Abstract
Background
Periodontitis is a chronic, polymicrobial inflammatory disease that degrades connective tissue and alveolar bone and results in tooth loss. Oxidative stress has been linked to the onset of periodontal tissue breakdown and systemic inflammation, and the success of antiresorptive treatments will rely on how effectively they can ameliorate periodontal disease–induced oxidative stress during oral infection.
Methods
Rats were infected with polybacterial inoculum consisting of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, as an oral lavage every other week for 12 weeks. Daily subcutaneous injections of enoxacin, bisenoxacin, alendronate, or doxycycline were administered for 6 weeks after 6 weeks of polybacterial infection in rats. The serum levels of oxidative stress parameters and antioxidant enzymes, including glutathione peroxidase, superoxide dismutase, and catalase, were evaluated in each of the infected, treated, and sham-infected rats.
Results
Rats infected with the periodontal pathogens displayed a five-fold increase in the oxidative stress index compared with controls as a result of increased levels of serum oxidants and decreases in total antioxidant activity. The overall decrease in antioxidant activity occurred despite increases in three important antioxidant enzymes, suggesting an imbalance between antioxidant macromolecules/small molecules production and antioxidant enzyme levels. Surprisingly, the bone-targeted antiresorptives bis-enoxacin and alendronate inhibited increases in oxidative stress caused by periodontitis. Bis-enoxacin, which has both antiresorptive and antibiotic activities, was more effective than alendronate, which acts only as an antiresorptive.
Conclusion
To the best of the authors’ knowledge, this is the first study to demonstrate that the increased oxidative stress induced by periodontal infection in rats can be ameliorated by bone-targeted antiresorptives.
Keywords: Alendronate, bacteria, periodontal, enoxacin, lipid peroxidation, oxidative stress, periodontal diseases
Periodontal disease is a common, chronic polymicrobial immunoinflammatory disease initiated by a complex subgingival bacterial biofilm and results in the inflammatory breakdown of tooth-supporting tissues, including the gingivae, periodontal ligament, and alveolar bone. In addition to eventual tooth loss, periodontal disease has been linked to various systemic illnesses, including atherosclerosis, cardiovascular disease, diabetes, rheumatoid arthritis, Alzheimer disease, and adverse pregnancy outcomes.1–6 One potential mechanism by which periodontal disease can manifest its systemic effects is through the generation of systemic oxidative stress (SOS).7 In recent years, strong evidence emerged to implicate reactive oxygen species (ROS) as the cause of oxidative stress and lipid peroxidation (LPO) in the pathogenesis of periodontal disease in humans.7–10 Free radical–induced LPO and the effect of ROS were implicated in the pathogenesis of several pathologic disorders.11 Mammals contain an array of antioxidant defense mechanisms consisting of non-enzymatic and enzymatic antioxidants to protect themselves, remove harmful ROS as soon as they are formed, and prevent their deleterious effects.12 If the level of oxidants outweighs the level of antioxidants, cells will be under oxidative stress.12 The presence of excess reactive oxidants is thought to provide a fertile soil for the progression of various diseases. If periodontal disease stimulates SOS, this could plausibly accelerate disease progression and provide a mechanism by which SOS can cause or enhance distant systemic disease. Several studies reported the role of antioxidant enzymes, including glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), in periodontitis in humans9,10,13–15 and rats.16
Subgingival bacteria and their products, such as lipopolysaccharides and proteases, are responsible for initiating the production of cytokines that are responsible for tissue breakdown in periodontal disease. Pathogens, such as Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia, interact with the host and may lead to systemic inflammation characterized by the induction of proinflammatory cytokines, chemokines, and an enhanced immune response, resulting in an increase in the number and activity of polymorphonucleocytes (PMNs). These PMNs produce the ROS superoxide (O2−) via the respiratory burst mechanism as part of the defense response to infection.17 The inflammatory response also stimulates increased osteoclastic bone resorption, which degrades the alveolar bone supporting the teeth in the infected area. Thus, production of ROS constitutes a component of the bone-resorptive process during periodontal disease.
Enoxacin (ENX) is an oral broad-spectrum fluoroquinolone antibacterial agent effective against many Gram-positive and -negative bacteria,18 and it also possesses anticancer properties because of its ability to enhance microRNA activity.19 ENX also blocks osteoclastogenesis and bone resorption without altering the expression levels of numerous osteoclast-specific proteins.20–22 ENX was very recently shown to reduce titanium particle–induced osteolysis via suppression of the c-Jun N-terminal kinase (JNK) signaling pathway.22 Bis-enoxacin (BE) is a bisphosphonate ester of ENX. Like ENX, BE blocks osteoclastogenesis and bone resorption without altering the expression levels of numerous osteoclast-specific proteins. BE has antiresorptive activity and inhibits osteoclast differentiation and bone resorption in vitro. It inhibits orthodontic tooth movement, an osteoclast-dependent process, and alveolar bone loss triggered by experimental periodontitis in rats.23,24
BE, like other bisphosphonates, adheres to mineralized matrix at sites of osteoclast resorption and inhibits osteoclast formation and bone resorption in a manner that resembles the mechanism by which ENX functions.23 In addition to its antiresorptive activity, BE is also an antibiotic.25 Alendronate (ALN) is a nitrogen-containing bisphosphonate that is a potent inhibitor of bone resorption. It blocks the prenylation of the Rho family guanosine triphosphatases (including Rho, Ras-related C3 botulinum toxin substrate 1 [Rac], and cell division cycle 42) in osteoclasts after it is mobilized by osteoclast activity. 26 ALN does not have antibiotic activity. Doxycycline (DOX) is amember of the tetracycline antibiotics group and is used to treat a variety of infections. It was reported that low doses of DOX decrease attachment loss by decreasing the levels of prostaglandins and phospholipase A2 and inhibiting the production and activation of the matrix metalloproteinases. 27
Because BE has both antiresorptive and antibiotic activities, in a previous study, BE was tested as a therapeutic agent to prevent alveolar bone resorption in a polybacterial rat model of periodontitis and found it to be extremely effective.24 In this current study, SOS was examined in the serum of rats infected with periodontal pathogens and treated with BE, ALN, DOX, and ENX, as well as in shaminfected and/or untreated control rats to confirm whether periodontal disease stimulates SOS. The ability of these four drugs to control SOS induced by periodontal pathogens was also examined.
MATERIALS AND METHODS
Bacterial Strains and Microbial Inocula
P. gingivalis FDC 381, T. denticola ATCC 35404, and T. forsythia ATCC 43037 were grown anaerobically at 37°C, and the inoculum was prepared as described previously.28,29 Bacterial cell concentrations for each species were enumerated, and bacteria were suspended in reduced transport fluid. P. gingivalis was mixed with an equal quantity of T. denticola and T. forsythia. The bacterial suspension was mixed with an equal volume of 4% sterile carboxymethylcellulose.||
Oral Infection and Oral Sampling
Forty-eight female Sprague-Dawley rats (10 weeks old) were obtained from the supplier.¶ Water was given ad libitum, and the rats were fed powdered normal chow.#28,29 All rat procedures were performed in accordance with the approved protocol (protocol no. 201004367) guidelines set forth by the Institutional Animal Care and Use Committee of the University of Florida. The University of Florida has an Assurance with the Office of Laboratory Animal Welfare and follows Public Health Service policy, the Animal Welfare Act and Animal Welfare Regulations, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The University of Florida is also accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. In addition, adequate measures were taken to minimize pain or discomfort to rats during oral bacterial infection and plaque sampling. Rats were administered 0.05 mg/mL kanamycin in their drinking water, followed by administration of 0.12% chlorhexidine gluconate mouthrinse to inhibit microorganisms endogenous in the rat oral cavity. Polymicrobial inocula containing P. gingivalis, T. denticola, and T. forsythia of 109 cells were administered as oral lavage every other week for 12 weeks to establish periodontal infection, whereas sham-infected control rats received sterile 4% carboxymethylcellulose only. Oral plaque samples were collected after every bacterial infection cycle by swabbing the oral cavity with sterile cotton tips.**
Treatment Groups
Forty-eight Sprague-Dawley rats were randomly divided into eight groups as follows: 1) polybacterial infection with P. gingivalis, T. denticola, and T. forsythia; 2) polybacterial infection plus treatment with BE (5 mg · kg−1 · d−1);23 3) polybacterial infection plus treatment with BE (25 mg · kg−1 · d−1);23 4) polybacterial infection plus treatment with ALN (1 mg · kg−1 · d−1);30 5) polybacterial infection plus treatment with ALN (10 mg · kg−1 · d−1);30 6) polybacterial infection plus treatment with ENX (5 mg · kg−1 · d−1);21,23 7) polybacterial infection plus treatment with DOX (5 mg/d);31 and 8) shaminfected and untreated controls. A daily subcutaneous injection of these treatments was administered for 6 weeks after 6 weeks of initial infection. The lower-dose 5 mg/kg mixture of BE powder was suspended in sterile PBS, whereas the higher dose of 25 mg/kg was more difficult to dilute and therefore was suspended in 5% ethanol. ENX (5 mg/kg) powder was suspended in 0.1 M NaOH. Both 1 mg/kg ALN mixture and the 5 mg/d DOX mixture were suspended in sterile PBS. After 12 weeks of bacterial infection, rats were euthanized, and blood, jaws, and internal organs (heart, spleen, liver, kidney, lung, and brain) were collected for analysis.29 Serum was separated, stored at −20°C, and used for oxidative stress analysis in this study.
Analysis of Biochemical Parameters
Polybacterial pathogen–infected, sham-infected, and infected and ENX-, BE-, ALN-, or DOX-treated rat serums were used to determine the level of the antioxidant/oxidant status and oxidative stress (total antioxidant status [TAS], total oxidant status [TOS], and oxidative stress index [OSI]), LPO product malondialdehyde (MDA), and antioxidant enzymes GPx, SOD, and CAT.
Analysis of TAS
Serum TAS levels from six rats in each of the groups 1 through 8 were determined using a commercially available assay kit.†† Briefly, the method is based on bleaching of color from a stable 2,2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS) radical cation by antioxidants. Antioxidants in the sample reduced the dark green–colored ABTS radical to a colorless reduced ABTS form.16,32
Measurement of TOS
Serum TOS levels from six rats in each of the eight groups were determined using a commercially available assay kit.‡‡ The method is based on oxidation of ferrous ion to ferric ion. The oxidation reaction is prolonged by enhancer molecules present in the reaction medium. The ferric ion makes a colored complex with chromogen in an acidic medium. The color intensity is related to the total amount of oxidant molecules present in the sample at 660 nm.16,32,33
Measurement of OSI
OSI for six rats in each of the eight groups was calculated using the percentage ratio of TOS-to-TAS. OSI value was calculated using the following formula: OSI = [(TOS, μmol/L)/(TAS, mmol Trolox equivalent/L)].16
Analysis of LPO Levels
This method is based on the reaction of MDA in the samples with thiobarbituric acid (TBA) to generate the MDA–TBA adduct. The MDA–TBA adduct can be quantified by the colorimetric method at 532 nm by a spectrophotometer.34 This assay was performed using the spectrophotometric assay kit.§§
Measurement of GPx Activity
Quantification of GPx activity is based on reducing cumene hydroperoxide with GPx while oxidizing glutathione (GSH) to glutathione disulfide (GSSG). The generated GSSG is reduced to GSH with the consumption of nicotinamide adenine dinucleotide phosphate (NADPH) by glutathione reductase (GR). The decrease of NADPH measured at 340 nm is proportional to GPx activity.35 GPx activity was measured from sera from six rats in each group using spectrophotometric assay kits according to the instructions of the manufacturer. Briefly, GPx activity was measured through a coupled reaction with GR. The method was based on oxidized GSH produced when cumene hydroperoxide is reduced by GPx. The decrease of NADPH was accompanied by a decrease in absorbance at 340 nm, which provided the spectrophotometric means of monitoring. Negative control experiments were performed by deleting the sample or substrate.
Measurement of SOD Activity
SOD activity was quantified from sera of six rats in the eight groups using spectrophotometric assay kits according to the instructions of the manufacturer. Briefly, the SOD assay used a highly water-soluble tetrazolium salt (WST-1) that produced a water-soluble formazan dye that is reduced by superoxide anion. The rate of the reduction with a superoxide anion was linearly related to the xanthine oxidase activity and was inhibited by SOD. The inhibition activity of SOD was determined by a colorimetric procedure using a spectrophotometric reader at 450 nm, and SOD activity was expressed as inhibition rate percentage.36
Measurement of CAT Activity
CAT activity was quantified from sera from six rats in each of eight groups using spectrophotometric assay kits according to the instructions of the manufacturer. Briefly, CAT was first reacted with hydrogen peroxide (H2O2) to produce water and oxygen. The unconverted H2O2 was then reacted with a probe|||| to produce a product, which was measured by colorimetry at 570 nm.36 CAT activity was expressed as nanomoles of H2O2 decomposed.
Statistical Analyses
All data were presented as mean ± SD. An unpaired, two-tailed Student t test was used to compare two independent groups. Statistical software was used for analysis,¶¶ and P <0.05 was considered significant.
RESULTS
Experimental Periodontal Disease Increases SOS But Elevates Antioxidant Parameters
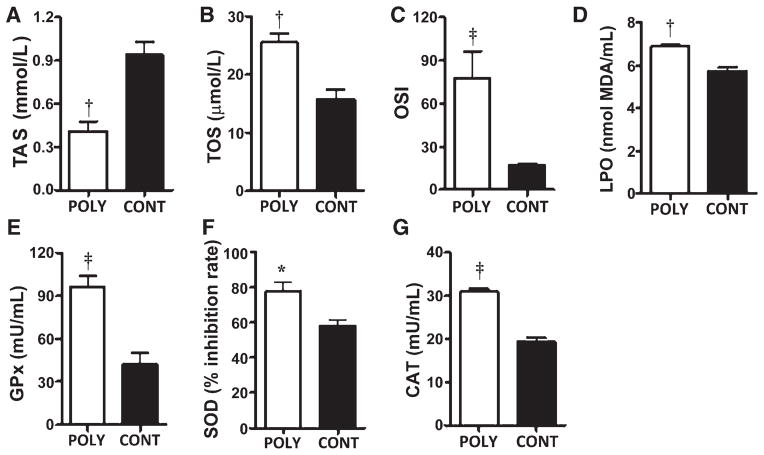
To determine whether periodontal disease triggered SOS in rats, the serum levels of TAS, TOS, specific markers of oxidation (LPO), and levels of antioxidant enzymes (GPx, SOD, and CAT) were evaluated. The OSI was calculated as the ratio of TOS and TAS measurements. Polybacterial oral infection significantly (P <0.01) decreased serum TAS levels in rats compared with sham-infected rats (Fig. 1A). In contrast, polybacterial infection significantly (P <0.01) increased serum TOS levels in rats compared with sham-infected rats (Fig. 1B). OSI levels were significantly higher (P <0.001) (approximately five-fold) in polybacterial-infected rats than in the sham-infected rats (Fig. 1C). Consistent with increased TOS, it was found that serum LPO was also significantly higher (P <0.01) in infected rats compared with shaminfected rats (Fig. 1D). Similarly, serum levels of GPx, SOD, and CAT were all significantly elevated (P <0.001, P <0.05, and P <0.001, respectively) in polybacterial-infected rats compared with sham-infected rats (Figs. 1E through 1G).
Figure 1.
Polybacterial infection–induced SOS and antioxidant enzymes in rats. Rat serum levels of TAS (A), TOS (B), OSI (C), LPO (D), GPx (E), SOD (F), and CAT (G). The error bars represent mean ± SD for each group. *P <0.05, †P <0.01, ‡P <0.001 versus sham-infected rats. POLY = polybacterial infection; CONT = shaminfected control.
Bone-Targeted Antiresorptives Reduce Elevated SOS Triggered by Periodontal Disease
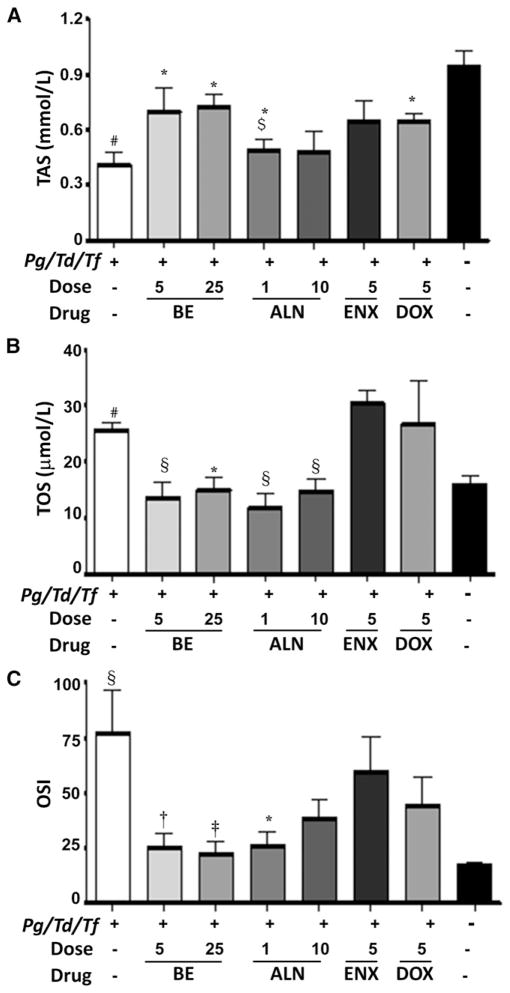
Both BE and ALN were reported to reduce alveolar bone resorption stimulated by experimental periodontal disease in rats.24 Using the same study rats, polybacterial infection–induced SOS was examined in rat serum, as was the effect of bone-targeted antiresorptives and antibiotics in altering the SOS by measuring total antioxidant capacity (TAS). Polybacterial infection significantly (P <0.01) decreased serum TAS levels in rats compared with shaminfected and untreated rats (group 8) (Fig. 2A). In contrast, treatment with BE (5 and 25mg · kg−1 · d−1), ALN (1 mg · kg−1 · d−1), and DOX significantly (P <0.05) enhanced TAS levels in rats relative to infected and untreated rats (group 1). When comparing antioxidant levels between treatments with BE and ALN, BE (5 mg · kg−1 · d−1) enhanced TAS levels more efficiently (P <0.05) than ALN (1 mg · kg−1 · d−1). ENX was ineffective at increasing antioxidants (Fig. 2A). Furthermore, infection-induced SOS was examined, as was the effect of bone-targeted antiresorptives and antibiotics in altering the SOS in rat serum by measuring TOS levels (Fig. 2B). As expected and quite the opposite of serum TAS levels, polybacterial infection significantly (P <0.01) increased serum TOS levels in rats compared with those in sham-infected and untreated rats (group 8). In contrast, treatment with BE (5 and 25mg) and ALN (1 and 10 mg) significantly (P <0.05 and P <0.01, respectively) inhibited/decreased TOS levels in rats compared with infected and untreated rats (group 1). TOS levels were not significantly different from those of sham-infected and untreated rats (Fig. 2B). Neither ENX nor DOX antibiotics significantly reduced TOS levels. OSI levels were significantly higher (P <0.01) in polybacterial-infected rats than in the sham-infected and untreated rats. Both BE and ALN significantly (P <0.01) prevented increased OSI resulting from periodontal disease (Fig. 2C).
Figure 2.
Effect of subcutaneous injections of ENX (5mg · kg−1 · d−1), BE (5 and 25 mg · kg−1 · d−1), ALN (1 and 10 mg · kg−1 · d−1), or DOX (5 mg/d) on the serum TAS (A), TOS (B), and OSI (C). The error bars represent mean ± SD for each group. Pg = P. gingivalis; Td = T. denticola; Tf = T. forsythia. *P <0.05, †P <0.01 versus infected untreated; ‡P <0.05 versus 5 mg BE; §P <0.01 versus uninfected untreated.
Effects of Therapeutic Agents on LPO
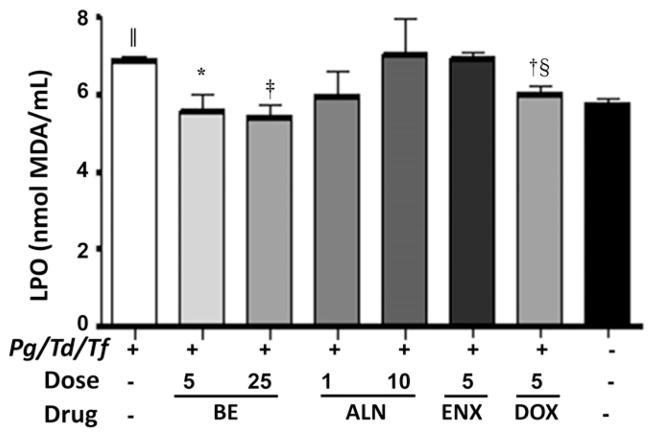
LPO is a measure of the presence of reactive oxidants in the blood. Polybacterial infection with three bacteria significantly (P <0.001) induced LPO in rats compared with uninfected and untreated rats. In contrast, BE at a lower dose (5 mg · kg−1 · d−1) and a higher dose (25 mg · kg−1 · d−1) significantly (P <0.05 and P <0.001, respectively) reduced LPO levels in rats compared with infected and untreated rats (group 1), whereas ALN (both doses) and ENX had no inhibitory effect on LPO induced by polybacterial infection (Fig. 3). Furthermore, DOX, an antibiotic, significantly (P <0.01) reduced LPO levels in rats compared with infected and untreated rats (group 1). Similarly, DOX-treated rats significantly (P <0.01) reduced LPO levels in rats compared with ENX (Fig. 3).
Figure 3.
Effect of subcutaneous injections of ENX (5mg · kg−1 · d−1), BE (5 and 25 mg · kg−1 · d−1), ALN (1 and 10 mg · kg−1 · d−1), or DOX (5mg/d) on serum LPO. The error bars represent mean ± SD for each group. Pg = P. gingivalis; Td = T. denticola; Tf = T. forsythia. *P <0.05, †P <0.01, ‡P <0.001 versus infected untreated; §P <0.01 versus 5 mg ENX; || P <0.001 versus uninfected untreated.
Effects of Therapeutic Agents on Antioxidant Enzymes Found in Blood
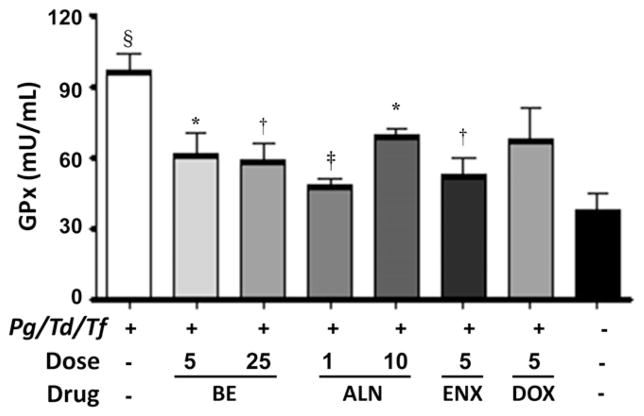
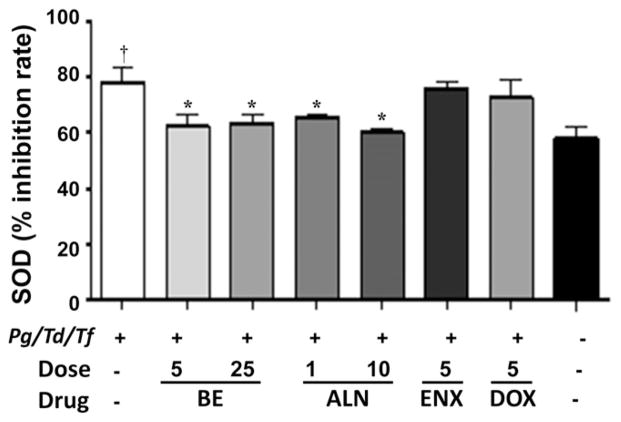
The antioxidant enzymes GPx, SOD, and CAT, which are intracellular ROS-preventive enzymes, play an important role in periodontal disease. GPx activity was significantly (P <0.001) elevated in rats infected with periodontal bacteria compared with shaminfected rats. All three therapeutic agents, BE, ALN, and ENX, decreased serum levels of GPx significantly (P <0.05, P <0.01, and P <0.001, respectively) compared with infected and untreated rats (Fig. 4). However, the GPx levels in the treated group are higher than in sham-infected rats. Similarly, SOD activity was significantly (P <0.05) elevated in rats infected with periodontal bacteria compared with sham-infected rats (Fig. 5). The administration of BE and ALN decreased serum levels of SOD significantly (P <0.05) compared with infected and untreated rats (Fig. 5). Similar to GPx and SOD, CAT activity was significantly (P <0.001) elevated in rats infected with periodontal bacteria compared with sham-infected rats (Fig. 6). The administration of BE, ALN, ENX, and DOX decreased serum levels of CAT significantly (P <0.01, P <0.001, P <0.05, and P <0.01 respectively) compared with infected and untreated rats. In addition, antibiotic DOX treatment decreased CAT activity significantly (P <0.001) compared to ENX (Fig. 6).
Figure 4.
Effect of subcutaneous injections of ENX (25 mg · kg−1 · d−1), BE (5 and 25 mg · kg−1 · d−1), ALN (1 and 10 mg · kg−1 · d−1), or DOX (5mg/d) on serum GPx activity. The error bars represent mean± SD for each group. Pg= P. gingivalis; Td = T. denticola; Tf= T. forsythia.*P <0.05, †P <0.01, ‡P <0.001 versus infected untreated; §P <0.001 versus uninfected untreated.
Figure 5.
Effect of subcutaneous injections of ENX (5mg · kg−1 · d−1), BE (5 and 25 mg · kg−1 · d−1), ALN (1 and 10 mg · kg−1 · d−1), or DOX (5mg/d) on serum SOD activity. The error bars represent mean ± SD for each group. Pg = P. gingivalis; Td = T. denticola; Tf = T. forsythia. *P <0.05 versus infected untreated; †P <0.05 versus uninfected untreated.
Figure 6.
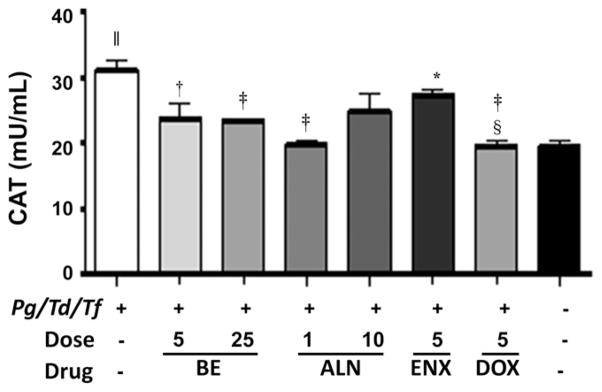
Effect of subcutaneous injections of ENX (5mg · kg−1 · d−1), BE (5 and 25mg · kg−1 · d−1), ALN (1 and 10mg · kg−1 · d−1), or DOX (5mg/d) on serum CAT activity. The error bars represent mean ± SD for each group. Pg = P. gingivalis; Td = T. denticola; Tf = T. forsythia. *P <0.05, †P <0.01, ‡P <0.001 versus infected untreated; §P <0.001 versus 5 mg ENX; ||P <0.001 versus uninfected untreated.
DISCUSSION
To the best of the authors’ knowledge, this is the first study to show that bone-targeted bisphosphonates efficiently reduce the SOS associated with periodontal disease. The present results are consistent with previous studies that showed that periodontal disease is associated with chronic inflammation and increases SOS.7,37 In healthy organisms, there is a balance between oxidants and antioxidants. If the balance is disrupted, cells will be under oxidative stress, which results in the activation of free radical–scavenging enzymes to neutralize the toxic effects of ROS, which include damage to DNA, LPO, amino acid oxidation, and inactivation of enzymes attributable to oxidation of cofactors.37 To counter these deleterious effects, elevated levels of ROS induce increased expression of antioxidant enzymes, such as GPx, SOD, and CAT. These enzymes are part of the machinery that protects cells against the harmful effects of ROS.
Our previous study examined the effects of therapeutic agents on alveolar bone resorption in periodontal disease and other indicators of the effectiveness of the therapeutic agents in vivo.24 BE and ALN were found to be effective at preventing alveolar bone resorption associated with periodontal disease, and neither DOX nor ENX significantly inhibited periodontal disease–induced alveolar bone resorption.24 In the current study, it is found that experimental periodontal disease significantly increased TOS and OSI, the levels of LPO, and GPx, CAT, and SOD activities, which shows the continuous activation of the immune response during the chronic inflammatory process.7,34,37 A significant decrease in TAS level in infected rats was observed; however, the BE and ALN treatment groups showed increased TAS levels and decreased oxidative stress. The overall decrease in antioxidant activity occurred despite increases in three important antioxidant enzymes, suggesting that these increases were more than offset by decreases in antioxidant macromolecules or small molecules. Decreased levels of antioxidant enzymes and LPO in the BE and ALN treatment groups can be construed as the result of decreased oxidative stress and an increased immune response in these groups by treatment. Decreased levels of TOS again support this idea in these groups.
Both BE and ALN efficiently reduced the levels of serum oxidants. Neither DOX nor ENX affected the levels of oxidants. BE also efficiently increased serum antioxidant levels through reduction of presumably secondary levels of antioxidant enzymes. Neither ENX nor DOX was effective at reducing the TOS associated with periodontal disease, although DOX modestly raised antioxidants. Overall, both BE and ALN reduced OSI, which is an effective indicator of the oxidant–antioxidant balance. These data suggest that OSI is likely associated with bone resorption itself or with intercellular communication associated with the bone microenvironment.
The nitrogen-containing bisphosphonate minodronate was shown previously to reduce advanced glycation end product–induced vascular adhesion molecule-1 expression in endothelial cells by suppressing ROS.38 It was hypothesized that minodronate had an effect on endothelial cell production of ROS as a result of disruption of prenylation of Rac, a component of NADPH oxidase in endothelial cells.
Previous studies showed increased levels of LPO in serum, saliva, or gingival crevicular fluid in patients with periodontitis compared with control groups.15,37,39 The present results are consistent with those findings. Increased LPO levels were found in the infected group, and the levels of LPO were significantly decreased in groups treated with BE and DOX, suggesting that treatment protects against cellular damage by inhibiting LPO. Previous studies reported differing results about the role of GPx activity in patients with periodontitis compared with healthy controls.9,10,13,14 GPx has an important role in cellular defense against toxic LPO products, and GPx activity provides protection against oxidative stress to the cell. CAT is another antioxidant enzyme of the defense system and helps to detoxify H2O2 in the host system. Increased levels of CAT activity were also found in a previous study of patients with periodontitis.9 The observed increase in CAT activity in the infected rats could be attributed to elevated oxidative damage via ROS. In addition, increased SOD activity was reported in two studies of patients with periodontitis and control groups.9,15
The present authors became interested in ENX because of its ability to block a binding interaction between vacuolar H+-adenosinetriphosphatase (VATPase) and microfilaments.20 This interaction was targeted based on data indicating that it is crucial for the specialized function of osteoclasts.40–42 BE also blocks this interaction.23 The present data support the hypothesis that both ENX and BE block osteoclast function by inhibiting this interaction.21,23 However, it is not yet known whether BE, like ENX, has the ability to block JNK signaling or stimulate microRNA activity. JNK is activated by oxidative stress and stimulates forkhead box O (FoxO) signaling. 43–45 Inhibition of the JNK pathway could reduce FoxO signaling, which would reduce the expression of CAT and SOD, consistent with the present results. Whether general stimulation of microRNAs would have effects on oxidative stress is not known, but it was proposed that microRNA dysregulation may play a vital role in the induction of oxidative stress.46 Additional study will be required to uncover the mechanism(s) involved in the reduction of SOS associated with BE and ALN.
CONCLUSIONS
To the best of the authors’ knowledge, this is the first study to show that bone-directed bisphosphonates reduce SOS stimulated by periodontal disease. Two types of bisphosphonates were effective. ALN, a classic nitrogen-containing bisphosphonate, reduced SOS but was less effective than BE. A previous study23 suggested that BE inhibits osteoclast formation and bone resorption by competitively blocking an interaction between the B2-subunit of V-ATPase and microfilaments. However, it is also known that BE is an antibiotic, which could contribute to its therapeutic effect. In addition, preliminary studies suggest that BE, like ENX, stimulates microRNA activity (Taylor Capasso, Dontreyl Holsey and LSH, Department of Orthodontics, University of Florida College of Dentistry, and Edward K. Chan, Department of Oral Biology, University of Florida College of Dentistry; unpublished data). The effects of generally stimulating microRNA activity are impossible to predict at this point. It is possible that bone-directed agents can be used therapeutically to ameliorate SOS associated with periodontal disease in addition to preventing alveolar bone resorption, but given the link between bisphosphonates (and antiresorptives in general) and osteonecrosis of the jaw, caution must be exercised.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research Grant R21 DE019862. Drs. Oktay and Chukkapalli contributed equally to this work.
Footnotes
Sigma-Aldrich, St. Louis, MO
Harlan, Indianapolis, IN.
Teklad Global Diet, Harlan.
VWR, Radnor, PA
Rel Assay Diagnostics, Gaziantep, Turkey
Lipid Peroxidation (MDA) Fluorometric/Fluorometric Assay Kit, BioVision, Mountain View, CA.
OxiRed, BioVision.
OxiRed, BioVision.
Prism for Windows v.5.0, GraphPad Software, San Diego, CA.
The authors report no conflicts of interest related to this study.
References
- 1.Lockhart PB, Bolger AF, Papapanou PN, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 2.Paquette DW, Brodala N, Nichols TC. Cardiovascular disease, inflammation, and periodontal infection. Periodontol 2000. 2007;44:113–126. doi: 10.1111/j.1600-0757.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 3.Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J Periodontol. 2013;84(Suppl 4):S135–S152. doi: 10.1902/jop.2013.1340013. [DOI] [PubMed] [Google Scholar]
- 4.Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: A systematic review. J Dent Res. 2013;92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- 5.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimers Dement. 2008;4:242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Scannapieco FA, Bush RB, Paju S. Periodontal disease as a risk factor for adverse pregnancy outcomes. A systematic review. Ann Periodontol. 2003;8:70–78. doi: 10.1902/annals.2003.8.1.70. [DOI] [PubMed] [Google Scholar]
- 7.D’Aiuto F, Nibali L, Parkar M, Patel K, Suvan J, Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241–1246. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–151. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 9.Panjamurthy K, Manoharan S, Ramachandran CR. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell Mol Biol Lett. 2005;10:255–264. [PubMed] [Google Scholar]
- 10.Patel SP, Pradeep AR, Chowdhry S. Crevicular fluid levels of plasma glutathione peroxidase (eGPx) in periodontal health and disease. Arch Oral Biol. 2009;54:543–548. doi: 10.1016/j.archoralbio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Saintot M, Astre C, Pujol H, Gerber M. Tumor progression and oxidant-antioxidant status. Carcinogenesis. 1996;17:1267–1271. doi: 10.1093/carcin/17.6.1267. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning) Free Radic Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- 13.Borges I, Jr, Moreira EA, Filho DW, de Oliveira TB, da Silva MB, Fröde TS. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediators Inflamm. 2007;2007:45794. doi: 10.1155/2007/45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz AS, Kalekar MG, Benjamin T, Suryakar AN, Prakashan MM. Effect of nonsurgical periodontal therapy and some oxidative stress markers in patients with chronic periodontitis: A biochemical study. Indian J Clin Biochem. 2013;28:374–380. doi: 10.1007/s12291-012-0283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients before and after periodontal therapy. Aust Dent J. 2010;55:70–78. doi: 10.1111/j.1834-7819.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 16.Yağan A, Kesim S, Liman N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2014;85:478–489. doi: 10.1902/jop.2013.130138. [DOI] [PubMed] [Google Scholar]
- 17.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17:411–416. doi: 10.4103/0972-124X.118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafalsky V, Andreeva I, Rjabkova E. Quinolones for uncomplicated acute cystitis in women. Cochrane Database Syst Rev. 2006;19:CD003597. doi: 10.1002/14651858.CD003597.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melo S, Villanueva A, Moutinho C, et al. Small molecule enoxacin is a cancer-specific growth inhibitor that acts by enhancing TAR RNA-binding protein 2-mediated microRNA processing. Proc Natl Acad Sci USA. 2011;108:4394–4399. doi: 10.1073/pnas.1014720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostrov DA, Magis AT, Wronski TJ, et al. Identification of enoxacin as an inhibitor of osteoclast formation and bone resorption by structure-based virtual screening. J Med Chem. 2009;52:5144–5151. doi: 10.1021/jm900277z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toro EJ, Zuo J, Ostrov DA, et al. Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis. J Biol Chem. 2012;287:17894–17904. doi: 10.1074/jbc.M111.280511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Qu X, Wu C, et al. The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials. 2014;35:5721–5730. doi: 10.1016/j.biomaterials.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Toro EJ, Zuo J, Guiterrez A, et al. Bis-enoxacin inhibits bone resorption and orthodontic tooth movement. J Dent Res. 2013;92:925–931. doi: 10.1177/0022034513501876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera MF, Chukkapalli SS, Velsko IM, et al. Bisenoxacin blocks rat alveolar bone resorption from experimental periodontitis. PLoS One. 2014;9:e92119. doi: 10.1371/journal.pone.0092119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herczegh P, Buxton TB, McPherson JC, 3rd, et al. Osteoadsorptive bisphosphonate derivatives of fluoroquinolone antibacterials. J Med Chem. 2002;45:2338–2341. doi: 10.1021/jm0105326. [DOI] [PubMed] [Google Scholar]
- 26.Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of the distribution of 3H-alendronate and 3H-etidronate in rat and mouse bones. Bone. 1996;19:281–290. doi: 10.1016/8756-3282(96)00182-2. [DOI] [PubMed] [Google Scholar]
- 27.Krakauer T, Buckley M. Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob Agents Chemother. 2003;47:3630–3633. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesavalu L, Sathishkumar S, Bakthavatchalu V, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivera MF, Lee JY, Aneja M, et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One. 2013;8:e57178. doi: 10.1371/journal.pone.0057178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit ACF, Wronski TJ. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 2010;16:674–685. doi: 10.1111/j.1601-0825.2010.01677.x. [DOI] [PubMed] [Google Scholar]
- 31.Karimbux NY, Ramamurthy NS, Golub LM, Nishimura I. The expression of collagen I and XII mRNAs in Porphyromonas gingivalis-induced periodontitis in rats: The effect of doxycycline and chemically modified tetracycline. J Periodontol. 1998;69:34–40. doi: 10.1902/jop.1998.69.1.34. [DOI] [PubMed] [Google Scholar]
- 32.Erel O. Anovel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Miricescu D, Totan A, Calenic B, et al. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol Scand. 2014;72:42–47. doi: 10.3109/00016357.2013.795659. [DOI] [PubMed] [Google Scholar]
- 35.Siu PM, Pei XM, Teng BT, Benzie IF, Ying M, Wong SH. Habitual exercise increases resistance of lymphocytes to oxidant-induced DNA damage by upregulating expression of antioxidant and DNA repairing enzymes. Exp Physiol. 2011;96:889–906. doi: 10.1113/expphysiol.2011.058396. [DOI] [PubMed] [Google Scholar]
- 36.Branchetti E, Sainger R, Poggio P, et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masi S, Salpea KD, Li K, et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011;50:730–735. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Yamagishi S, Matsui T, Nakamura K, Takeuchi M. Minodronate, a nitrogen-containing bisphosphonate, inhibits advanced glycation end product-induced vascular cell adhesionmolecule-1 expression in endothelial cells by suppressing reactive oxygen species generation. Int J Tissue React. 2005;27:189–195. [PubMed] [Google Scholar]
- 39.Akalin FA, Baltacioğlu E, Alver A, Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J Clin Periodontol. 2007;34:558–565. doi: 10.1111/j.1600-051X.2007.01091.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen SH, Bubb MR, Yarmola EG, et al. Vacuolar H+-ATPase binding to microfilaments: Regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem. 2004;279:7988–7998. doi: 10.1074/jbc.M305351200. [DOI] [PubMed] [Google Scholar]
- 41.Lee BS, Gluck SL, Holliday LS. Interaction between vacuolar H(+)-ATPase and microfilaments during osteoclast activation. J Biol Chem. 1999;274:29164–29171. doi: 10.1074/jbc.274.41.29164. [DOI] [PubMed] [Google Scholar]
- 42.Zuo J, Jiang J, Chen SH, et al. Actin binding activity of subunit B of vacuolar H+-ATPase is involved in its targeting to ruffled membranes of osteoclasts. J Bone Miner Res. 2006;21:714–721. doi: 10.1359/jbmr.060201. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Zhou Y, Graves DT. FOXO transcription factors: Their clinical significance and regulation. Biomed Res Int. 2014;2015:925350. doi: 10.1155/2014/925350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrogini E, Almeida M, Martin-Millan M, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartell SM, Kim HN, Ambrogini E, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5:3773. doi: 10.1038/ncomms4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nat Rev Endocrinol. 2013;9:228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]