Abstract
Despite widespread recognition that the physiological systems underlying stress reactivity are well coordinated at a neurobiological level, surprisingly little empirical attention has been given to delineating precisely how the systems actually interact with one another when confronted with stress. We examined cross-system response proclivities in anticipation of and following standardized laboratory challenges in 664 4- to 14-year-olds from four independent studies. In each study, measures of stress reactivity within both the locus coeruleus-norepinephrine system (i.e., the sympathetic and parasympathetic branches of the autonomic nervous system) and the corticotrophin releasing hormone system (i.e., the hypothalamic-pituitary-adrenal axis) were collected. Latent profile analyses revealed six distinctive patterns that recurred across the samples: moderate reactivity (average cross-system activation; 52%-80% of children across samples), parasympathetic-specific reactivity (2%-36%), anticipatory arousal (4%-9%), multisystem reactivity (7%—14%), hypothalamic-pituitary-adrenal axis specific reactivity (6%-7%), and underarousal (0%-2%). Groups meaningfully differed in socioeconomic status, family adversity, and age. Results highlight the sample-level reliability of children’s neuroendocrine responses to stress and suggest important cross-system regularities that are linked to development and prior experiences and may have implications for subsequent physical and mental morbidity.
Exposure to chronic stress and adversity, especially early in life, has been convincingly shown to augment risks for physical and mental health problems, not only in childhood, but also across the human life span (Hertzman & Boyce, 2010; McEwen, 1998; Obradović, 2012; Taylor, Lerner, Sage, Lehman, & Seeman, 2004). A primary pathway through which adversity exerts this influence is via changes in stress-responsive biological systems, especially the sympathetic and parasympathetic branches of the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal (HPA) axis, and their target tissues (Berntson, Cacioppo, & Quigley, 1993; Cicchetti & Rogosch, 2012; Porges, 2007; Sapolsky, Romero, & Munck, 2000).
Stress-induced neurobiological responses in these channels have evolved to guide adaptive and essential responses to environmental challenge, and an impressive body of research has elaborately delineated the processes underlying these responses (De Kloet, Fitzsimons, Datson, Meijer, & Vreugdenhil, 2009; Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Porges, 2007). Within seconds of stressor onset, for instance, activation of the sympathetic nervous system (SNS) readies the organism for action by producing a broad array of catecholamine-mediated fight or flight responses, inducing functional changes in target organs (Cacioppo et al., 1998). Concomitant withdrawal or deactivation of parasympathetic neurotransmitter signaling can amplify SNS responses, or activation of the parasympathetic nervous system (PNS) can serve as a “brake” on the sympathetic effects, restoring homeostatic control of end-organ function and counterregulating excitation (Porges, 2007). The HPA axis is also activated, though with a range of slower, transcription-mediated neuroendocrine effects, which can lead to both suppressive and excitatory influences that further allow the organism to adapt to and recover following stress exposure (Sapolsky et al., 2000).
In contrast, heightened or prolonged responses, including activation of either the SNS or the HPA axis, as well as prolonged deactivation of the PNS, can confer increased risk for a range of physical and mental health morbidities (e.g., Essex, Boyce, et al., 2002; Taylor et al., 2004). These risks have been identified across the life span, but they are believed to be profoundly influenced by heightened responses that began in early childhood, when the systems were still developing and becoming calibrated to environmental demands (Alkon, Boyce, Davis, & Eskenazi, 2011; Miller et al., 2009). A large body of work has revealed associations between dysregulated reactivity of the ANS and HPA axis and a host of negative outcomes (e.g., Beauchaine, Neuhaus, Brenner, & Gatzke-Kopp, 2010; Boyce et al., 2001; El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008; Obradović, Bush, & Boyce, 2011). However, a majority of this work, particularly in children, has focused on one or two stress-responsive systems and has rarely taken into account the complex nature of coordination across multiple systems. This research has also not considered how different facets of this coordination may increase risk for negative health consequences (for a discussion of this issue, see Beauchaine, 2009). The purpose of the current research was to comprehensively investigate patterns of physiological arousal and reactivity across systems and development, and thus lay the foundation for continued research on the varied ways in which stress-responsive systems are coordinated and how this coordination may relate to subsequent health morbidities.
We are certainly not the first to propose cross-system coordination of stress responses. Several theoretical accounts have eloquently described different ways in which multiple physiological systems interact when exposed to stress and challenge. McEwen and colleagues (e.g., McEwen, 2006; McEwen & Gianaros, 2010; McEwen & Stellar, 1993) proposed the construct of allostatic load as a measurable biological cost of chronic, multisystem efforts to maintain homeostatic balance under conditions of chronic stress. Sapolsky et al. (2000) discussed the combined and closely cooperative functions of stress reactivity systems, pointing out complementarity in the timing and duration of responses, the systems’ cross-regulatory functions at peripheral and central levels, and the systems’ separable and balanced effects on target organs.
Other models have suggested multiple ways in which the systems are coordinated, with some types of coordination likely conferring subsequent risk. Berntson et al. (1993), for example, developed a two-dimensional model of autonomic space, whereby different combinations of activation and deactivation of the sympathetic and parasympathetic systems to challenge lead to several patterns of responses.1 The most well-studied patterns include two that are complimentary: coactivation (sympathetic activation combined with parasympathetic activation) and coinhibition (parasympathetic deactivation combined with sympathetic deactivation); and two that are opposing: reciprocal sympathetic activation (heightened arousal, induced by sympathetic activation combined with parasympathetic deactivation) and reciprocal parasympathetic activation (low arousal, induced by parasympathetic activation combined with sympathetic deactivation). Bauer, Quas, and Boyce (2002) similarly argued for the existence of a two-dimensional model, but they identified the sympathetic system and HPA axis as key contributors, particularly in relation to stress regulation in childhood. Specifically, building on Sapolsky et al.’s (2000) detailed description of the HPA axis’s ability to exert inhibitory pressure on stress-induced SNS responses, Bauer et al. argued that cross-system response tendencies can be reciprocal or opposing. Reciprocity following stress exposure, as reflected in high HPA axis activity serving as a regulatory check on high SNS activation, indicates well-coordinated biological response tendencies, whereas activation of one system without the other, that is, opposing responses, indicates dysregulation or poor coordination.
Empirical research testing these theoretical models has taken one of several methodological approaches (Alkon et al., 2003; Gordis, Granger, Susman, & Trickett, 2006; Salomon, Matthews, & Allen, 2000). In developmental studies, for example, children have often been assigned to groups according to median splits or cross-products on measures of sympathetic (e.g., preejection period [PEP], blood pressure, or alpha amylase) and parasympathetic (e.g., respiratory sinus arrhythmia or vagal tone) reactivity or HPA axis (e.g., salivary cortisol levels) reactivity to laboratory stressors (Gordis, Feres, Olezeski, Rabkin, & Trickett, 2010; Kroenke et al., 2011). Groups have then been compared across various predictors or outcomes. With such an approach, Salomon et al. (2000) found that youth, ages 7–8 through midteens, classified as co-inhibitors according to Berntson et al.’s SNS–PNS model, tended to come from families with higher levels of conflict and hostility than did youth classified as co-activators. Somewhat different results were reported by El Sheikh et al. (2009), who found that, in later childhood (i.e., ages 8–10 years), co-inhibitors and co-activators were at greater risk for behavior problems following exposure to family conflict than were reciprocal sympathetic or parasympathetic co-activators.
Studies testing Bauer et al.’s (2002) model of cross-system response tendencies have typically relied on cross-products between SNS and HPA axis markers of reactivity (Gordis, Granger, Susman, & Trickett, 2008; Kroenke et al., 2011; Lisonbee, Pendry, Mize, & Gwynn, 2010). Again, the findings vary: Gordis et al. (2006) found that reciprocal HPA axis–SNS responses to a laboratory stressor, as reflected in high levels of salivary cortisol (an index of HPA axis activation) and low salivary alpha amylase (an index of SNS activation) relative to baseline markers of both responses, were associated with low levels of aggressive tendencies in 11- to 13-year-olds, while concurrently low levels of cortisol and salivary alpha amylase to the same stressor were associated with high aggressive tendencies. Hastings et al. (2011) found somewhat similar results in 11-year-olds who completed a surprise speech task in the laboratory while samples of cortisol and peripheral markers of autonomic arousal (heart rate and blood pressure) were collected. High cortisol and blood pressure predicted lower concurrent internalizing problems, while high cortisol and low blood pressure predicted higher internalizing symptoms (a similar interaction was observed for externalizing symptoms between cortisol and heart rate). However, when behavioral problems were examined 2 years later, for girls, concurrently high cortisol and heart rate now predicted larger increases in internalizing symptoms, whereas for boys, reciprocal patterns of low cortisol and high heart rate predicted larger increases in such symptoms. Finally, Koss et al. (2013) compared physiological response and behavior problems in 7- to 9-year-olds who had been exposed to varying level of family conflict. Results again diverged. Children who exhibited high SNS responses followed by low HPA axis responses were at greatest risk for behavior problems (relative to children who exhibited low SNS responses, or high SNS and high HPA axis responses), but only when they came from families with parents reporting high levels of marital conflict. When parents reported low levels of conflict, children with high SNS and high HPA axis responses were at greatest risk for behavior problems.
Despite inconsistencies in specific findings, the results to date nonetheless highlight that (a) different individuals exhibit varying patterns of reactivity across several stress-responsive physiological systems, and (b) these patterns may be functionally linked to specific phenotypic behavioral outcomes. To understand these potential links better though, several critical issues need to be addressed. First, there is the need to consider not only reactivity to stress but also basal levels of arousal within and across systems. Like reactivity, baseline arousal also varies substantially between individuals and has implications for physical and mental health morbidities (Bruce, Fisher, Pears, & Levine, 2009; Calkins, 1997; Essex, Klein, Cho, & Kalin, 2002; Hinnant & El-Sheikh, 2009). Second, and perhaps more critical, is the need to expand models of cross-system coordination to consider more than two biological components or axes. Both branches of the ANS, as well as the HPA axis, contribute to regulatory functions following stress exposure (Cannon, 1932; Sapolsky et al., 2000), suggesting that optimal response measurements would involve concurrent examination of these systems. Any such examination should ideally include markers central to the systems’ responses themselves rather than only markers considered peripheral indices of autonomic arousal (e.g., heart rate and blood pressure) that can be affected by factors unrelated to stress (Faes, De Neeling, Kingma, Ten Voorde, & Karemaker, 1995; Gellman et al., 1990). Third, another issue, which has been virtually ignored in extant empirical research, concerns how cross-system response tendencies are shaped by developmental processes. The role that development plays has implications not only for conceptualizations of patterns of reactivity but also for understanding how and when cross-system patterns of responses confer increased or decreased risk (Alkon et al., 2011; Loman & Gunnar, 2010; Lupien, McEwen, Gunnar, & Heim, 2009).
More specifically, with regard to the need for attending to basal levels of arousal, relatively few multisystem investigations of physiological responses have included baseline measures in their analytic approach. Ignoring baseline levels of arousal may be appropriate when studying adults in laboratory settings, who have typically been given ample time (e.g., 30 min) to achieve comparable levels of relaxation and hence baseline states of arousal. However, such approaches are neither appropriate nor feasible in children, given that their baseline levels of arousal, even during well-controlled laboratory relaxation activities, vary considerably (Alkon et al., 2011; Essex, Klein, et al., 2002). Some studies have adjusted for individual differences in basal arousal, for instance, by calculating adjusted difference scores or by computing area under the curve (Harkness, Stewart, & Wynne-Edwards, 2011; Quas, Murowchick, Bensadoun, & Boyce, 2002). However, such statistical approaches fail to address the possibility, which has been substantiated by numerous studies, that basal levels of arousal per se are linked to prior experiences and are predictive of subsequent health (e.g., El Sheikh et al., 2009; Lupien, King, Meaney, & McEwen, 2001; Quevedo, Johnson, Loman, LaFavor, & Gunnar, 2012; Tarullo & Gunnar, 2006). A few investigations have relied on multilevel modeling procedures in their analyses that take into account children’s baseline arousal and hence adjust for initial conditions (e.g., El-Sheikh, Keller, & Erath, 2007; Quas, Yim, Edelstein, Cahill, & Rush, 2011; Shirtcliff & Essex, 2008). However, these studies have not incorporated, in a complex manner, multiple systems’ response tendencies concurrently. Given that an individual’s baseline levels of arousal can directly affect the absolute level of reactivity that the individual can achieve (Berntson, Uchino, & Cacioppo, 1994; Lovallo, 1975; Wilder, 1958) and given that baseline levels of arousal have direct implications for subsequent risk, basal arousal needs to be considered when children’s cross-system functioning is examined (Burt & Obradović, 2012).
For the issue of multisystem models, none of the theoretical models that argue for the existence and importance of cross-system patterns of physiological stress response have elaborated on how more than two biological systems operate in conjunction with one another. Berntson, Cacioppo, Quigley, and Fabro (1994), for example, did not consider the role of HPA axis activation, directly or in coordination with autonomic indices, despite the importance of HPA arousal and reactivity in moderating activation of other stress-responsive systems and predicting morbidities (Gunnar, Tout, de Haan, Pierce, & Stanbury, 1997; Heim & Nemeroff, 1999; McEwen, 1998; Sapolsky et al., 2000; Torpy & Chrousos, 1996). Likewise, Bauer et al. (2002) did not take into account the regulatory function of the parasympathetic system, which may be critical in abating responses driven by sympathetic activation for some children, but for others may augment the already heightened levels of reactivity in other systems (Beauchaine, 2009; Davies, Sturge-Apple, Cicchetti, Manning, & Zale, 2009).
Recently, Del Giudice, Ellis, and Shirtcliff (2011) described an adaptive calibration model, in which they theorize how different constellations of early childhood experiences may give rise to varying patterns of baseline arousal and stress-induced activation of the HPA axis, as well as both branches of the ANS. The researchers argue for four primary patterns of response tendencies: children labeled as “sensitive” exhibit moderate to high sympathetic and HPA axis responses at baseline and following stress exposure and concurrently high parasympathetic activation. In terms of childhood experiences, sensitive children theoretically come from safe family environments and have warm family relationships. Children in a second group, termed “buffered,” are believed to have been exposed to moderate environmental stress, with their physiological responses reflected in moderate levels of arousal and reactivity across the sympathetic and parasympathetic branches of the ANS and the HPA axis. A third typology, “vigilant,” is characterized by children exhibiting parasympathetic deactivation and sympathetic and HPA axis activation to challenge, a pattern that likely results from exposure to chronically stressful environments requiring children to be constantly prepared for action or coping. Fourth, an “unemotional” pattern, indicated by low reactivity, particularly of the sympathetic system and HPA axis, results from extreme stress exposure or low empathy and emotional responsiveness, especially in males.
In a preliminary test of their model, the researchers examined patterns of stress reactivity across the two branches of the ANS, at baseline and following completion of a laboratory stressor, in 8- to 10-year-olds (Del Giudice, Benjamin Hinnant, Ellis, & El-Sheikh, 2012). Although only two systems were considered, some evidence suggestive of the patterns emerged. The largest number of children was classified as buffered, and smaller numbers were classified as sensitive, unemotional, and vigilant. Higher levels of family stress were evident in the unemotional and vigilant groups, as would be expected. However, more frequent negative family interactions were also common in the sensitive group. This was unexpected. Although these preliminary findings are intriguing, HPA axis activation was not considered concurrently, limiting inferences that can be drawn about cross-system coordination or the overall model itself. Moreover, Del Giudice et al.’s (2011) model only minimally distinguishes sympathetic and HPA axis reactivity, despite markedly different functions and response times across these systems. Nuanced variations between them may be critical in relation to prior experience and subsequent risk.
A final important limitation to existing models and empirical work on cross-system responsivity concerns the lack of direct consideration of developmental changes. This is not to say that models fail to acknowledge that development may be important. Several models directly suggest that early stress exposure alters how neurobiological stress-responsive pathways are established and calibrates physiological systems’ response tendencies to specific environmental input (Boyce & Ellis, 2005; Del Giudice et al., 2012; Evans & Kim, 2007; Gunnar & Herrera, 2013). These suggestions have been supported by an extensive body of work revealing links between early stress exposure and later physiological reactivity (Gunnar & Quevedo, 2007; Loman & Gunnar, 2010; Rogosch, Dackis, & Cicchetti, 2011), with early chronic stress exposure, particularly before ages 5 or 6 years, being linked to dysregulation in baseline arousal and reactivity of the ANS and HPA, at least when individual systems have been studied. With regard to cross-system coordination, it may not be until after this early developmental period of heightened sensitivity to environmental conditions, for instance, by middle childhood, that integrated patterns of responses across systems emerge (e.g., Del Giudice et al., 2012). If these integrated patterns of responses emerge in middle childhood, particularly in response to exposure to normative, perhaps relatively mild, environmental stress, the patterns may well reflect a high degree of coordination, or perhaps symmetry as described by Bauer et al. (2002) or sensitivity as described by Del Giudice et al. (2011). Moreover, the patterns may then undergo little further developmental variation, at least in the absence of dramatic changes in environmental input.
By contrast, in light of substantial reorganization of neurological and biological stress responses across the pubertal transition (Dahl & Gunnar, 2009; Forbes & Dahl, 2010), cross-system coordination may be relatively unstable until after puberty onset. Extant studies of multiple systems’ responses to date have either not directly examined developmental changes in how the systems operate in conjunction with one another or focused on a restricted age range, usually in middle to late childhood, precluding insight into the stability, or lack thereof, in patterns of ANS and HPA axis responses across development.
In order to ascertain the ways in which physiological systems are coordinated in response to stress exposure, a comprehensive, empirical investigation of basal arousal and reactivity across both branches of the ANS and the HPA axis is needed. Such an investigation must include children spanning a wide age range to evaluate whether patterns emerge consistently across development. Finally, the investigation needs to be broadly exploratory in order to capture unique interactive patterns of responses, potentially reflective of both adaptive and maladaptive forms of responding to environmental conditions.
The present study constitutes such an investigation. We examined arousal and reactivity of the ANS and the HPA axis in four separate samples of children, ranging in age from 4 to 14 years. We tested whether reliable subgroups existed within each sample that exhibited unique patterns of responses, whether those subgroups were similar across samples, and whether the subgroups differed across development, at least across childhood up to the beginning of the pubertal transition. We further examined whether family adversity variables predicted subgroup membership in meaningful ways.
In each sample, children completed standardized, stress-inducing laboratory assessment tasks while measures of autonomic and adrenocortical responses were collected. Measures included PEP, an index of sympathetic influence on the cardiac cycle (Cacioppo, Uchino, & Berntson, 1994); respiratory sinus arrhythmia (RSA), a measure of parasympathetic regulation of cardiac chronotropy (Cacioppo et al., 1994; Porges, 2007); heart rate (HR), an integrated signal reflecting multiple components of cardiac activity; and salivary cortisol, an indicator of HPA axis activation (Kirschbaum & Hellhammer, 1994). Across the studies, the precise laboratory stress tasks that children completed varied, but the same autonomic and HPA axis measures were collected, which enabled us to subject each sample’s data to comparable analyses and identify subgroups. If the groups overlapped substantially, findings would suggest important regularities in responses that may have implications for children’s mental health and adaptation.
Because such an investigation has never been conducted before, we were wary of advancing strong predictions about the anticipated subgroups. Nonetheless, on theoretical grounds, we generated several tentative hypotheses. First, insofar as the normative experience for a majority of children growing up in Western society (i.e., the children in our samples) does not include extreme stress exposure, we expected a large group of children to exhibit moderate levels of both arousal and reactivity, consistent with models of biological sensitivity to environmental demands (Boyce & Ellis, 2005; Del Giudice et al., 2011). Second, given reciprocal feedback loops among the stress-responsive systems and their components (Sapolsky et al., 2000), we anticipated that a subset of children would exhibit a pattern suggestive of coordinated reciprocity: moderate to high levels of activation of the SNS, paired with countervailing activation of the HPA axis as well as heightened activation (i.e., regulation) of the PNS (Bauer et al., 2002; Boyce & Ellis, 2005; Gordis et al., 2008). Third, a small set of children, it was hypothesized, would exhibit heightened reactivity across all systems (i.e., high activation of the SNS and the HPA axis, and high deactivation of the PNS), a pattern suggested by studies in which reactivity in multiple systems have each independently been linked to early childhood stress and suggested by Del Giudice et al. (2011) among children exposed to chronic threats in their environment. Fourth, as has been noted in some studies of physiological responses in maltreated and other high-risk samples, a pattern of underarousal, in both baseline and reactivity, was anticipated in a subset of children (El-Sheikh et al., 2008; Raine, 2002; Shirtcliff, Granger, Booth, & Johnson, 2005). Fifth, beyond our heuristic group expectations, we explored whether age differences emerged in the proportion of children in the different cohorts, speculating that perhaps a larger proportion of older children and adolescents relative to younger children would show reciprocal patterns of reactivity, given potential age differences in cross-system coordination.
Method
Peers and Wellness Study (PAWS)
Participants
A total of 324 4- to 6-year-olds involved in a longitudinal study of family social status, biological responses to adversity, and health served as participants (Obradović et al., 2011). Children were recruited from kindergarten classrooms in public schools in an urban area in Northern California. Ethnicity varied (19% African American, 11% Asian, 43% European/White, 4% Latino, 22% multiethnic, and 2% other). All parents provided written consent to participate (additional demographic details are located in Table 1).
Table 1.
Sample characteristics for each of the cohorts
| PAWS | WSFW | SMP | HMP | |
|---|---|---|---|---|
| N | 324 | 120 | 109 | 111 |
| Age (years) | M = 5.31 (SD = 0.32) | M = 7.27 (SD = 0.24) | M = 10.37 (SD = 2.60) | M = 12.35 (SD = 0.55) |
| Range = 4.75–6.28 | Range = 6.80–7.80 | Range = 7.01–15.08 | Range = 11.09–13.05 | |
| Gender (% girls) | N = 157 girls (49%) | N = 73 girls (61%) | N = 57 girls (52%) | N = 58 girls (52%) |
| Parents with college degree | 75% | 59%a | 66% | NA |
| Annual household income | Mdn = $80,000–$99,999 | Mdn = $49,000 | Mdn = $60,001–$100,000 | NA |
| Study tasks | Range = <$10,000–>$400,000 | Range = <$10,000–>$200,000 | Range = <$15,000–>$200,000 | |
| Lab challenges | 15-min stress paradigm | 15-min stress paradigm | TSST-M | 1. Star tracing/reaction time tasks |
| 2. TSST-C | ||||
| Baseline activities | Neutral activities | Neutral stories | Casual conversation | 1. Relaxing video |
| 2. Casual conversation | ||||
| Measures | ||||
| Autonomic nervous system PEP (SNS), RSA (PNS), HR |
Collected continuously during reactivity protocol and neutral activities |
Collected continuously during reactivity protocol and neutral stories |
Collected continuously during casual conversation and TSST-M |
Collected continuously during start tracing and reaction time task and video |
| HPA axis Salivary Cortisol |
Collected before and after reactivity protocol |
Collected before and after reactivity protocol |
Collected before and repeatedly after TSST-M |
Collected before and repeatedly after TSST-C |
Note: The stress paradigm included a brief social interview, the digit span, a sensation test, and watching emotional videos (Boyce et al., 2001; Quas et al., 2004). PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project; TSST, Trier Social Stress Test (Kirschbaum et al., 1993); SNS, sympathetic nervous system; PEP, preejection period; RSA, respiratory sinus arrhythmia; HR heart rate; HPA, hypothalamic-pituitary-adrenal. The TSST-M (Yim et al., 2010) required children give a speech about themselves and complete mental arithmetic. The TSST-C (Buske-Kirschbaum et al., 1997) required children answer questions about a story and complete a mental arithmetic.
For WSFW, the percentage corresponds to mothers.
Procedures and measures
Testing times were scheduled at children’s schools in the afternoons to control for diurnal changes in circulating cortisol. The stress paradigm, a reactivity protocol, was administered in the fall, after children were settled into their school year. The protocol began with children being introduced to the research assistant (RA) and equipment, the latter of which consisted of electrodes connected to an impedance cardiography system (Biopac MP150) to collect continuous autonomic data. Once these were placed on the child and he/she had adjusted to the equipment and the RA, an initial saliva sample was collected. Next, the child individually completed a standardized reactivity protocol (Boyce et al., 2001), composed of four age-appropriate, ecologically valid challenges. These included a social task, which contained questions about the child’s likes and dislikes; a cognitive task, which required the child to repeat digits read by the RA; a sensory task, for which the child tasted an unfamiliar substance; and an emotional task, which involved the child watching emotionally evocative video clips (the protocol’s development is described in full in Alkon et al., 2003). Each challenge lasted 1–3 min and was preceded by a motor activity-matched baseline activity. After the final task was complete, a second saliva sample was collected (see Table 1 for details).
The autonomic data were cleaned and edited for artifact using MINDWARE Software (Columbus, OH), and then averaged first into 1-min epochs within each task and baseline activity. From these, averages were created for each of the four challenges and four baseline activities for PEP, RSA, and HR responses, and finally, the task and baseline scores were averaged again to create one of each for PEP, RSA, and HR. The saliva samples were frozen for storage and later assayed in duplicate for cortisol using a commercial immunoassay with chemiluminescence detection (Cortisol Luminescence Immunoassay, IBL-Hamburg, Hamburg, Germany). Additional information regarding the physiological data coding, editing, and assaying can be found in Obradović, Bush, Stamperdahl, Adler, and Boyce (2010).
While children were taking part in the larger study, parents completed measures of their background and functioning. Background information included their child’s age and ethnicity, as well as their family annual income and the highest educational attainment of both parents. From the measures of income and education, a composite index of socioeconomic status (SES) was created by averaging the standardized mean of the highest level of education attained by a parent and family annual income. Parents also completed measures of their mental health, financial stress, parental stress, and marital/relationship functioning, both in the fall of children’s participation in the larger study and again in the spring (Obradović et al., 2010, 2011). For mental health, parents completed the 20-item Center for Epidemiological Studies Depression Scale (α = 0.81; Radloff, 1977). Financial stress was indexed via parents’ responses to 4 items from Essex, Boyce, et al. (2002) that asked about their feelings regarding money problems, difficulty paying bills, and limited opportunities because of lack of finances (α = 0.81). To capture parents’ own feelings of stress, their responses to 5 items about their feelings of being overwhelmed with parenting duties, juggling conflicting obligations, and lacking time to rest or pursue desired activities were averaged (α = 0.79) Finally, parents’ responses to the O’Leary-Porter Overt Hostility Scale (α = 0.72) were included as a measure of marital stress. Questions ask about such topics as how often parents openly argue, display physical and verbal hostility, and criticize each other in the presence of their children (Johnson & O’Leary, 1987; Porter & O’Leary, 1980). An overall index of chronic stress was created by averaging parents’ standardized scores on the four measures in the fall and spring. This created a single cross-time marker of chronic family adversity that could be included as a predictor of subgroup reactivity differences.
Wisconsin Study of Families and Work (WSFW)
Participants
One hundred twenty 6- and 7-year-olds who were taking part in a study of children’s reactivity and functioning participated (Boyce et al., 2006; Essex et al., 2006). The children were recruited from a large, longitudinal investigation of maternal well-being, family, work, and children’s development taking place in a university town in the Midwest (Klein, Hyde, Essex, & Clark, 1998). The sample was selected to include equal numbers of children with high (comparable internalizing and externalizing problems) and low reported symptoms based on an assessment when children were 5 years of age. Most (90%) children were Caucasian; the remaining were multiethnic (see Table 1 for further details).
Procedures and measures
The laboratory assessment protocol was highly similar to that in the PAWS study. Thus, following parental consent, children completed a protocol composed of four developmentally appropriate challenges while continuous autonomic measures were collected via an electrocardiogram. Differences in procedures between this study and PAWS included the following. In WSFW, the protocol was administered in a mobile laboratory at children’s homes. The baseline activity consisted of listening to neutral stories, before and after the protocol was complete (Table 1). Autonomic data were collected using a Minnesota Impedance Cardiograph Model 304B, and the autonomic editing software was ANSUITE (Columbus, OH). PEP, RSA, and HR task scores were created as they were in PAWS. Baseline arousal was computed from participants’ mean response while listening to the first 3-min story that was read to them. Saliva samples were collected before and after the protocol. The delay between samples was comparable to that in PAWS (20 min). Samples were then frozen for storage and later assayed for cortisol (for further details, see Boyce et al., 2006). Assays were conducted using the Pantex (Santa Monica, CA) 1251 Cortisol RIA Kit modified for saliva. All samples were assayed in duplicate, and results were considered acceptable if they achieved a coefficient of variation of <25% for cortisol concentrations of <0.0552 g/dl or <15% for concentrations of <90.0552 g/dl. Repeat assays were performed on all samples not meeting these criteria.
Children completed sessions in either the morning or the afternoon. Children who completed the protocol in the morning had cortisol levels according to the sample collected after the protocol ended, in what was considered their task response, than children who completed the protocol in the afternoon, t (117) = 2.59, p = .01. However, the two groups’ baseline Cortisol levels, as reflected in their preprotocol levels, and their cortisol reactivity (task minus baseline) scores did not vary depending on the time of day of the session, ts (116–117) < 1.28, ns. Nonetheless, to account for subtle circadian variations in circulating cortisol levels, cortisol scores were standardized separately for children in the morning and the afternoon session groups.
Stress and Memory Project (SMP)
Participants
This sample included 109 7- to 14-year-olds who completed a study of stress and memory (Quas et al., 2011). Families lived in suburban neighborhoods in Southern California and were recruited via a marketing firm specializing in obtaining diverse samples for scientific research. Children with chronic health problems, on medication, or who had anxiety of public speaking or math were excluded. Children’s ethnicity varied (8% African American, 1% Asian and Middle Eastern, 45% Caucasian, 9% Hispanic, and 36% multiethnic), as did household income and parental education (see Table 1).
Procedures and measures
Sessions took place in a laboratory between 1:30 and 5:30 p.m. and consisted of children completing a modified version of the Trier Social Stress Test (TSST-M; Yim, Quas, Cahill, & Hayakawa, 2010). The TSST (Kirschbaum, Pirke, & Hellhammer, 1993) is a widely used laboratory protocol that requires participants to complete a speech and arithmetic task in front of neutral observers. Numerous studies have found that the TSST and TSST-M induces physiological, behavioral, and self-reported arousal in children as young as 7–8 years, adolescents, and adults (Buske-Kirschbaum et al., 1997; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009).
Following parental consent, and after an initial minute acclimation period, children were introduced to the physiological equipment, which consisted of a BIONEX electrocardiograph and eight spot electrodes. These were placed on children to collect autonomic data. After a 5-min adjustment period to become comfortable with the autonomic equipment, children completed a baseline activity (engaging in a casual conversation with a familiar RA) and then provided a saliva sample (samples were collected with the Salivette sampling device, Sarstedt, Nümbrecht, Germany). Next, children were escorted into an adjacent room to complete the 15-min TSST-M, which consisted of a 2-min instruction period, a 3-min preparation period, a 5-min speech about themselves, and a 5-min math task (for details, see Yim et al., 2010). Children’s autonomic responses were recorded continuously throughout the TSST-M. Immediately after the TSST-M ended, children completed a second, identical baseline activity. Additional saliva samples were collected 1, 10, 20, 30, 45, 60, and 75 min after the end of the TSST-M. Procedure details are presented in Table 1.
Once collected, samples were frozen at −70 °C until assayed. Cortisol was determined by a commercially available enzyme immunoassay (ELISA, IBL-America, Minneapolis, MN). Samples were assayed in duplicate. Children’s cortisol levels from the initial (pre-TSST-M) time point were considered baseline scores. Five children were missing these scores, and the average of their final three samples (which did not significantly differ for others from their pre-TSST-M value) was substituted. The largest of the 10-, 20-, or 30-min posttask scores was taken as a child’s task response. Reactivity scores reflected the difference between children’s task and baseline cortisol levels.
Participants’ autonomic data were edited for artifact using MINDWARE (Columbus, OH). PEP, RSA, and HR were computed in 1-min epochs. Then, averages were created for the first baseline activity to index baseline arousal and the combined speech and math tasks to index their task response. One child’s PEP baseline score was missing. The child’s post-TSST-M baseline score was substituted. Participants’ baseline values were subtracted from their task responses to obtain reactivity scores.
Hearts and Minds Project (HMP)
Participants
This sample included 111 11- and 12-year-olds in an urban public school in northwestern Canada whose parents consented to a science study on stress regulation. Ethnicity and family income were not available because data were collected exclusively from children while at school, although given the demographics of the region where the study was conducted, a majority was presumed to be Caucasian.
Procedures and measures
Children completed two afternoon sessions (delay = 2 weeks) in a mobile laboratory. In Session 1, children were affixed with electrodes and connected to a BIOPAC MP150 electrocardiograph to obtain autonomic data. Then they watched a 5-min relaxing video (baseline activity), which was followed by a tracing (tracing an image in a mirror; buzzer sounded when children erred) and reaction time (pressing a button when distinct sounds are heard among many) task, widely used procedures to induce autonomic responses in children (El-Sheikh et al., 2008). Children’s autonomic responses were extracted in 1-min epochs and then edited using Mindware Software (Columbus, OH). The average of their responses across the two challenging tasks and the average of their responses across the baseline activity were then computed (see Table 1).
In Session 2, children completed the TSST—Child (TSST-C; Buske-Kirschbaum et al., 1997), which required they finish a story and complete mental arithmetic in front of one observer. Saliva samples were collected using a dental role (Sullivan Dental Products, St. Laurent, Quebec) before the TSST-C began (baseline) and 10 and 25 min after the TSST-C ended. Samples were frozen at −20 °C for storage and later assayed for quantitative cortisol using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LCC, Philadelphia, PA) at the University of British Columbia Weinberg Laboratory. Intra- and interassay coefficients of variation were 2.92% and 3.41%, respectively. The higher of the 10- and 25-min sample values served as the child’s task response.
Results
Main measures and analytic plan
In each study, all children completed laboratory challenges and baseline activities while measures of ANS (SNS and PNS) and HPA axis responses were collected. Table 1 provides for a more direct comparison, across samples, of the procedures, measures, and demographic characteristics. As is evident, the precise challenges and baseline activities varied across studies, as did children’s ages. In addition, as noted above, the hardware and software used to collect, edit, and assay the physiological data varied. These variations can affect the raw physiological scores and the magnitude of children’s responses and can lead to differences in cross-system response tendencies that are due to idiosyncratic variations across samples. Laboratory environments have been shown to act as a confound in psychobiological and biomarker research (Crabbe, Wahlsten, & Dudek, 1999; Lewejohann et al., 2006). Thus, it is inappropriate to collapse all children into a single cohort and compare responses directly in a single analysis. Instead, because all studies collected identical measures of PEP, RSA, HR, and cortisol during laboratory challenges and during baseline activities, we were able to use an identical statistical approach with each sample and detect potentially important and meaningful cross-system response tendencies in a clean and unconfounded manner. We were then able to visually compare the patterns in each sample to identify patterns of similarities and differences across them in response tendencies.
For each child in each sample, eight standardized physiological scores were calculated: four baseline arousal scores and four reactivity scores, computed for PEP, RSA, HR, and Cortisol. PEP and RSA scores were further reversed so that higher scores on each are indicative of greater arousal. For the three autonomic measures, data were normally distributed, with no evidence of skewness or kurtosis. Cortisol was skewed. Baseline and reactivity scores were thus log transformed prior to their inclusion in the analyses. Finally, some scores were extremely high or low (e.g., standardized scores fell >3 SD above or <−3 SD below the mean) but were still biologically plausible, while a smaller number of scores fell into a range of being no longer physiologically possible (>5 or <−5 SD). The latter scores, which occurred less than .1 % of the time, were excluded from the analyses. By including plausible outliers, we were able to determine whether important but very small subsets of children existed who exhibited extreme patterns of response tendencies.
Scores in each sample were entered into latent profile analysis (LPA), a form of finite mixture modeling that identifies potential unobserved subgroups among a set of indicators (for a review, see Muthén, 2008). Models were fitted within a structural equation framework, and missing data were estimated using full information maximum likelihood using Mplus 6.11 (http://www.statmodel.com). Four fit indices were then evaluated for each cohort. These included Akaike information criterion (AIC) and Bayesian information criterion (BIC), statistical information criteria for which decreasing values indicate better fit (the number of classes is determined when values no longer decrease); entropy, a measure indicating average classification accuracy when assigning participants to classes (values closer to 1 indicate greater precision); and the bootstrap-likelihood ratio test (BLRT), which compares class enumeration between K and K–1 classes (p < .05 suggests that K classes fit the data better than K–1; p > .05 reveals that neither K nor K–1 classes is necessarily better). With psychological data, it is rare for different fit indices to converge on a single, best fitting model (Nagin & Odgers, 2010). Thus, as is often done in psychological research, including studies of physiological response tendencies (Del Giudice et al., 2012), we evaluated the combination of the four fit indices (see Table 2), in conjunction with the sub-group profile plots, to identify the best models and most likely number of classes or subgroups in each data set. When the indices were not uniform in suggesting a particular fit, we relied most heavily on BIC and BLRT, given evidence from simulation studies suggesting that these indices are more stable that other indices with smaller data sets (see Nagin & Odgers, 2010; Nylund, Asparouhov, & Muthén, 2007, for a discussion of fit indices).
Table 2.
Latent profile analyses results
| Classes | Log Likelihood | AIC | BIC | Entropy | BLRT |
|---|---|---|---|---|---|
| PAWS | |||||
| 1 | −3344.53 | 6721.05 | 6781.55 | ||
| 2 | −3340.77 | 6585.89 | 6680.41 | 0.61 | p < .001 |
| 3 | −3310.22 | 6531.49 | 6660.033 | 0.76 | p < .001 |
| 4 | −3306.85 | 6488.81 | 6651.38 | 0.76 | p < .001 |
| 5 | −3296.34 | 6449.84 | 6646.44 | 0.79 | p < .001 |
| 6 | −3267.95 | 6434.18 | 6664.09 | 0.80 | p < .001 |
| 7 | −3231.74 | 6414.26 | 6678.92 | 0.75 | NA |
| WSFW | |||||
| 1 | −1248.65 | 2529.30 | 2573.90 | ||
| 2 | −1248.65 | 2469.87 | 2539.55 | 0.67 | p < .001 |
| 3 | −1218.18 | 2404.73 | 2499.01 | 0.82 | p < .001 |
| 4 | −1184.72 | 2371.71 | 2491.57 | 0.79 | NA |
| SMP | |||||
| 1 | −1162.34 | 2356.70 | 2399.97 | ||
| 2 | −1128.99 | 2305.99 | 2373.76 | 0.91 | p < .001 |
| 3 | −1106.90 | 2262.39 | 2353.46 | 0.92 | p < .001 |
| 4 | −1092.48 | 2246.85 | 2362.57 | 0.93 | p < .001 |
| 5 | −1077.72 | 2231.47 | 2371.42 | 0.90 | p < .087 |
| HMP | |||||
| 1 | −1179.38 | 2390.75 | 2434.10 | ||
| 2 | −1147.77 | 2345.55 | 2413.29 | 0.65 | p < .001 |
| 3 | −1126.26 | 2320.52 | 2412.64 | 0.72 | p < .001 |
| 4 | −1105.77 | 2297.53 | 2414.04 | 0.86 | p < .001 |
| 5 | −1152.47 | 2269.70 | 2410.53 | 0.88 | p < .001 |
| 6 | −1179.38 | 2258.14 | 2423.42 | 0.90 | NA |
Note: The best fitting models across all fit indices are in bold. PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project; AIC, Akaike information criterion; BIC, Bayesian information criterion. On both criteria, lower values indicate better fit. Entropy refers to the average classification accuracy when assigning participants to trajectory classes (values closer to 1 indicate greater precision). BLRT, bootstrap likelihood ratio test. The BLRT compares the classification between K and K-l classes; values <.05 indicate that K classifications fit the data better than K-l classes, and values >.05 indicate that K classes are not necessarily better. NA, models for which the likelihood ratios could not be replicated, so the BLRT results are not trustworthy.
Raw data (baseline arousal and reactivity) for each emergent subgroup are presented in Table 3. Analyses of variance (ANOVAs) were conducted within each study to identify significant differences across subgroups. Groups with too few members were not included. ANOVAs and chi-squared analyses further tested whether subgroups differed in age, gender, and SES. Table 4 shows the subgroups’ age and gender distribution. Finally, for the largest cohort (PAWS), ANOVAs investigated whether the subgroups could be distinguished based on family adversity.
Table 3.
Mean scores for the eight indicators for each subgroup identified via latent profile analyses
| Baseline Arousal Raw Scores |
Reactivity (Difference) Scores |
|||||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | PEP | RSA | HR | Cortisol (log) | PEP | RSA | HR | Cortisol (log) |
| PAWS | ||||||||
| Moderate reactivity | 77.46a | 6.44a | 100.05a | 0.66a | 0.02a | 0.08a | 1.36a | −0.12a |
| Parasympathetic-specific reactivity | 80.43b | 7.88b | 88.13b | 0.65a | −0.36b | −0.11b | 2.85b | −0.04b |
| Anticipatory arousal | 76.73a | 4.90c | 116.73c | 0.81b | 0.34a | 0.38c | 0.45c | −0.01bc |
| Multisystem reactivity | 78.38ab | 6.72ad | 103.05d | 0.31c | −1.23c | −0.09ab | 1.16ac | 0.48d |
| HPA-specific reactivity | 81.64b | 7.03d | 96.09e | 0.49d | 0.33a | 1.08a | 1.84a | 0.07c |
| Underaroused | 79.54ab | 8.82e | 79.44f | 0.63abd | 4.32d | −0.14ab | 4.44d | −0.02abc |
| WSFW | ||||||||
| Moderate reactivity | 84.01a | 5.40a | 97.22a | −1.00a | 1.63a | 0.15a | −0.45a | 0.08a |
| Parasympathetic-specific reactivity | 80.92b | 6.91b | 85.32b | −0.93a | 0.72b | −0.14b | 0.96b | 0.07a |
| Anticipatory arousal | 79.75ab | 3.19c | 113.18c | −0.42b | 0.15ab | 1.02c | −2.01a | −0.21b |
| Multisystem reactivity | — | — | — | — | — | — | — | — |
| HPA-specific reactivity | — | — | — | — | — | — | — | — |
| Underaroused | — | — | — | — | — | — | — | — |
| SMP | ||||||||
| Moderate reactivity | 108.82 | 6.01a | 95.48a | 0.62a | 0.96a | 0.30a | 1.67a | 0.21a |
| Parasympathetic-specific reactivity | 110.12 | 5.26 | 105.37 | 0.43 | −1.20 | −1.38 | 30.08 | 0.89 |
| Anticipatory arousal | — | — | — | — | — | — | — | — |
| Multisystem reactivity | 118.18 | 6.52b | 85.02b | 0.47a | −7.49b | −0.16b | 12.96b | 0.53b |
| HPA-specific reactivity | 101.04 | 5.80a | 99.06a | −0.51b | 4.74a | 0.35a | 0.86c | 1.07c |
| Underaroused | — | — | — | — | — | — | — | — |
| HMP | ||||||||
| Moderate reactivity | 84.52 | 6.15a | 85.39a | −0.93 | −1.25a | −0.42a | 1.39a | 0.19a |
| Parasympathetic-specific reactivity | 84.06 | 7.58b | 76.68b | −0.94 | −2.36a | −1.69b | 8.01b | 0.22a |
| Anticipatory arousal | 82.70 | 4.75c | 99.53c | −1.01 | −2.46a | 0.75c | −4.93c | 0.39b |
| Multisystem reactivity | 84.69 | 5.58d | 92.59d | −0.88 | −8.89b | −1.15d | 10.74d | 0.42b |
| HPA-specific reactivity | — | — | — | — | — | — | — | |
| Underaroused | 84.12 | 6.69 | 66.28 | −0.77 | −7.17 | 0.64 | 31.62 | −0.12 |
Note: Raw scores are reported for all variables. PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project; HPA, hypothalamic-pituitary-adrenal. For preejection period (PEP) and respiratory sinus arthythmia (RSA), larger values correspond to greater sympathetically or parasympatheticalty induced arousal, respectively. HR, Heart rate. Cortisol is reported in nanomoles per liter (nmol/1), log transformed. Analyses of variance compared group means within each sample. For significant main effects (all Fs < 3.63, ps < .01), different subscript letters correspond to groups that differed significantly (subgroups with small ns were not included) according to post hoc comparisons (ps < .05).
Table 4.
Characteristics of the subgroups
| N | Mean Posterior Prob. (Range) | Number of Girls | Mean Age (SD) | |
|---|---|---|---|---|
| PAWS | ||||
| Moderate reactivity | 180 (52%) | 0.86 (0.44–0.99) | 89 (49%) | 5.31 (0.32) |
| Parasympathetic-specific reactivity | 79 (24%) | 0.86 (0.42–1.00) | 32 (41%) | 5.31 (0.31) |
| Anticipatory arousal | 19 (7%) | 0.90 (0.51–1.00) | 13 (68%) | 5.14 (0.31) |
| Multisystem reactivity | 22 (7%) | 0.88 (0.45–1.00) | 13 (59%) | 5.36 (0.34) |
| HPA-specific reactivity | 18 (6%) | 0.80 (0.46–0.98) | 8 (44%) | 5.24 (0.28) |
| Underaroused | 6 (2.0%) | 0.96 (0.80–1.00) | 2 (33%) | 5.48 (0.34) |
| WSFW | ||||
| Moderate reactivity | 72 (60%) | 0.90 (0.52–1.00) | 44 (61%) | 7.27 (0.24) |
| Parasympathetic-specific reactivity | 43 (36%) | 0.91 (0.54–1.00) | 28 (65%) | 7.28 (0.25) |
| Anticipatory arousal | 5 (4%) | 0.91 (0.57–1.00) | 1 (20%) | 7.15 (0.25) |
| Multisystem reactivity | — | — | — | — |
| HPA-specific reactivity | — | — | — | — |
| Underaroused | — | — | — | — |
| SMP | ||||
| Moderate reactivity | 87 (80%) | 0.96 (0.51–1.00) | 45 (52%) | 10.46 (2.58)a |
| Parasympathetic-specific reactivity | 2 (2%) | 1.00 (NA) | 1 (50%) | 12.52 (0.80) |
| Anticipatory arousal | — | — | — | — |
| Multisystem reactivity | 12 (11%) | 0.94 (0.57–1.00) | 7 (58%) | 12.43 (2.30)b |
| HPA-specific reactivity | 8 (7%) | 0.98 (0.91–1.00) | 4 (50%) | 9.45 (2.36)a |
| Underaroused | — | — | — | — |
| HMP | ||||
| Moderate reactivity | 67 (60%) | 0.94 (0.53–1.00) | 35 (52%) | 12.42 (0.55) |
| Parasympathetic-specific reactivity | 18 (16%) | 0.93 (0.58–1.00) | 7 (39%) | 12.27 (0.56) |
| Anticipatory arousal | 10 (9%) | 0.92 (0.76–1.00) | 5 (50%) | 12.11 (0.56) |
| Multisystem reactivity | 15 (14%) | 0.89 (0.54–1.00) | 10 (67%) | 12.24 (0.53) |
| HPA-specific reactivity | — | — | — | — |
| Underaroused | 1 (1%) | 1.00 (NA) | 1 (100%) | 13.10 (NA) |
Note: PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project; HPA, hypothalamic–pituitary–adrenal; Posterior Prob., the mean probability of children assigned to each subgroup actually belonging to that subgroup. HPA, For SMP, subgroups significantly differed in age, F (2,104) = 4.10, p = .019. Different subscripts denote significant age differences according to post hoc tests (ps ≤ .05).
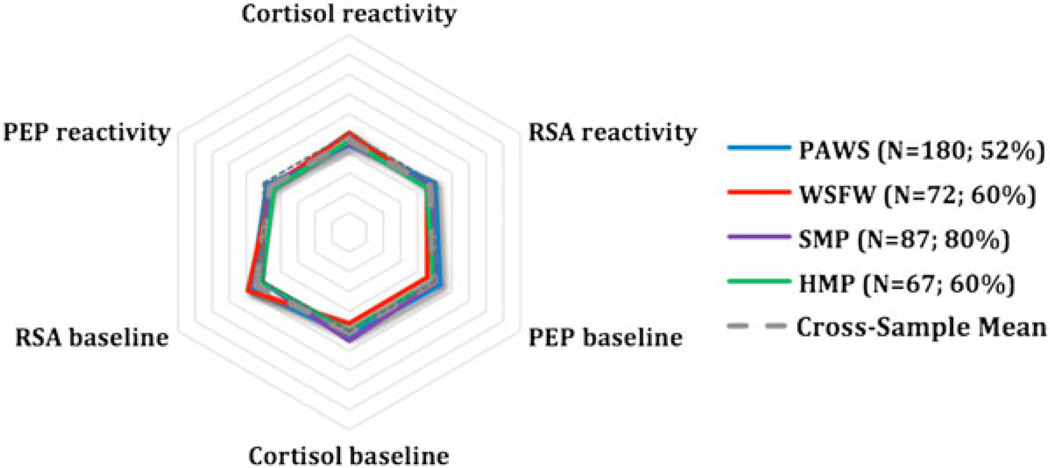
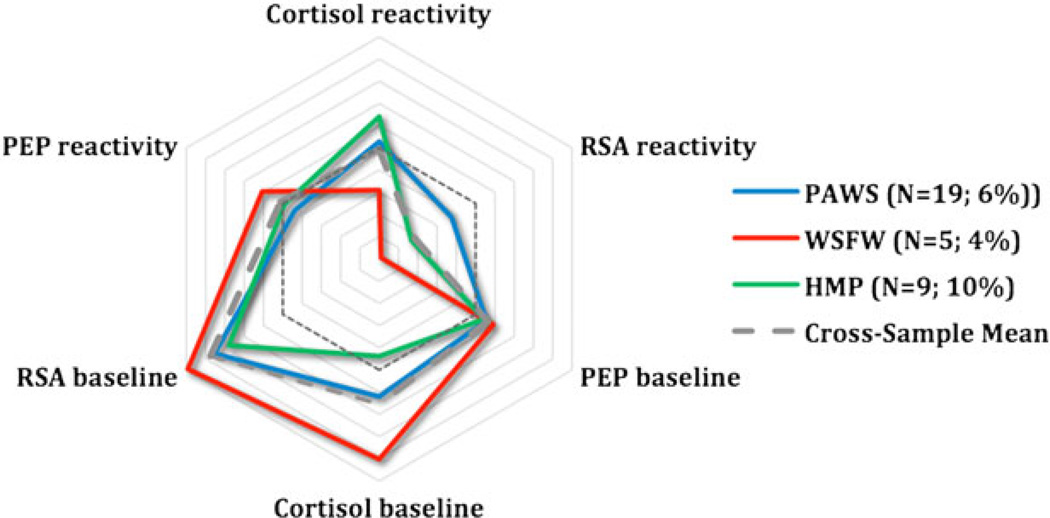
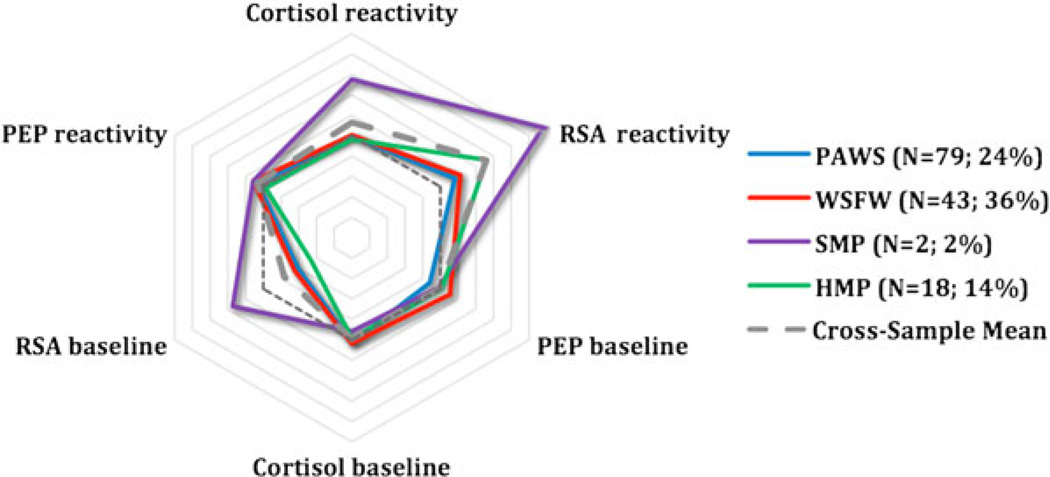
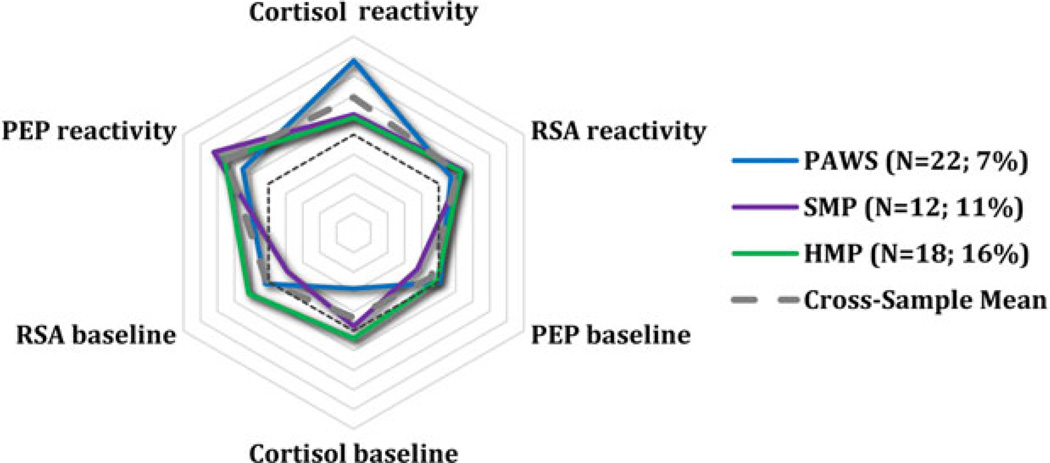
To provide a visual representation of group trends, the standardized scores on three main parameters (PEP, RSA, and cortisol) were plotted in a radial format for each identified subgroup across the four samples. PEP and RSA scores were reversed so that, for all scores, larger values correspond to greater arousal or reactivity. This visual representation reveals the often remarkable degree of consistency in arousal and reactivity of the subgroups across cohorts. The studies are depicted in different colors, with the average across studies shown in gray. The reference line (dashed), which reflects 0 scores for all parameters, is also shown. HR was not plotted because its inclusion did not further differentiate the cohorts and because HR scores were strongly collinear with RSA scores.
PAWS
LPA
The PAWS sample was the largest, most diverse group. As such, it represented the ideal sample on which to carry out the initial LPA analyses and evaluate whether subgroups reliably emerged from the different physiological markers of arousal and reactivity. Results of LPA are presented in Table 2. When all of the fit indices were evaluated concurrently, it was determined that the six-class model most closely fit the data. BIC values ceased to decrease once the number of classes increased to five, though entropy was slightly higher for the six-class solution. The fourth index, BLRT, was significant when comparing five to six classes, but also six to seven classes. However, the model was less stable with seven classes, and because of the convergence across indices at the six class solution, this was determined to best represent the data.
The largest percentage of children showed small to moderate responses across systems, at baseline and in response to laboratory stressors (Table 4), a moderately reactive group, as predicted. These children responded to the laboratory assessment, but their responses were not especially large, as suggested by their scores falling close to the mean across physiological markers at baseline and to the assessment tasks. A second and also sizable group of children exhibited a pattern of what could be called parasympathetic-specific reactivity. These children evidenced exaggerated reactivity across several measures, most notably in terms of parasympathetic deactivation (i.e., increased arousal) during the assessment tasks relative to their baseline arousal. Post hoc comparisons of RSA scores (Table 3) did confirm that this group differed markedly from the others in level of parasympathetic deactivation during the tasks.
The remaining subgroups identified in the model were substantially smaller: 6.5% appeared highly aroused across systems at baseline and reacted only minimally to the stressor itself, a pattern suggestive of anticipatory arousal; 7.2% exhibited large responses to the laboratory task across all parameters (sympathetic, parasympathetic, and HPA axis) and hence were considered multisystem reactive; 5.9% responded most vigorously in terms of HPA axis reactivity and were labeled HPA-specific reactivity; and 2% appeared minimally responsive across systems in terms of baseline arousal and stress reactivity, that is, the children seemed to be under-aroused.
Posterior probabilities were examined to evaluate the appropriateness of the six-class solution. These probability values reflect how well each child fit his or her assigned subgroup; a heuristic cutoff of 0.80 is often used to infer that individuals fit their subgroup well (e.g., Kudel et al., 2006). Probability means ranged from 0.80 to 0.96 across subgroups, indicating a high degree of certainty of classification of individual children into their respective groups (Table 4).
Predictors of group membership
Subgroups were compared in demographic features of gender, age, and family SES and in family adversity. The groups did not differ significantly in age, although the range was fairly restricted. Nor were boys and girls differentially distributed across groups (see Table 4 for age and gender distribution across subgroups). However, the subgroups did differ in SES, calculated as the mean of standardized scores based on parents’ highest education level and annual income, F (5, 284) = 2.56, p = .027. Children in the underaroused subgroup came from substantially lower SES families (M = −1.31) than children in the other subgroups (post hoc tests ps ≤ .01; moderate reactivity M = −0.01, parasympathetic-specific reactivity M = 0.10, anticipatory arousal = −0.12, multisystem reactivity M = 0.30, and HPA-specific reactivity, M = −0.07). A one-way group ANOVA conducted predicting the adversity index also revealed group differences, F (4, 223) = 2.48, p = .05. The adversity index was calculated as a composite standardized score across measures of parental well-being, income stress, parenting stress, and marital conflict over time. Children in the underaroused group were excluded from this analysis because of missing data from a few participants (given the group’s already small size, even a few missing measures in the adversity index affected the ability to include the group in analyses). Post hoc tests indicated that children classified as multisystem reactive (M = 0.44) had higher adversity scores than children classified as moderately reactive (M = 0.03), parasympathetic-specific reactive (M = −0.11), anticipatory aroused (M = −0.17), and HPA-specific reactive (M = −0.23, ps < .05). Together, these analyses indicate that both underarousal and high levels of cross-system reactivity to laboratory stressors may well be linked to a history of family stress, as reflected in SES or chronic family adversity.
Converging evidence: Profiles in WSFW, SMP, and HMP
To assess whether meaningful subgroups emerged in the three smaller data sets, we subjected each of the cohorts’ physiological data to LPA. Again, because of differences in the precise procedures, ages, and methods used to collect the physiological data, it was not appropriate to collapse the cohorts into a single data set. For each cohort, we selected the number of classes based on the fit indices for that cohort. By not forcing each of the data sets to conform to the same number of groups that emerged in PAWS, we were able independently to evaluate whether similar subgroups existed in the three separate cohorts. For each one, we examined the posterior probabilities and conducted post hoc analyses to test for differences in the subgroups’ demographic characteristics. Finally, we plotted the groups in radial format, along with those from PAWS, to provide a visual comparison of pattern overlap across cohorts.
When the WSFW data were considered, a three-class solution was selected as the best fitting model (Table 2). Entropy and BLRT both suggested that the three-class model represented the best fit: entropy was highest and BLRT was significant. AIC and BIC decreased from two to three classes, but also from three to four classes, indicating that the four-class model could also fit. However, the BLRT results were no longer replicating with four classes, and the three-class model was thus selected. Moreover, the three groups bore a remarkable similarity to those in PAWS: 60% evidenced a moderately reactive pattern of responses; 36% displayed a pattern of parasympathetic-specific reactivity, and 4% displayed high levels of anticipatory arousal (see Table 4). Mean posterior probabilities for the three groups were also quite high, ranging from 0.90 to 0.91 (Table 4), suggesting children fit quite well into their respective subgroups.
The consistency of the patterns between PAWS and WSFW is also immediately and visibly evident in Figures 1–3, which show the subgroups response tendencies across the samples. Post hoc analyses comparing the groups across demographic characteristics revealed no age, gender, or SES differences (the latter calculated as the composite of standardized scores for parental income and education). However, SES variability was low in this cohort relative to PAWS.
Figure 1.
Moderate reactivity. PEP, preejection period; RSA, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project.
Figure 3.
Anticipatory arousal. PEP, preejection period; RSA, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; HMP, Hearts and Minds Project.
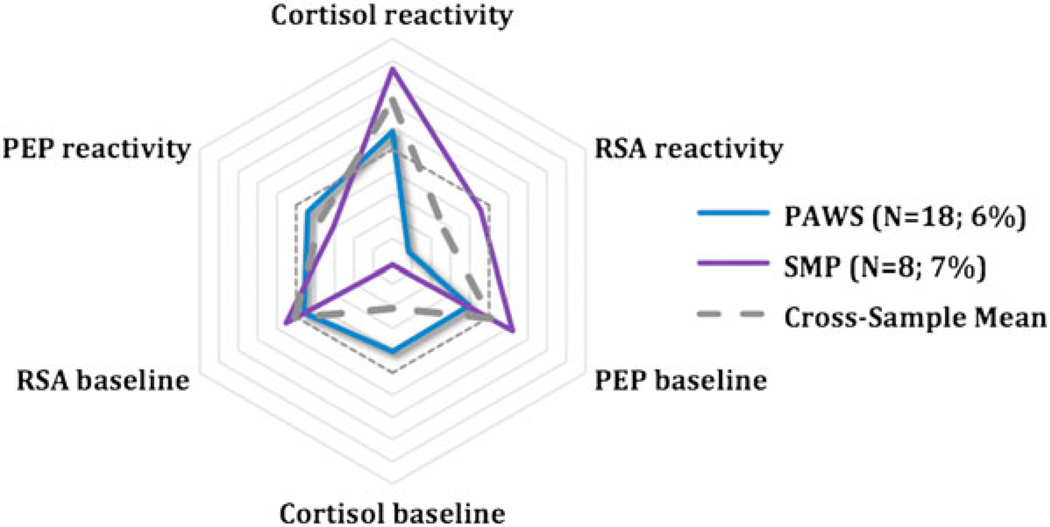
Next, data from the SMP were examined. The four-class model best fit the data. The BLRT indicated a four-class model was significantly better than a three-class model. Classification accuracy, according to entropy, was highest, and AIC values ceased decreasing at four classes (Table 2). BIC continued to decrease until five classes, but the BLRT was no longer significant. Posterior probabilities, which ranged from 0.94 to 1.0 (Table 4), confirmed that the four subgroups captured individual children’s response proclivities quite well.
The largest percentage of children (80%) fell into the moderately reactive group, similar to the prior two cohorts (see Figure 1). Smaller percentages were classified as parasympathetic-specific reactive (2%), multisystem reactive (12%), and HPA-specific reactivity, all classifications that overlapped substantially with PAWS, as is evident in Figures 2, 4, and 5. When we compared the moderately reactive, HPA-specific reactive, and multisystem reactive subgroups in demographic features (the parasympathetic-specific reactivity subgroup was not included because of its small sample size), no differences emerged in gender or SES. However, the groups differed in age (Table 4). This is perhaps not surprising, because the age range in the study (7–14 years) was sizably larger than that in the other studies. According to post hoc tests, children in the multisystem reactivity subgroup were significantly older than were children in the moderately reactive and HPA-specific reactivity subgroups, the latter of whom were the youngest age group. Children in the parasympathetic-specific reactivity group also seemed to be slightly older.
Figure 2.
Parasympathetic-specific reactivity. PEP, preejection period; RS A, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; WSFW, Wisconsin Study of Families and Work; SMP, Stress and Memory Project; HMP, Hearts and Minds Project.
Figure 4.
Multisystem reactivity. PEP, preejection period; RSA, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; SMP, Stress and Memory Project; HMP, Hearts and Minds Project.
Figure 5.
Hypothalamic-pituitary-adrenal (HPA) specific reactivity. PEP, preejection period; RSA, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; SMP, Stress and Memory Project.
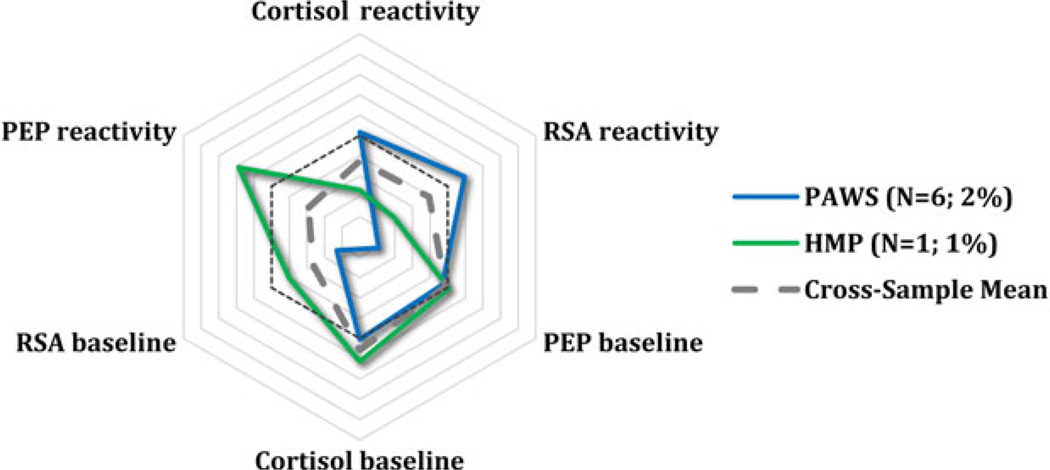
Finally, the HMP sample was examined. A five-class model was selected as the best fitting model. Classification accuracy was highest, both the AIC and BIC values ceased to decrease after five classes, and BLRT was significant (Table 2). Two subgroups overlapped with those of all the other cohorts: 60% displayed moderate reactivity and 16% displayed parasympathetic-specific reactivity. In addition, 9% displayed a pattern tending toward anticipatory arousal (similar to PAWS and WSFW; Figure 3) and 14% displayed a pattern consistent with multisystem reactivity subgroup seen in the PAWS and SMP cohorts (Figure 4). Only one participant was classified separately from these. She seemed to exhibit particularly low cortisol and RSA arousal and reactivity, similar to the underaroused children in PAWS (Figure 6), but she also had especially high heart rate reactivity. Thus, while we have labeled her underaroused, we are tentative in this classification. Posterior probabilities means ranged from 0.89 to 1.0, indicating that each child fit their group quite well. Finally, post hoc analyses revealed no gender or age differences across subgroups. SES data were not available.
Figure 6.
Underaroused. PEP, preejection period; RSA, respiratory sinus arrhythmia; PAWS, Peers and Wellness Study; HMP, Hearts and Minds Project.
Discussion
Although it is well established that biological stress reactivity plays a role in conferring increased risk for poor health and maladaptive outcomes across the life span, empirical findings testing precisely how reactivity leads to risk remain mixed. Some studies have found that additive linear associations and two-way interactions between different markers of physiological reactivity predict negative outcomes, whereas others report that low reactivity is associated with elevated risk or that baseline levels and not reactivity per se predict risk (El Sheikh et al., 2009; Obradović, 2012; Salomon et al., 2000; Weiner, 1992). A primary reason for the varied findings may be that empirical research has paid minimal attention to responses across neurobiological systems, including whether differential patterns exist in the ways in which the systems’ responses are coordinated when exposed to challenge. We took a novel approach in our efforts to advance understanding, in a significant and meaningful manner, of cross-system patterns of stress reactivity in childhood. We specifically applied LPA to biological data collected from multiple systems, both at baseline and in response to well-established laboratory challenges across four separate samples of children, ranging from 4.75 to slightly over 15 years of age.
What is perhaps most remarkable is the emergence of several meaningful subgroups of children in each sample, subgroups that overlapped in compelling manners across the studies. In the largest cohort, the largest number of subgroups emerged. However, virtually all of these subgroups were replicated in at least one of the other cohorts. The subgroups exhibited unique patterns in critical stress-responsive neurobiological circuits, were visibly consistent across studies, and differed in meaningful ways across demographic and experiential characteristics. Had only one system, simple linear interactions, or only markers of reactivity been examined, the evident patterns would not have been detected.
That the response constellations were visibly apparent and at times proportional across samples is even more impressive given significant differences in the samples’ ages and ethnic distribution, family SES, parents’ educational attainment, the location where each sample was collected, and in the types of laboratory stressors completed and the hardware and software used to collect the data. The most common pattern was the predicted moderately reactive subgroup (see Figure 1). None of the stress-responsive systems showed hyper- or hyporesponsivity in anticipation of or during the challenges, reflecting considerable intersystem balance and a potentially adaptive integration of biological responsivity when faced with challenge (Glassman, 1973). Of note, it is not that these children failed to exhibit a response, as is evidenced by their mean reactivity scores presented in Table 3. Instead, their responses were modulated and well coordinated, a type of buffered pattern (Del Giudice et al., 2011) that may be acquired over time when children are raised in environments characterized by low levels of familial conflict, stressors, or challenges (Boyce & Ellis, 2005; Obradović, 2012). The moderately reactive subgroup was not only sizable but also evident in all samples, and may be indicative of what could be characterized as an age-appropriate response proclivity among typically developing Western samples of children, at least samples that have not endured extreme trauma or deprivation. The size and existence of the moderately reactive groups may also be, in part, a function of the statistical approach, which involved standardizing scores, resulting ipso facto in large number of children being assigned to this group.
A pattern suggestive of parasympathetic-specific reactivity, although not directly predicted, was also evident in all samples, with between 2% and 36% of the children being classified as such (Figure 2). These children were reactive to the laboratory challenges, though nearly exclusively through deactivation (withdrawal) of parasympathetic cardiac chronotropy. Because PNS deactivation is often more easily evoked in laboratory stress paradigms than are SNS and HPA axis reactivity, variations in parasympathetic responsivity may be an especially sensitive indicator of predisposition to arousal and reactivity in childhood. This predisposition, which is similar to Del Giudice et al.’s (2011) adaptive calibration model’s “sensitive” pattern, could result from children being raised in low stress environments who hence become calibrated to respond easily and quickly to environmental input. The ability to mount an appropriate response, largely via parasympathetic withdrawal, to mild environmental demands has been linked to enhanced attention and sociability (Doussard-Roosevelt, Montgomery, & Porges, 2003; Porges, 2007). At the same time, though, excessive parasympathetic deactivation (i.e., withdrawal) has also been linked to poor emotion regulation, internalizing symptoms, and low levels of social functioning (Beauchaine, Gatzke-Kopp, & Mead, 2007). Understanding the conditions under which parasympathetic reactivity emerges in the absence of systematic variations in arousal and reactivity in other stress-responsive systems as well as the consequences of heightened reactivity of only this specific system are both critical areas that need to be explored in subsequent work.
In three of the samples, a pattern of anticipatory arousal was evident in between 4% and 9% of the children, see Figure 3. Multiple systems were activated before the laboratory challenges commenced, and responses to the actual challenges were somewhat blunted. Mounting an anticipatory response could be seen as adaptive, insofar as it reflects a preparatory response learned over time, although it may also be interpreted as maladaptive because it demonstrates poor regulatory capacity or chronic and persistent dysregulation (Boyce et al., 2001), perhaps as a result of an accrual of allostatic load (McEwen, 1998; Sapolsky et al., 2000). Of note, in the PAWS cohort, children in the anticipatory arousal subgroup were not from families with high levels of stress or adversity, raising important questions as to the function of unprovoked multisystem arousal, particularly when coupled with blunted reactivity, in children whose response tendencies are still stabilizing.
Emerging in three cohorts was also a tendency toward exaggerated reactivity across the biological measures, that is, multisystem reactivity, including 7%-14% of the children (Figure 4). We had predicted such a group would emerge and be reflective of heightened vigilance among children who have grown up amid chronic threats in their environment (Del Giudice et al., 2011). In support of such a possibility, in PAWS, the multisystem reactive children came from families with higher scores on the composite adversity index, a pattern that parallels prior research revealing links between early life adversity and physiological dysregulation (Cicchetti & Rogosch, 2001; Natsuaki et al., 2009; Obradović et al., 2010). What we did not identify, though, was a separate group of children that exhibited a vigorous SNS response, for instance, followed by HPA axis activation, which we had anticipated would be reflective of a sophisticated form of cross-system reciprocity or a high degree of regulation in the face of challenge (Bauer et al., 2002; Roozendaal, Hahn, Nathan, Dominique, & McGaugh, 2004; Sapolsky et al., 2000). Instead, our multisystem reactivity subgroup exhibited heightened responses in both the SNS and the PNS, as well as the HPA axis.
Despite substantial overlap in some emergent patterns, important differences also emerged. In two cohorts, smaller, unique subgroups were also evident. An HPA-axis specific reactivity subgroup emerged in PAWS and SMP with 6% and 14% of children, respectively. This group was most notable in SMP, with these children exhibiting robust HPA axis responses to the laboratory challenge, concurrent with an almost dampened response in both branches of the ANS (Figure 5). Of interest, in SMP, these children tended to be younger in age than children in that sample who exhibited heightened parasympathetic reactivity or reactivity across systems. Developmental changes are quite robust in terms of children’s understanding of social evaluation, perceptions of threat and embarrassment, ability to cope with threat or challenge, and biological reactivity to stressors (Alkon et al., 2011; Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001; Gunnar, Wewerka, et al., 2009; Ruble, Boggiano, Feldman, & Loebl, 1980). These certainly could have contributed to early exaggerated responses within one system, as well as a gradual developmental shift to more global reaction tendencies. Hints of this pattern were also evident in PAWS, though, suggesting that other developmental processes may also have been at play.
Finally, in PAWs, the largest and youngest cohort included in the current study, a very small percentage of children, 2%, was nonresponsive or underaroused, both at baseline and in response to laboratory stressors (Figure 6). One child in HMP may also have exhibited this pattern. In PAWS, the underaroused children had significantly lower family SES than did the other subgroups. Perhaps these children had encountered a larger range of other potentially stressful experiences, rendering the laboratory assessment innocuous or unchallenging by comparison. Alternatively, exposure to mild chronic stress, which often accompanies low SES, may already be compromising these children’s general response tendencies. Del Giudice et al. (2012) identified a much larger percentage of underaroused children in their sample. However, HPA axis activation was not also examined. Thus, it is not known as to how many of the children would have remained classified as underaroused across all parameters. Overall, the prevalence and meaning of these smaller but quite interesting subgroups need to be determined.
Although comparable physiological measures were collected in each sample, the laboratory assessment procedures themselves varied and may have affected children’s responses and the emergent subgroups. Children in the SMP and HMP, for instance, completed TSST-like procedures, requiring that they give a speech and solve math problems in front of neutral observers. The TSST is a widely used and particularly effective protocol to induce high levels of HPA activation in older children, adolescents, and young adults (Dickerson & Kemeny, 2004; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004; Yim et al., 2010). The laboratory assessment protocol in the PAWS and WSFW cohorts, in contrast, might be milder, in that children simply watched emotionally evocative videoclips, repeated numbers, answered questions about themselves, and tasted an unfamiliar substance (Alkon et al., 2003; Quas, Carrick, Alkon, Goldstein, & Boyce, 2006). However, it is not clear that administering the TSST-M, or a similar type of procedure, would have led to a comparable experience across age. Younger children have a more limited understanding of social evaluation, the primary feature in the TSST that is believed to underlie its effectiveness at inducing arousal (Dickerson & Kemeny, 2004), and hence may not react as robustly, at least until the ages when social evaluation becomes more important, such as across the pubertal transition (Gunnar, Wewerka, et al., 2009; Yim et al., 2010). Instead, and insofar as younger children tend to rely more on others to facilitate coping with novel, emotionally arousing experiences (Altshuler & Ruble, 1989; Zimmer-Gembeck & Skinner, 2011), emotionally evocative videos and social interactions may be sufficient to induce substantial reactivity in younger children. Overall, what the different laboratory assessment protocols mean to children and how they affect the different systems’ responsiveness need to be determined in order to evaluate, in a comprehensive manner, patterns of stress reactivity in the ANS and the HPA axis across childhood.
Beyond discussing the meaning and implications of the similarities and differences in the patterns of responses that emerged across the four cohorts, two other important issues deserve mention. One concerns developmental changes in how the ANS and corticotrophin releasing hormone system systems function together when exposed to threat. The other concerns the nature of our statistical approach and its implications both for interpreting our findings and for future research. Turning to development, we had speculated that perhaps, with age, stronger patterns of reciprocity (e.g., high SNS and HPA axis activation, as well as high PNS activation/regulation) would emerge, as the systems gradually calibrate to environmental input (Del Giudice et al., 2011). Some hints that this was occurring emerged. For instance, the SMP had the widest age range and, as such, was the most likely to uncover age-related differences in the subgroups. Children classified as multisystem reactive were significantly older than children classified as moderately and HPA-specific reactive, with the latter children then tending to be the youngest. The HMP sample was the oldest on average, with this cohorts mean age being comparable to that of children in SMP classified as multisystem reactive. The HMP sample also had a subset of children who exhibited a multisystem pattern of responses, suggesting perhaps a developmental shift in cross-system regulatory abilities. This greater sophistication in coordination across system, for instance, with HPA axis activation, serving a regulatory function in controlling vigorous autonomic responses (Bauer et al., 2002; Roozendaal et al., 2004; Sapolsky et al., 2000), may come online with development. Of course, at times, multisystem reactivity may be indicative of chronic arousal or allostatic load, conferring risk for frequent, severe, or chronic stress-related morbidities, an interpretation supported by evidence from Del Giudice et al. (2012). They found higher levels of family conflict in “vigilant” children, characterized by heightened sympathetic (though not parasympathetic) responses to laboratory challenges. Consistent with the latter view, the other cohort in which the multisystem reactive pattern emerged was PAWS, and the children in this subgroup had higher scores on the family adversity index than did the other children in that sample. Perhaps, therefore, the meaning of multiple systems responding to environmental stress varies developmentally. As additional research is conducted testing cross-system integration and reactivity, larger samples with children across wide age ranges must be included to ascertain not only how coordination varies with development but also the functional significance of these variations.
In the present investigation, we relied on LPA to extract potentially meaningful subgroups, that is, latent classes, from a large set of continuous predictors. Although inherently exploratory, LPA enabled us to detect patterns in a much richer way than we would have been able to do without the use of the procedure. We did detect unique patterns of responses in the largest samples, patterns that supported our initial hypotheses and that were then replicated in the smaller samples. Thus, hypotheses guided our work and were subsequently partially supported. Moreover, the emergent patterns were differentiated based on familial factors. Nonetheless, these classifications will likely need further refinement as more empirical evidence is accumulated. This evidence will need to address not only the replicability of the groups but also whether the identified “groups” truly reflect unique, qualitatively different response tendencies or instead differ in a more continuous manner across the response dimensions of interest. Prior models suggest that cross-system reactive tendencies are continuous (Bauer et al., 2002), even when described, in functional terms, as categorical groups. We suspect that a similar phenomenon may be occurring here. At the same time. though, it may also be that some groups operate in categorically different manners, with accompanying neurological and phenotypic differences, similar to that observed with highly inhibited children (Kagan, 2011).
Our approach of applying LPA and identifying discrete subgroups in the cohorts may also have led to overestimations in the number of different patterns of responses across cohorts. That is, some of the different “subgroups” identified may instead reflect different quantitative response tendencies on some dimensions. For example, in the PAWS sample, we elected to examine the larger number of six subgroups rather than five, but the fit indices only minimally favored the former. Had we only included five subgroups, the HPA-specific reactivity subgroup would have been merged with the moderate reactivity subgroup. These groups, therefore, may reflect small continuous differences across the physiological parameters, more than separable subgroups, a possibility in need of further empirical investigation. More generally, both inter-and cross-class variability need to be taken into account in subsequent research, as will other behavioral (e.g., self-regulation and aggression) and neurobiological markers (e.g., serotonergic activity), all of which may lead to different constellations of responses with different phenotypic consequences (Boyce & Ellis, 2005; Cicchetti & Rogosch, 2012). Likewise, and although the cohorts varied some in risk factors for morbidities, all were composed of community-based samples of children. Patterns will need to be confirmed and extended in clinical and other high-risk populations (Cicchetti, 2010).
In summary, despite clear evidence that multiple biological processes are involved, directly and interactively, in anticipating, responding to, and recovering from stressful psychological challenges, a vast majority of the reactivity literature, particular that focused on developmental populations, has sought to isolate potential effects of one or at most two systems on reactivity or adaptation. We took a novel approach in this research, methodologically and statistically, and examined patterns of responses across multiple neurobiological channels. We identified strikingly similar patterns of coherent response tendencies in four diverse cohorts that varied by location of study, demographics, developmental stage, and methodological approach. Our findings reveal a complex, symphonic network of central and peripheral, neural and neuroendocrine components that play a role both in children’s basal levels of arousal and in their adaptive responses to environmental threats and challenges. Precisely how this network is organized varies across children and is modulated by the social context within which children are reared. The next step is to document more clearly the developmental and experiential underpinnings of this complexity and their combined links to subsequent maladaptive outcomes.
Acknowledgments
This work was funded by National Institute of Mental Health Grants R01-MH62320, R01-MH044340, P50-MH069315, P50-MH084051, and R24-MH081797; by National Science Foundation Grant BCS-0721377; and by the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development.
Footnotes
A variety of terms has been used to refer to whether changes in sympathetic and parasympathetic responsivity lead to increases or decreases in arousal or reactivity. For consistency, in the current paper, we refer simply to the direction of the response (activation or deactivation) when describing changes in each system, regardless of whether the direction induces greater or reduced arousal or reactivity per se. Activation of the PNS (indicated by higher RSA values, sometimes referred to as parasympathetic regulation or parasympathetic tone) is reflective of reduced arousal, and activation of the SNS is reflective of increased arousal. Conversely, deactivation of the PNS (lower RSA values, sometimes referred to as parasympathetic withdrawal) reflects increased arousal, whereas deactivation of the SNS reflects decreased arousal.
References
- Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. Journal of Developmental & Behavioral Pediatrics. 2011;32:668. doi: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Altshuler JL, Ruble DN. Developmental changes in children’s awareness of strategies for coping with uncontrollable stress. Child Development. 1989;60:1337–1349. doi: 10.1111/j.1467-8624.1989.tb04007.x. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23:102. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP. Some difficulties in interpreting psychophysiological research with children. Monographs of the Society for Research in Child Development. 2009;74:80–88. doi: 10.1111/j.1540-5834.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Brenner SL, Gatzke-Kopp L. Ten good reasons to consider biological processes in prevention and intervention research. Development and Psychopathology. 2010;20:745. doi: 10.1017/S0954579408000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin. 1993;114:296. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and psychophysiological response. Psychophysiology. 1994;31:44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Uchino BN, Cacioppo JT. Origins of baseline variance and the law of initial values. Psychophysiology. 1994;31:204–210. doi: 10.1111/j.1469-8986.1994.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ. Autonomic reactivity and psychopathology in middle childhood. British Journal of Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt KB, Obradovic J. The construct of psychophysiological reactivity: Statistical and psychometric issues. Developmental Review. 2012;33:29–57. [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free Cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: The reactivity hypothesis. Annals of the New York Academy of Sciences. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. New York: Norton; 1932. [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: A multilevel perspective. World Psychiatry. 2010;9:145–154. doi: 10.1002/j.2051-5545.2010.tb00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Neuroendocrine regulation and emotional adaptation in the context of child maltreatment. Monographs of the Society for Research in Child Development. 2012;77:87–95. [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wads-worth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127:87–127. [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Manning LG, Zale E. Children’s patterns of emotional reactivity to conflict as explanatory mechanisms in links between interpartner aggression and child physiological functioning. Journal of Child Psychology and Psychiatry. 2009;50:1384–1391. doi: 10.1111/j.1469-7610.2009.02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet E, Fitzsimons C, Datson N, Meijer O, Vreugdenhil E. Glucocorticoid signaling and stress-related limbic susceptibility pathway: About receptors, transcription machinery and microRNA. Brain Research. 2009;1293:129–141. doi: 10.1016/j.brainres.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Benjamin Hinnant J, Ellis BJ, El-Sheikh M. Developmental programming of children’s physiological responsivity— Adaptive patterns of stress responsivity: A preliminary investigation. Developmental Psychology. 2012;48:775. doi: 10.1037/a0026519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and Cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Montgomery LA, Porges SW. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43:230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008;56:601–611. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Keller PS, Erath SA. Marital conflict and risk for child maladjustment over time: Skin conductance level reactivity as a vulnerability factor. Journal of Abnormal Child Psychology. 2007;35:715–727. doi: 10.1007/s10802-007-9127-2. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: Pathways involving interactions between parasympathetic and sympathetic nervous system activity. Monographs of the Society for Research in Child Development. 2009;74:vii. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood: II. Developing the Mac-Arthur Health & Behavior Questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WTB, Goldsmith HH, Klein MH, et al. Exploring risk factors for the emergence of children’s mental health problems. Archives of General Psychiatry. 2006;63:1246. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Faes TJC, De Neeling NND, Kingma R, TenVoorde BJ, Karemaker JM. On the quantification of heart rate changes in autonomic function tests: Relations between measures in beats per minute, seconds and dimensionless ratios. Clinical Science. 1995;89:557–564. doi: 10.1042/cs0890557. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellman M, Spitzer S, Ironson G, Llabre M, Saab P, Pasin RDC, et al. Posture, place, and mood effects on ambulatory blood pressure. Psychophysiology. 1990;27:544–551. doi: 10.1111/j.1469-8986.1990.tb01972.x. [DOI] [PubMed] [Google Scholar]
- Glassman RB. Persistence and loose coupling in living systems. Behavioral Science. 1973;18:83–98. [Google Scholar]
- Gordis EB, Feres N, Olezeski CL, Rabkin AN, Trickett PK. Skin conductance reactivity and respiratory sinus arrhythmia among maltreated and comparison youth: Relations with aggressive behavior. Journal of Pediatric Psychology. 2010;35:547–558. doi: 10.1093/jpepsy/jsp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary Cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and behavior. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Herrera AM. The Oxford handbook of developmental psychology: Vol. 2. Self and other. Oxford: Oxford University Press; 2013. The development of stress reactivity: 3. A neurobiological perspective; p. 45. [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stanbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31:65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, et al. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2011;23:1149. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biological Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annual Review of Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- Hinnant JB, El-Sheikh M. Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of Abnormal Child Psychology. 2009;37:1049–1061. doi: 10.1007/s10802-009-9341-1. [DOI] [PubMed] [Google Scholar]
- Johnson PL, O’Leary KD. Parental behavior pattern and conduct problems in girls. Journal of Abnormal Child Psychology. 1987;15:573–581. doi: 10.1007/BF00917242. [DOI] [PubMed] [Google Scholar]
- Kagan J. Three lessons learned. Perspectives on Psychological Science. 2011;6:107–113. doi: 10.1177/1745691611400205. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klein MH, Hyde JS, Essex MJ, Clark R. Maternity leave, role quality, work involvement, and mental health one year after delivery. Psychology of Women Quarterly. 1998;22:239–266. [Google Scholar]
- Koss KJ, George MRW, Cummings EM, Davies PT, El-Sheikh M, Cicchetti D. Asymmetry in children’s salivary cortisol and alpha-amylase in the context of marital conflict: Links to children’s emotional security and adjustment. Developmental Psychobiology. 2013 doi: 10.1002/dev.21156. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradović J, Lin J, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosomatic Medicine. 2011;73:533–540. doi: 10.1097/PSY.0b013e318229acfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudel I, Farber SL, Mrus JM, Leonard AC, Sherman SN, Tsevat J. Patterns of responses on health-related quality of life questionnaires among patients with HIV/AIDS. Journal of General Internal Medicine. 2006;21:S48–S55. doi: 10.1111/j.1525-1497.2006.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Gortz N, et al. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes, Brain and Behavior. 2006;5:64–72. doi: 10.1111/j.1601-183X.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- Lisonbee JA, Pendry P, Mize J, Gwynn EP. Hypothalamic-pituitary-adrenal and sympathetic nervous system activity and children’s behavioral regulation. Mind, Brain, and Education. 2010;4:171–181. [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Bio-behavioral Reviews. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W. The cold pressor test and autonomic function: A review and integration. Psychophysiology. 1975;12:268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal Cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the life span on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Seminars in medicine of the Beth Israel Deaconess Medical Center: Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience. 2006;8:367. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093. [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B. Latent variable hybrids: Overview of old and new models. Advances in Latent Variable Mixture Models. 2008;1:1–24. [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxier C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child & Adolescent Psychology. 2009;38:513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14:535–569. [Google Scholar]
- Obradović J. How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology. 2012;24:371–387. doi: 10.1017/S0954579412000053. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23:101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter B, O’Leary KD. Marital discord and childhood behavior problems. Journal of Abnormal Psychology. 1980;8:287–295. doi: 10.1007/BF00916376. [DOI] [PubMed] [Google Scholar]
- Quas JA, Carrick N, Alkon A, Goldstein L, Boyce WT. Children’s memory for a mild stressor: The role of sympathetic activation and parasympathetic withdrawal. Developmental Psychobiology. 2006;48:686–702. doi: 10.1002/dev.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Murowchick E, Bensadoun Jr, Boyce WT. Predictors of children’s cortisol activation during the transition to kindergarten. Journal of Developmental & Behavioral Pediatrics. 2002;23:304–313. doi: 10.1097/00004703-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Quas JA, Yim IS, Edelstein RS, Cahill L, Rush EB. The role of cortisol reactivity in children’s and adults’ memory of a prior stressful experience. Developmental Psychobiology. 2011;53:166–174. doi: 10.1002/dev.20505. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development. 2012;36:19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: Consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23:1107. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, Dominique JF, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. Journal of Neuroscience. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruble DN, Boggiano AK, Feldman NS, Loebl JH. Developmental analysis of the role of social comparison in self-evaluation. Developmental Psychology. 1980;16:105. [Google Scholar]
- Salomon K, Matthews KA, Allen MT. Patterns of sympathetic and parasympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37:842–849. [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal Cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72:1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Chrousos GP. The three-way interactions between the hypothalamic-pituitary-adrenal and gonadal axes and the immune system. Bailliere’s Clinical Rheumatology. 1996;10:181. doi: 10.1016/s0950-3579(96)80014-8. [DOI] [PubMed] [Google Scholar]
- Weiner H. Perturbing the organism: The biology of stressful experience. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Wilder J. Modern psychophysiology and the law of initial value. American Journal of Psychotherapy. 1958;12:199–221. doi: 10.1176/appi.psychotherapy.1958.12.2.199. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayakawa CM. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 2010;35:241–248. doi: 10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Zimmer-Gembeck MJ, Skinner EA. Review: The development of coping across childhood and adolescence: An integrative review and critique of research. International Journal of Behavioral Development. 2011;35:1–17. [Google Scholar]