Abstract
Thirty-day readmission (30-DR) has become an important quality-of-care measure. Allogeneic hematopoietic cell transplantation (allo-HCT) presents a medical setting with higher readmission rates. We analyzed factors affecting 30-DR and its impact on patient outcomes and on health care costs in 91 patients who underwent reduced-toxicity conditioning (RTC) allo-HCT with fludarabine and busulfan. The patient cohort was divided into 2: the readmission group (R-gp) or the no-readmission group (NR-gp). Overall, 38% (n = 35) required readmission with a median time to readmission of 14 days. In multivariate analysis, only documented infection during the index admission predicted 30-DR, P =.01. With a median follow-up of 18 months (range, 1 to 69) for surviving patients, the 2-year overall survival was 49% and 58% in the R-gp and NR-gp respectively, P =.48. The 1-year nonrelapse mortality in R-gp and NR-gp was 18% and 13% respectively, P =.43. The median post-transplantation hospital charges in the R-gp and NR-gp were $85,115 (range, $32,015 to $242,519) and $45,083 (range, $10,715 to $485,456), P = .0002. In conclusion, only documented infections during the index hospitalization influenced 30-DR after RTC allo-HCT. Although 30-DR did not adversely affect mortality or survival, it was associated with significantly increased 100-day post-transplantation hospital charges, thus supporting its role as a quality-of-care measure in allo-HCT patients.
Keywords: Allogeneic hematopoietic cell, transplantation, Thirty-day readmission, Health care cost, Reduced-toxicity conditioning
INTRODUCTION
Readmission to the hospital within 30 days of discharge from a prior admission has emerged as an important topic of discussion and debate within the medical community. Centers for Medicare and Medicaid Services (CMS) considers 30-day readmission (30-DR) as a quality-of-care indicator and has recently implemented the Hospital Readmission Reduction Program with proposed penalization of hospitals with high rates of risk-adjusted readmissions [1]. Review of the claims data of a large cohort of Medicare patients suggests approximately 20% readmission rates for its beneficiaries and also noted that only 10% of such readmissions were planned [2]. Published data pertaining to hospital readmissions show significant heterogeneity in readmission rates based on geographical location, diagnosis, severity of illness, and socioeconomic status [1-7]. Various strategies, including improving transition of care, effective discharge planning, immediate postdischarge telephone encounter, and short-term clinic follow-up, have been identified as potential measures to decrease readmissions [5,8-10]. Readmission rates alone maybe a crude gauge of quality-of-care, as studies have shown that, although it does adversely affect health care costs and quality of life, increased 30-DR in heart failure patients was associated with lower 30-day mortality, possibly because of increased use of hospital resources [11-13].
Allogeneic hematopoietic cell transplantation (allo-HCT) is a widely used therapeutic strategy in the management of various hematologic disorders. Although potentially curative, allo-HCT’s therapy-related morbidity is significant, and the readmission rates in allo-HCT patients are higher than those of their peers, ranging from 39% to 51% [14-17]. A handful of prior reports have shown that infections during index admission, HCT-comorbidity index (HCT-CI), donor type, stem cell source, and conditioning regimen may predict the risk of readmission in allo-HCT recipients [14,15,18]. The effect of readmission on survival is more contentious, with some reports suggesting inferior survival [14,15], whereas 1 report in the pediatric population showed better survival in the readmission group [18]. The impact of 30-DR after allo-HCT on health care costs is not known. In this study, we analyzed 30-DR rates and its predictors in a cohort of patients with hematologic malignancies who underwent reducedtoxicity conditioning (RTC) allo-HCT. We also evaluated the impact of 30-DR on mortality, survival, and health care costs of allo-HCT recipients.
MATERIALS AND METHODS
Patient Population
Ninety-four consecutive patients underwent peripheral blood allo-HCT after RTC with fludarabine/busulfan between August 2007 and December 2012 at our transplantation center. Three patients who died before discharge from the index transplantation admission were excluded. The remaining 91 patients are the subjects of this report. The cohort was divided into 2 groups based on whether they were readmitted within 30 days of discharge after index transplantation admission: the readmission group (R-gp, n = 35) and the no readmission group (NR-gp, n = 56). The conditioning regimen consisted of intravenous fludarabine (total dose, 150 to 160 mg/m2) and busulfan (total dose, 6.4 mg/Kg or 12.8 mg/Kg) with or without thymoglobulin (total dose, 6.0 mg/Kg). High-resolution HLA typing was done at the allele level for class-I (HLA - A, - B, - C) and class II (HLA-DRB1) molecules as described previously [19]. Graft-versus-host-disease (GVHD) prophylaxes included a calcineurin inhibitor (tacrolimus or cyclosporine) combined with either mycophenolate mofetil or short-course methotrexate [20]. As standard institutional practice, patients received antibacterial, antiviral (acyclovir or valacyclovir), and anti-fungal (fluconazole) prophylaxis.
All patients were admitted to the bone marrow transplantation service for the conditioning regimen and allograft infusion, and they remained inpatient (IP) until neutrophil recovery and resolution of early complications. After discharge, patients were monitored daily by the bone marrow outpatient service until day +100. Data pertaining to patient demographics, disease- and transplantation-related parameters, and patient outcomes are prospectively maintained by the dedicated transplantation data manager at our center. Readmission and hospital charges were retrospectively obtained from department of decision support. The study was approved by the institutional review board and protocol review and monitoring committee at our institution.
Study Definitions
Thirty-day readmission was defined as any patient who required inpatient admission within 30 days of discharge from the index transplantation admission for any reason. The primary objective was to evaluate the factors predicting 30-DR in patients undergoing RTC allo-HCT. The lists of variables utilized in our analysis are shown in Tables 1 and 2 and include patient-, disease-, and transplantation-related factors and caregiver support available. The hospital charges up to day +100 after transplantation incurred after discharge from index transplantation admission in the 2 groups were collected and analyzed to identify the effect of readmission on health care costs. The impact of 30-DR on progression free survival (PFS), overall survival (OS), relapse rate (RR), and nonrelapse morality (NRM) was evaluated. Neutrophil recovery was defined as first of 3 consecutive days to an absolute neutrophil count (ANC) ≥ .5 × 109/L, after post-transplantation nadir and platelet recovery as first of 7 consecutive days to platelet count ≥ 20 × 109/L without platelet transfusion. OS was defined as the time to death from any cause from the date of transplantation. Death and relapse/progression were considered events for PFS. Surviving patients were censored at time of last follow-up. NRM was defined as death from any cause other than disease progression or relapse.
Table 1.
Baseline Patient Characteristics
| Characteristic | Readmission (n = 35) | Not Readmitted (n = 56) | P Value |
|---|---|---|---|
| Age, median (range) | 56 (17-72) | 54 (22-68) | .23 |
| Male | 21 (60) | 34 (61) | .99 |
| Diagnosis | .93 | ||
| ALL/AML/MDS | 23 (65.7) | 39 (70) | |
| CLL/CML | 2 (5.7) | 3 (5) | |
| Hodgkin/NHL/Others | 10 (28.6) | 14 (25) | |
| Disease risk* | .18 | ||
| Low | 16 (45.7) | 24 (43) | |
| Intermediate | 3 (8.6) | 13 (23) | |
| High | 16 (45.7) | 19 (34) | |
| Disease status | .49 | ||
| Chemosensitive | 23 (66) | 41 (73) | |
| Refractory disease | 12 (34) | 15 (27) | |
| Prior number of therapy, median (range) | 2 (1-6) | 2 (0-6) | .65 |
| Prior radiation therapy | 2 (6) | 8 (14) | .31 |
| Prior autologous transplantation | 2 (6) | 6 (11) | .71 |
| KPS, median (range) | 80 (60-100) | 85 (70-100) | .44 |
| HCT-CI, median (range) | 2 (0-7) | 1 (0-5) | .31 |
| Busulfan dose | .12 | ||
| High | 27 (77) | 34 (61) | |
| Low | 8 (23) | 22 (39) | |
| Patients receiving thymoglobulin | 23 (66) | 31 (55) | .38 |
| Donor type | |||
| Unrelated | 19 (54) | 28 (50) | .83 |
| Matched sibling | 16 (46) | 28 (50) | |
| HLA mismatch† | .99 | ||
| Allele level | 1 (2) | 3 (5) | |
| Antigen level | 1 (2) | 1 (2) | |
| Infused CD34 cell dose‡, median (range) | 6.5 (2.7-12.8) | 6.5 (1.8-15.1) | .98 |
| Infused CD3 cell dose§, median (range) | 31.3 (9.6-58.5) | 32.4 (11.5-94.5) | .48 |
| GVHD prophylaxis | .83 | ||
| MTX + calcineurine inhibitor | 22 (63) | 33 (59) | |
| MMF + calcineurine inhibitor | 13 (37) | 23 (41) | |
| Caregiver | .13 | ||
| Spouse | 17 (49) | 37 (66) | |
| Other | 18 (51) | 19 (34) | |
| Number of caregivers | .99 | ||
| 1 | 30 (86) | 47 (84) | |
| 2+ | 5 (14) | 9 (16) | |
| Documented unreliable caregiver | 3 (9) | 5 (9) | .99 |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CLL, chronic lymphoblastic leukemia; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; KPS, Karnofsky performance status; HCT-CI, hematopoietic cell transplantation-comorbidity index; ATG, antithymocyte globulin; HLA, human leukocyte antigen; GVHD, graft versus host disease; MTX, methotrexate; MMF, mycophenolate mofetil.
Data presented are n (%) unless otherwise indicated.
American Society for Blood and Marrow Transplantation: standard criteria [26].
High-resolution HLA typing at the allele level for A, B, C, and DRB-1 for all patients.
Cell dose × 106/kg patient body weight.
Cell dose × 107/kg patient body weight.
Table 2.
Post-transplantation Outcomes
| Outcome | Readmission (n = 35) | Not Readmitted (n = 56) | P Value |
|---|---|---|---|
| Neutrophil recovery*, median (range) | 14.5 (5-27) | 17 (5-23) | .09 |
| Platelet recovery†, median (range) | 13 (7-44) | 12 (7-19) | .21 |
| Acute GVHD, time to onset, median (range), d | 40.5 (14-279) | 45 (12-137) | .97 |
| Acute GVHD, grade II-IV | 11 (31) | 12 (21) | .11 |
| Length of stay for index admission, median (range), d | 25 (20-38) | 26 (14-73) | .68 |
| Documented infection during index admission, n (%) | 14 (40) | 11 (20) | .05 |
| Time to readmission, median (range), d | 14 (1-29) | NA | |
| Time to readmission | |||
| ≤ 7 days, n (%) | 8 (23) | NA | |
| > 7 but ≤ 30 days, n (%) | 27 (77) | NA | |
| Cause of readmission‡, n (%) | |||
| Documented infection | 12 (31.6) | NA | |
| Fever without documented infection | 6 (15.8) | NA | |
| Cardiovascular | 6 (15.8) | NA | |
| Respiratory | 4 (10.5) | NA | |
| Gastrointestinal | 4 (10.5) | NA | |
| GVHD | 3 (7.9) | NA | |
| Other§ | 3 (7.9) | NA | |
| Greater than 1 cause of readmission, n (%) | 3 (9) | NA | |
| Length of readmission stay, median (range), d | 3 (1-34) | NA | |
| Follow-up surviving patients, median (range), d | 480 (71-2005) | 532 (31-2080) |
GVHD indicates graft-versus-host disease; PFS, progression-free survival.
Defined as absolute neutrophil count (ANC) > 500 × 3 days.
Defined as platelets > 20 × 7 days without transfusion support.
Three patients were readmitted for multiple reasons, all causes were included.
Includes neurologic, genitourinary, and musculoskeletal complications.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics. Categorical data were described using contingency tables including counts and percentages. Continuously scaled measures were summarized with descriptive statistical measures (ie, mean [± SD] or median [range]). Fisher’s exact test and Wilcoxon’s rank-sum test were used to compare categorical and continuous variables, respectively. The univariate and multivariable logistic regression models were used to assess the risk factors for readmission. Survival curves were estimated using the Kaplan-Meier method and survival between readmission groups was compared using a 2-sided log-rank test. The cumulative incidences of NRM and RR were estimated by considering these 2 events as competing risks [21]. Cox proportional hazards model was constructed for potential variables predicting 30-DR, using a limited backward selection procedure. Variables considered in the model were those significant at α = .20 level from the univariable model. Variables remaining in the fi8/29/2015nal model were significant at α = .05 level. Estimates for hazard ratios and corresponding 95% confidence intervals (CI) were obtained for each significant prognostic factor. All P values are 2 sided. Statistical analyses were carried out using SAS 8.2, SPLUS, version 2000 (Insightful Corp., Seattle, WA) and R statistical software (Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
The baseline characteristics of the patient cohort (n = 91) are shown in Table 1. All patients received T cell–replete, unmanipulated, peripheral blood allografts after RTC with fludarabine/busulfan from matched sibling (n = 44) or unrelated (n = 47) donors, P = .83. There were no differences between the R-gp and NR-gp with regards to age, gender, race, and caregiver status. The proportion of refractory disease in the R-gp and NR-gp was 34% (n = 12) and 27% (n = 15) respectively, P = .49. No difference was noted in the number of prior therapies, including previous radiation or autografting between the groups (P > .1).
Thirty-day Readmissions
A total of 35 patients (38%) were readmitted within 30 days of discharge after index transplantation admission, with a median time to readmission of 14 days (range, 1 to 29) (Table 2). Documented infection (n = 12) was the leading cause of readmission followed by cardio-pulmonary complications (n = 10), fever without a documented infection (n = 6), gastrointestinal issues (n = 4), and GVHD (n = 3). Documented infections leading to readmissions included central line–associated bloodstream infections (n = 4), BK-virus hemorrhagic cystitis (n = 3), Clostridium difficile colitis (n = 2), and 1 case each of bacterial urinary tract infection, viral pneumonitis, and cytomegalovirus reactivation. The median length of stay after readmission was 3 days (range, 1 to 34). Eight (23%) of the 35 readmission occurred within a week of index discharge. The main causes for early readmissions were fever (n = 3) and cardio-pulmonary complications (n = 3).
We analyzed the data to identify risk factors that may predict 30-DR after initial discharge. The variables used and the results of the univariate analysis are shown in Table 3. Social factors, such as the type of primary caregiver (spouse versus others) and the number of caregivers, did not affect readmission risk. In multivariate analysis only, documented infections during index admission predicted 30-DR, (40% versus 20%; odds ratio, 5.24; 95% CI, 1.42 to 19.32; P =.01). A proportional hazards regression analysis was performed to identify risk factors for documented infections during the initial hospital stay. None of the variables tested, including use of antithymocyte globulin in the conditioning regimen, were found to be significantly associated with infections during index transplantation stay (P > .05) (Supplementary Table 1S).
Table 3.
Univariate Analysis of Risk Factors for Readmissions
| Risk Factor | Odds Ratio* (95% CI) | P Value |
|---|---|---|
| Age (per 10-year increments) | 1.01 (.97-1.05) | .54 |
| Gender | .97 (.41-2.30) | .95 |
| Diagnosis (acute leukemia/MDS versus others) | .84 (.34-2.06) | .70 |
| Disease risk | ||
| Low | - | - |
| Intermediate | .35 (.09-1.41) | .08 |
| High | 1.26 (.50-3.16) | .12 |
| Disease status (chemosensitive versus refractory) | .70 (.28-1.75) | .45 |
| Prior number of therapy | 1.01 (.67-1.52) | .95 |
| Prior radiation therapy | .36 (.07-1.82) | .22 |
| Prior autologous transplantation | .51 (.10-2.66) | .42 |
| KPS | .98 (.93-1.03) | .37 |
| HCT-CI | 1.22 (.97-1.53) | .10 |
| High busulfan dose (high versus low) | 2.18 (.84-5.67) | .11 |
| GVHD prophylaxis (MMF versus MTX) | .85 (.36-2.02) | .71 |
| Pretransplantation ATG | 1.55 (.65-3.71) | .33 |
| Donor type (sibling versus unrelated) | .84 (.36-1.96) | .69 |
| HLA mismatch | 3.0 (.08-107.45) | .55 |
| Infused CD34 cell dose | .97 (.85-1.12) | .69 |
| Infused CD3 cell dose | .98 (.95-1.02) | .30 |
| Days to neutrophil recovery | .95 (.84-1.08) | .41 |
| Days to platelet recovery | 1.11 (.97-1.26) | .13 |
| Acute GVHD, median days to onset (range) | 1.00 (.99-1.02) | .81 |
| Severity of acute GVHD (< grade II versus ≥ grade II) | 1.68 (.65-4.38) | .29 |
| Length of stay for index admission | .99 (.93-1.05) | .78 |
| Infection during index admission | 2.72 (1.06-7.01) | .04 |
| Caregiver (spouse versus other) | .49 (.21-1.15) | .10 |
| Number of caregivers (1 versus > 1) | 1.15 (.35-3.76) | .82 |
CI indicates confidence interval; MDS, myelodysplastic syndrome; KPS, Karnofsky performance status; ATG, antithymocyte globulin; HCT-CI, hematopoietic cell transplantation–comorbidity index; MMF, mycophenolate mofetil; MTX, methotrexate; HLA, human leukocyte antigen; GVHD, graft-versus-host disease.
Odds ratio of greater than 1 implies risk factor more in the readmission group compared to nonreadmission group.
Mortality and Survival
The median follow-up for surviving patients for the entire cohort was 521 days (range, 31 to 2080). At last follow-up, 51.4% (n = 18) in the R-gp and 60.7% (n = 34) in the NR-gp were alive. The estimated 1-year and 2-year OS in the R-gp and NR-gp were 58% and 67% and 49% and 58%, respectively (Figure 1); log-rank P value = .48. The 1-year PFS was 50% and 50.3% in the R-gp and NR-gp, respectively; P = .8 (Figure 2). The 100-day NRM was 0% and 3.5% in R-gp and NR-gp respectively (P = .43) and the corresponding 1-year NRM and RR were 18% and 13% (P = .43) and 32% and 37% (P = .79), respectively (Figure 3).
Figure 1.
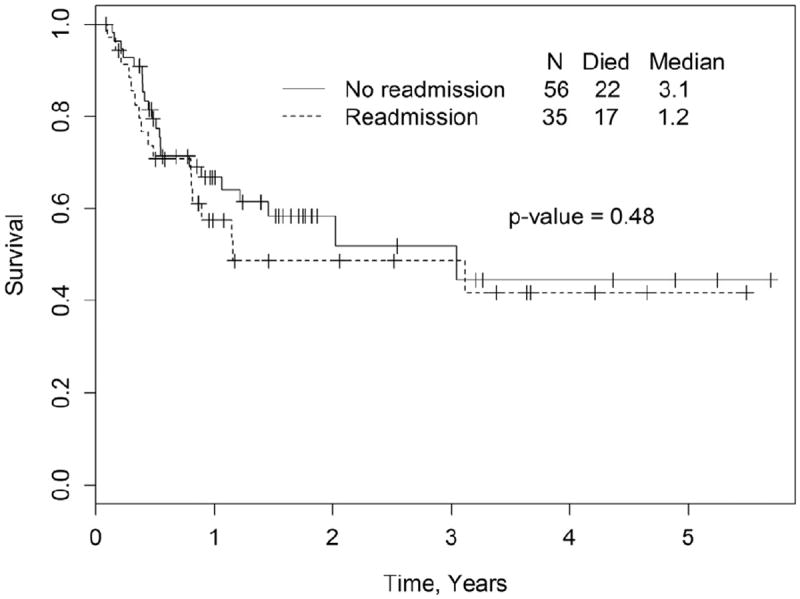
Kaplan-Meier estimates of overall survival (OS) in the readmission group (R-gp) and no-readmission group (NR-gp), P = .48 by log-rank test.
Figure 2.
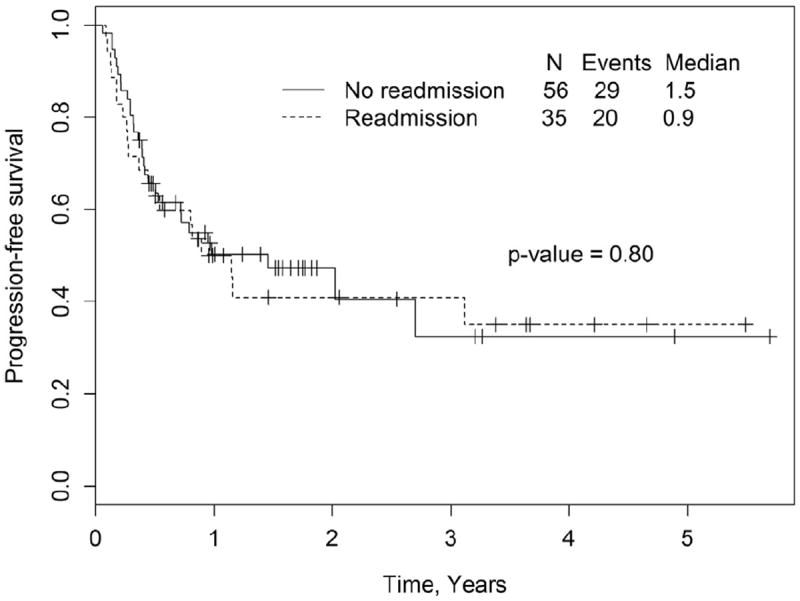
Kaplan-Meier estimates of progression-free survival (PFS) in the readmission group (R-gp) and no-readmission group (NR-gp), P = .8 by log-rank test.
Figure 3.
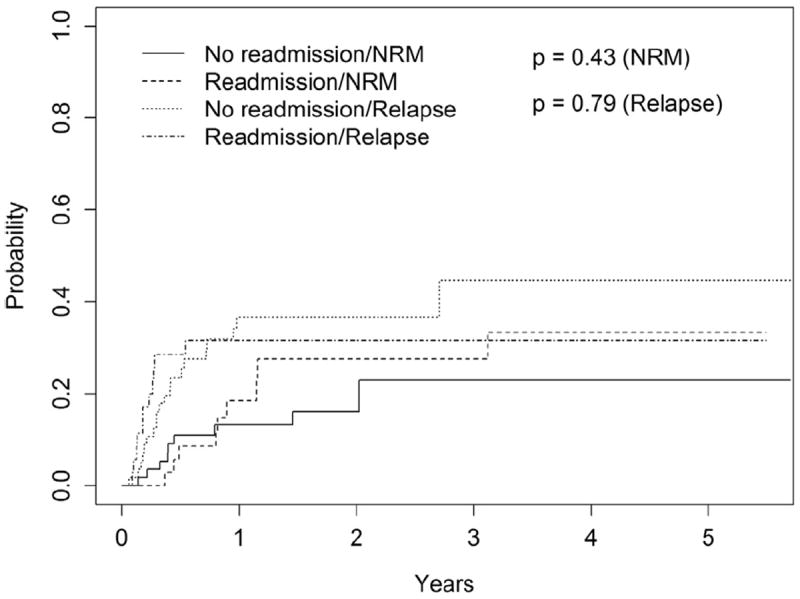
Cumulative incidence curves of non-relapse mortality (NRM) and relapse rate (RR) in the readmission group (R-gp) and no-readmission group (NR-gp).
Health Care Costs
Data for health care cost analysis was extracted from electronic medical records and billing up to 100 days after allograft and does not include hospital charges incurred during the index admission (Table 4). Limited information pertaining to outpatient (OP) costs was available before 2008 because of an institution-wide change in billing record-keeping. OP hospital charges were not available for 3 patients in R-gp and 5 patients in NR-gp. One patient was excluded from the NR-gp cost analysis, as the patient was discharged from the index admission on hospice and no additional information was available. The mean IP charges in the R-gp and NR-gp were $45,982 (range, $5997 to $210,669) and $24,292 (range, 0 to $442,248), respectively; P < .0001. The OP charges did not differ between the 2 groups, P = .22. The mean post-transplantation 100-day total hospital charges incurred by the R-gp and NR-gp were $93,925 (range, $32,015 to $242,519) and $69,143 (range, $10,715 to $485,456), respectively; P = .0002.
Table 4.
Hospital Charges
| Hospital Charges* | Readmission
|
Not Readmitted
|
P Value | ||
|---|---|---|---|---|---|
| n | Median/Mean (range) | n | Median/Mean (range) | ||
| Inpatient charges | 32 | 25,698.73/45,982.41 (5,997.15-210,669.08) | 54 | 0/24,292.30 (0-442,248.04) | <.0001 |
| Outpatient charges | 32 | 43,280.43/47,942.85 (0-118,513.37) | 50 | 37,834.77/42,421.10 (10,714.78-82,711.17) | .22 |
| Total charges | 32 | 85,115.45/93,925.26 (32,014.86-242,519.35) | 50 | 45,083.09/69,142.63 (10,714.78-485,456.08) | .0002 |
Includes only post-transplantation charges after discharge from index admission and up to day +100. All charges in US dollars
DISCUSSION
In this study, we evaluated the rates and predictors of 30-DR in patients undergoing RTC allo-HCT, as well as its effect on survival and health care costs, and we make several observations. First, readmission rates even after RTC allo-HCT are high (38%). Second, documented infections during index hospitalization are strong predictors of 30-DR; hence, identifying a patient population of interest, for future quality improvement efforts. Third, with limitations of our sample size in mind, 30-DR did not appear to impact OS, PFS, RR, or NRM after transplantation. Fourth (and most notably), 30-DR was significantly associated with increased post-transplantation health care costs, justifying its place as a quality-of-care measure in allo-HCT population.
As lawmakers and health care professionals aim to improve the quality of health care in the United States, the implementation of all-cause 30-DR as an index of poor quality-of-care has sparked widespread debate. It may be erroneous to hold patients with different diagnoses and treatment plans to the same standards. As recently reported in abstract form, readmission rates among oncology patients were found to be approximately 32% higher than those of their peers with other disease conditions. Among those, patients with hematological malignancies and those undergoing HCT had a significantly higher readmission rate (46%) [16]. Our study, specific to allo-HCT patients, had a 30-DR of 38% and is comparable to other single-institution reports [14,15,17]. This is in stark contrast to reports of autologous HCT that show readmission rates of 14% and exemplifies the limitation of instituting the same quality-of-care model for different treatment protocols and programs [22].
Allogeneic HCT recipients constitute a unique cohort of patients with significantly higher risk of cytopenias, GVHD, prolonged immunosuppression, and infections, including those associated with central venous catheters. Bejanyan et al., in their report of allo-HCT after myeloablative conditioning, found documented infections, higher HCT-CI, and total body irradiation (TBI)–conditioning as risk factors for increased 30-DR [14]. In contrast, our report evaluated patients receiving peripheral blood HCT after lower intensity and/or toxicity conditioning with fludarabine/busulfan and found only documented infections as a predictor for 30-DR. This difference between the studies is likely explained by higher therapy-related morbidity associated with ablative conditioning with TBI in high-risk patients, which leads to increased readmissions. Dungarwalla et al., in their abstract, reported readmissions by day +100 (as opposed to 30-DR) in reduced-intensity conditioning allo-HCT and found no predictors for rehospitalizations, but they noted infections as the major cause for readmissions [15]. In our study, 30-DR did not affect the NRM, RR, PFS, or OS. In the Bejanyan study, 30-DR increased the risk of all-cause mortality, leading to inferior OS. This variance is probably explained by the fact that in their study, the 2 predictive factors for readmission, ablative conditioning with TBI and higher HCT-CI, may have negatively influenced survival [23,24]. It is also possible that the relatively small sample size in our study prevented detection of a significant difference in OS and NRM.
Health care cost is increasingly becoming a major determinant in health care policy. The influence of 30-DR on hospital costs after HCT has not been previously reported, to our knowledge. In our study, 30-DR significantly increased the post-transplantation IP hospital charges as well the overall 100-day post-transplantation charges. It may be noted that the OP charges were similar in both groups. The significantly escalated IP cost found in our analysis provides support to using 30-DR as a quality-of-care parameter in patients undergoing allo-HCT. It is, however, important to point out that our analysis utilized hospital charges, and it may not be reflective of charges at other centers. Also, these charges are not identical to actual institutional reimbursement. We decided to estimate cost on hospital charges, in order to circumvent large reimbursement variations across various payers (CMS, Medicaid, private insurance, self pay, etc.) and variations across state lines. In October 2012, CMS implemented a plan to reduce Medicare payments for Inpatient Prospective Payment System for hospitals with excess readmissions. Excess readmissions are measured as a ratio, by dividing a hospital’s number of “predicted” 30-DR for heart attack, heart failure, and pneumonia by the number that would be “expected,” based on an average hospital with similar patients. A ratio greater than 1 indicates excess readmissions. However, it remains unclear how and if this metric would, in the future, be applicable to the HCT population [25].
In our institution, the HCT program has an elaborate and well-organized discharge process. Discharge planning is instituted at least 5 days before the planned day of release, all medications are reconciled, and printed copies of medication lists are provided. Caregivers undergo formal discharge training classes by the transplantation coordinators. All patients residing more than 30 minutes from the cancer center are discharged to an apartment complex physically attached to the hospital building with a 24-hour caregiver. They follow up daily in the OP infusion center and are seen by a health care professional at least 1 to 2 times a week until day +100. Most well-established centers have similar infrastructure for intensive post-discharge follow-up. Under such circumstances, it may be argued that the high 30-DR in allograft patients is due to the inherent risks associated with the procedure itself and does not necessarily reflect poor planning or lack of appropriate follow-up. However, studies like ours will help further identify high-risk patients; for example, those with documented infection during index stay who may benefit from closer outpatient follow-up (eg, every other day midlevel provider visit), predischarge infectious disease consultation, etc. Among our patients with 30-DR, 4 were admitted because of central line-associated blood-stream infections and 2 because of C. difficile colitis. It is possible that meticulous line care, early removal of central venous catheters, and proper hand washing precautions could have prevented some of these readmissions. A possible adverse consequence of penalizing hospitals in such cases is delayed discharge from index admission, which will likely have deleterious effects on health care costs. In our analysis, readmissions did increase the health care costs significantly, thus placing substantial financial burden on the health care system. Policies to minimize readmissions are perhaps warranted. The importance of utilizing a risk-adapted approach, taking into consideration relevant patient and treatment-related factors, in appraising quality-of-care utilizing 30-DR as an indicator cannot be over emphasized [14].
Notwithstanding its retrospective nature and sample size, this single-institution study evaluated readmission rates and factors in peripheral blood allograft after RTC with fludarabine/busulfan. Our readmission rates are similar to other reports, but failed to show a significant adverse effect of 30-DR on mortality and survival. Noting the financial burden caused by readmissions, its utilization as quality-of-care indicator is justified. Although efforts to minimize readmissions are necessary, it must be remembered that in complex health care situations such as allo-HCT, all readmissions are not avoidable.
Supplementary Material
Acknowledgments
Financial disclosure: J.Z. and S.W. acknowledge the support from NIH grant U54GM104942.
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2013.12.559.
Conflict of interest statement: There are no conflicts of interest to report.
References
- 1.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013;6:1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare Fee-for-Service Program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Ross JS, Chen J, Lin Z, Bueno H, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz H, Lin Z, Keenan P, et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309:587–593. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez A, Greiner M, Fonarow G, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 6.Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. N Engl J Med. 2009;361:2637–2645. doi: 10.1056/NEJMsa0904859. [DOI] [PubMed] [Google Scholar]
- 7.Lindenauer PK, Normand S-LT, Drye EE, et al. Development, validation, and results of a measure of 30-day readmission following hospitalization for pneumonia. J Hosp Med. 2011;6:142–150. doi: 10.1002/jhm.890. [DOI] [PubMed] [Google Scholar]
- 8.Jack B, Chetty V, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman EA, Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556–557. doi: 10.1046/j.1532-5415.2003.51186.x. [DOI] [PubMed] [Google Scholar]
- 10.Jha AK. BOOST and readmissions: thinking beyond the walls of the hospital. J Hosp Med. 2013;8:470–471. doi: 10.1002/jhm.2069. [DOI] [PubMed] [Google Scholar]
- 11.Epstein AM. Revisiting readmissionsdchanging the incentives for shared accountability. N Engl J Med. 2009;360:1457–1459. doi: 10.1056/NEJMe0901006. [DOI] [PubMed] [Google Scholar]
- 12.Ong MK, Mangione CM, Romano PS, et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2:548–557. doi: 10.1161/CIRCOUTCOMES.108.825612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? N Engl J Med. 2010;363:297–298. doi: 10.1056/NEJMc1001882. [DOI] [PubMed] [Google Scholar]
- 14.Bejanyan N, Bolwell BJ, Lazaryan A, et al. Risk factors for 30-day hospital readmission following myeloablative allogeneic hematopoietic cell transplantation (allo-HCT) Biol Blood Marrow Transplant. 2012;18:874–880. doi: 10.1016/j.bbmt.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Dungarwalla M, Brennan J, Kulkarni S, et al. Duration of initial admission and subsequent readmission in the first 100 days following reduced intensity conditioning allogeneic stem cell transplantation. ASH Annu Meet Abstr Blood. 2007;110:1999. [Google Scholar]
- 16.Walters R, Kidin L, Roquemore J, et al. Thirty-day all-cause readmission rate at a cancer center as a quality of care measure. J Clin Oncol ASCO Qual Care Symp Abstr. 2012;30(34_supplement):247. [Google Scholar]
- 17.Grant M, Cooke L, Bhatia S, Forman SJ. Discharge and unscheduled readmissions of adult patients undergoing hematopoietic stem cell transplantation: implications for developing nursing interventions. Oncolology Nurs Forum. 2005;32:E1–E8. doi: 10.1188/05.onf.e1-e8. [DOI] [PubMed] [Google Scholar]
- 18.Jaing T-H, Tsay P-K, Yang C-P, et al. Evaluation of readmission in children receiving allogeneic hematopoietic stem cell transplantation: an institutional experience. Transplant Proc. 2008;40:3643–3645. doi: 10.1016/j.transproceed.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 19.Bray RA, Hurley CK, Kamani NR, et al. National Marrow Donor Program HLA matching guidelines for unrelated adult donor hematopoietic cell transplants. Biol Blood Marrow Transplant. 2008;14(9 Suppl):45–53. doi: 10.1016/j.bbmt.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Hamadani M, Craig M, Phillips GS, et al. Higher busulfan dose intensity does not improve outcomes of patients undergoing allogeneic haematopoietic cell transplantation following fludarabine, busulfan-based reduced toxicity conditioning. Hematol Oncol. 2011;29:202–210. doi: 10.1002/hon.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Mohan SR, Rybicki L, Smith SD, et al. Analysis of readmission after autologous HCT: predictive factors and clinical consequences. Blood. 2010;116:932. Abstract. [Google Scholar]
- 23.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared to TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation–specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007 Dec 15;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. [November 27, 2013];Readmission Reduction. [Internet]. Available from: http://www.medicare.gov/hospitalcompare/readmission-reduction-program.html.
- 26. [October 10, 2013];American Society for Blood and Marrow Transplantation: Standard criteria. Available at: http://www.asbmt.org/displaycommon.cfm?an=1&subarticlenbr&=35.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


