Abstract
We developed a semiquantitative job exposure matrix (JEM) for workers exposed to polychlorinated biphenyls (PCBs) at a capacitor manufacturing plant from 1946 to 1977. In a recently updated mortality study, mortality of prostate and stomach cancer increased with increasing levels of cumulative exposure estimated with this JEM (trend p values=0.003 and 0.04, respectively). Capacitor manufacturing began with winding bales of foil and paper film, which were placed in a metal capacitor box (pre-assembly), and placed in a vacuum chamber for flood-filling (impregnation) with dielectric fluid (PCBs). Capacitors dripping with PCB residues were then transported to sealing stations where ports were soldered shut before degreasing, leak testing, and painting. Using a systematic approach, all 509 unique jobs identified in the work histories were rated by predetermined process- and plant-specific exposure determinants; then categorized based on the jobs’ similarities (combination of exposure determinants) into 35 job exposure categories. The job exposure categories were ranked followed by a qualitative PCB exposure rating (baseline, low, medium, and high) for inhalation and dermal intensity. Category differences in other chemical exposures (solvents, etc.) prevented further combining of categories. The mean of all available PCB concentrations (1975 and 1977) for jobs within each intensity rating was regarded as a representative value for that intensity level. Inhalation (in microgram per cubic milligram) and dermal (unitless) exposures were regarded as equally important. Intensity was frequency adjusted for jobs with continuous or intermittent PCB exposures. Era-modifying factors were applied to the earlier time periods (1946–1974) because exposures were considered to have been greater than in later eras (1975–1977). Such interpolations, extrapolations, and modifying factors may introduce non-differential misclassification; however, we do believe our rigorous method minimized misclassification, as shown by the significant exposure–response trends in the epidemiologic analysis.
Keywords: Polychlorinated biphenyls (PCB), Job exposure matrix (JEM)
Introduction
Polychlorinated biphenyls (PCBs) are synthetic chemicals consisting of 209 structurally different chlorobiphenyl congeners with 1–10 chlorines. PCBs were produced commercially from 1929 to 1977 (Smith and Brown 1987) and contained a mixture of 50–90 congeners. The principal product used in the USA was sold under the trade name “Aroclor”. PCBs were widely used as a dielectric fluid in transformers and electrical capacitors due to their high stability, dielectric properties, and resistance to oxidation (Silberhorn et al. 1990; Smith and Brown 1987). PCBs have long half-lives in both humans and the environment (Maroni et al. 1981; Phillips et al. 1989; Silberhorn et al. 1990). PCBs were banned from US production and distribution in 1978 due to concerns about the persistence of PCBs in the environment and potential health risks (Smith and Brown 1987).
Inhalation, dermal, and oral routes of exposure contribute to the absorption of PCBs; however, the importance of each route is debated. In vitro skin studies have shown PCBs not to penetrate skin (Schmid et al. 1992). While as much as 80 % of levels commonly seen in adipose tissue from PCB exposed capacitor workers were due to inhalation, dermal absorption accounted for up to 20 % (Wolff 1985). Another study of capacitor manufacturing workers concluded that exposures to PCBs by the dermal route were the predominant contributor to body burden (Lees et al. 1987).
A review of data gaps pertaining to PCB carcinogenicity has been published recently (Ward et al. 2010). Much of the epidemiologic literature has been reviewed (Faroon et al. 2001; Golden et al. 2003; Golden and Kimbrough 2009). The lack of congruity (Robertson and Ruder 2010) in the cohort results may be due to all occupational PCB exposure having been to mixtures of congeners, with the proportion of each congener varying from batch to batch (Hopf et al. 2009). There are so many PCB congeners, some co-planar and some not, some estrogenic and some not (Robertson and Ruder 2010), and some congener enantiomers have effects on the induction of CYP P450 and some not (Ali and Aboul-Enein 2004). Therefore, it seems plausible that a variety of tumor types could arise from exposure to various congeners, or their metabolites. The International Agency for Research on Cancer (IARC) has, in Monograph Volume 100 F, classified PCB congener 126 as carcinogenic to humans (group 1; Baan et al. 2009). The carcinogenicity of all PCB congeners is being evaluated by an IARC working group in February 2013.
One cohort of electrical capacitor manufacturing workers at a plant located in upstate New York has been included in several cancer mortality studies, although yielding inconsistent results. Table 1 lists the mortality cohort studies published. The original National Institute for Occupational Safety and Health (NIOSH) retrospective cohort study (Brown and Jones 1981), later updated several times (Brown 1987; Prince et al. 2006a) included only highly PCB exposed workers (n=2,567; 10 % of the overall work force) and excluded workers with exposures to trichloroethylene (TCE), a liver carcinogen (U.S. EPA 2011; http://www.epa.gov/iris/subst/0199.htm#carc). “Highly exposed” was defined as a minimum of 3 months of employment working in areas of the plant with the heaviest exposure to PCBs. These highly exposed jobs were identified by the company and the unions, and confirmed by area and personal PCB air concentrations. No associations were found between cancer and duration of employment in a PCB exposed job. The NIOSH expanded cohort (n=6,941) included all those who worked at least 3 months at the plant (Prince et al. 2006b). The analysis of this cohort, which used the job exposure matrix described in this report, also included another plant of capacitor manufacturing workers. The statistics in Table 1 are for this plant only.
Table 1.
Epidemiologic cohort studies of the upstate New York capacitor manufacturing cohort
| Reference | N | Eligibility criteria | Update through |
PCB exp | Entire Cohort Cause, deaths, SMR (CI)a |
Highly exposedb Cause, deaths, ratio (CI) |
|---|---|---|---|---|---|---|
| Brown and Jones (1981) | 968 (385 women, 583 men) |
≥3 months work 1946–1976, possible PCB exposure, no TCE exposure |
1976 | 1977 personal air samples 24–393 μg/m3 area samples 3–476 μg/m3 |
All 73, SMR 0.95 (0.8–1.1) | |
| Cancer 13, SMR 0.8 (0.4–1.3) | ||||||
| Rectal ca 1, SMR 1.4 (0.0–12) | ||||||
| Liver ca 1, SMR 2.4 (0.0–20) | ||||||
| Brown (1987) | 981: (383 women, 593 men) |
≥3 months work 1946–1976 | 1982 | 1977 personal air samples 24–393 μg/m3 area samples 3–476 μg/m3 |
All 116, SMR 0.9 (0.7–1.1) | |
| Cancer 18, SMR 0.6 (0.3–0.9) | ||||||
| Rectal ca 1, SMR 1.2 (0.0–10) | ||||||
| Liver ca 1, SMR 0.7 (0.0–5.4) | ||||||
| Prince et al. (2006a, b) | 973 | ≥3 months work 1946–1977 with direct PCB exposure (impregnation, sealing, testing capacitors)+no solvent exposure |
1998 | 1977 personal air samples 24–393 μg/m3 area samples 3–476 μg/m3 |
All 1586, SMR 0.87 (0.8–0.9) | All 310, SMR 0.95(0.9–1.1) |
| All cancer 473, SMR 0.95 (0.9–1.0) | All cancer 71, SMR 0.8 (0.6–1.0) | |||||
| Pancreas ca 21, SMR 0.9 (0.6–1.4) | Pancreas ca 25, SMR 0.95 (0.6–1.4) | |||||
| Rectal ca 11, SMR 1.2 (0.6–2.2) | Rectal ca 1, SMR 0.6 (0.0–3.3) | |||||
| Liver ca 9, SMR 0.8 (0.4–1.5) | Liver ca 4, SMR 1.9 (0.5–4.9) | |||||
| Intestinal ca 53. SMR 1.2 (0.9–1.6) | Intestinal ca 8. SMR 1.0 (0.4–2.0) | |||||
| Kidney ca 10, SMR 0.9 (0.4–1.8) | Kidney ca 2, SMR 1.1 (0.3–3.8) | |||||
| Prostate ca 21, SMR 1.0 (0.6–15) | Prostate ca 5, SMR 1.4 (0.5–3.3) | |||||
| Lymph-hemat 10, SMR 0.7 (0.3–1.3) | Lymph-hemat 10, SMR 0.7 (0.3–1.3) | |||||
| Ruder et al. (submitted) |
8,727 (3,792 women; 4,935 men) |
>1 day work 1946–1977 | 2008 | 1977 personal air samples 24–393 μg/m3 area samples 3–476 μg/m3 |
Prostate ca 47 deaths, SMR 1.2 (0.9–1.6) | Prostate ca cum exp quartiles : <45,000 unit-days, 11 deaths, SRRb 1.0 |
| 45,000–150,000 unit –days, 11 deaths, SRR 1.5 |
||||||
| 150,000–1, 300,000 unit –days, 11 deaths, SRR 1.4 |
||||||
| Stomach ca 17 deaths, SMR 0.6 (0.4–0.97) | 1, 300,000+unit–days, 11 deaths, SRR 3.1 | |||||
| p (trend)=0.0026 | ||||||
| Stomach ca cum exp quartiles: <110,000 unit days, 3 deaths, SRR 1.0 |
||||||
| 110,000–340,000 unit days, 4 deaths, SRR 4.4 |
||||||
| 340,000–790,000 unit days, 3 deaths, SRR 5.1 |
||||||
| 790,000+ unit days, 4 deaths, SRR 4.8 | ||||||
| P (trend) 0.044 | ||||||
| Nicholson et al. (1987) | 729 (322 women, 447 men) |
≥5years employment beginning before 1954 |
1982 | All cause men 43, SMR 0.95 (0.7–1.3)d | ||
| All cancer 37, SMR 0.97 (0.7–1.3) | ||||||
| pancreas ca 1, SMR 0.5 (0.0–4.0) | ||||||
| rectal ca 2, SMR 1.7 (0.2–7.2) | ||||||
| Intestinal ca 2, SMR 0.5 (0.1–2.0) | ||||||
| Liver ca 0 | ||||||
| Lymph-hemat 6, SMR 1.9 (0.7–4.3) | ||||||
| Taylor et al. (1988) | 6,292 (2,691 women, 3,601 men) |
≥3 months work 1946–1975 | 1980 | 1975 air samples geometric mean (GM) 679 μg/m3 direct exposure areas; GM 260 μg/m3 indirect exposure areas; 1977 air samples GM 310 μg/m3 direct exposure areas; GM 27 μg/m3 indirect exposure areas; 1977 personal air samples GM 168 μg/m3 direct exposure areas |
All 510, SMR 0.8 (0.8, 0.9) | |
| All cancer 136, SMR 0.9 (0.8–1.1) | ||||||
| Pancreas ca 10, SMR 1.6 (0.8–2.9) | ||||||
| Rectal ca 7, SMR 2.0 (0.8–4.3) | ||||||
| Intestinal ca 18, SMR 1.5 (0.9–2.3) | ||||||
| Liver ca 3, SMR 1.2 (0.2–3.8) | ||||||
| Lymph-hemat 10, SMR 0.7 (0.3–1.3 | ||||||
| Kimbrough et al. (1999) | 7,075 (3,013 women, 4,062 men) |
≥3 months work 1946–1977 | 1946–1993 | High exp: filling & impregnating 1975 air samples 227–1,500 μg/m3 |
All 1,195, SMR 0.8 (0.7–0.8); | All 966, SMR 0.9 (0.8–0.9) |
| All cancer 353, SMR 0.9 (0.8–1.0) | All cancer 278, SMR 1.0 (0.8–1.1) | |||||
| Rectal ca 10, SMR 1.3 (0.6–2.5) | Rectal ca 7, SMR 1.2 (0.5–2.6) | |||||
| Liver ca 5, SMR 0.9 (0.3–2.0) | Liver ca 4, SMR 0.9(0.2–2.4) | |||||
| Kimbrough et al. (2003) | 7,075 (3,013 women, 4,062 men) |
≥3 months work 1946–1977 | 1946–1998 | Hi exp: Filling & impregnating 1975 air samples 227–1,500 μg/m3 |
All, 1,654, SMR 0.9 (0.85–0.93) | All 1,333, SMR 1.0 (0.9–1.0) |
| All cancer 492 SMR 1.0 (0.9–1.1) | All cancer 381, SMR 1.0 (0.9–1.2) | |||||
| Rectal ca 12, SMR 1.3 (0.7–2.3) | Rectal ca 8, SMR 1.2 (0.5–2.4) | |||||
| Liver ca 9, SMR 0.8 (0.4–1.5) | Liver ca 8, SMR 1.0 (0.4–1.9) |
Standardized mortality ratio (SMR)
The definition of “highly exposed” varies from study to study
Standardized relative risk (SRR) compares rates across quantiles of exposure
The Nicholson et al. (1987) data were not published but appear in a report issued by the Ontario Ministy of Labor. The table with the data on women workers duplicates the observed and expected all causes values presented for men in the previous table; this likely represents an inadvertent copying of the data for men, so is not presented here
At the same time, Brown was updating the original NIOSH cohort report, another cancer mortality study (Nicholson et al. 1987), including workers (n=788) with employment beginning prior to 1954 for a period of at least 5 years (Golden et al. 2003), reported no increased cancer risk with duration of employment. An expanded cohort study (6,292 workers) included workers with all levels of exposure (as opposed to only workers in highly PCB exposed jobs) who were employed for a minimum of 3 months between 1946 and 1975 (Taylor et al. 1988). Exposures were characterized as either indirect or direct, and further subdivided into high (PCB air contact and frequent dermal exposure), medium (PCB air contact and occasional dermal contact), and low (PCB air contact only) exposures. No exposure–response was detected. Exposure–response trends were also absent in another retrospective cohort mortality study with a longer follow-up conducted among 7,075 of these workers (Kimbrough et al. 1999). Here, jobs were classified as incurring high (direct dermal contact and inhalation exposures such as filling, impregnation, repair, and moving PCB-filled capacitors) or low (inhalation limited to background levels) exposures. No significant cancer mortality increases were seen in the high exposed group, nor did SMRs increase with length of cumulative employment and latency. The results were similar in the 5-year mortality update (Kimbrough et al. 2003).
The lack of exposure–response in most of these epidemiological studies could be related to the crude PCB exposure assessments. Precision of a cohort study, especially where dichotomous exposures are used, can be reduced due to nondifferential exposure misclassification, which will bias the estimates toward the null (Armstrong 1998). Results for the highly exposed (Prince et al. 2006a) and expanded NIOSH cohort (Prince et al. 2006b; Table 1) show that even focusing on “highly exposed” without evaluating cumulative exposures, does not result in accurate exposure–response. There is a need to include a spectrum of exposure to demonstrate exposure response.
Improving the exposure assessment by using a job exposure matrix (JEM) can improve relative risk estimates (Teschke et al. 2002). We therefore reconstructed the historical exposure by developing a plant-specific JEM for this cohort of former capacitor manufacturing workers exposed to PCBs. Our JEM lists all jobs on one axis and the agents (PCB, other chemical exposures such as TCE) on another. The cells of the matrix indicate the presence, intensity, and frequency of exposure to PCBs in a specific job. We also included calendar periods as a third axis. This JEM was used in a cancer mortality study of this cohort combined with another manufacturing cohort showing an exposure response. Analyses for a recent update (3) showed that prostate and stomach cancer mortality in the New York cohort increased with cumulative exposure estimated with the JEM (trend p values=0.003 and 0.04, respectively). Without the JEM, using duration of employment as the proxy of exposure, no exposure–response relationship had been detected.
The plant-specific JEM described here included all jobs performed at the plant in all eras, which allowed the inclusion of all those who ever worked at the plant; it also included continuous exposure estimates based on available plant air concentration measurements rather than excluding all those not working in high PCB exposure jobs (Brown 1987; Brown and Jones 1981) or using duration of employment as the exposure estimate and stratifying by exposure group (Kimbrough et al. 1999, 2003; Taylor et al. 1988). The JEM increased the number of job exposure categories from three (high, medium, and low exposed) to 33, perhaps most importantly it distinguished between dermal and inhalation and additional chemical exposures.
This PCB cohort is unusual with regard to its limited exposures to other chemicals. Only a small number of workers were exposed to low toluene concentrations in the painting area, TCE in the degreasing area, and metals (lead, aluminum, and iron) in the soldering area. The most challenging part of developing this JEM was the wealth of qualitative data that we converted to semiquantitative data. Here, we describe a systematic approach in converting qualitative data into qualitative exposure ratings and then anchoring these to the few PCB air concentration measurements available to produce semiquantitative exposure values.
Methods
Plant and process description
The plant had two facilities located 1 mile apart; facility A manufactured small PCB-filled capacitors starting in 1946 and from 1957 facility B began manufacturing PCB-filled power capacitors. The electrical capacitor manufacturing began with winding paper, foil, or plastic film together. The winding operations were interrupted to permit insertion of metal strips for external electrical connections. These sections were subsequently loaded into metal casings in an enclosed dust free room. A capacitance test was then performed. The capacitors were then loaded into a processing chamber to be vacuum dried under elevated temperature. Impregnation followed immediately; warm dielectric fluid (PCB) was admitted under vacuum to the vacuum dried capacitor to enable complete filling. PCB oil was prefiltered with fuller’s earth primarily to reduce moisture; however, later, it was shown that contaminants such as polychlorinated dibenzofurans (PCDFs) were also efficiently removed. After impregnation, the capacitors were dripping wet with PCB oil. Terminals were attached, often by soldering, to the openings of the capacitors. The capacitors were then checked for leaks. If a capacitor failed the leak test, it was removed from the assembly line and sent to salvage and repair. Salvage and repair of large capacitors that did not meet test specifications involved drilling to drain the PCB, removing the cover seal, and manually removing and repairing the wet components. The leak-free capacitors were soldered shut before being washed free of oil. The clean and dry capacitors were then tested for capacitance before painting. The last manufacturing step was packing and shipping the capacitors.
In a plant report from 1981, PCB exposures were described: “As a result of volatilization, condensation, dripping and spillage, the capacitor impregnation, sealing and salvage operations created local environments where significant portions of all exposed surfaces were wet with PCBs, and where air levels in the immediate vicinity of such surfaces could become saturated with PCB vapor.” There were considerable opportunities for both dermal and inhalation exposures among individuals performing jobs related to the manufacturing operations, and also opportunities for inhalation exposures among those working in nearby operations. Physical layouts within the plant changed repeatedly and records of many of the layouts were unavailable. In certain areas of the plant, there was also the possibility for exposure to dusts, oils, solvents, and heavy metals.
The two facilities differed in several aspects; at facility A, racks of warm, wet, small flood-filled capacitors were transported from the heated chambers to the sealing station on dollies by “movemen”. In the mid-1950s, a carousel system with an automatic feed apparatus was installed. After the capacitors were impregnated, they were taken to an area where the openings that were used for impregnation were sealed by crimping. During this step, the capacitors were covered with residual PCBs remaining on the metal casing and the entire crimping area was heavily laden with the oil. In later eras, most of the capacitors were soldered rather than crimped. Facility A used mainly a phosphate detergent and heated water to clean the PCB-filled capacitors.
At facility B, originally, the capacitors were filled manually through ports in their tops, which allowed for spillage and dermal contact. In 1960, closed systems were instituted with an automated manifold filling system with electrical controls. In addition, capacitors were also flood-filled at this facility. The valves were removed manually after filling and the tops cleaned of any excess PCBs. A degreasing agent, TCE, was used. The production area was split across several floors, separated into rooms. Impregnation was located on the first floor and used large flood-filling chambers which were evacuated and heated to 150 °C. From the 1970s on and at the time of the NIOSH 1976 survey, 90 % of the capacitors were filled by inserting a hose into a special valve on the top of the unit, which seemed to have decreased the exposures from previous manual filling methods, while 10 % were still being impregnated by the flood-fill method. The capacitors were brought up to the second floor by conveyors for heat soak (80–100 °C) to stabilize the capacitors. After being cooled, the capacitors were leak tested.
The greatest sources of airborne PCBs were areas where heated PCB processes were completely (example soldering) or partially open (example treat room). High PCB exposures were also associated with nonheated processes (example: movemen work, crimping operations). The potential for dermal uptake in these jobs was also high and potentially even higher than via inhalation.
Air concentrations
NIOSH investigators conducted an industrial hygiene survey in April 1977(Jones 1977). Personal air concentrations were measured for those in jobs considered highly PCB exposed (facility A: n=19; range, 24–396 μg/m3; facility B: n=12; range, 50–316 μg/m3) and area air concentrations were measured in areas considered to be at low PCB exposure (assembly, shipping, winding, can, and cover manufacturing) (facility A: n=6; range, 45–476 μg/m3; facility B: n=7; range, 3–41 μg/m3). In addition to PCBs, exposure levels were determined for other chemicals, including TCE, toluene, methyl isobutyl ketone, lead, zinc, tin, aluminum, and iron.
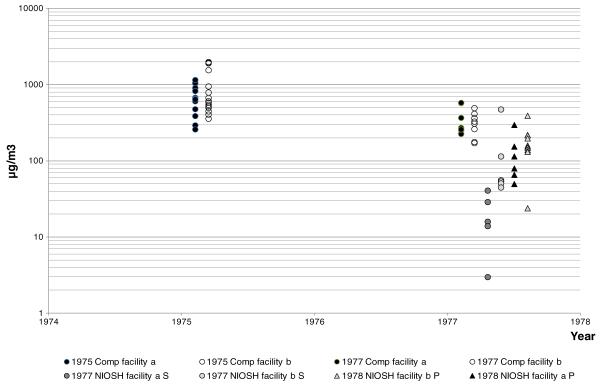
Prior to the NIOSH survey, area air samples had been collected by the company in the capacitor production departments in both facilities in October 1975 (method description missing) (facility A: n = 15; range, 360–2,000 μg/m3 and facility B: n = 15; range, 260–1,160 μg/m3). These PCB air concentrations were much higher than those from the NIOSH 1977 survey. The company also measured PCB air concentrations in April 1977 in other highly exposed areas: manifold fill area, treat tank area, salvage and repair and leak testing (facility A: n=8; range, 227–582 μg/m3 and facility B: n=8; range, 172–496 μg/m3). Two of these samples were collected in the same highly exposed area in both years (1975 and 1977), and showed a decline of 80 %, while levels of samples from the other workstations declined between 30 and 60 %. The reason for this large reduction could have been “the new production techniques recently initiated” as noted in the NIOSH survey. The company continued to monitor PCB air concentrations in both plants in the 1980s after they stopped using PCB, and these showed marked declines and low PCB air concentrations in previously high exposure areas. As opposed to the 1975–1977 measurements, these later measurements showed significant differences between the facilities both in mean levels and range levels. The explanation offered by the company was that more hidden deposits of PCBs; i.e., areas saturated with PCBs from spills over the years, were located in Facility A. Company and NIOSH air concentration measurements for 1975–77 are shown in Fig. 2. These were used in developing the JEM.
Fig. 2.
Air concentration measurements 1975 and 1977 by the company and NIOSH. Comp company measurements, S area air samples, P personal samples
PCB use over time
Commercial PCBs are generally mixtures of many different chlorinated biphenyls, manufactured to meet operational specification, and may vary chemically from batch to batch. The commercial PCBs used at this plant were Aroclors, manufactured by Monsanto Company. The four digits following the name indicated the weight percent of chlorine. Aroclor 1254 contained 54 % chlorine by weight (w/w; mostly four to six chlorine atoms), and Aroclor 1242 contained 42 % w/w (mostly two to five chlorine atoms). Aroclor 1016 was a distillate of Aroclor 1242 containing 41 % w/w (mostly two to four chlorine atoms). Facility A used Aroclor 1254 from 1946 to 1950, Aroclor 1242 from 1950 to 1971, and Aroclor 1016 from 1971 to 1977. Facility B used Aroclor 1254 from 1952 to 1955, Aroclor 1242 from 1955 to 1971, and Aroclor 1016 from 1971 to 1977. These years were indicators of change, as the substitution from one Aroclor to another was gradual over several months. Also there were some indications that from 1956 to 1959 the plant reverted back to using Aroclor 1254; however, this was unconfirmed.
Development of the JEMs
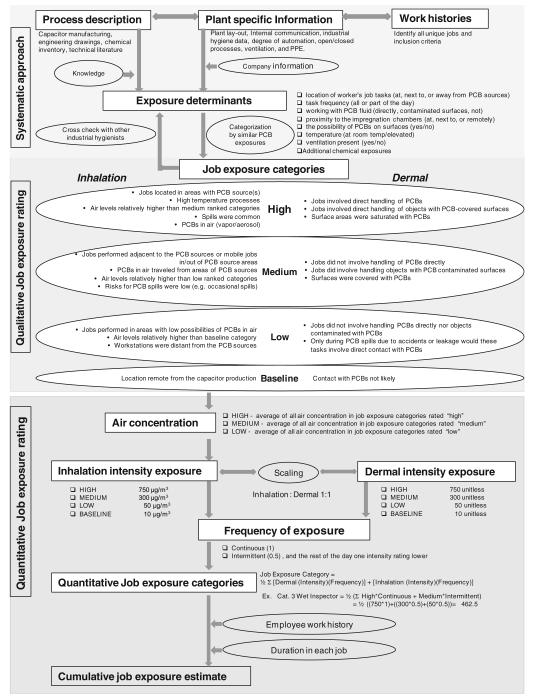
A flowchart of the process we used to develop the plant-specific semiquantitative JEM is presented in Fig. 1. The method used here to develop the JEM built on three previous methods (Astrakianakis et al. 1998; Kauppinen and Partanen 1988; Lewis et al. 1997) and was adjusted according to available data.
Fig. 1.
Flow chart of the development of the JEM
First, a systematic approach was used to organize all the available data such as plant-specific process description and work histories. To understand this plant’s manufacturing processes, it was necessary to acquire knowledge about the general capacitor manufacturing process and how it had evolved from 1946 until 1977. The company could not provide us with pictures; however, photographs from a technical book describing capacitor and transformer manufacturing in the 1930s (Marbury 1949) were useful during discussions with the company. The plant-, facility- and time-specific layouts were then studied to understand the flow of the manufacturing process. Here, it was helpful to have the 1980s PCB air concentrations to determine where PCB sources were located in each facility. The production process was divided into departments identified with two-digit codes. This code was also the two first digits of the four-digit codes identifying different jobs in the work history records. A frequency plot of department codes indicated changes in departments and jobs over time. With some investigating, the reasons for these observed changes, such as increase in production or installing new equipment, could usually be found in internal communication documents, insurance letters, or meeting minutes.
After cleaning the data, we were left with 509 unique jobs that were fairly well described, including how they changed (were eliminated, redefined, or renamed) over time. The layouts over time did not indicate the presence of any exhaust ventilation (the company confirmed this). Based on the available information, we defined the appropriate factors that could affect possible PCB exposures (exposure determinants) (Stewart 1999; Stewart and Stewart 1994) within the plant and in a job. Using exposure determinants to systematize available data for each job has been shown to be efficient (Stewart 1999). The exposure determinants are given in Fig. 1.
Second, jobs with similar PCB exposures as determined by the similarly rated exposure determinants were assigned to a common job exposure category. The job exposure rating was performed by one industrial hygienist (IH), and crosschecked by a second experienced IH. The job exposure categories thus accounted for differences in exposures across jobs and across facilities.
Third, each job exposure category was ranked according to PCB exposures. The major factors governing the differences across job exposure categories were dermal, inhalation, and additional chemical exposures. We used qualitative evaluation of inhalation and dermal exposure (high, medium, and low) to rank the categories relative to baseline. The qualitative evaluations for each intensity group are given in Fig. 1. Job exposure categories with similar qualitative rating but with different additional chemical exposures were not combined, but kept separate because other chemical exposures such as TCE and other potential carcinogens could contribute to exposure misclassification in the epidemiological studies.
Fourth, the qualitative exposure rating began by assessing the frequency and intensity of dermal and inhalation exposures. We defined exposure frequency as dichotomous: PCB tasks performed continuously (continuous) or sporadically (intermittent) throughout the day. The frequency rating was assessed for the highest possible PCB intensity exposure. Thus for the intermittent frequency rating, the rest of the day had less PCB exposures and was assigned an intensity rating lower. Inhalation intensity depended on proximity to the PCB sources (changed with layout changes), degree of automation (changed considerably in later years), ventilation (no local exhaust ventilation, only general ventilation and cross-drafts created with doors and windows), and use of personal protective equipment. Dermal intensity considered degree of contact with PCBs such as handling open PCB-filled containers or touching PCB-contaminated surfaces (Fig. 1).
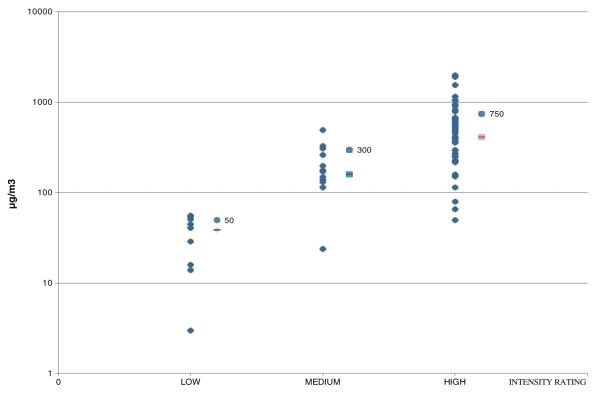
Fifth, the qualitative PCB exposure rankings for each job exposure category were replaced by quantitative values. We assigned continuous exposure to be 1. Intermittent exposure was assigned 1/2 to account for time when other tasks with lower PCB exposures were performed. Using all available PCB air concentrations (Fig. 2), the approximate means for each qualitative inhalation intensity ranking was assigned (Fig. 3). Because there were not a lot of samples, mean PCB levels measured for a particular exposure category were applied to all jobs in that category.
Fig. 3.
Available PCB air concentrations by exposure group rating: high, medium, and low PCB exposures. Group GMs (low ( ), medium (
), medium ( ), high (
), high ( )) and their given exposure ratings (
)) and their given exposure ratings ( with concentration value) are also indicated
with concentration value) are also indicated
Skin exposures were assessed for a few jobs with the wipe sampling technique; however, these were insufficient to assign the dermal intensity exposure ratings. We therefore assigned high, medium, low, and baseline dermal values equivalent to the corresponding high, medium, low, and baseline inhalation intensity values, except that the dermal values were unitless. This is not to say that absorbed dose from a dermal PCB exposure with a given numerical value would be equal to that of an inhalation exposure with the same value.
Sixth, the job exposure category value for either route of PCB exposures (inhalation and dermal) was the product of frequency and intensity. For categories assigned intermittent exposure (higher exposure during part of the day, lower exposure the rest of the day), this calculation was performed once for the initial higher intensity rating (1°) and again for the secondary intensity rating (2°), which was set 1 level lower than the initial intensity rating to account for exposures during the rest of the day. Each job exposure category was described with a dermal and an inhalation exposure value, separately. For example: the mean for inhalation intensity was 300 μg/m3 for medium intensity and 50 μg/m3 for low intensity. The value calculated for Laborers’ rated inhalation intensity “medium” with intermittent frequency was [(300×1/2)+(50×1/2)]=175 μg/m3.
Seventh, production changes (layout, equipment, Aroclor type, and the awareness of industrial hygiene) made over the years, which influenced PCB exposures, were incorporated into the JEM using an era-modifying factor. Factors that governed the decision of choosing an era were installation of the manifold system (~1958), change of Aroclor usage (from Aroclor 1254 to 1242), implementation of hygienic measures and ventilation system (~1975). PCB exposure was regarded as having been higher in the earlier years.
Cumulative exposure estimates using the JEMs
Cumulative exposure at time t was calculated by summing the product of the duration of exposure in each job and the exposure level for the job as assigned by the JEM over all jobs worked prior to time t. The complete JEM for this plant is shown in Table 2.
Table 2.
Assigned frequency and intensity (primary and secondary) for the inhalation and dermal JEMs, including calculated exposure levels [concentrations] for each JEM and average of both JEMs
| Job exposure category | Dermal exposure (unitless) | Inhalation exposure (μg/m3) | Additional exposure | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Description | Frequencya | Intensityb |
Exposure levelc | Frequencya | Intensityb |
Exposure levelc | |||
| 1° | 2° | 1° | 2° | ||||||
| PCB capacitor manufacturing | |||||||||
| Pre-assembly | |||||||||
| 01 Winding | L | 1 | B | 30 | L | 1 | B | 30 | |
| 23 Assemble capacitors | L | I | B | 30 | M | I | L | 175 | |
| 02 Dry inspector | M | I | L | 175 | L | I | B | 30 | |
| 04 Dry operator | M | I | L | 175 | L | C | 50 | ||
| 21 Setup and operate machine | L | I | B | 30 | L | I | B | 30 | Machine grease |
| 17 Welders | L | I | B | 30 | L | I | B | 30 | Welding fumes |
| 18 Tester | M | C | 300 | M | C | 300 | |||
| Impregnation | |||||||||
| 03 Wet inspector | H | C | 750 | M | I | L | 175 | ||
| 05 Wet operator | H | C | 750 | H | C | 750 | |||
| 26 Treat room operators | H | C | 750 | H | C | 750 | |||
| 28 Seal and crimping | H | C | 750 | H | C | 750 | |||
| 12 Laborers | H | I | M | 525 | M | I | L | 175 | |
| 13 Movemen | H | C | 750 | M | C | 300 | |||
| 25 Solderers | H | C | 750 | H | C | 750 | Solder fumes | ||
| 22 Degreasers and painters | M | C | 300 | M | C | 300 | TCE and VOC in paint | ||
| 32 Detergent washer | M | C | 300 | M | C | 300 | Soap and water | ||
| 19 Leak tester | H | C | 750 | H | C | 750 | |||
| Post-assembly | |||||||||
| 15 Salvage and repair | H | C | 750 | H | C | 750 | |||
| 09 Drivers | H | I | M | 525 | L | I | B | 30 | Exhaust |
| 31 Material handler | M | I | L | 175 | M | I | L | 175 | |
| 16 TEAM | M | I | L | 175 | L | I | B | 30 | |
| 11 PEC | M | I | L | 175 | M | I | L | 175 | |
| 06 Stock workers | M | I | L | 175 | L | I | B | 30 | |
| 27 Metal plating | L | I | B | 30 | L | I | B | 30 | Metal fumes, metal oxides and acids |
| 29 Check workers | L | I | B | 30 | M | I | L | 175 | |
| Nonmanufacturing | |||||||||
| 07 Toolcrib | M | I | L | 175 | L | I | B | 30 | Oil and machine grease |
| 14 Clerks | M | I | L | 175 | L | I | B | 30 | |
| 30 Leaders | L | C | 50 | L | C | 50 | |||
| 08 Facility watch workers | L | C | 50 | L | C | 50 | |||
| 24 Battery workers | L | I | B | 30 | L | I | B | 30 | |
| 10 Boilers | L | C | 50 | L | C | 50 | |||
| 20 Office workers | B | C | 10 | B | C | 10 | |||
TCE trichloroethylene; VOC volatile organic compounds; TEAM tool associated jobs, elastomers, apprentice, machinist or mill; PEC plant engineering craftsmen
Frequency of exposure (I intermittent, C continuous)
Intensity of exposure (H high, M medium, L low, B baseline)
Exposure level was calculated using weights of H for high, M for medium, 50 for low, and 10 for baseline. For continuous exposure, the exposure intensity was assigned to the primary intensity level. For intermittent exposures, is assigned to the primary intensity level and the remaining half was assigned to the secondary intensity level. Exposure levels for employment in the first part of the production period (January 1, 1946 to December 31, 1960) were estimated to be 10% higher; second part of the production period (January 1, 1961 to December 31, 1975) were estimated to be 20% higher
Results
Based on all available PCB exposure and manufacturing information, we identified eight exposure determinants (Fig. 1): worker’s location during task, task frequency, direct or indirect PCB exposure, proximity to impregnation chambers, PCBs on surfaces (or not), ambient temperature, ventilation (or not), and additional chemical exposures (considering solvents/metals/detergents and chemical state fumes/vapors/aerosols; or not). Jobs rated similarly using these exposure determinants were combined into a common job exposure category. All 509 unique jobs were categorized into 33 job exposure categories (cat.). Two additional categories, for which we had no exposure information, were created: salaried workers (cat. 34) and 11 undefined jobs (cat. 35). Neither of these categories was listed in Table 2.
The job exposure categories were arranged from lowest to highest exposure, with the lowest exposure category (office workers) as the “baseline”, followed by increasing PCB intensity exposures “low”, “medium”, and “high” (see Fig. 1).
For example, the highest PCB exposures for both routes of entry were assigned to “treat room operators”. Workers in this category produced small capacitors, continuously handling capacitors covered with warm residual PCBs. Appropriate use of personal protective equipment (PPE) such as gloves, aprons, safety glasses, and shoes, which were supplied to the employees by the company, would reduce a worker’s exposure if used properly. However, information regarding PPE use was insufficient to be able to be used to adjust the job exposure category ratings.
In pre-assembly, the exposure levels were mostly intermittent with low-to-medium intensity for both inhalation and dermal exposures. Three job exposure categories had the same qualitative rating (cat. 1, 17, 21); however, they were not combined due to differences in other chemical exposures: machine grease and welding fume (Table 2). In impregnation, most categories were rated with continuous PCB exposure with medium-to-high intensity. Because the impregnation processes involve heating, inhalation was given high intensity for half of the categories but not for nonheated processes. Dermal exposures associated with impregnation were rated high for eight of the ten categories because areas were extensively contaminated with PCB on all surfaces and tools. Several categories with the same overall ratings were not combined because of other chemical exposures such as solder fumes, TCE, volatile organic compounds in paint, detergents, and soaps (Table 2).
In post-assembly, the work was not only diverse but also variable with respect to work activities. This was reflected in the spread of PCB intensity rating (low-to-high) with mostly intermittent frequency of PCB exposures (Table 2). In nonmanufacturing, the intensity rating was baseline-to-low with generally higher ratings for dermal route of PCB exposures (Table 2).
Air concentration measurements were only collected for a short time during the tale end (1975–1977) of the period of PCB use. Because of the 5 to 20+years latency between exposure to a chemical carcinogen and diagnosis of cancer (Goldsmith 1987), and because PCB exposures were probably higher during the early years of PCB production (1946–1960), details of exposures during the early production era would be the most interesting to analyze. However, there are no air concentration data for this era (or through 1974). The capacitor flood-filling process was prone to large spills. The work area where PCB was handled directly would have become saturated with PCB probably soon after production started. It is highly likely that spills and work processes would have saturated floors and surfaces of equipment early, while ceilings and walls might have taken longer to become saturated. For example, hot ovens were opened and vapors of PCB evaporated upwards, and condensed on the relatively cold ceiling where they would be deposited. The higher PCB concentrations in the earlier era were due to spills, less restrictive work practices, or higher volume of use, while saturation of porous surfaces offgassing PCBs increased over time. To reflect historical changes that would influence the PCB concentrations, we multiplied the estimates for the first production era (1-1-1946 to 12-31-1960) by 1.20 to reflect an estimated exposure level 20 % higher than in the 1976–1977 era; for the second production era (January 1, 1961 to December 31, 1975) the multiplier was 1.10, to reflect an exposure level estimated to have been 10 % higher than in 1976–1977.
Discussion
We developed a semiquantitative JEM based on a plethora of qualitative data and only limited air concentration data. This JEM performed well in the epidemiological study showing exposure response in overall cancer mortality. This exposure response was again detected for prostate and stomach cancer mortality in this particular cohort (three). Here, we discuss how we handled incompleteness of the data set and what estimates were necessary to cover job exposure categories with little or no data.
Despite the lack of complete and valid exposure data, especially for important historical periods, which is common in retrospective exposure assessments (Seixas and Checkoway 1995), we also encountered a lack of useful information and little or no data available on certain jobs. Error is inevitably introduced in the process of estimating an exposure from observed or measured data or information. In our historical reconstruction, we have tried to reduce these errors using a systematic approach, simple algorithms, and extrapolation, as discussed below.
The first definition (assumption) was that a unique job was the grouping factor for best predicting exposures. In every collapsing step, information gets lost. Therefore, it is beneficial to categorize jobs systematically by a set of exposure determinants (Stewart 1999), creating categories as similar as possible with respect to PCBs and other exposures. However, it is important to bear in mind that a job may seem stationary, but due to the changes in the plant the exposure could have changed radically over time. Grouping by job is quite common as this information is generally readily available. Others (Benke et al. 2000; Dick et al. 2010; Hyland et al. 2010) have refined this by grouping by task, which may represent an additional dimension of the JEM; however, we did not have such detailed information. But we did group generic tasks such as machine operating, maintenance, or cleaning into categories as recommended by others (Benke et al. 2000). Some jobs were further categorized under the assumption that they had indistinguishable exposures, or because there was insufficient detail in the exposure data to allow them to be kept separate. We deemed the information sufficient to develop 35 job exposure categories, as opposed to previous studies of the plant which classified all jobs into three groups (Kimbrough et al. 1999, 2003; Nicholson et al. 1987; Taylor et al. 1988). However, these three groups did not produce an exposure–response in the exposure stratified epidemiological studies (Table 1).
During the PCB production process, contaminant PCDFs were also formed (Bowes et al. 1975). PCDFs are dioxin-like carcinogens (IARC 1997). PCDF contamination was low at this plant as the recycled PCBs from the flood-filling chambers were treated with fuller’s earth, which removed the contaminant, a benefit not known at the time. This was confirmed at a later time (Brown 1987).
Due to absence of quantitative data, our evaluation of exposure started with an ordinal scale rather than with direct quantitative estimates of exposure intensity. It has been shown that subjective raters are able to rank exposure levels with some validity but that there is a high degree of variability between raters (Kromhout et al. 1987). Factors which might influence the validity and reliability of experts’ assessment include the agents being assessed and the expertise of the assessors (Kromhout et al. 1987). To increase inter-rater agreement, we therefore performed a cross-check when categorizing each job into job exposure categories. One experienced industrial hygienist would rate each of the jobs based on the exposure determinants; ratings were checked by the second industrial hygienist, who had extensive experience in retrospective exposure assessments. Discrepancies were resolved through discussion and consensus.
Limited measurement data might not be used for statistical purposes but can allow a calibration of the estimates to be less biased (Teschke et al. 2002). The decisions of how many, and which dimensions should be included for a particular study depend primarily on the extent of the data available and a judgment of the importance of each factor in representing exposures. The constraint of statistical modeling is the number of categories that may be used (estimation of the coefficient by least squares statistical modeling requires at least as many data points as categories) unless there are at least several data points for each parameter estimated. If not, the parameter estimates will be very unstable (Seixas and Checkoway 1995). Although 74 air concentration samples were available, which could have allowed for a statistical approach given 35 job exposure categories, the samples were collected only at two time points (1975 and 1977). Moreover, the later samples were collected after production was reduced and ventilation was installed. Discarding the 1977 data would leave 30 air concentration samples, which were not randomly sampled throughout the plant but rather targeted for high exposure areas. We therefore used a simpler approach; the PCB air concentration means were adopted to represent PCB levels for each inhalation intensity rating (high, medium, and low). We cannot say that an alternative approach of rescaling the dimensions into a smaller number of categories and with a statistical model for estimation could not have proved equally useful.
Assigning values to dermal exposure estimates was a very difficult part in constructing the JEM. Surface wipes of capacitor manufacturing workers showed extensive PCB contamination (Wolff 1985); however, dermal exposure estimates based on surface wipes may contain substantial measurement errors (Tulve et al. 2011). Discrepancies in the evaluation of dermal absorption of PCBs exist. An invitro skin study showed PCBs not to penetrate skin (Schmid et al. 1992), while in an earlier study (Lees et al. 1987), biological monitoring of workers performing capacitor repairs with PCBs showed extensive dermal PCB exposures. PCB exposures have recently been determined among transformer repair and salvage workers in China, and again showed significant dermal PCB exposures (Xing et al. 2011). Based on the occupational studies, we decided that both routes of exposures—dermal and inhalation—were equally important. The scaling was therefore kept equal. We did not estimate dermal absorption based on exposed surface area and skin penetration rates, which is common in risk assessments, because sufficient information was not available.
Since the exposure assessment was independent from diagnosis of disease any exposure misclassification introduced was probably nondifferential meaning that the probability of misclassification was the same for all workers (Blair and Stewart 1992). Nondifferential misclassification tends to disrupt exposure–response trends and diminish a confidence that a causal association exists (Blair et al. 2007). We have chosen to scale the job exposure categories from baseline. The rating was dichotomous for frequency, but continuous for intensity, both for inhalation and dermal exposures. By assigning a job the mean exposure of its job exposure category, the precision of the exposure estimate was increased and misclassification should have been reduced.
Interpolation and extrapolation of exposure data with a simple algorithm was used to fill the “empty” cells in our matrix. Available PCB air concentrations were divided into the three intensity levels (high, medium, and low) and the means were set as the estimates for exposure. Thus given the particular nature of the available data, we used the most specific information available to extrapolate to matrix cells with little or no information. Other authors have recommended using a stepwise algorithm that uses marginal means across various dimensions of the matrix to estimate exposures where data are missing (Seixas and Checkoway 1995), which is a similar to what we used.
For outcomes with short indication and latency periods, measurements of current exposures may serve as reasonable surrogates for exposure in the disease induction period; however, for PCBs with both short (lower chlorinated PCBs; Phillips et al. 1989) and long (higher chlorinated PCBs; Seegal et al. 2011; Wolff et al. 1992) biological half-lives current levels can provide only limited information on historical exposures. To reflect this, the JEM was assessed for potential daily PCB exposures in each job by route of exposures, and then the historical evolutions of PCB exposures were applied using modifying era factors. For example high, medium, and low intensity exposures for years in which no data were available (1946–1974) were estimated based on the 1975 data with corrective factors to account for higher exposure in the earlier eras. We used the 1975 data to extrapolate and provide estimates for earlier time periods. These era modifying factors were subjective estimates (110 and 120 %) but were anchored in the significance of specific engineering changes occurring within the facilities, department, and jobs. These modifying factors may introduce bias, which makes the interpretation of exposure–disease relationship more difficult (Armstrong 1990; Dosemeci et al. 1990; Steenland et al. 2000). Validation of the JEM could not be performed directly as part of this plant was demolished. Nevertheless, given all the uncertainties involved in observational retrospective epidemiology, it is ultimately in the exposure—response analysis that the validity of the exposure assessment is shown. Our JEM performed well in the mortality study of this capacitor manufacturing cohort, showing increased prostate and stomach cancer mortality with cumulative exposure estimated with the JEM (trend p value=0.003 and 0.04, respectively) (Ruder et al, Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: a ten-year update, Under Review).
Although the JEM cannot be used directly by other studies because of plant process differences, the approach of developing the JEM will be applicable to any facility. This systematic methodology first selected pertinent exposure determinants based on the agents’ intrinsic properties and work tasks performed in the facility. Then, an ordinal ranking of possible exposures was performed before grouping similarly rated (i.e., the exposure determinants were similar) jobs into job exposure categories. This approach could be applied for any facility. It is important to anchor the industrial hygienists’ rankings with agents’ measured concentration data if these are available.
Conclusion
We developed a semiquantitative JEM based on a plethora of qualitative data and only limited air concentration data. This JEM performed well in the epidemiological studies showing exposure response in prostate and stomach cancer mortality (Ruder et al, Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: a ten-year update, Under Review). As is common in historical exposure reconstruction the data set was incomplete. However, in developing the JEM, we overcome this problem by using a systematic approach when rating all unique job codes by predetermined process- and plant-specific exposure determinants, followed by categorizing the jobs based on their similarly rated exposure determinants into job exposure categories. Ranking the job exposure categories ordinally and then using their overall PCB exposure rating, the lowest exposure job exposure category was identified and given the intensity value baseline, while all others were rated low, medium, and high for inhalation and dermal intensity, both exposures deemed equally important for PCBs. Other chemical exposures (e.g. TCE, toluene, etc.) prevented the job exposure categories to be collapsed further. Inter-rater agreement was achieved by one industrial hygienist crosschecking the primary exposure rater and differences being resolved through consensus. We used PCB air concentrations to anchor the intensity rating. Estimates were necessary to cover job exposure categories with no measurement data, therefore, we calculated the mean of PCB air concentrations for each intensity rating, and assigned this value to all job exposure categories rated the same (e.g., the PCB concentration mean of all job exposure categories rated high were given the same value). Inhalation intensity had measurement units (in microgram per cubic milligram) while dermal intensity was unitless. Each intensity rating was frequency adjusted based on whether the exposure was continuous or intermittent throughout the day. Era-modifying factors were applied to the earlier time periods (1946–1974) because they were regarded as more exposed compared to available PCB air concentrations measured later (1975–1977). These interpolations, extrapolations, and modifying factors may have introduced nondifferential misclassification. Using a systematic approach, exposure determinants, ordinal ranking, similar job exposure categories, and anchoring the industrial hygienists’ rankings with PCB air concentration data; we do believe we have minimized the misclassification. The validity of the JEM was shown in the recent cancer mortality study of this capacitor manufacturing cohort, showing increased prostate and stomach cancer mortality with cumulative exposure estimated with the JEM (trend p value=0.003 and 0.04, respectively; (Ruder et al, Mortality among 24,865 workers exposed to polychlorinated biphenyls (PCBs) in three electrical capacitor manufacturing plants: a ten-year update, Under Review)).
Highlights.
PCB exposure assessments based on job description
All jobs from 1946 to 1977 assessed for PCB exposures
Measures of quantitative intensity and frequency of PCB exposures
Both inhalation and dermal route of exposures assessed
Abbreviations
- EPA
Environmental Protection Agency
- GM
Geometric mean
- IARC
International Agency for Research on Cancer
- IH
Industrial hygienist
- JEM
Job exposure matrix
- NIOSH
National Institute for Occupational Safety and Health
- PCB
Polychlorinated biphenyls
- PCDF
Polychlorinated dibenzofurans
- PPE
Personal protective equipment
- SMR
Standardized mortality ratio
- SRR
Standardized relative risk
- TCE
Trichloroethylene
Footnotes
Disclaimers
Mention of company names or products does not constitute endorsement by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Contributor Information
Nancy B. Hopf, Institute for Work and Health (IST), Route de la Corniche 2, 1066, Epalinges-Lausanne, Switzerland
Avima M. Ruder, Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH), 4676 Columbia Parkway, Cincinnati, OH 45226, USA
Martha A. Waters, Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH), 4676 Columbia Parkway, Cincinnati, OH 45226, USA
References
- Ali I, Aboul-Enein HY. Chiral pollutants: distribution, toxicity and analysis by chromatography and capillary electrophoresis. Wiley; Chichester: 2004. ISBN 0-470-86780-9. [Google Scholar]
- Armstrong BG. The effects of measurement errors on relative risk regressions. Am J Epidemiol. 1990;132(6):1176–1184. doi: 10.1093/oxfordjournals.aje.a115761. [DOI] [PubMed] [Google Scholar]
- Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55(10):651–656. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrakianakis G, Anderson JTL, Keefe AR, Bert JL, Lea N, Fanga R. Job–exposure matrices and retrospective exposure assessment in the pulp and paper industry. Appl Occup Environ Hyg. 1998;13(9):663–670. doi:10.1080/1047322x.1998.10390135. [Google Scholar]
- Baan R, Grosse Y, Straif K, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part F: chemical agents and related occupations. Lancet Oncol. 2009;10(12):1143–1144. doi: 10.1016/s1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- Benke G, Sim M, Fritschi L, Aldred G. Beyond the job exposure matrix (JEM): the task exposure matrix (TEM) Ann Occup Hyg. 2000;44(6):475–482. [PubMed] [Google Scholar]
- Blair A, Stewart PA. Do quantitative exposure assessments improve risk estimates in occupational studies of cancer? Am J Ind Med. 1992;21(1):53–63. doi: 10.1002/ajim.4700210108. [DOI] [PubMed] [Google Scholar]
- Blair AP, Stewart PP, Lubin JHP, Forastiere FMD. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med. 2007;50(3):199–207. doi: 10.1002/ajim.20281. [DOI] [PubMed] [Google Scholar]
- Bowes CW, Mulvihill MJ, Simoneit BR, Burlingame AL, Risebrough RW. Identification of chlorinated dibenzofurans in American polychlorinated biphenyls. Nat Biotechnol. 1975;256(5515):305–307. doi: 10.1038/256305b0. [DOI] [PubMed] [Google Scholar]
- Brown DP. Mortality of workers exposed to polychlorinated biphenyls—an update. Arch Environ Health. 1987;42(6):333–339. doi: 10.1080/00039896.1987.9934355. [DOI] [PubMed] [Google Scholar]
- Brown DP, Jones M. Mortality and industrial hygiene study of workers exposed to polychlorinated biphenyls. Arch Environ Health. 1981;36(3):120–129. doi: 10.1080/00039896.1981.10667615. [DOI] [PubMed] [Google Scholar]
- Dick FD, Semple SE, van Tongeren M, Miller BG, Ritchie P, Sherriff D, Cherrie JW. Development of a task–exposure matrix (TEM) for pesticide use (TEMPEST) Ann Occup Hyg. 2010;54(4):443–452. doi: 10.1093/annhyg/meq014. [DOI] [PubMed] [Google Scholar]
- Dosemeci M, Wacholder S, Lubin JH. Does nondifferential misclassification of exposure always bias a true effect toward the null value? Am J Epidemiol. 1990;132(4):746–748. doi: 10.1093/oxfordjournals.aje.a115716. [DOI] [PubMed] [Google Scholar]
- Faroon OM, Keith S, Jones D, De Rosa C. Carcinogenic effects of polychlorinated biphenyls. Toxicol Ind Health. 2001;17(2):41–62. doi: 10.1191/0748233701th098oa. [DOI] [PubMed] [Google Scholar]
- Golden R, Kimbrough R. Weight of evidence evaluation of potential human cancer risks from exposure to polychlorinated biphenyls: an update based on studies published since 2003. Crit Rev Toxicol. 2009;39(4):299–331. doi: 10.1080/10408440802291521. [DOI] [PubMed] [Google Scholar]
- Golden R, Doull J, Waddell W, Mandel J. Potential human cancer risks from exposure to PCBs: a tale of two evaluations. Crit Rev Toxicol. 2003;33(5):543–580. [PubMed] [Google Scholar]
- Goldsmith JR. The importance of time in cancer epidemiology: testicular cancer as an example. Public Health Rev. 1987;15(3):195–214. [PubMed] [Google Scholar]
- Hopf NB, Waters MA, Ruder AM. Cumulative exposure estimates for polychlorinated biphenyls using a job-exposure matrix. Chemosphere. 2009;76(2):185–193. doi: 10.1016/j.chemosphere.2009.03.058. doi:10.1016/j.chemosphere.2009.03.058. [DOI] [PubMed] [Google Scholar]
- Hyland RA, Yates DH, Benke G, Sim M, Johnson AR. Occupational exposure to asbestos in New South Wales, Australia (1970–1989): development of an asbestos task exposure matrix. Occup Environ Med. 2010;67(3):201–206. doi: 10.1136/oem.2008.039347. [DOI] [PubMed] [Google Scholar]
- IARC Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monogr Eval Carcinog Risks Hum. 1997;69:1–631. [PMC free article] [PubMed] [Google Scholar]
- Jones M. In: Industrial Hygiene Survey of Westinghouse Electric Corporation, Bloomington, Indiana. Centers for Disease Control and Prevention NIfOSaH, editor. National Institute for Occupational Safety and Health; Cincinnati: 1977. [Google Scholar]
- Kauppinen T, Partanen T. Use of plant- and period-specific job–exposure matrices in studies on occupational cancer. Scand J Work Environ Health. 1988;14(3):161–167. doi: 10.5271/sjweh.1936. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD, Doemland ML, LeVois ME. Mortality in male and female capacitor workers exposed to polychlorinated biphenyls. J Occup Environ Med. 1999;41(3):161–171. doi: 10.1097/00043764-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD, Doemland ML, Mandel JS. A mortality update of male and female capacitor workers exposed to polychlorinated biphenyls. J Occup Environ Med. 2003;45(3):271–282. doi: 10.1097/01.jom.0000052959.59271.59. [DOI] [PubMed] [Google Scholar]
- Kromhout H, Oostendorp Y, Heederik D, Boleij JS. Agreement between qualitative exposure estimates and quantitative exposure measurements. Am J Ind Med. 1987;12(5):551–562. doi: 10.1002/ajim.4700120509. [DOI] [PubMed] [Google Scholar]
- Lees PS, Corn M, Breysse PN. Evidence for dermal absorption as the major route of body entry during exposure of transformer maintenance and repairmen to PCBs. Am Ind Hyg Assoc J. 1987;48(3):257–264. doi: 10.1080/15298668791384715. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Bell GM, Cordingley N, Pearlman ED, Rushton L. Retrospective estimation of exposure to benzene in a leukaemia case–control study of petroleum marketing and distribution workers in the United Kingdom. Occup Environ Med. 1997;54(3):167–175. doi: 10.1136/oem.54.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbury RE. Power capacitors. 1st edn McGraw-Hill; New York: 1949. [Google Scholar]
- Maroni M, Colombi A, Cantoni S, Ferioli E, Foa V. Occupational exposure to polychlorinated biphenyls in electrical workers. I. Environmental and blood polychlorinated biphenyls concentrations. Br J Ind Med. 1981;38(1):49–54. doi: 10.1136/oem.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WJ, Seidman H, Selikoff IJ. Report to the Industrial Disease Standards Panel, Ontario Ministry of Labor. Ontario Ministry of Labor; Ontario: 1987. Mortality experience of workers exposed to polychlorinated biphenyls during manufacture of electrical capacitors. [Google Scholar]
- Phillips DL, Smith AB, Burse VW, Steele GK, Needham LL, Hannon WH. Half-life of polychlorinated biphenyls in occupationally exposed workers. Arch Environ Health. 1989;44(6):351–354. doi: 10.1080/00039896.1989.9935905. [DOI] [PubMed] [Google Scholar]
- Prince MM, Hein MJ, Ruder AM, Waters MA, Laber PA, Whelan EA. Update: cohort mortality study of workers highly exposed to polychlorinated biphenyls (PCBs) during the manufacture of electrical capacitors, 1940–1998. Environ Health. 2006a;5:13. doi: 10.1186/1476-069X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MM, et al. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Environ Health Perspect. 2006b;114(10):1508–1514. doi: 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LW, Ruder AM. Polychlorinated biphenyls (PCBs) identification of research needs to resolve the carcinogenicity of high-priority IARC carcinogens. IARC; Lyon, France: 2010. pp. 166–183. [Google Scholar]
- Schmid P, Bühler F, Schlatter C. Dermal absorption of PCB in man. Chemosphere. 1992;24(9):1283–1292. doi:10.1016/0045-6535(92)90053-t. [Google Scholar]
- Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, Todd AC, Parsons P, Molho ES, Higgins DS, Factor SA, Marek KL, Seibyl JP, Jennings DL, McCaffrey RJ. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J Expo Sci Environ Epidemiol. 2011;21(3):234–246. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- Seixas NS, Checkoway H. Exposure assessment in industry specific retrospective occupational epidemiology studies. Occup Environ Med. 1995;52(10):625–633. doi: 10.1136/oem.52.10.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberhorn EM, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20(6):440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Smith AB, Brown DP. Polychlorinated biphenyls in the workplace. In: Waid JS, editor. PCBs and the environment. CRC Press; Boca Raton: 1987. pp. 63–82. [Google Scholar]
- Steenland K, Deddens JA, Zhao S. Biases in estimating the effect of cumulative exposure in log-linear models when estimated exposure levels are assigned. Scand J Work Environ Health. 2000;26(1):37–43. doi: 10.5271/sjweh.508. [DOI] [PubMed] [Google Scholar]
- Stewart P. Challenges to retrospective exposure assessment. Scand J Work Environ Health. 1999;25(6):505–510. doi: 10.5271/sjweh.473. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Stewart PA. Occupational case–control studies: I. Collecting information on work histories and work-related exposures. Am J Ind Med. 1994;26(3):297–312. doi: 10.1002/ajim.4700260304. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Stelma JM, Auger I, Lawrence CE. The relation of occupational polychlorinated biphenyl exposure to cancer and total mortality. Harvard School of Public Health; Boston: 1988. [Google Scholar]
- Teschke K, Olshan AF, Daniels JL, De Roos AJ, Parks CG, Schulz M, Vaughan TL. Occupational exposure assessment in case–control studies: opportunities for improvement. Occup Environ Med. 2002;59(9):575–593. doi: 10.1136/oem.59.9.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulve NS, Egeghy PP, Fortmann RC, Xue J, Evans J, Whitaker DA, Croghan CW. Methodologies for estimating cumulative human exposures to current-use pyrethroid pesticides. J Expo Sci Environ Epidemiol. 2011;21(3):317–327. doi: 10.1038/jes.2010.25. [DOI] [PubMed] [Google Scholar]
- Ward EM, Schulte PA, Straif K, Hopf NB, Caldwell JC, Carreón T, DeMarini DM, Fowler BA, Goldstein BD, Hemminki K, Hines CJ, Pursiainen KH, Kuempel E, Lewtas J, Lunn RM, Lynge E, McElvenny DM, Muhle H, Nakajima T, Robertson LW, Rothman N, Ruder AM, Schubauer-Berigan MK, Siemiatycki J, Silverman D, Smith MT, Sorahan T, Steenland K, Stevens RG, Vineis P, Zahm SH, Zeise L, Cogliano VJ. Research recommendations for selected IARC-classified agents. Environ Health Perspect. 2010;118(10):1355–1362. doi: 10.1289/ehp.0901828. doi:10.1289/ehp.0901828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS. Occupational exposure to polychlorinated biphenyls (PCBs) Environ Health Perspect. 1985;60:133–138. doi: 10.1289/ehp.8560133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Fischbein A, Selikoff IJ. Changes in PCB serum concentrations among capacitor manufacturing workers. Environ Res. 1992;59(1):202–216. doi: 10.1016/s0013-9351(05)80240-3. [DOI] [PubMed] [Google Scholar]
- Xing GH, Liang Y, Chen LX, Wu SC, Wong MH. Exposure to PCBs, through inhalation, dermal contact and dust ingestion at Taizhou, China—a major site for recycling transformers. Chemosphere. 2011;83(4):605–611. doi: 10.1016/j.chemosphere.2010.12.018. [DOI] [PubMed] [Google Scholar]