMed] [Google Scholar]
- 82.Yu KT, Mills S, Thompson N, Cunanan C. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia. 2003;44(5):724–726. doi: 10.1046/j.1528-1157.2003.41302.x. [DOI] [PubMed] [Google Scholar]
- 83.Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: A randomized controlled trial. J Child Neurol. 2007;22(10):1191–1197. doi: 10.1177/0883073807306248. [DOI] [PubMed] [Google Scholar]
- 84.Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: A pilot study. Neurology. 2006;67(2):340–342. doi: 10.1212/01.wnl.0000224880.35053.26. [DOI] [PubMed] [Google Scholar]
- 85.Tiamkao S, Sawanyawisuth K, Chancharoen A. The efficacy of intravenous sodium valproate and phenytoin as the first-line treatment in status epilepticus: a comparison study. BMC Neurol. 2013;13:98. doi: 10.1186/1471-2377-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chitsaz A, Mehvari J, Salari M et al. A comparative assessment the efficacy of intravenous infusion of sodium valproate and phenytion in the treatment of status epilepticus. Int J Prev Med. 2013;4(Suppl 2):S216–221. [PMC free article] [PubMed] [Google Scholar]
- 87.Malamiri RA, Ghaempanah M, Khosroshahi N et al. Efficacy and safety of intravenous sodium valproate versus phenobarbital in controlling convulsive status epilepticus and acute prolonged convulsive seizures in children: a randomised trial. Eur J Paediatr Neurol. 2012;16(5):536–541. doi: 10.1016/j.ejpn.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 88.Brigo F, Storti M, Del Felice A et al. IV valproate in generalized convulsive status epilepticus: a systematic review. Eur J Neurol. 2012;19(9):1180–1191. doi: 10.1111/j.1468-1331.2011.03606.x. [DOI] [PubMed] [Google Scholar]
- 89.Brigo F, Igwe SC, Nardone R et al. A common reference-based indirect comparison meta-analysis of intravenous valproate versus intravenous phenobarbitone for convulsive status epilepticus. Epileptic Disord. 2013;15(3):314–323. doi: 10.1684/epd.2013.0601. [DOI] [PubMed] [Google Scholar]
- 90.Rossetti AO, Bromfield EB. Determinants of success in the use of oral levetiracetam in status epilepticus. Epilepsy Behav. 2006;8(3):651–654. doi: 10.1016/j.yebeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Patel NC, Landan IR, Levin J et al. The use of levetiracetam in refractory status epilepticus. Seizure. 2006;15(3):137–141. doi: 10.1016/j.seizure.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Rupprecht S, Franke K, Fitzek S et al. Levetiracetam as a treatment option in non-convulsive status epilepticus. Epilepsy Res. 2007;73(3):238–244. doi: 10.1016/j.eplepsyres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 93.Goraya JS, Khurana DS, Valencia I et al. Intravenous levetiracetam in children with epilepsy. Pediatr Neurol. 2008;38(3):177–180. doi: 10.1016/j.pediatrneurol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med. 2009;10(4):505–510. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirmani BF, Crisp ED, Kayani S, Rajab H. Role of intravenous levetiracetam in acute seizure management of children. Pediatr Neurol. 2009;41(1):37–39. doi: 10.1016/j.pediatrneurol.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 96.Standish JC, Hilmas E, Falchek SJ. Levetiracetam for the treatment of pediatric status epilepticus: a case series. J Pediatr Neurol. 2011;9(2):195–201. [Google Scholar]
- 97.McTague A, Kneen R, Kumar R et al. Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: Experience from a children's hospital. Seizure. 2012;21(7):529–534. doi: 10.1016/j.seizure.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 98.Hofler J, Trinka E. Lacosamide as a new treatment option in status epilepticus. Epilepsia. 2013;54(3):393–404. doi: 10.1111/epi.12058. [DOI] [PubMed] [Google Scholar]
- 99.Shiloh-Malawsky Y, Fan Z, Greenwood R, Tennison M. Successful treatment of childhood prolonged refractory status epilepticus with lacosamide. Seizure. 2011;20(7):586–588. doi: 10.1016/j.seizure.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Jain V, Harvey AS. Treatment of refractory tonic status epilepticus with intravenous lacosamide. Epilepsia. 2012;53(4):761–762. doi: 10.1111/j.1528-1167.2012.03419.x. [DOI] [PubMed] [Google Scholar]
- 101.Temkin NR, Dikmen SS, Anderson GD et al. Valproate therapy for prevention of posttraumatic seizures: a randomized trial. J Neurosurg. 1999;91(4):593–600. doi: 10.3171/jns.1999.91.4.0593. [DOI] [PubMed] [Google Scholar]
- 102.Gerstner T, Teich M, Bell N et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47(7):1136–1143. doi: 10.1111/j.1528-1167.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 103.Embacher N, Karner E, Wanschitz J et al. Acute encephalopathy after intravenous administration of valproate in non-convulsive status epilepticus. Eur J Neurol. 2006;13(10):e5–6. doi: 10.1111/j.1468-1331.2006.01394.x. [DOI] [PubMed] [Google Scholar]
- 104.Novy J, Patsalos PN, Sander JW, Sisodiya SM. Lacosamide neurotoxicity associated with concomitant use of sodium channel-blocking antiepileptic drugs: a pharma-codynamic interaction? Epilepsy Behav. 2011;20(1):20–23. doi: 10.1016/j.yebeh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 105.Bleck T, Cock H, Chamberlain J et al. The established status epilepticus trial 2013. Epilepsia. 2013;54(suppl 6):89–92. doi: 10.1111/epi.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinnar S, Pellock JM, Moshe SL et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38(8):907–914. doi: 10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 107.Wu YW, Shek DW, Garcia PA et al. Incidence and mortality of generalized convulsive status epilepticus in california. Neurology. 9 2002;58(7):1070–1076. doi: 10.1212/wnl.58.7.1070. [DOI] [PubMed] [Google Scholar]
- 108.Moshe SL. Brain injury with prolonged seizures in children and adults. J Child Neurol. 1998;13(suppl 1):S3–S6. doi: 10.1177/0883073898013001021. discussion S30–32. [DOI] [PubMed] [Google Scholar]
- 109.Sahin M, Menache CC, Holmes GL, Riviello JJ., Jr. Prolonged treatment for acute symptomatic refractory status epilepticus: outcome in children. Neurology. 12 2003;61(3):398–401. doi: 10.1212/01.wnl.0000073139.53008.f2. [DOI] [PubMed] [Google Scholar]
- 110.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83(3):323–331. [PubMed] [Google Scholar]
- 111.Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14(9):602–609. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 112.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42(11):1461–1467. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 113.Kim SJ, Lee DY, Kim JS. Neurologic outcomes of pediatric epileptic patients with pentobarbital coma. Pediatr Neurol. 2001;25(3):217–220. doi: 10.1016/s0887-8994(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 114.Fountain NB, Fugate JE. Refractory status epilepticus: what to put down: the anesthetics or the patient? Neurology. 25 2014;82(8):650–651. doi: 10.1212/WNL.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 115.Claassen J, Hirsch LJ, Mayer SA. Treatment of status epilepticus: a survey of neurologists. J Neurol Sci. 15 2003;211(1–2):37–41. doi: 10.1016/s0022-510x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 116.Holtkamp M, Masuhr F, Harms L et al. The management of refractory generalised convulsive and complex partial status epilepticus in three european countries: a survey among epileptologists and critical care neurologists. J Neurol Neurosurg Psychiatry. 2003;74(8):1095–1099. doi: 10.1136/jnnp.74.8.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43(2):146–153. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 118.Young RS, Ropper AH, Hawkes D et al. Pentobarbital in refractory status epilepticus. Pediatr Pharmacol (New York) 1983;3(2):63–67. [PubMed] [Google Scholar]
- 119.Lowenstein DH, Aminoff MJ, Simon RP. Barbiturate anesthesia in the treatment of status epilepticus: Clinical experience with 14 patients. Neurology. 1988;38(3):395–400. doi: 10.1212/wnl.38.3.395. [DOI] [PubMed] [Google Scholar]
- 120.Rashkin MC, Youngs C, Penovich P. Pentobarbital treatment of refractory status epilepticus. Neurology. 1987;37(3):500–503. doi: 10.1212/wnl.37.3.500. [DOI] [PubMed] [Google Scholar]
- 121.Yaffe K, Lowenstein DH. Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology. 1993;43(5):895–900. doi: 10.1212/wnl.43.5.895. [DOI] [PubMed] [Google Scholar]
- 122.Kinoshita H, Nakagawa E, Iwasaki Y et al. Pentobarbital therapy for status epilepticus in children: Timing of tapering. Pediatr Neurol. 1995;13(2):164–168. doi: 10.1016/0887-8994(95)00114-u. [DOI] [PubMed] [Google Scholar]
- 123.Holmes GL, Riviello JJ., Jr. Midazolam and pentobarbital for refractory status epilepticus. Pediatr Neurol. 1999;20(4):259–264. doi: 10.1016/s0887-8994(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 124.Barberio M, Reiter PD, Kaufman J et al. Continuous infusion pentobarbital for refractory status epilepticus in children. J Child Neurol. 2012;27(6):721–726. doi: 10.1177/0883073811424941. [DOI] [PubMed] [Google Scholar]
- 125.Mirski MA, Williams MA, Hanley DF. Prolonged pentobarbital and phenobarbital coma for refractory generalized status epilepticus. Crit Care Med. 1995;23(2):400–404. doi: 10.1097/00003246-199502000-00028. [DOI] [PubMed] [Google Scholar]
- 126.Lee WK, Liu KT, Young BW. Very-high-dose phenobarbital for childhood refractory status epilepticus. Pediatr Neurol. 2006;34(1):63–65. doi: 10.1016/j.pediatrneurol.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 127.Osorio I, Reed RC. Treatment of refractory generalized tonic-clonic status epilepticus with pentobarbital anesthesia after high-dose phenytoin. Epilepsia. 1989;30(4):464–471. doi: 10.1111/j.1528-1157.1989.tb05327.x. [DOI] [PubMed] [Google Scholar]
- 128.Krishnamurthy KB, Drislane FW. Relapse and survival after barbiturate anesthetic treatment of refractory status epilepticus. Epilepsia. 1996;37(9):863–867. doi: 10.1111/j.1528-1157.1996.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 129.Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: Effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62(11):1698–1702. doi: 10.1001/archneur.62.11.1698. [DOI] [PubMed] [Google Scholar]
- 130.Schreiber JM, Gaillard WD. Treatment of refractory status epilepticus in childhood. Curr Neurol Neurosci Rep. 2011;11(2):195–204. doi: 10.1007/s11910-010-0170-y. [DOI] [PubMed] [Google Scholar]
- 131.Van Ness PC. Pentobarbital and EEG burst suppression in treatment of status epilepticus refractory to benzodiazepines and phenytoin. Epilepsia. 1990;31(1):61–67. doi: 10.1111/j.1528-1157.1990.tb05361.x. [DOI] [PubMed] [Google Scholar]
- 132.Chicella M, Jansen P, Parthiban A et al. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med. 2002;30(12):2752–2756. doi: 10.1097/00003246-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 133.Bledsoe KA, Kramer AH. Propylene glycol toxicity complicating use of barbiturate coma. Neurocrit Care. 2008;9(1):122–124. doi: 10.1007/s12028-008-9065-z. [DOI] [PubMed] [Google Scholar]
- 134.Miller MA, Forni A, Yogaratnam D. Propylene glycol-induced lactic acidosis in a patient receiving continuous infusion pentobarbital. Ann Pharmacother. 2008;42(10):1502–1506. doi: 10.1345/aph.1L186. [DOI] [PubMed] [Google Scholar]
- 135.Barnes BJ, Gerst C, Smith JR et al. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006;26(1):23–33. doi: 10.1592/phco.2006.26.1.23. [DOI] [PubMed] [Google Scholar]
- 136.Parker MG, Fraser GL, Watson DM, Riker RR. Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy. Intensive Care Med. 2002;28(1):81–84. doi: 10.1007/s00134-001-1125-1. [DOI] [PubMed] [Google Scholar]
- 137.Zar T, Yusufzai I, Sullivan A, Graeber C. Acute kidney injury, hyperosmolality and metabolic acidosis associated with lorazepam. Nat Clin Pract Nephrol. 2007;3(9):515–520. doi: 10.1038/ncpneph0573. [DOI] [PubMed] [Google Scholar]
- 138.Rivera R, Segnini M, Baltodano A, Perez V. Midazolam in the treatment of status epilepticus in children. Crit Care Med. 1993;21(7):991–994. doi: 10.1097/00003246-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 139.Koul RL, Raj Aithala G, Chacko A et al. Continuous midazolam infusion as treatment of status epilepticus. Arch Dis Child. 1997;76(5):445–448. doi: 10.1136/adc.76.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Igartua J, Silver P, Maytal J, Sagy M. Midazolam coma for refractory status epilepticus in children. Crit Care Med. 1999;27(9):1982–1985. doi: 10.1097/00003246-199909000-00043. [DOI] [PubMed] [Google Scholar]
- 141.Ozdemir D, Gulez P, Uran N et al. Efficacy of continuous midazolam infusion and mortality in childhood refractory generalized convulsive status epilepticus. Seizure. 2005;14(2):129–132. doi: 10.1016/j.seizure.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 142.Morrison G, Gibbons E, Whitehouse WP. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32(12):2070–2076. doi: 10.1007/s00134-006-0362-8. [DOI] [PubMed] [Google Scholar]
- 143.Koul R, Chacko A, Javed H, Al Riyami K. Eight-year study of childhood status epilepticus: Midazolam infusion in management and outcome. J Child Neurol. 2002;17(12):908–910. [PubMed] [Google Scholar]
- 144.Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17(2):106–110. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 145.Hayashi K, Osawa M, Aihara M et al. Efficacy of intravenous midazolam for status epilepticus in childhood. Pediatr Neurol. 2007;36(6):366–372. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 146.Claassen J, Hirsch LJ, Emerson RG et al. Continuous EEG monitoring and mid-azolam infusion for refractory nonconvulsive status epilepticus. Neurology. 25 2001;57(6):1036–1042. doi: 10.1212/wnl.57.6.1036. [DOI] [PubMed] [Google Scholar]
- 147.Ferlisi M, Shorvon S. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(Pt 8):2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 148.Fernandez A, Lantigua H, Lesch C et al. High-dose midazolam infusion for refractory status epilepticus. Neurology. 28 2014;82(4):359–365. doi: 10.1212/WNL.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin. 2013;29(2):239–257. doi: 10.1016/j.ccc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Kumar A, Bleck TP. Intravenous midazolam for the treatment of refractory status epilepticus. Crit Care Med. 1992;20(4):483–488. doi: 10.1097/00003246-199204000-00009. [DOI] [PubMed] [Google Scholar]
- 151.Naritoku DK, Sinha S. Prolongation of midazolam half-life after sustained infusion for status epilepticus. Neurology. 2000;54(6):1366–1368. doi: 10.1212/wnl.54.6.1366. [DOI] [PubMed] [Google Scholar]
- 152.Harrison AM, Lugo RA, Schunk JE. Treatment of convulsive status epilepticus with propofol: case report. Pediatr Emerg Care. 1997;13(6):420–422. doi: 10.1097/00006565-199712000-00019. [DOI] [PubMed] [Google Scholar]
- 153.Brown LA, Levin GM. Role of propofol in refractory status epilepticus. Ann Pharmacother. 1998;32(10):1053–1059. doi: 10.1345/aph.17367. [DOI] [PubMed] [Google Scholar]
- 154.Rossetti AO, Reichhart MD, Schaller MD et al. Propofol treatment of refractory status epilepticus: A study of 31 episodes. Epilepsia. 2004;45(7):757–763. doi: 10.1111/j.0013-9580.2004.01904.x. [DOI] [PubMed] [Google Scholar]
- 155.Parviainen I, Uusaro A, Kalviainen R et al. Propofol in the treatment of refractory status epilepticus. Intensive Care Med. 2006;32(7):1075–1079. doi: 10.1007/s00134-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 156.Rossetti AO, Milligan TA, Vulliemoz S et al. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care. 2011;14(1):4–10. doi: 10.1007/s12028-010-9445-z. [DOI] [PubMed] [Google Scholar]
- 157.van Gestel JP, Blusse van Oud-Alblas HJ, Malingre M et al. Propofol and thiopental for refractory status epilepticus in children. Neurology. 2005;65(4):591–592. doi: 10.1212/01.wnl.0000173066.89001.f9. [DOI] [PubMed] [Google Scholar]
- 158.Timpe EM, Eichner SF, Phelps SJ. Propofol-related infusion syndrome in critically ill pediatric patients: Coincidence, association, or causation? J Pediatr Pharmacol Ther. 2006;11(1):17–42. doi: 10.5863/1551-6776-11.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barclay K, Williams AJ, Major E. Propofol infusion in children. BMJ. 1992;305(6859):953. doi: 10.1136/bmj.305.6859.953. author reply 953–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cray SH, Robinson BH, Cox PN. Lactic acidemia and bradyarrhythmia in a child sedated with propofol. Crit Care Med. 1998;26(12):2087–2092. doi: 10.1097/00003246-199812000-00046. [DOI] [PubMed] [Google Scholar]
- 161.Wolf A, Weir P, Segar P et al. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet. 24 2001;357(9256):606–607. doi: 10.1016/S0140-6736(00)04064-2. [DOI] [PubMed] [Google Scholar]
- 162.Withington DE, Decell MK, Al Ayed T. A case of propofol toxicity: Further evidence for a causal mechanism. Paediatr Anaesth. 2004;14(6):505–508. doi: 10.1111/j.1460-9592.2004.01299.x. [DOI] [PubMed] [Google Scholar]
- 163.Culp KE, Augoustides JG, Ochroch AE, Milas BL. Clinical management of cardiogenic shock associated with prolonged propofol infusion. Anesth Analg. 2004;99(1):221–226. doi: 10.1213/01.ANE.0000117285.12600.C1. [DOI] [PubMed] [Google Scholar]
- 164.Guitton C, Gabillet L, Latour P et al. Propofol infusion syndrome during refractory status epilepticus in a young adult: Successful ecmo resuscitation. Neurocrit Care. 2011;15(1):139–145. doi: 10.1007/s12028-010-9385-7. [DOI] [PubMed] [Google Scholar]
- 165.Mayette M, Gonda J, Hsu JL, Mihm FG. Propofol infusion syndrome resuscitation with extracorporeal life support: A case report and review of the literature. Ann Intensive Care. 2013;3(1):32. doi: 10.1186/2110-5820-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37(12):3024–3030. doi: 10.1097/CCM.0b013e3181b08ac7. [DOI] [PubMed] [Google Scholar]
- 167.Baumeister FA, Oberhoffer R, Liebhaber GM et al. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics. 2004;35(4):250–252. doi: 10.1055/s-2004-820992. [DOI] [PubMed] [Google Scholar]
- 168.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107(5):1689–1703. doi: 10.1213/ane.0b013e3181852595. [DOI] [PubMed] [Google Scholar]
- 169.Walder B, Tramer MR, Seeck M. Seizure-like phenomena and propofol: a systematic review. Neurology. 2002;58(9):1327–1332. doi: 10.1212/wnl.58.9.1327. [DOI] [PubMed] [Google Scholar]
- 170.Zubair S, Patton T, Smithson K et al. Propofol withdrawal seizures: Non-epileptic nature of seizures in a patient with recently controlled status epilepticus. Epileptic Disord. 2011;13(1):107–110. doi: 10.1684/epd.2011.0404. [DOI] [PubMed] [Google Scholar]
- 171.Dorandeu F, Dhote F, Barbier L et al. Treatment of status epilepticus with ketamine, are we there yet? CNS Neurosci Ther. 2013;19(6):411–427. doi: 10.1111/cns.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sheth RD, Gidal BE. Refractory status epilepticus: response to ketamine. Neurology. 1998;51(6):1765–1766. doi: 10.1212/wnl.51.6.1765. [DOI] [PubMed] [Google Scholar]
- 173.Rosati A, L'Erario M, Ilvento L et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012;79(24):2355–2358. doi: 10.1212/WNL.0b013e318278b685. [DOI] [PubMed] [Google Scholar]
- 174.Gaspard N, Foreman B, Judd LM et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498–1503. doi: 10.1111/epi.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gofrit ON, Leibovici D, Shemer J et al. Ketamine in the field: The use of ketamine for induction of anaesthesia before intubation in injured patients in the field. Injury. 1997;28(1):41–43. doi: 10.1016/S0020-1383(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 176.Kofke WA, Young RS, Davis P et al. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71(5):653–659. doi: 10.1097/00000542-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 177.Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61(8):1254–1259. doi: 10.1001/archneur.61.8.1254. [DOI] [PubMed] [Google Scholar]
- 178.Fugate JE, Burns JD, Wijdicks EF et al. Prolonged high-dose isoflurane for refractory status epilepticus: Is it safe? Anesth Analg. 2010;111(6):1520–1524. doi: 10.1213/ANE.0b013e3181f6da34. [DOI] [PubMed] [Google Scholar]
- 179.Visser NA, Braun KP, Leijten FS et al. Magnesium treatment for patients with refractory status epilepticus due to polg1-mutations. J Neurol. 2011;258(2):218–222. doi: 10.1007/s00415-010-5721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kahriman M, Minecan D, Kutluay E et al. Efficacy of topiramate in children with refractory status epilepticus. Epilepsia. 2003;44(10):1353–1356. doi: 10.1046/j.1528-1157.2003.11803.x. [DOI] [PubMed] [Google Scholar]
- 181.Blumkin L, Lerman-Sagie T, Houri T et al. Pediatric refractory partial status epilepticus responsive to topiramate. J Child Neurol. 2005;20(3):239–241. doi: 10.1177/08830738050200031701. [DOI] [PubMed] [Google Scholar]
- 182.Akyildiz BN, Kumandas S. Treatment of pediatric refractory status epilepticus with topiramate. Childs Nerv Syst. 2011;27(9):1425–1430. doi: 10.1007/s00381-011-1432-y. [DOI] [PubMed] [Google Scholar]
- 183.Perry MS, Holt PJ, Sladky JT. Topiramate loading for refractory status epilepticus in children. Epilepsia. 2006;47(6):1070–1071. doi: 10.1111/j.1528-1167.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 184.Bragatti JA, Torres CM, Netto CB et al. Topiramate is effective for status epilepticus and seizure control in neuraminidase deficiency. Arq Neuropsiquiatr. 2011;69(3):565–566. doi: 10.1590/s0004-282x2011000400031. [DOI] [PubMed] [Google Scholar]
- 185.Takeoka M, Holmes GL, Thiele E et al. Topiramate and metabolic acidosis in pediatric epilepsy. Epilepsia. 2001;42(3):387–392. doi: 10.1046/j.1528-1157.2001.04500.x. [DOI] [PubMed] [Google Scholar]
- 186.Ozer Y, Altunkaya H. Topiramate induced metabolic acidosis. Anaesthesia. 2004;59(8):830. doi: 10.1111/j.1365-2044.2004.03884.x. [DOI] [PubMed] [Google Scholar]
- 187.Fakhoury T, Murray L, Seger D et al. Topiramate overdose: clinical and laboratory features. Epilepsy Behav. 2002;3(2):185–189. doi: 10.1006/ebeh.2002.0339. [DOI] [PubMed] [Google Scholar]
- 188.Lynch MJ, Pizon AF, Siam MG, Krasowski MD. Clinical effects and toxicokinetic evaluation following massive topiramate ingestion. J Med Toxicol. 2010;6(2):135–138. doi: 10.1007/s13181-010-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 190.Wheless JW. Treatment of refractory convulsive status epilepticus in children: other therapies. Semin Pediatr Neurol. 2010;17(3):190–194. doi: 10.1016/j.spen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 191.Rogawski MA, Loya CM, Reddy K et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(suppl 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Granata T, Fusco L, Gobbi G et al. Experience with immunomodulatory treatments in rasmussen's encephalitis. Neurology. 2003;61(12):1807–1810. doi: 10.1212/01.wnl.0000099074.04539.e0. [DOI] [PubMed] [Google Scholar]
- 193.Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (Norse) syndrome: outcomes with early immunotherapy. Seizure. 2013;22(3):217–220. doi: 10.1016/j.seizure.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 194.Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23(3):689–696. doi: 10.1016/j.nbd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 195.Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12(4):367–372. doi: 10.1097/00003246-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 196.Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9(2):189–197. doi: 10.1007/s12028-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 197.Rossetti AO. What is the value of hypothermia in acute neurologic diseases and status epilepticus? Epilepsia. 2011;52(suppl 8):64–66. doi: 10.1111/j.1528-1167.2011.03241.x. [DOI] [PubMed] [Google Scholar]
- 198.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome p450 enzyme system. Crit Care Med. 2007;35(9):2196–2204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 199.Empey PE, de Mendizabal NV, Bell MJ et al. Therapeutic hypothermia decreases phenytoin elimination in children with traumatic brain injury. Crit Care Med. 2013;41(10):2379–2387. doi: 10.1097/CCM.0b013e318292316c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weiner RD, Coffet CE, Fochtmann L. The Practice of ECT: Recommendations for Treatment, Training and Privileging. 2nd ed. Washington, DC: American Psychiatric Press; 2001. [Google Scholar]
- 201.Sackeim HA. The anticonvulsant hypothesis of the mechanisms of action of ECT: current status. J ECT. 1999;15(1):5–26. [PubMed] [Google Scholar]
- 202.Lambrecq V, Villega F, Marchal C et al. Refractory status epilepticus: electroconvulsive therapy as a possible therapeutic strategy. Seizure. 2012;21(9):661–664. doi: 10.1016/j.seizure.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 203.Cervenka MC, Hartman AL, Venkatesan A et al. The ketogenic diet for medically and surgically refractory status epilepticus in the neurocritical care unit. Neurocrit Care. 2011;15(3):519–524. doi: 10.1007/s12028-011-9546-3. [DOI] [PubMed] [Google Scholar]
- 204.Cobo NH, Sankar R, Murata KK et al. The ketogenic diet as broad-spectrum treatment for super-refractory pediatric status epilepticus: challenges in implementation in the pediatric and neonatal intensive care units. J Child Neurol. 2014 doi: 10.1177/0883073813516192. [DOI] [PubMed] [Google Scholar]
- 205.Nabbout R, Mazzuca M, Hubert P et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51(10):2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 206.Nam SH, Lee BL, Lee CG et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011;52(11):e181–184. doi: 10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 207.Thakur KT, Probasco JC, Hocker SE et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 25 2014;82(8):665–670. doi: 10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Winston KR, Levisohn P, Miller BR, Freeman J. Vagal nerve stimulation for status epilepticus. Pediatr Neurosurg. 2001;34(4):190–192. doi: 10.1159/000056018. [DOI] [PubMed] [Google Scholar]
- 209.Patwardhan RV, Dellabadia J, Jr., Rashidi M et al. Control of refractory status epilepticus precipitated by anticonvulsant withdrawal using left vagal nerve stimulation: a case report. Surg Neurol. 2005;64(2):170–173. doi: 10.1016/j.surneu.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 210.O'Neill BR, Valeriano J, Synowiec A et al. Refractory status epilepticus treated with vagal nerve stimulation: case report. Neurosurgery. 2011;69(5):E1172–1175. doi: 10.1227/NEU.0b013e318223b979. [DOI] [PubMed] [Google Scholar]
- 211.Hauptman JS, Mathern GW. Vagal nerve stimulation for pharmacoresistant epilepsy in children. Surg Neurol Int. 2012;3(suppl 4):S269–274. doi: 10.4103/2152-7806.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rossignol E, Lortie A, Thomas T et al. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure. 2009;18(1):34–37. doi: 10.1016/j.seizure.2008.06.010. [DOI] [PubMed] [Google Scholar]


