Abstract
OBJECTIVES: With increasing complexity of critical care medicine comes an increasing need for multidisciplinary involvement in care. In many institutions, pharmacists are an integral part of this team, but long-term data on the interventions performed by pharmacists and their effects on patient care and outcomes are limited. We aimed to describe the role of pediatric clinical pharmacists in pediatric intensive care unit (PICU) practice.
METHODS: We retrospectively reviewed the records of pharmacy interventions in the PICU at the Mayo Clinic in Rochester, Minnesota, from 2003-2013, with a distinct period of increased pharmacist presence in the PICU from 2008 onward. We compared demographic and outcome data on patients who did and who did not have pharmacy interventions during 2 periods (2003–2007 and 2008–2013).
RESULTS: We identified 27,773 total interventions by pharmacists during the 11-year period, of which 79.8% were accepted by the clinical team. These interventions were made on 10,963 unique PICU admissions and prevented 5867 order entry errors. Pharmacists' interventions increased year over year, including a significant change in 2008. Patients who required pharmacy involvement were younger, sicker, and had longer intensive care unit, hospital, and ventilator duration. Average central line infections and central line entry rates decreased significantly over the study period.
CONCLUSIONS: Increased pharmacist presence in the PICU is associated with increased interventions and prevention of adverse drug events. Pharmacist participation during rounds and order entry substantially improved the care of critically sick children and should be encouraged.
INDEX TERMS: adverse drug reaction, catheter-related infections, medication errors, pediatric intensive care units, pharmacists
INTRODUCTION
Multidisciplinary involvement in patient care in the intensive care unit (ICU) has been shown to decrease hospital errors and improve patient outcomes. A multidisciplinary approach accounts for the complex modern critical care practice and the role of different participants in critical care delivery. Performing daily medical rounds by a multidisciplinary team has previously been shown to decrease mortality among medical ICU patients.1 Such a model has also been endorsed by the American College of Critical Care Medicine Task Force on models of critical care delivery.2 Although there is empirical support for a multidisciplinary approach to care, the existing literature does not agree on either specific attributes of the multidisciplinary team or the optimal team size.
Pharmacists are considered an integral part of the team, although their level of involvement in the critical care practice is variable.3 A few small studies have shown that a pharmacist's involvement in critical care rounds is associated with fewer adverse effects4 and alone may be associated with lower mortality among ICU patients.5 The American Academy of Pediatrics in 2003 proposed that inclusion of a pharmacist in the critical care team can help decrease medication errors.6 To date, however, descriptions in the pediatric literature of a clinical pharmacist's role in this team have remained limited. A recently published study conducted during 8 months in a pediatric ICU (PICU) described the number and types of interventions performed by pharmacists per patient.7 Because of the short duration of this study, it was not possible to demonstrate an effect of pharmacist interventions on outcomes or of pharmacist staffing on the number and types of interventions.
With transitions occurring in the field of clinical pharmacy, it is important to define the role of the clinical pharmacist on the multidisciplinary team and to highlight the value of the position, which includes enhancing the safety and quality of patient care, in addition to financial savings. Clinical pharmacists have been a part of the PICU team at our institution since 2003, with evolution of their role and involvement over time. The pharmacists working in the PICU have either completed a pediatric pharmacy residency or departmental pediatric pharmacy training. In 2008, a significant change was implemented: pharmacist coverage increased, allowing for presence of a clinical pharmacist in the unit from 7 AM to 5 PM on weekdays. This provided us with 2 distinct periods in which to analyze the effects of clinical pharmacists in PICU practice. The primary objective of this study was to delineate the clinical pharmacist's role in a pediatric critical care practice through retrospective review of interventions. Of specific interest were the types of interventions performed, their effects on patient care, and the extrapolated cost savings.
METHODS
This study was approved by our institutional review board. Our critical care and hospital pharmacy services divisions maintain records of quality metrics. Since January 2003, pharmacists have recorded their clinical interventions and outcomes of those interventions in an institutionally developed pharmaceutical care database (named P-Care). Data concerning pharmacists' interventions and changes in therapy due to their interventions were retrospectively extracted from the P-Care database in yearly increments via a reporting functionality that is available within the system.
A clinical intervention was defined as any recommendation the pharmacist made to the patient care team regarding a change to a patient's medication therapy or monitoring of their medication therapy. At the time the pharmacist documented a clinical intervention in P-Care, the system automatically recorded the patient demographic information, nursing unit/bed, date/time, pharmacist, and service. When recording each clinical intervention, the pharmacist categorized the type of clinical intervention, assigned a clinical severity rating, indicated whether the intervention was secondary to an ordering error, provided a brief narrative of the intervention, and documented whether the intervention resulted in a change in medication therapy or monitoring of that therapy. Each clinical intervention captured was designated as 1 of 6 intervention types: drug-dosing regimen, drug interaction or incompatibility, drug monitoring, drug route/method of administration, drug selection, and medication profile/order clarification.
We defined these interventions as follows: 1) drug-dosing regimen: a recommendation to change a medication dose, frequency, or duration of therapy; 2) drug interaction or incompatibility: a suggestion to change secondary to a drug-drug interaction, drug-food interaction, or drug-laboratory interaction, and drug-drug physical incompatibility; 3) drug monitoring: any suggestion associated with the laboratory monitoring of drug therapy; 4) drug route/method of administration: a proposed change in the route or method of administration; 5) drug selection: a recommendation to use an alternative medication for various reasons including allergy, intolerance, contraindication, drug shortage, duplicate therapy, formulary issue, not optimal for indication, inappropriate dosage form, indication with no medication ordered, medication not needed, restricted medication, and therapeutic interchange; and 6) medication profile/order clarification: an intervention required to further clarify an order or the patient medication profile because of incompleteness, illegibility, contrary to policy, written for wrong patient, or inappropriate procedures followed when using electronic order entry.
A clinical severity rating was also assigned to each clinical intervention. To minimize interprovider variability in assigning the impact, guidelines with examples were available to the clinical pharmacist.
Minor impact on patient outcome: the intervention has minimal health consequences for the patient. Examples: incomplete information on the medication order, inappropriate dosage form, illegible or incomplete medication order, drug shortage intervention, intravenous (IV) to oral (PO) route conversion, drug-cost savings.
Moderate impact on patient outcome: the intervention results in improved patient outcome, but the overall health consequences are considered to be non–life-threatening. Examples: dose adjustment for disease state or serum concentration, medication needed but not ordered and is not a life-threatening omission, medication selection optimized, electrolyte therapy for asymptomatic patients with abnormal laboratory results, and drug-monitoring issues.
Life-threatening or potentially life-threatening health consequences: the intervention removes a potentially life-threatening situation for the patient. Examples: history of anaphylaxis to an ordered medication; 10-fold overdose ordered; overdose of chemotherapy; duplication of therapy, which may have life-threatening consequences such as both heparin and low-molecular-weight heparin ordered; inappropriate route such as PO medication ordered to be given IV; life-threatening drug interactions; subtherapeutic or supratherapeutic antibiotic dosing or suboptimal antibiotic selection for a septic patient.
Data were collected from an institutional critical care data repository—ICU datamart, which contains near–real-time copies of the pertinent ICU patient information beginning in 20038—along with the critical care process and outcome information from January 1, 2003, through December 31, 2013. During the first 5 years, our staffing model allowed for a pharmacist to be present during rounds, approximately 2.5 hr/day; in addition we estimate another 2.5 hours of time was dedicated to profiling pediatric orders (total pediatric pharmacist involvement of 5 hr/day), with limited information technology support to clinically intervene in real time. Beginning in 2008, a full-time pediatric clinical pharmacist was dedicated solely to the PICU for 10 hours every day. Thus, data were compared for 2 periods: 2003-2007 and 2008-2013. Demographic and outcome data also were compared for patients who did and who did not have pharmacy interventions in the 2 periods. Standard statistical tests (t test for continuous data and χ2 test for categorical data) were used to compare and analyze the data. Data analysis were performed by using JMP statistical software (SAS Institute, Inc, Cary, NC). p < 0.05 was considered statistically significant.
RESULTS
Pharmacy Total Interventions
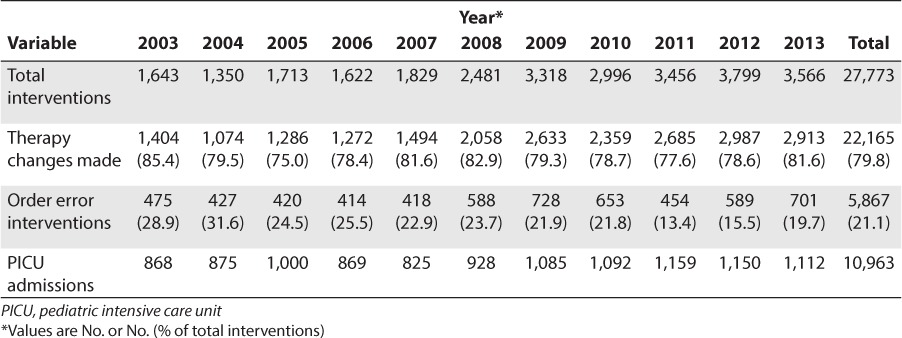
During the 11-year study period, pharmacists made 27,773 clinical interventions in the PICU, of which 22,165 (79.8%) resulted in changes in medication therapy or therapy monitoring (Table 1). These interventions were made on a total of 10,963 PICU admissions. As the pharmacist role in the PICU multidisciplinary practice evolved and became more defined over time, the number of total interventions increased from 1,643 in 2003 to 3,566 in 2013 (p < 0.01). The PICU admissions also increased significantly, from 868 in 2003 to 1,112 in 2013 (p < 0.01). The year-over-year increase in pharmacy interventions is statistically significant (p < 0.001), even after controlling for the increase in PICU admissions, as determined by a bivariate fit model of total interventions minus PICU admissions by time. The increased pharmacist working hours (10 hours vs. 5 hours) from 2008 onwards, had a positive correlation with total interventions (r = 0.75, p = 0.01; controlled for PICU admission). Each extra hour of pharmacist presence in the PICU led to 191.5 additional interventions (p = 0.01) (data not shown). During the study period, pediatric pharmacists prevented 5867 order entry errors. The exact number of adverse drug events (ADEs) prevented is difficult to estimate from our data. However, on the basis of a previous study,9 we believe that up to 18% of order entry errors may have resulted in an ADE. Clinical pharmacist presence in the PICU has thereby resulted in the prevention of 1,056 ADEs in the past 11 years. Although the total interventions increased, the percentage of interventions that were order errors decreased over time from 28.9% in 2003 to 19.7% in 2013 (Table 1).
Table 1.
Pharmacist Interventions and Associated Variables
Pharmacy Intervention Categories
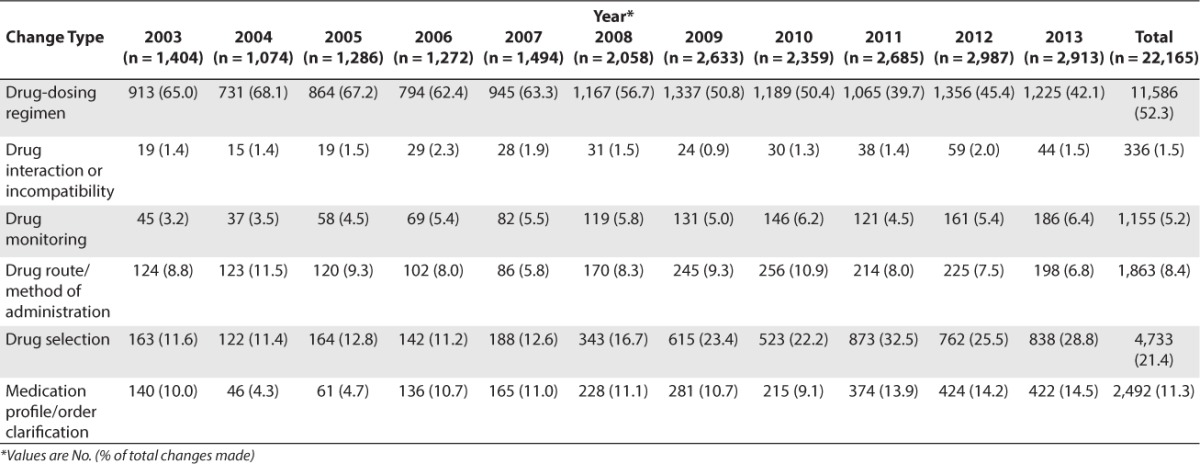
We documented 22,165 accepted therapy changes by pharmacy recommendations during the study period (Table 2). The 2 most common change categories were changes in drug dose regimen (11,586 [52.3%]) and appropriate drug selection (4,733 [21.4%]). Drug interaction or incompatibility (336 [1.5%]) and drug monitoring (1,155 [5.2%]) were least often recommended. The drug dosing interventions as a percentage of total interventions decreased from 65% in 2003 to 42% in 2013 (Table 2).
Table 2.
Categories of Pharmacy Interventions That Resulted in Therapy Changes
Effects on Patient Care
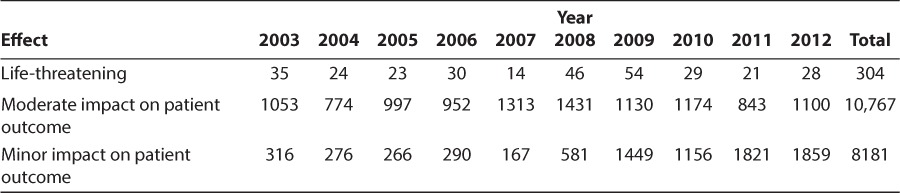
The perceived effects on patient safety from the therapy changes described in Table 1, as evaluated by the pediatric pharmacists, were categorized as “life-threatening,” “moderate impact on patient outcome,” or “minor impact on patient outcome.” Of 19,252 interventions implemented (2003 to 2012), 304 (1.6%) were deemed to be life-threatening or to potentially have life-threatening consequences. Most interventions were deemed as having a moderate impact on patient outcome (10,767; 55.9%) (Table 3).
Table 3.
Pharmacists' Perceived Effect of Intervention on Patient Outcomes
Effect of Change in Pediatric Pharmacist Staffing Model
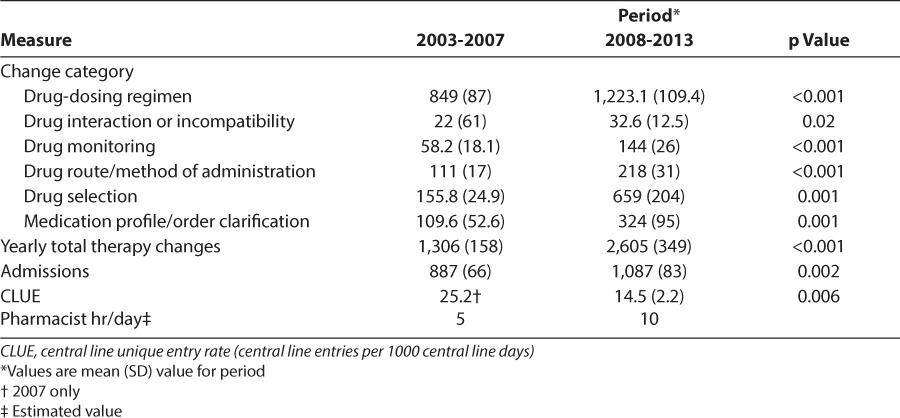
To assess the effect of the increased pharmacy staffing model, we compared the data on pharmacy interventions during 2 distinct periods: 2003-2007 and 2008-2013. The mean (SD) number of clinical interventions accepted increased from the first period (1,306 [158]) to the second period (2,605 [349]) (p < 0.001). Significant increases were also noted in all categories of pharmacy interventions, including drug-dosing recommendation, drug interactions, drug monitoring, route and method of administration, drug selection, and profile or order clarification (Table 4).
Table 4.
Effect of Increased Pharmacy Presence on Number of Interventions
Patient Safety and Outcomes
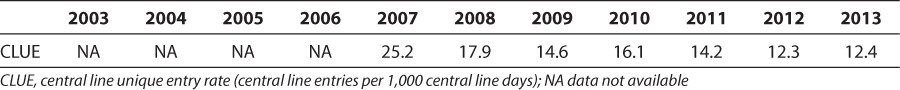
Pharmacists' recommendation of IV to PO conversion of medications can affect central line entry rate. From 2007 to 2013, we collected data on the number of central line entries, after a quality initiative in our unit aiming to coordinate laboratory draws and medication administration. The number of central line unique entries (CLUEs; total central line entries/patient observations) decreased from 25.2 in 2007 to 12.4 per 1,000 central line days in 2013 (p = 0.006) (Table 5). The mean (SD) CLUE rate from 2008 to 2013 was 14.5 (2.2), which was also significantly decreased from the 2007 value (p = 0.006, t test assuming equal variance) (Table 4). The yearly average number of changes in drug route/method of administration intervention by the pharmacist for 2003 to 2007 was 111 and doubled to 218 for the years 2008-2012, a significant increase (p < 0.001). The year-over-year increase in drug route administration intervention showed a strong negative correlation with the CLUE rates on univariate analysis (r = −0.83, p = 0.026) (Table 4).
Table 5.
Central Line Infections and Entry Rates Over the Course of Study Period
Pharmacy Intervention and Patient Demographics
To better identify the categories of patients who most often require pharmacy interventions, we compared the demographics and outcome of all patients with any pharmacy intervention (7,069, 64.4% of all admissions) with those who had no pharmacy interventions (3,894, 35.5% of all admissions). Patients who required more pharmacy interventions were significantly younger at admission (7.3 years vs. 8.1 years; p < 0.001) and had longer ICU (4.3 days vs. 1.1 days; p < 0.001) and hospital stay (11.5 days vs. 3.6 days; p < 0.001). Patients who required more pharmacy interventions also were on mechanical ventilation significantly longer (4.8 days vs. 0.8 days; p < 0.001) and had more deaths during the study period (150 vs. 27; p < 0.001). There was no statistically significant difference in mortality rate between those without and those with pharmacy interventions (1% vs. 2%; p = 0.55) (data not shown).
DISCUSSION
To our knowledge, this study is the largest to report data showing that having a pediatric clinical pharmacist dedicated to the PICU promotes the care of patients. We showed that increased pharmacist presence in the PICU is positively associated with increased interventions and prevention of order entry errors. Patients who are sicker and younger require more pharmacist involvement in their management.
Clinical pharmacy is a relatively younger branch of pharmacy, having branched out of general pharmacy in the mid 1960s. Clinical pharmacists are focused on patient pharmacotherapy rather than drug product dispensing. The need for specialized clinical pharmacists arose out of dissatisfaction with old practice norms and the pressing need for a health professional with a comprehensive knowledge of the therapeutic use of drugs.10 The American College of Clinical Pharmacists defines clinical pharmacy as “the area of pharmacy concerned with the science and practice of rational medication use.”11 Clinical pharmacists are the experts in therapeutic use of medications and a primary source of scientifically valid information and advice regarding the safe, appropriate, and cost-effective use of medications.12 Depending on the institution and staffing, the role of a clinical pharmacist can vary from medication consulting to medication prescribing. Clinical pharmacists not only improve drug safety13 but also can help to decrease health care costs,14 improve quality of pharmacotherapy,15 act as a liaison between pharmacy and other departments,16 and refine a patient's drug knowledge.17
Although clinical pharmacists have been a part of the ICU team for many years, description of their involvement has been limited to small studies with limited time frames. Laro-chelle and colleagues7 previously described the extent and nature of pharmacy interventions in the PICU. During 8 months they evaluated 893 interventions on 159 patients (5.5 interventions per patient). Our rate of pharmacy interventions was 2.5 interventions per patient, although it ranged from 1.9 in 2003 to 3.2 in 2013. Dosing recommendations were the most common type of intervention in the study of Larochelle and colleagues7 (28.8%) as in our study (52.7%). Our percentage may be higher because Larochelle et al7 considered dosing recommendations and pharmacokinetics (21.4%) separately. The medical team's acceptance of the pharmacist's recommendation was lower in our study (79.8%) than in other studies in literature (98% in Larochelle et al7; 95.8% in Strong and Tsang18). Our study data were self-reported by the pharmacist over a much longer period, which may explain the low acceptance rates. Moreover, we try to foster a culture of open discussion in the PICU. The pharmacists are encouraged to bring forth any idea that they feel merits consideration by the team, even if it is an idea that may not have a high likelihood of being implemented, but something the team should keep in mind. These conversations are recorded as interventions.
Medication error rates as reported in the pediatric literature are much higher than in the adult population, possibly because most available drugs have been developed for use in adults.19 The rate of potentially dangerous medication errors is 3 times higher for pediatric patients than for adult patients.9 Drugs used for children are often unlicensed and used off-label because of lack of appropriate strengths or suitable formulations. Many drugs with a narrow therapeutic index can have serious adverse effects if used with incorrect dosing. These challenges provide unique opportunities for pharmacists to improve the quality of care for pediatric patients. In a before-after study in adult ICU patients, Leape et al20 showed a decrease in preventable ADEs by 66%. A similar study by Kaushal et al21 in a PICU also showed a reduction in the rate of serious medication errors from 29 per 1,000 patient days to 6 per 1,000 patient days within 3 months. In an observational study in pediatric and adult in-patients, the rate of pharmacist interventions per 10,000 orders written was 75.3 for children and only 4.8 for adults.22 The corresponding incidence of drug-related problems per 10,000 orders was 165 for children, compared with 8.7 for adults.
In this study, pharmacists in our ICU identified and corrected 5,867 medication order errors in 10,963 patients (0.53 errors/patient). It is not possible from our study to calculate how many of the order errors may have led to an ADE, but a previous study9 reported 115 potential ADEs among 616 medication order errors (18%). That study also found that 79% of the potential ADEs occurred during drug ordering, and a unit-based clinical pharmacist could have prevented 94% of the potential ADEs. Extrapolating their rates to our study, we believe that clinical pharmacists may have prevented 1,056 ADEs. We have shown that pharmacists influence various aspects of care in the PICU. Improvement in many of these outcomes is closely related to other quality improvement efforts, which thus limits our ability to accurately predict pharmacist impact on cost-effectiveness. Although the actual cost savings may be much more, ADE prevention has been shown in previous studies to result in the highest cost-benefit ratio.23
In our study, the percentage of interventions that are order errors decreased over time, although the total number of interventions increased. This may be related to more organized computerized pharmacy order entry, as well as other electronic decision support tools—such as electronic Micromedex (Truven Health Analytics, Greenwood Village, CO), which is easily available while ordering medications, and Internet access on all computers—that have been implemented over the years. The clinical pharmacist's role has also evolved from only medication order correction to being more involved in decision making and therapeutics. The overall decrease in the CLUE rates in the past 11 years is probably due to multiple factors and measures implemented in our unit over the years. We hypothesize that pharmacist intervention in intravenous to oral drug conversions led to a decrease in CLUE rates, but this needs to be verified in an independent, sufficiently powered study.
In our study, patients who had pharmacy interventions had longer ICU and hospital stays. They also had more ventilator days. In contrast, other studies have shown a favorable outcome with pharmacist presence in the PICU.24 However, in the absence of baseline disease severity scores or mortality risk scores, it is not possible to compare patients with and without pharmacy interventions. We hypothesize that, in our study, pharmacist interventions were only needed in sicker and younger patients. In fact, the mean age of patients who had pharmacy interventions was significantly younger than that of the patients without interventions.
Our study has similar limitations to those of previous studies. The interventions were self-reported by the intervening pharmacist, which may lead to bias and underreporting of interventions owing to time constraints or omission of activities not deemed important in the pharmacist's opinion. We also did not have a defined control phase, with no pharmacist presence; this limited our ability to quantify the impact.
On the basis of our study and previous studies, we believe that the dedication of pharmacist time, the improved clinical expertise of the pharmacist in PICU patient care, and the physical presence on the unit working in a multidisciplinary fashion with physicians and providers all result in optimization of pharmacotherapy. In view of the substantial impact of pharmacists in the care of critically sick children, pharmacist participation during rounds and order entry should be encouraged. Future studies should evaluate more innovative use of the pharmacy resources in drug prescribing and error prevention.
ABBREVIATIONS
- ADE
adverse drug event
- CLUE
central line unique entry
- ICU
intensive care unit
- IV
intravenous
- PICU
pediatric intensive care unit, PO, oral
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Kim MM, Barnato AE, Angus DC et al. The effect of multidisciplinary care teams on intensive care unit mortality [erratum in: Arch Intern Med. 2010;170(10):867] Arch Intern Med. 2010;170(4):369–376. doi: 10.1001/archinternmed.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brilli RJ, Spevetz A, Branson RD. The American College of Critical Care Medicine Guidelines for the Definition of an Intensivist and the Practice of Critical Care Medicine: critical care delivery in the intensive care unit: defining clinical roles and the best practice model. Crit Care Med. 2001;29(10):2007–2019. doi: 10.1097/00003246-200110000-00026. et al; American College of Critical Care Medicine Task Force on Models of Critical Care Delivery. [DOI] [PubMed] [Google Scholar]
- 3.Sanghera N, Chan PY, Khaki ZF et al. Interventions of hospital pharmacists in improving drug therapy in children: a systematic literature review. Drug Saf. 2006;29(11):1031–1047. doi: 10.2165/00002018-200629110-00003. [DOI] [PubMed] [Google Scholar]
- 4.Kucukarslan SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018. doi: 10.1001/archinte.163.17.2014. [DOI] [PubMed] [Google Scholar]
- 5.Bond CA, Raehl CL, Franke T. Clinical pharmacy services and hospital mortality rates. Pharmacotherapy. 1999;19(5):556–564. doi: 10.1592/phco.19.8.556.31531. [DOI] [PubMed] [Google Scholar]
- 6.Stucky ER, American Academy of Pediatrics Committee on Drugs; American Academy of Pediatrics Committee on Hospital Care Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431–436. doi: 10.1542/peds.112.2.431. [DOI] [PubMed] [Google Scholar]
- 7.Larochelle JM, Ghaly M, Creel AM. Clinical pharmacy faculty interventions in a pediatric intensive care unit: an eight-month review. J Pediatr Pharmacol Ther. 2012;17(3):263–269. doi: 10.5863/1551-6776-17.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach: a team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28(11):42, 44–45. [PubMed] [Google Scholar]
- 9.Kaushal R, Bates DW, Landrigan C et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 10.Miller RR. History of clinical pharmacy and clinical pharmacology. J Clin Pharmacol. 1981;21(4):195–197. doi: 10.1002/j.1552-4604.1981.tb05699.x. [DOI] [PubMed] [Google Scholar]
- 11.American College of Clinical Pharmacy. The definition of clinical pharmacy. Pharmacotherapy. 2008;28(6):816–817. doi: 10.1592/phco.28.6.816. [DOI] [PubMed] [Google Scholar]
- 12.Perez A, Doloresco F, Hoffman JM. ACCP: economic evaluations of clinical pharmacy services: 2001–2005. Pharmacotherapy. 2009;29(1):128. doi: 10.1592/phco.29.1.128. et al; American College of Clinical Pharmacy. [DOI] [PubMed] [Google Scholar]
- 13.Fortescue EB, Kaushal R, Landrigan CP et al. Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;111(4, pt 1):722–729. doi: 10.1542/peds.111.4.722. [DOI] [PubMed] [Google Scholar]
- 14.Bailey TC, Ritchie DJ, McMullin ST et al. A randomized, prospective evaluation of an interventional program to discontinue intravenous antibiotics at two tertiary care teaching institutions. Pharmacotherapy. 1997;17(2):277–281. [PubMed] [Google Scholar]
- 15.Gibson JT, Alexander VL, Newton DS. Influence on medication therapy of increased patient services by pharmacists in a pediatric hospital. Am J Hosp Pharm. 1975;32(5):495–500. [PubMed] [Google Scholar]
- 16.Lal LS, Anassi EO, McCants E. Documentation of the first steps of pediatric pharmaceutical care in a county hospital. Hosp Pharm. 1995;30(12):1107–1108. [PubMed] [Google Scholar]
- 17.Nazareth I, Burton A, Shulman S et al. A pharmacy discharge plan for hospitalized elderly patients: a randomized controlled trial. Age Ageing. 2001;30(1):33–40. doi: 10.1093/ageing/30.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Strong DK, Tsang GW. Focus and impact of pharmacists' interventions. Can J Hosp Pharm. 1993;46(3):101–108. [PubMed] [Google Scholar]
- 19.World Health Organization. Promoting safety of medicines for children [Internet] c2007. http://www.who.int/medicines/publications/essentialmedicines/Promotion_safe_med_childrens.pdf. Accessed November 25, 2014.
- 20.Leape LL, Cullen DJ, Clapp MD et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit [erratum in: JAMA. 2000;283(10):1293] JAMA. 1999;282(3):267–270. doi: 10.1001/jama.282.3.267. [DOI] [PubMed] [Google Scholar]
- 21.Kaushal R, Bates DW, Abramson EL et al. Unit-based clinical pharmacists' prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm. 2008;65(13):1254–1260. doi: 10.2146/ajhp070522. [DOI] [PubMed] [Google Scholar]
- 22.Chan DS, Kotzin DA. Adult vs pediatric clinical intervention trends: a four year retrospective report. J Pediatr Pharm Pract. 1998;3:144–149. [Google Scholar]
- 23.Anderson SV, Schumock GT. Evaluation and justification of clinical pharmacy services. Expert Rev Pharmacoecon Outcomes Res. 2009;9(6):539–545. doi: 10.1586/erp.09.57. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Zhang L, Huang L et al. Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial. PLoS One. 2012;7(1):e30856. doi: 10.1371/journal.pone.0030856. [DOI] [PMC free article] [PubMed] [Google Scholar]


