Abstract
Despite early positive response to platinum-based chemotherapy, the majority of ovarian carcinomas develop resistance and progress to fatal disease. Protein phosphatase 2A (PP2A) is a ubiquitous phosphatase involved in the regulation of DNA damage response and cell cycle checkpoint pathways. Recent studies have shown that LB100, a small molecule inhibitor of PP2A, sensitizes cancer cells to radiation-mediated DNA damage. We hypothesized that LB100 could sensitize ovarian cancer cells to cisplatin treatment. We performed in vitro studies in SKOV-3, OVCAR-8, and PEO1, 4, and 6 ovarian cancer lines to assess cytotoxicity potentiation, cell-death mechanism(s), cell cycle regulation, and DNA damage response signaling. In vivo studies were conducted in an intraperitoneal metastatic mouse model using SKOV-3/f-Luc cells. LB100 sensitized ovarian carcinoma lines to cisplatin-mediated cell death. Sensitization via LB100 was mediated by abrogation of cell cycle arrest induced by cisplatin. Loss of the cisplatin-induced checkpoint correlated with decreased Wee1 expression, increased cdc2 activation, and increased mitotic entry (p-histone H3). LB100 also induced constitutive hyperphosphorylation of DNA damage response proteins (BRCA1, Chk2, γH2AX), altered the chronology and persistence of JNK activation, and modulated the expression of 14-3-3 binding sites. In vivo, cisplatin sensitization via LB100 significantly enhanced tumor growth inhibition and prevented disease progression after treatment cessation. Our results suggest that LB100 sensitizes ovarian cancer cells to cisplatin in vitro and in vivo by modulation of the DNA damage response pathway and cell cycle checkpoint abrogation.
Keywords: cisplatin, drug resistance, PP2A, ovarian cancer, cell cycle arrest
Introduction
Ovarian cancer is the fifth leading cause of cancer death in women, taking the lives of over 14,000 patients in the United States in 2013 (1). Due to non-specific early symptoms and unreliable screening measures, most patients present with late-stage disease and a poor (less than 20%) chance of long-term survival (2). Current standard of treatment involves maximal debulking followed by combination chemotherapy consisting of a platinum-based compound and a taxane (3). Although patients may have an initial positive response, most eventually develop multidrug resistance and die of progressive cancer (4).
Cisplatin [cis-[PtCl2(NH3)2]] is a platinum-based drug that is commonly used in the treatment of ovarian cancer. Cisplatin acts by forming DNA crosslinks that lead to double strand break (DSB) formation as a consequence of innate repair mechanisms. The consequent DSB accumulation and stalled DNA fork progression result in apoptosis of sensitive cells (5). Despite its high potency, however, cisiplatin is associated with potential toxicities, including nephrotoxicity, nausea/vomiting, neurotoxicity, and ototoxicity, limiting the effective dose that can be employed (6). Cisplatin resistance in ovarian cancer is also common, and has been reported to involve an increase in tolerance and/or repair of DNA adducts as well as a failure of apoptotic pathway activation (7, 8). Importantly, greater than 90% of ovarian cancers harbor inactivating mutations of p53, and lack the ability to arrest the cell cycle at the G1/S phase junction (9, 10). Therefore, these cancers respond to DNA damage via S and G2/M phase arrests, allowing DNA damage repair. Previous studies have shown that the disruption of the critical S and G2/M phase checkpoints can sensitize cells to cisplatin (11).
DNA damage response is facilitated by a highly integrated and complex series of phosphorylation and dephosphorylation events regulated by key kinases and phosphatases, respectively. It has been shown that constitutive phosphorylation of intermediates within the response signaling pathways is a barometer of the critical cellular processes that determine whether the cell will repair the damaged DNA or induce apoptotic cell death (12–15). The serine/threonine kinases ATM and ATR are the primary coordinators of cellular responses to DNA damage. These kinases are activated following double strand break induction or a stalled DNA replication fork and are implicated in regulating DNA repair, cell cycle checkpoints, and apoptotic signaling. ATM/ATR directly and indirectly exert these effects by controlling the phosphorylation of downstream target proteins such as BRCA1, H2AX, Chk1, and Chk2 (15). Furthermore, increased and constitutive phosphorylation of numerous other non-ATM/ATR pathway signaling proteins may be correlated with the extent of apoptotic induction. For example, sustained SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) activation following cisplatin treatment plays a role in both extrinsic and mitochondrial apoptosis (16). Thus, inducing constitutive phosphorylation via targeted inhibition of phosphatases prior to the initiation of DNA damaging therapy may enhance cytotoxic efficacy.
Protein phosphatase 2A (PP2A) is a ubiquitous serine/threonine phosphatase that regulates numerous proteins of both ATM/ATR-dependent and -independent response pathways (17). Pharmacologic inhibition of PP2A has previously been shown to sensitize cancer cells to radiation-mediated DNA damage via constitutive phosphorylation of various signaling proteins, such as p53, γH2AX, PLK1 and Akt, resulting in cell cycle deregulation, inhibition of DNA repair, and apoptosis (18).
LB100 (Fig. 1A) is a small molecule derivative of the natural product cantharadin with significantly less toxicity (19). Previous pre-clinical studies have shown that LB100 enhanced the cytotoxic effects of temozolomide, doxorubicin, and radiation therapy against glioblastoma (GBM), metastatic pheochromocytoma, and pancreatic cancer, respectively (18–20). Although the exact mechanism by which LB100 inhibits PP2A function has not yet been deduced, the overall potentiation of DNA damage therapy seems to derive from abrogation of cell cycle arrest despite DNA damage. LB100 is currently undergoing a phase I study in combination with docetaxel for the treatment of solid tumors (21). Given the importance of platinum agents for use in clinical treatment of ovarian cancer as well as the well-established literature implicating cisplatin as a potent DNA-damaging agent, we hypothesized that LB100 could enhance the effectiveness of cisplatin treatment in ovarian cancer model systems.
Figure 1.
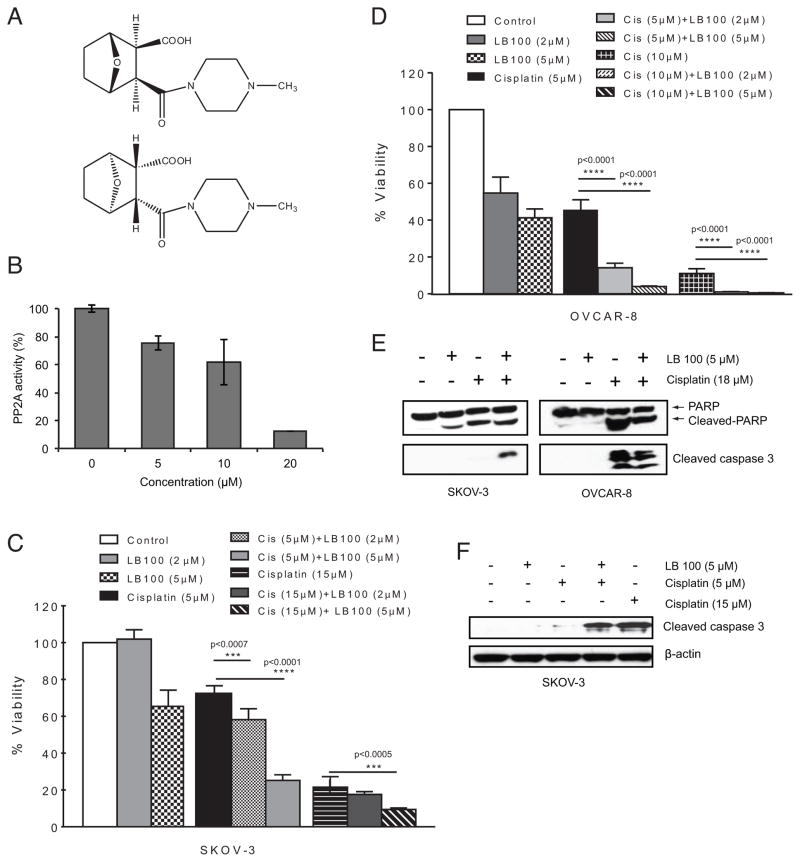
Inhibition of PP2A by LB100 sensitizes ovarian cancer cells to cisplatin cytotoxicity. A, Structure of LB100, used as the racemate. B, Dose-dependent inhibition of PP2A activity following 2 h LB100 treatment in SKOV-3 cells. B and C, MTT assay after 72 h treatment showing increased cytotoxicity in SKOV-3 cells (C) and OVCAR-8 cells (D) for both IC25 and IC75 doses of cisplatin when cells were pre-treated with LB100 compared to either drug alone. E, Western blots following 48 h treatment shows apoptosis via cleaved PARP and cleaved caspase-3. F, Western blot following 72 h treatment with cisplatin shows that pre-treatment with LB100 enhances apoptosis induced with a sub-lethal dose (IC25) of cisplatin.
In order to test this hypothesis, in vitro studies were performed in various ovarian carcinoma cell lines. LB100-dependent effects on cellular PP2A activity, cytotoxic potentiation, cell cycle modulation, apoptosis and activation of DNA damage signaling and repair pathways were investigated. Additionally, possible additive or synergistic effects of LB100 on cisplatin treatment were determined. LB100-induced cisplatin sensitization was further determined in vivo in a metastatic ovarian cancer model.
Materials and Methods
Cell lines, cell culture, and drug solutions
Luciferase-expressing cells were generated by infecting SKOV-3 cells with pCLNCX-luciferase retrovirus (SKOV-3-Luc) as previously reported (22) and cultured in McCoy’s 5A medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum and 100 units/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, and 292 μg/mL L-glutamine (BioWhittaker, Walkersville, MD). Human OVCAR-8 ovarian cancer cells were provided by the National Cancer Institute (part of the NCI-60 collection). The PEO1, PEO4, and PEO6 ovarian cancer cell lines have previously been characterized (23) and were kindly provided by Dr. Ian Goldlust (National Center for Advancing Translational Sciences, Shady Grove, MD). All the PEO cells and OVCAR-8 cells were cultured in RPMI medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 100 units/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, and 292 μg/mL L-glutamine (BioWhittaker). Characterization and maintenance of HEK 293 human embryonic kidney cell lines overexpressing Pgp, MRP1, or ABCG2; and the parental human epidermoid carcinoma cell line KB-3-1 and the cisplatin-resistant KB-CP.5 cells have been described previously (11, 24). All cell lines were thawed immediately prior to experimentation, and cell lines were characterized using short tandem repeat profiling. Cisplatin was purchased from Sigma-Aldrich (St Louis, MO) and prepared as a 3.32 mM stock solution dissolved in sterile saline (0.9% sodium chloride) (25). Solutions for injection were prepared immediately prior to administration. LB100, a water-soluble small-molecule inhibitor of PP2A was provided by Lixte Biotechonology Holdings, Inc ADD (East Setauket, NY) and was diluted in sterile PBS prior to administration.
PP2A phosphatase activity assay
Ovarian cancer cells were grown to 80% confluence in 100 mm dishes and treated with LB100 as indicated and prepared as described previously (18). Following treatment for 2 h, cells were washed twice with cold PBS (pH 7.4) and lysed in lysis buffer (20 mM imidazole-HCL, 2 mM EDTA, 2 mM EGTA, pH 7.0) supplemented with protease inhibitors (Thermo Scientific, Rockford, IL) for 30 minutes on ice. Cell lysates were sonicated for 10 s then centrifuged at 2,000 x g for 5 min. Supernatants were assayed with the PP2A Phosphatase Assay Kit (Millipore, Billerica, MA) according to the manufacturer’s instructions. Experiments were performed in triplicate, and the data are presented as a percent mean of relative PP2A activity compared to control ± SD.
MTT assay
Cell survival was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Invitrogen) assay. Cells were seeded at a density of 5,000 cells per well in 96-well plates and incubated at 37°C in humidified 5% CO2 for 24 hours. The 50% inhibitory concentration (IC50) values were defined as the drug concentrations required to reduce cell numbers to 50% of the untreated control. For IC50 determination, serially diluted LB100 or cisplatin was added to give the intended final concentrations. MTT assays were carried out according to the manufacturer’s instructions (Molecular Probes, Eugene, OR). Absorbance values were determined at 570nM on a Spectra Max 250 spectrophotometer (Molecular Devices, Sunnyvale, CA). All MTT assays were performed in triplicate. In order to determine if LB100 could enhance the cytotoxic effect of cisplatin, cells were pretreated with LB100 for 1 h prior to the addition of cisplatin. Cells were treated with both drugs for 72 h. Cell viability was analyzed via the MTT assay as described above. Experiments were performed in triplicate, and the data are presented as a percent mean ± SD.
Production of stable NT-shRNA and PP2A-C-shRNA expressing SKOV-3 and OVCAR-8 cells
To stably knockdown expression of the catalytic subunit of PP2A, PP2A-C, a pLKO.1-puro plasmid-based shRNA targeting the sequence: TGGAACTTGACGATACTCTAA (clone ID: TRCN0000002483, Sigma-Aldrich) was employed (PP2AC-shRNA). Additionally, a non-targeting shRNA plasmid (NT-shRNA) that targets no known human sequence was utilized as a control. A primer containing the target sequence (CTGGTTACGAAGCGAATCCTT) along with a stem loop followed by the reverse target sequence was annealed to a complimentary primer and inserted into the EcoRI and AgeI sites of the pLKO.1-puro plasmid (Addgene number 10878). Lentiviral particles were produced via Lipofectamine 2000 (Invitrogen)-mediated triple transfection of 293T cells with either the PP2A-C-shRNA or the NT-shRNA along with the lentiviral envelope plasmid (pMD2.G, Addgene number 12259) and the lentiviral packaging plasmid (psPAX2, Addgene number 12260). Target cells (SKOV-3 and OVCAR-8 human ovarian cancer cell lines) were transduced with either PP2A-C-shRNA or NT-shRNA containing lentiviral particles in the presence of [8 μg/mL] polybrene and stable cells were selected using [2 μg/mL] puromycin.
Cell-cycle analysis
SK-OV-3 and OVCAR 8 cells were treated with LB100, cisplatin, or LB100 plus cisplatin at indicated concentrations for 24 and 48 h. For cell-cycle analysis, cells were washed with PBS and fixed overnight in ice-cold 70% ethanol and stored at 4°C. Cells were then centrifuged and resuspended in 100 U RNAse (Sigma-Aldrich), and incubated at 37°C for 20 min. Propidium idodide solution (Invitrogen, 500 μL, 50μg/mL in DPBS) was added to each tube and incubated in the dark at 4°C overnight. Flow cytometry analysis was performed with CellQuestPro and data analysis was completed with ModFit LT. All data is in triplicate and presented as a percent mean ± SD.
Immunoblotting
Whole cell and homogenized tumor tissues were lysed in NP-40 lysis buffer [50 mM Tris/HCl, pH 7.4, 150 mM NaCl and 1% Nonidet P40, supplemented with Complete Protease Inhibitor Cocktail tablets and PhosStop phosphatase inhibitors (Roche, Indianapolis, IN)] and prepared as previously described (26). Total cellular proteins (40 μg) were separated on 12% or 15% SDS/PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon).. The membrane was then blocked for 1 hour at room temperature in 5% (w/v) non-fat milk in TBS-Tween-20 and probed overnight with primary antibodies followed by anti-rabbit or anti-mouse IgG-horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA) in blocking buffer for 1 h. Membranes were subsequently incubated in Immobilon Western Blot Chemiluminescent HRP Substrate (Millipore) and developed on biomax XAR film (Kodak). Primary antibodies were purchased from Cell Signaling Technology: γH2AX (Ser139), p-Wee1 (Ser 642), Wee1, p-cdc2 (Tyr15), p-BRCA1 (Ser1524), p-Chk1 (Ser345), p-Chk1 (Ser317), Chk-1, phospho-Chk2 (Thr68), PP2A-C, PP2A-A, cleaved caspase-3 (Asp175), cleaved PARP (Asp214), p-histone H3 (Ser10), p-ATR (Ser428), and p-(Ser) 14-3-3 binding motif.
In vivo intraperitoneal ovarian cancer model
Five- to seven-week-old female nude athymic mice (NCR nu/nu) were obtained from NCI (Frederick, MD), maintained in accredited animal facilities and used as stipulated by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, in accordance with institutional reviews (http://oacu.odnih.gov). 106 SKOV-3/f-Luc cells were suspended in 100 μL PBS and injected into the intraperitoneal (i.p.) cavity. After four days, the mice were randomized into four groups (4–5 animals per group): vehicle control (PBS), LB100 (1.5 mg/kg, i.p.), cisplatin (1.5 mg/kg, i.p.), and LB100 plus cisplatin (same doses as administered alone). Dose and treatment schedule were established based on the activity of each agent reported in previous studies (18, 27). Following tumor inoculation, mice were dosed on days 4, 6, 8, 10, 12 and 14. For the combination group, LB100 was administered 1 h prior to cisplatin. Tumor growth was measured twice a week via bioluminescence imaging (BLI) as previously described (28). D-Luciferin (3mg/100 μL PBS) was administered via i.p. injection. Relative intensity of the BLI signal for each mouse was calculated by dividing the total luminescence for each session by the total luminescence measured on the first day of treatment. Mice were continuously observed until indicated euthanasia endpoints. Toxicity of the treatment regimens was assessed by the degree of weight loss and the overall health status was continuously monitored by a veterinarian on staff. For ex vivo western blot analysis, four tumor bearing mice were treated with either saline (control), LB100 (1.5 mg/kg), cisplatin (2.5 mg/kg), or LB100 (1.5 mg/kg) + cisplatin (2.5 mg/kg). After 4 h, mice were euthanized and tumors were rapidly dissected from the intraperitoneal cavity, snap-frozen in liquid nitrogen, and lysed as described above.
Statistical analysis
Statistical analysis was performed using the software GraphPad Prism 6 (GraphPad Software, USA). Mean values are reported as mean ± standard deviation, and a two-tailed unpaired t test was performed to assess statistical significance. Statistical significance was passed at two-sided p<0.05.
Results
Ovarian cancer cell line sensitivity to LB100 and cisplatin
In order to characterize the effects of LB100 and cisplatin on ovarian carcinoma cells in vitro, six ovarian cell lines were tested. SKOV-3 and OVCAR-8 cells have previously been described as p53 null and harboring an inactivating p53 mutation, respectively (29). Both cell lines have also been characterized as intrinsically resistant to cisplatin (30–32). The PEO cell lines (PEO-1s, PEO-1m, PEO-4 and PEO-6) were generated from the same patient prior to chemotherapy (PEO-1s and PEO-1m) and following the development of clinical cisplatin resistance (PEO-4 and PEO-6). The PEO-1 cell lines carry BRCA2 missense (m) and STOP (s) mutations (23).
The 50% inhibitory concentration (IC50) of each compound was determined using the MTT cytotoxicity assay (Table 1). Cell lines known to harbor intrinsic cisplatin resistance (SKOV-3, OVCAR-8) or acquired resistance (PEO-4, PEO-6) showed a 2- to 3-fold decreased sensitivity to cisplatin compared to PEO-1. SKOV-3 (IC50 = 10.1 ± 1.8 μM) was 2-fold less sensitive to LB100 compared to the other ovarian lines (average IC50 = 5.7 μM), which correlated with the level of PP2A protein expression (Supplementary Fig. S1), suggesting cell line-specific sensitivity to PP2A inhibition depends in part on the overall protein level. While ATP-binding cassette (ABC) efflux transporters have been shown to impact efficacy of candidate small-molecule therapeutics (33), no information exists on whether this is the case for LB100. When HEK 293 human embryonic kidney cell lines overexpressing Pgp, MRP1, or ABCG2 were treated with the same concentration of LB100, the IC50s of the transfected lines did not increase, and were similar to parental cells or in the presence of an inhibitor (tariquidar) (Supplementary Fig. S2). In contrast, these cell lines were resistant to known substrates for these transporters, such as paclitaxel for ABCB1, etoposide for ABCC1, and mitoxantrone for ABCG2 (data not shown). Cisplatin-resistant KB-CP.5 cells demonstrated two-fold increased resistance to LB100 (vs. 4.8-fold resistance to cisplatin) compared with parental KB-3-1 human adenocarcinoma cells, indicating minimal cross-resistance and potential therapeutic efficacy in cisplatin resistant cell lines (Supplementary Fig. S3).
Table 1.
IC50’s of cisplatin and LB100 on ovarian cancer cell lines.
| Cell Line | LB100 (μM) (SD) | Cisplatin (μM) (SD) |
|---|---|---|
| SK-OV3 | 10.1 (1.8) | 7.6 (1.6) |
| OVCAR8 | 5.5 (0.5) | 7.2 (2.3) |
| PEO1-Brca2 Missense | 6.2 (1.5) | 2.1 (0.4) |
| PEO1-Brca2 STOP | 6.9 (1.0) | 2.3 (0.3) |
| PEO4 | 5.0 (0.6) | 4.3 (1.8) |
| PEO6 | 5.1 (0.2) | 8.0 (1.9) |
LB100 sensitizes ovarian cancer cells to the cytotoxic effects of cisplatin in vitro
To determine whether PP2A inhibition with LB100 could sensitize ovarian cancer cells to the cytotoxic effects of cisplatin, we first assessed the effect of LB100 on PP2A enzymatic activity. Consistent with previous findings in other types of cancer cells (18, 19), LB100 alone caused a concentration-dependent decrease in PP2A enzymatic function in SKOV-3 cells (Fig. 1B). Next, we performed cytotoxicity assays on ovarian cancer lines using either IC25 (5 μM) or IC75 (15 μM) doses of cisplatin in the presence of 2 μM (<IC25) or 5 μM of LB100. LB100 pre-treatment (1 h) resulted in a significant decrease in cell viability compared to either treatment alone. In SKOV-3 cells, 5 μM (IC25) cisplatin alone resulted in 73 ± 2% viability compared with control, while the presence of 2 μM and 5 μM LB100 significantly potentiated cisplatin toxicity (58 ± 2% and 25 ± 1% viability, respectively) (Fig. 1C). This effect was observed for both low and high dose cisplatin concentrations in additional ovarian cell lines examined (Fig. 1D and Supplementary Fig. S4 A–C). Immunoblot analysis of LB100 pre-treatment in combination with cisplatin in SKOV-3 and OVCAR-8 showed increased levels of cleaved caspase-3 and cleaved PARP, indicating apoptosis as the mechanism of cell death (Fig. 1E). In SKOV-3 cells, LB100 sensitization greatly enhanced the expression of apoptotic factors in combination with an IC25 (5 μM) dose of cisplatin 72 h post treatment (Fig. 1F).
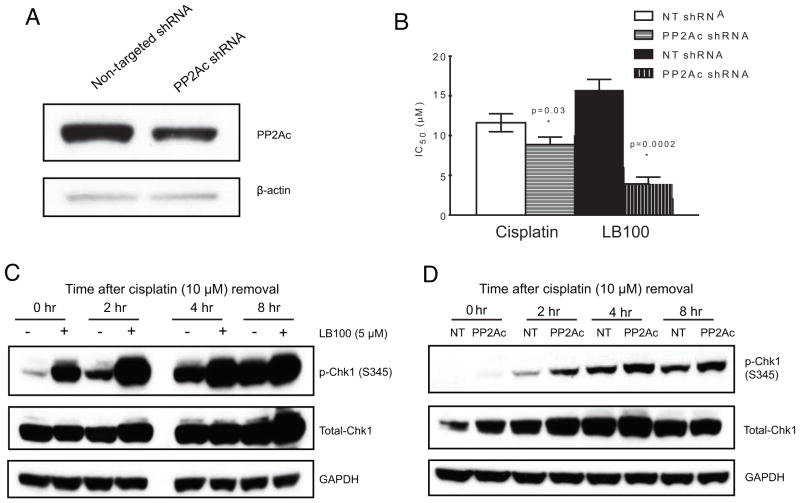
Since pharmacologic inhibition of PP2A via LB100 sensitized ovarian cancer cells to cisplatin, we investigated whether stable knockdown of expression of the catalytic subunit of PP2A (PP2A-C) might result in the same effect. Stable knockdown of PP2A-C was achieved in OVCAR-8 cells, with approximately 50% knockdown of PP2A-C expression compared to control, non-specific shRNA (Fig. 2A). Cisplatin and LB100 sensitivity were determined for OVCAR-8 PP2A-C shRNA-expressing cells and compared to the same non-specific shRNA control (Fig. 2B). Consistent with the pharmacologic sensitization induced by LB100, PP2A-C knockdown sensitized OVCAR-8 cells to cisplatin compared to non-specific control. As expected, sensitivity to LB100 was greatly enhanced in the PP2A-C knockdown cells compared to control (LB100 OVCAR-8 NT shRNA IC50 = 15.7 ± 1.3 μM, LB100 OVCAR-8 PP2A-C shRNA IC50 = 3.9 ± 0.9 μM, Fig. 2B). Conversely, stable expression of PP2A-C-specific shRNA in SKOV-3 cells resulted in vastly decreased numbers of viable cells (data not shown), highlighting that a baseline expression of PP2A is essential for cellular viability (34).
Figure 2.
Validation of PP2A-C as a mediator of cisplatin cytotoxicity. A, Western blot showing stable knockdown of PP2A-C in OVCAR-8 cells. B, Cell viability (MTT) assay demonstrating increased sensitivity to cisplatin and LB100 following PP2A-C knockdown. C and D, Western Blot showing hyperphosphorylation of Chk1 (S345) for up to 8 hrs following cisplatin washout in PP2A-C knockdown (C) and LB100 treated (D) OVCAR-8 cells.
Inhibition of PP2A by LB100 induces hyperphosphorylation of Chk1
PP2A activity has been associated with dephosphorylation of γH2AX, Chk2, and BRCA1 (12, 35). Chk1 is a central mediator of the DNA damage response and maintains the integrity of the genome by inducing S or G2/M cell cycle arrest and promoting DNA repair. Additionally, the functional integrity of Chk1 is maintained by continuous dephosphorylation of key serine residues such as S345, by PP2A (36). In order to assess whether inhibition of PP2A by LB100 could sensitize the DNA damage response pathway by inducing hyperphosphorylation of Chk1 at S345, OVCAR-8 cells were treated with cisplatin for 1 h with or without a 1 h pre-treatment with LB100, then incubated in media with or without LB100 for up to 8 h. LB100 significantly increased the phosphorylation of Chk1 at S345 for each time point following cisplatin treatment compared to cells incubated in media alone (Fig. 2C). To confirm whether this differential phosphorylation was due to decreased PP2A function, we performed the same experiment in the stable PP2A-C knockdown OVCAR-8 cells (Fig. 2D). Consistent with the pharmacologic data, decreased expression of PP2A-C resulted in hyperphosphorylation of Chk1 following cisplatin, compared to OVCAR-8 cells stably expressing control, non-targeting shRNA.
LB100 induces constitutive phosphorylation of key mediators in the DNA damage response pathway independent of ATR activation, allowing persistent DNA damage
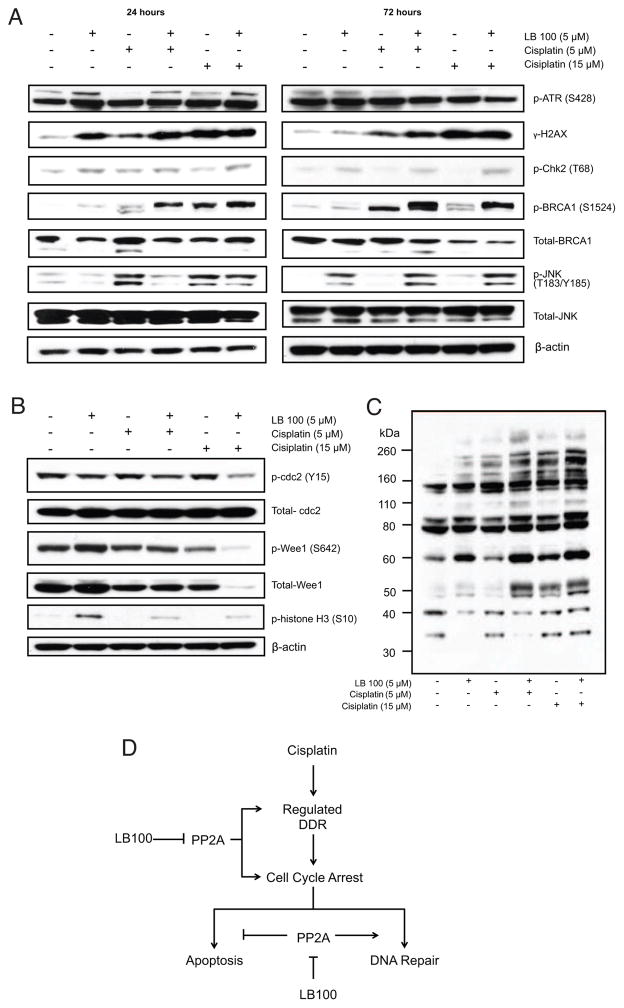
Persistent expression of γ-H2AX is an indicator of inadequate DNA damage repair (37), and its time-sensitive dephosphorylation is critical for maintaining the chronologic fidelity of repair initiation (13, 38). Furthermore, constitutive phosphorylation of BRCA1 and JNK has been shown to bias the cell towards apoptosis following induction of DNA damage (14, 16). In order to understand the potential mechanism by which LB100 pre-treatment sensitizes ovarian cancer cells to the effect of cisplatin, we compared the phosphorylation state of these key intermediaries of the DNA damage response pathway following treatment with of LB100 and cisplatin. Inhibition of PP2A enhanced the phosphorylation of γH2AX, Chk2, and BRCA1 at 24 h, and JNK at 72 h (Fig. 3A). These effects were independent of ATR activation (Fig. 3A top panel). LB100 plus cisplatin (5 μM) hyperphosphorylated γH2AX and BRCA1 at 24 and γH2AX, Chk2, and BRCA1 at 72 h compared to cisplatin (5 μM) alone. For the IC75 dose of cisplatin (15 μM), LB100 pre-treatment led to hyperphosphorylation of Chk2 and BRCA1, compared to cisplatin alone, while expression of γH2AX was similar for both groups. Phosphorylation levels of JNK were greater for 5 μM cisplatin, compared to 5 μM of both LB100 and cisplatin initially (24 h), but at 72 h the combination treatments resulted in greater JNK phosphorylation compared to both doses of cisplatin alone.
Figure 3.
Potential mechanisms of LB100 induced cisplatin sensitization in SKOV-3 cells. A, Western blot showing LB100 mediated regulation of DNA damage response proteins. B, Western blot of cell lysates collected after 24 h of treatment showing LB100-induced changes in the phosphorylation states of cell cycle related proteins. C, Western blot demonstrating differential generation of 14-3-3 p-Ser binding sites with LB100 alone or in combination with cisplatin. D, Simplified scheme highlighting potential mechanisms involved in LB100-induced cisplatin sensitization.
Cisplatin-induced cell cycle checkpoints are abrogated by LB100, which are mediated by changes in both Wee1 expression and cdc2 activation
Given the integral interactions between PP2A and numerous cell cycle checkpoint proteins, we assessed whether LB100 could abrogate cisplatin-induced cell cycle arrest. FACS analysis was performed on SKOV-3 and OVCAR-8 cells at both 24 and 48 h following treatment with various concentrations of both cisplatin and LB100 (Table 2). LB100 treatment alone caused SKOV-3 cells to progress through the G1 stage, resulting in a significantly higher percentage of cells in the G2/M phase. This LB100-mediated event was concentration-dependent [Cell fraction in G/2M (%): control (19.4 ± 0.9), LB100 (2 μM) (25.1 ± 0.8), LB100 (10 μM) (32.1± 1.6), LB100 (15μM) (33.9 ±1.4)]. In agreement with previous reports (39), cisplatin induced either slow S-phase progression/arrest (SKOV-3) or G2/M-phase arrest, which appeared over 48 h (OVCAR-8). When each cell line was pre-treated for 1 h with IC25 concentrations of LB100 (5 μM for SKOV-3, 2 μM for OVCAR-8), cell cycle arrest was abrogated at both 24 h and 48 h. In SKOV-3 cells, cisplatin alone resulted in 38 % of cells in the S-phase while pre-treatment with LB100 resulted in 25 % of S-phase cells at 48 h. In OVCAR-8 cells, cisplatin alone resulted in 79 % of cells in the G2/M-phase while pre-treatment with LB100 resulted in 67 % of S-phase cells at 48 h.
Table 2.
Cell cycle analysis of SKOV-3 and OVCAR8 cells
| SKOV-3 | OVCAR-8 | ||||||
|---|---|---|---|---|---|---|---|
| % G1 (SD) | % S (SD) | % G2/M (SD) | % G1 (SD) | % S (SD) | % G2/M (SD) | ||
| 24 Hour | |||||||
| Control (PBS) | 58.0 (0.9) | 21.1 (0.6) | 20.9 (0.8) | Control (PBS) | 39.8 (0.9) | 40.9 (0.5) | 19.3 (0.3) |
| LB100 (5 μM) | 51.4 (1.5) | 19.7 (0.8) | 28.9 (1.2) | LB100 (2 μM) | 45.4 (1.5) | 37.0 (1.6) | 17.6 (0.4) |
| Cisplatin (18 μM) | 49.8 (2.3) | 33.6 (1.8) | 16.6 (1.3) | Cisplatin (5 μM) | 4.6 (2.0) | 81.9 (1.6) | 13.4 (0.6) |
| Cisplatin (18 μM) + LB100 (5 μM) | 55.1 (1.2) | 26.6 (2.2) | 18.3 (2.9) | Cisplatin (5 μM) + LB100 (2 μM) | 18.5 (0.4) | 59.2 (1.0) | 22.3 (0.7) |
| 48 Hour | |||||||
| Control (PBS) | 58.0 (0.9) | 14.8 (0.5) | 19.4 (0.9) | Control (PBS) | 60.8 (0.9) | 28.0 (0.2) | 11.3 (0.3) |
| LB100 (5 μM) | 47.2 (1.9) | 30.2 (3.1) | 25.1 (0.8) | LB100 (2 μM) | 55.3 (0.4) | 29.9 (1.1) | 14.9 (1.5) |
| Cisplatin (18 μM) | 40.4 (0.8) | 38.4 (0.5) | 21.2 (1.0) | Cisplatin (5 μM) | 0.85 (0.3) | 20.7 (2.1) | 78.5 (2.2) |
| Cisplatin (18 μM) + LB100 (5 μM) | 50.4 (4.1) | 24.7 (1.6) | 25.0 (3.1) | Cisplatin (5 μM) + LB100 (2 μM) | 0.98 (0.2) | 32.6 (0.7) | 66.5 (0.5) |
Transition into mitosis is critically dependent on the activation state of the cdc2/cyclin B complex (40). Cdc2 is negatively regulated by the Wee1 kinase through an inhibitory phosphorylation on Y15 and is positively regulated by the cdc25C phosphatase via dephosphorylation at this same residue. Wee1 is phosphorylated (p-Wee1, Ser642) in an Akt/PKB-dependent fashion, such that when G2/M arrest occurs in cells p-Wee1 abundance increases (40). We assessed whether LB100-induced checkpoint abrogation and cell cycle progression are due to alterations of checkpoint protein function and/or expression. SKOV-3 cells were treated for 24 h with PBS (vehicle control), LB100 (5 μM), and cisplatin (5 μM or 15 μM) following 1 h pre-treatment with LB100 (5 μM). LB100 (5uM) slightly increased the phosphorylation of Wee-1 compared to control (Fig. 3B), and not observed at all when LB100 was added to either concentration of cisplatin or at lower LB100 concentrations (Supplementary Fig. S5A). Wee1 phosphorylation was nearly absent for the LB100 and cisplatin (15 μM) combination. Total Wee1 protein levels were also decreased for this treatment group, suggesting that degradation or decreased expression resulted in the observed reduction in phosphorylation levels. Decreased Wee1 expression was correlated with a decrease in p-cdc2 (Y15) for both doses of cisplatin when pre-treated with LB100 as well as LB100 alone, allowing cell cycle progression into mitosis, indicated by the strong increased expression of p-Histone H3 (41). Since the DNA damage response (DDR) and cell cycle checkpoint proteins are substrates for and regulated by the 14-3-3 family of chaperone proteins (42) and PP2A inhibition may increase available p-Ser binding domains, we next assessed whether LB100 enhances available p-Ser binding sites recognized by 14-3-3 proteins. In SKOV-3 cells following 24 h treatment with LB100 and cisplatin, LB100 alone or in combination with cisplatin (5 μM and 15 μM) altered the generation of p-Ser binding sites compared to control and cisplatin alone, respectively (Fig. 3C).
LB100 sensitizes tumor cells to cisplatin in vivo
We next investigated the biological efficacy of LB100-induced cisplatin sensitization in an in vivo mouse model of metastatic ovarian carcinoma. Tumors were established in female athymic nude mice via i.p. injection of SKOV-3 cells expressing firefly luciferase, recapitulating the peritoneal spread observed in the clinical setting (43). Mice were randomized into four groups [vehicle (PBS) control (n=4), LB100 (1.5 mg/kg) (n=5), cisplatin (1.5 mg/kg) (n=5), and LB100 (given 1 h prior to cisplatin) + cisplatin (n=5)] and treated six times, with drugs administered every other day, starting from four days after tumor inoculation. Following the final treatment, mice were observed until pre-determined health concerns necessitated euthanization. Dose and treatment schedules were determined from biologic profiles of each agent determined in previous studies (18, 19, 27) and disease progression was monitored by bioluminescence imaging (BLI).
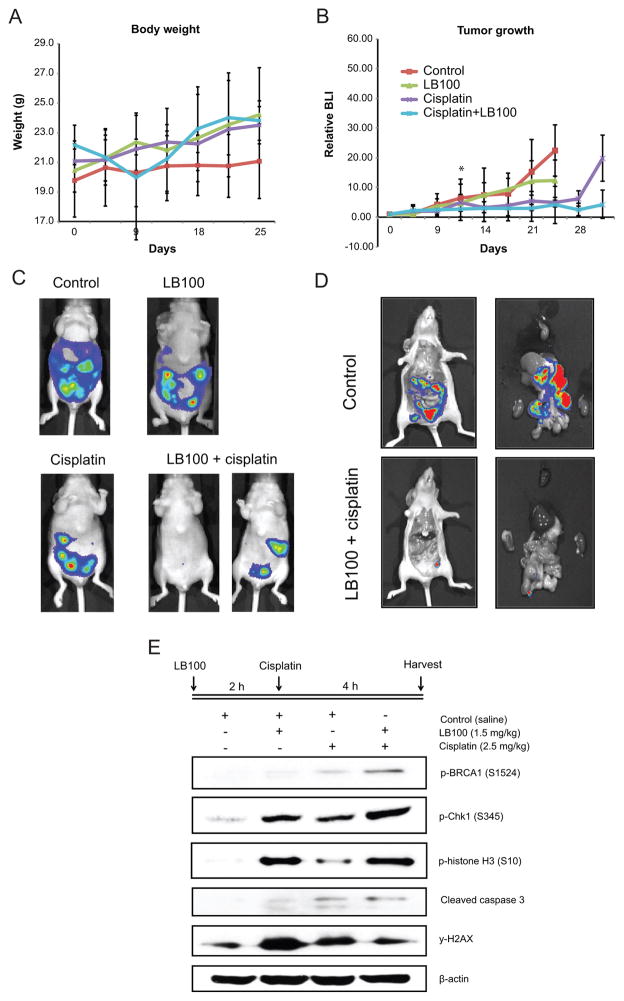
There was no significant difference in mean body weight among the four treatment groups, indicating minimal toxicity of the compounds (Fig. 4A). LB100 alone did not alter tumor growth, as assessed by BLI (Fig. 4B, 4C). On the other hand, cisplatin (relative intensity 5.0 ± 1.6) and the combination of cisplatin and LB100 (4.1 ± 6.3) significantly delayed disease progression by day 25 compared to vehicle (22.4 ± 8.7) and LB100 alone (12.4 ± 6.7). By day 35, the combination treatment significantly delayed tumor progression compared to cisplatin alone (4.3 ± 4.7 vs. 19.8 ± 7.9, p=0.03). By day 25 and day 28, massive ascites developed in the LB100 and control group respectively, necessitating euthanasia. Ex vivo analysis confirmed that the BLI signals were originating from the tumors (Fig. 4D).
Figure 4.
LB100 sensitizes SKOV-3 intraperitoneal xenografts to the cytotoxic effects of cisplatin. Mice bearing SKOV-3 intraperitoneal metastatic tumors were treated with PBS (vehicle control) (n=4), LB100 (1.5mg/kg) (n=5), cisplatin (1.5mg/kg) (n=5), or LB100 (1.5mg/kg 1 hr pre-cisplatin) + cisplatin (1.5mg/kg) (n=5) for 6 session given every other day. A, No significant difference in body weight indicates minimal toxicity. B, LB100+cisplatin combination treatment significantly slows tumor growth, as measured by bioluminescence signaling, compared to other treatment groups. Data is represented as mean ± SD of relative total photon flux compared to day 1 of treatment. C, Representative imaging of each treatment group. An average (left) and best (right) responder in the combination group is shown by comparison. D, ex-vivo imaging confirms that the signal obtained originates from tumor cells. E, Western blot obtained from ex-vivo tumor samples illustrates hyperphosphorylation of γH2AX, BRCA-1, Chk-1.
Next, we assessed whether the same molecular mechanisms observed in vitro were involved in the LB100-induced sensitization of intraperitoneal tumors to cisplatin. Consistent with the in vitro findings, LB100 alone induced hyperphosphorylation of BRCA1, Chk1, γH2AX, and p-histone H3 (Fig. 4E, Supplementary Fig. S5B). BRCA1 and Chk-1 phosphorylation was further enhanced with cisplatin and LB100 combination treatment compared to cisplatin alone. Combination treatment also resulted in hyperphosphorylation of histone H3 and caspase 3 cleavage, indicating progression into mitosis and enhanced apoptosis. In contrast to these findings with the tumor samples, LB100 did not induce hyperphosphorylation of γH2AX in the kidney (Supplementary Fig. S5C).
Discussion
The aim of our study was to assess whether LB100, a small-molecular inhibitor of PP2A that is currently undergoing a phase I trial for solid tumors (21), can sensitize pre-clinical models of ovarian cancer to cisplatin. Our results show that pre-treatment with LB100 enhances cisplatin-induced apoptosis for various ovarian cancer cells in vitro. This effect was observed for both low (IC25) and high (IC75) doses of cisplatin and was correlated with constitutive phosphorylation of key DNA damage response proteins leading to persistent DNA damage and abrogation of cell cycle arrest, culminating in apoptosis (schematically shown in Figure 3D). In our in vivo model of intraperitoneal metastatic ovarian carcinoma using the SKOV-3 cell line, we also observed delayed tumor growth when LB-100 was combined with cisplatin. Notably, at the protocol endpoint, the tumors in mice that were treated with LB100 and cisplatin did not relapse while mice treated with cisplatin alone eventually developed progressive disease.
The majority of ovarian cancers harbor inactivating mutations of p53 (9). Since p53 orchestrates the G1 to S phase cell cycle checkpoint, cancer cells with mutant p53 depend on G2/M arrest for maintaining genomic integrity following DNA damaging therapy (39). Entry from G2 into mitosis depends on the activation and nuclear localization of Cdc2/cyclin B, which is negatively regulated by Wee1 and Chk1 kinase and positively regulated by Cdc25C phosphatase. As such, cancer cells resistant to DNA damage often induce increased expression and function of G2/M checkpoint kinases in response to genotoxic stress and pharmacologic inhibition of these kinases can sensitize cancer cells to platinum compounds (11). Specific kinase inhibitors have clinical limitations, however, since resistant cells possess alternate pathways that can circumvent inhibition (44). On the other hand, ubiquitous Ser/Thr phosphatases such as PP2A are extensively involved in regulation of the DNA response pathway and potentially allow manipulation of multiple signaling pathways through the use of a single agent (45).
PP2A is an attractive target for DNA damage sensitization for many reasons. Extensive studies in Xenopus have shown that PP2A is induced as part of the DNA damage response and is involved in G2/M arrest (46). Thus, inhibition of PP2A leads to aberrant entry into mitosis, resulting in mitotic catastrophe and apoptosis. PP2A also regulates Chk1, a critical mediator of DDR, through a negative feedback loop that maintains Chk1 in a low-activity state during normal cell division, while priming it for rapid response upon DNA damage (36). This integral relationship is maintained by continuous phosphorylation and dephosphorylation of Chk1 (S345) (36, 47). Following DNA damage and DSB formation, ATM/ATR activates Chk1 via phosphorylation at S345, a site negatively regulated by PP2A-mediated dephosphorylation. Constitutive phosphorylation of S345 induces E3 ligase mediated ubiquitination and proteasomal degradation, and thus is critical for Chk1 protein stability (48). Our results show that pharmacologic and genetic inhibition of PP2A by LB100 and PP2A-C shRNA respectively, induces hyperphosphorylation of Chk1 (S345) without altering the phosphorylation state of other serine residues (Fig. S4B). It is possible, therefore, that the LB100-induced cisplatin sensitization may be in part due to deregulation of the negative feedback loop between PP2A and Chk1, rendering Chk1 less effective in the DNA response pathway.
Through its phosphatase activity, PP2A maintains the relative number and distribution of docking sites for chaperone proteins carrying specific phospho-Ser/Thr binding motifs, such as 14-3-3 and BRCA1 (49, 50). These docking sites exist on a vast array of proteins within the cell, ranging from DNA damage response factors to housekeeping proteins (51, 52). The 14-3-3 family of proteins bind to target proteins carrying specific p-Ser/Thr recognition sequences and have been demonstrated to affect the enzymatic activity, DNA-binding activity, sequestration, and protein-protein interactions of these target proteins (49). In our study, LB100-treated SKOV-3 cells showed widespread increased expression of p-Ser 14-3-3 binding motifs compared to control treatment (Fig. 3C), and showed altered phosphorylation states of proteins known to interact with 14-3-3, such as Wee1 and Chk1. Whether 14-3-3 proteins directly or indirectly affect the activity of these proteins following LB100 and cisplatin combination treatment is yet to be determined. Nonetheless, the results of our study show that LB100-induced modulation of cellular 14-3-3 motifs is correlated with cell cycle progression and enhanced apoptosis.
Our results also consistently showed that LB100, either alone or in combination with cisplatin, induces hyperphosphorylation of BRCA1 at distinct residues, which was maintained for 72 h. Previous studies have shown that the phosphorylation state of BRCA1 disrupts its interaction with Chk1 and may render cells more sensitive to caspase-3 mediated apoptosis (14, 53). BRCA1 contains C-terminal domains (BRCT) that bind to specific Ser/Thr residues and are integral for BRCA1-mediated DNA damage response. As such, the availability of the BRCT binding domain may be necessary for the proper coordinated response following induction of DNA damage leading to DNA repair (50). LB100-induced deregulation of Ser/Thr motif distribution, as shown in our study, may lead to redistribution of docking-proteins in a way that biases the cisplatin-induced DNA damage response pathway towards mitotic catastrophe and apoptosis.
In the context of LB100-induced constitutive phosphorylation of the DNA damage pathway and G2/M arrest abrogation, it will be of interest to assess the efficacy of LB100 in combination with other preclinical compounds such as inhibitors of Chk1, Wee-1, and PARP1, with and without chemo-radiation. Our results add to the growing literature regarding the efficacy of LB100, and illustrate a potential approach to enhancing cisplatin efficacy during the treatment of ovarian cancer. Given the proven, yet toxicity-limited, use of cisplatin in the clinical setting, we propose that LB100 may be an additive or dose-lowering agent that can enhance/maintain the cytotoxic effect of cisplatin without adding undue toxicity.
Supplementary Material
Acknowledgments
Financial support: This research was funded by the Intramural Research Program of the NIH, the National Cancer Institute, and the National Institute of Neurological Disorders and Stroke.
We would like to thank George Leiman for editorial assistance.
Abbreviations
- PP2A
Protein phosphatase 2A
- DSB
double strand break
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- i.p
intraperitoneal
- BLI
bioluminescence imaging
- IC50
inhibitory concentration
Footnotes
The authors declare no conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–82. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 4.Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–93. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 6.Wong E, Giandomenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–28. [PubMed] [Google Scholar]
- 8.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–21. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat Commun. 2014;5:3156. doi: 10.1038/ncomms4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouliot LM, Chen YC, Bai J, Guha R, Martin SE, Gottesman MM, et al. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Cancer Res. 2012;72:5945–55. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–9. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Chowdhury D. What goes on must come off: phosphatases gate-crash the DNA damage response. Trends Biochem Sci. 2011;36:569–77. doi: 10.1016/j.tibs.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin SA, Ouchi T. BRCA1 phosphorylation regulates caspase-3 activation in UV-induced apoptosis. Cancer Res. 2005;65:10657–62. doi: 10.1158/0008-5472.CAN-05-2087. [DOI] [PubMed] [Google Scholar]
- 15.Clemenson C, Marsolier-Kergoat MC. DNA damage checkpoint inactivation: adaptation and recovery. DNA Repair (Amst) 2009;8:1101–9. doi: 10.1016/j.dnarep.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, et al. Sustained activation of JNK/p38 MAPK pathways in response to cisplatin leads to Fas ligand induction and cell death in ovarian carcinoma cells. J Biol Chem. 2003;278:19245–56. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 17.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–4. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, et al. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res. 2013;19:4422–32. doi: 10.1158/1078-0432.CCR-13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Kovach JS, Johnson F, Chiang J, Hodes R, Lonser R, et al. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc Natl Acad Sci U S A. 2009;106:11697–702. doi: 10.1073/pnas.0905930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martiniova L, Lu J, Chiang J, Bernardo M, Lonser R, Zhuang Z, et al. Pharmacologic modulation of serine/threonine phosphorylation highly sensitizes PHEO in a MPC cell and mouse model to conventional chemotherapy. PLoS One. 2011;6:e14678. doi: 10.1371/journal.pone.0014678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung VMA. Phase I study of LB-100 with docetaxel in solid tumors. 2013 Available from: http://www.clinicaltrials.gov/ct2/show/NCT01837667.
- 22.Wei BR, Hoover SB, Ross MM, Zhou W, Meani F, Edwards JB, et al. Serum S100A6 concentration predicts peritoneal tumor burden in mice with epithelial ovarian cancer and is associated with advanced stage in patients. PLoS One. 2009;4:e7670. doi: 10.1371/journal.pone.0007670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–72. [PubMed] [Google Scholar]
- 24.Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol Ther. 2005;4:187–94. [PubMed] [Google Scholar]
- 25.Hall MD, Telma KA, Chang KE, Lee TD, Madigan JP, Lloyd JR, et al. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin and Other Platinum Complexes. Cancer Res. 2014;74:3913–22. doi: 10.1158/0008-5472.CAN-14-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madigan JP, Bodemann BO, Brady DC, Dewar BJ, Keller PJ, Leitges M, et al. Regulation of Rnd3 localization and function by protein kinase C alpha-mediated phosphorylation. Biochem J. 2009;424:153–61. doi: 10.1042/BJ20082377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13:4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 28.Bakhsheshian J, Wei BR, Chang KE, Shukla S, Ambudkar SV, Simpson RM, et al. Bioluminescent imaging of drug efflux at the blood-brain barrier mediated by the transporter ABCG2. Proc Natl Acad Sci U S A. 2013;110:20801–6. doi: 10.1073/pnas.1312159110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debernardis D, Sire EG, De Feudis P, Vikhanskaya F, Valenti M, Russo P, et al. p53 status does not affect sensitivity of human ovarian cancer cell lines to paclitaxel. Cancer Res. 1997;57:870–4. [PubMed] [Google Scholar]
- 30.Kelland LR, Murrer BA, Abel G, Giandomenico CM, Mistry P, Harrap KR. Ammine/amine platinum(IV) dicarboxylates: a novel class of platinum complex exhibiting selective cytotoxicity to intrinsically cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1992;52:822–8. [PubMed] [Google Scholar]
- 31.Kelland LR, Sharp SY, O’Neill CF, Raynaud FI, Beale PJ, Judson IR. Mini-review: discovery and development of platinum complexes designed to circumvent cisplatin resistance. J Inorg Biochem. 1999;77:111–5. [PubMed] [Google Scholar]
- 32.Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, Joenje H, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–74. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 33.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–8. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 34.Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci U S A. 1998;95:12370–5. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlessi L, Buscemi G, Fontanella E, Delia D. A protein phosphatase feedback mechanism regulates the basal phosphorylation of Chk2 kinase in the absence of DNA damage. Biochim Biophys Acta. 2010;1803:1213–23. doi: 10.1016/j.bbamcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Mol Cell Biol. 2006;26:7529–38. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–94. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussenzweig A, Paull T. DNA repair: tails of histones lost. Nature. 2006;439:406–7. doi: 10.1038/439406a. [DOI] [PubMed] [Google Scholar]
- 39.Sorenson CM, Barry MA, Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990;82:749–55. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- 40.Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21:245–55. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci U S A. 1998;95:7480–4. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piwnica-Worms H. Cell cycle. Fools rush in Nature. 1999;401:535, 7. doi: 10.1038/44029. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton TC, Young RC, Louie KG, Behrens BC, McKoy WM, Grotzinger KR, et al. Characterization of a xenograft model of human ovarian carcinoma which produces ascites and intraabdominal carcinomatosis in mice. Cancer Res. 1984;44:5286–90. [PubMed] [Google Scholar]
- 44.Lovly CM, Shaw AT. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin Cancer Res. 2014;20:2249–56. doi: 10.1158/1078-0432.CCR-13-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wurzenberger C, Gerlich DW. Phosphatases: providing safe passage through mitotic exit. Nat Rev Mol Cell Biol. 2011;12:469–82. doi: 10.1038/nrm3149. [DOI] [PubMed] [Google Scholar]
- 46.Margolis SS, Perry JA, Forester CM, Nutt LK, Guo Y, Jardim MJ, et al. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127:759–73. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng A, Maller JL. Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene. 2010;29:5977–88. doi: 10.1038/onc.2010.371. [DOI] [PubMed] [Google Scholar]
- 48.Leung-Pineda V, Huh J, Piwnica-Worms H. DDB1 targets Chk1 to the Cul4 E3 ligase complex in normal cycling cells and in cells experiencing replication stress. Cancer Res. 2009;69:2630–7. doi: 10.1158/0008-5472.CAN-08-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hermeking H. The 14-3-3 cancer connection. Nat Rev Cancer. 2003;3:931–43. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad DH, Yaffe MB. 14-3-3 proteins, FHA domains and BRCT domains in the DNA damage response. DNA Repair (Amst) 2009;8:1009–17. doi: 10.1016/j.dnarep.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15:163–77. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt HC, Yaffe MB. Phospho-Ser/Thr-binding domains: navigating the cell cycle and DNA damage response. Nat Rev Mol Cell Biol. 2013;14:563–80. doi: 10.1038/nrm3640. [DOI] [PubMed] [Google Scholar]
- 53.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30:285–9. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.