Abstract
The B cell receptor (BCR) pathway plays a crucial role in the survival, proliferation, and trafficking of chronic lymphocytic leukemia (CLL) cells. Inhibitors of the key kinases in this pathway, including spleen tyrosine kinase (SYK), mammalian target of rapamycin (mTOR), phosphoinositide 3’-kinase (PI3K), and Bruton's tyrosine kinase (BTK), have been found in pre-clinical models to decrease CLL cell viability both directly and indirectly through modulation of the microenvironment. Recently, oral agents targeting each of these kinases have been explored in early phase clinical trials in patients with CLL. BCR pathway antagonists appear to be highly active in relapsed refractory CLL, independent of high-risk disease makers such as del(17p). These agents have shown a unique pattern of inducing early transient lymphocytosis which typically is associated with nodal response. Here, we review the biology of the BCR, the kinases within this pathway, and their interaction with the CLL microenvironment. We also discuss data from recent and ongoing clinical trials of BCR antagonists. We address the development of potential biomarkers for response to these agents such as ZAP-70, IGHV status, and CCL3, and discuss where these exciting new drugs may fit in the evolving landscape of CLL therapy.
Keywords: Lymphoid Leukemia, Signaling Therapies, Prognostication
INTRODUCTION
Although chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the Western world, the disease is incurable by conventional therapy[1]. A common clinical observation is that in response to chemotherapy, CLL cells in the peripheral blood are often effectively treated, yet disease persists in the lymph nodes and bone marrow[2]. These observations support the hypothesis that CLL is incurable because even though our current treatments are effective at putting the disease into remission, resistant cells remain in sanctuary sites in lymph nodes or bone marrow and lead to relapse. Until recently, little mechanistic evidence was available to support this hypothesis.
In the last few years, significant progress has been made in our understanding of the molecular pathogenesis underlying treatment resistance in the CLL microenvironment. Increasing evidence suggests that CLL cells in lymph nodes and bone marrow are protected from cell death by a variety of proliferative and prosurvival signals, such as CD40L, BAFF, APRIL, and adhesion molecules[3]. A key recent discovery is the critical role that the B cell receptor (BCR) pathway plays in this pathophysiology of CLL cells in the stromal microenvironment[4]. For example, lymph node-derived CLL cells were found to have a gene expression profile similar to activated B cells, and showed increased proliferation compared to samples from the same patients’ peripheral blood[5]. In addition to its role in promoting B cell proliferation and survival[5], BCR signaling also appears to be essential for CLL cell trafficking and interaction with the stromal microenvironment[5,6]. Recently, a variety of novel kinase inhibitors have been developed to target various components of the BCR pathway, including SYK[7], mTOR[8], PI3K[9], BTK[10], and others (Table I). These agents share a unique pattern of response that results in nodal reduction with redistribution lymphocytosis, which likely reflects microenvironment modulation. BCR pathway antagonists appear to be equally efficacious in the setting of traditional high-risk prognostic abnormalities such as del(17p). These oral therapies are also better tolerated than combination chemotherapy regimens in older, less-fit patients. Inhibitors of the BCR pathway have therefore generated significant excitement in the field by raising the possibility that they may bring us closer to curing CLL by providing a powerful new treatment option.
Table I.
B cell receptor (BCR) inhibitors and their clinical efficacy in chronic lymphocytic leukemia (CLL)
| Kinase | Function in CLL | Inhibitor(s) | Lymphocytosis Rate | Response Rate by 2008 IW CLL Criteria |
|---|---|---|---|---|
| Spleen tyrosine kinase (SYK) | Upstream amplifier of BCR signal through ITAM phosphorylation, mediates tonic BCR signaling | Fostamatinib (R788) | 9/11 patients (82%) | 6/11 patients (55%)* |
| Mamallian target of rapamycin (mTOR) | Downstream mediator of BCR signaling, regulates cell cycle and translation | Everolimus | 6/22 patients (27%) | 4/22 (18%) |
| Phosphoinositide 3’-kinase (PI3K) | Intermediary signaling between microenvironment and downstream pro-survival factors AKT and mTOR | Delta-Specific: CAL-101 (GS1101) | 32/55 patients (58%) | 13/55 patients (24%) |
| Pan-PI3K: SAR245408 (S08) | 3/5 patients (60%) | 0/5 patients (0%) | ||
| Bruton's tyrosine kinase (BTK) | Upstream mediator of stroma-mediated pro-survival signals through BCR pathway | PCI-32765 | 56/61 patients (91 %) | 41/61 patients (67%) |
| AVL-292 | TBD | TBD |
by lymphoma response criteria
Here, we review the biology of the BCR pathway and its component proteins, as well as data from recent and ongoing clinical trials of these agents. We also discuss where these exciting new drugs may fit in to the evolving landscape of CLL therapy.
OVERVIEW OF THE B CELL RECEPTOR PATHWAY
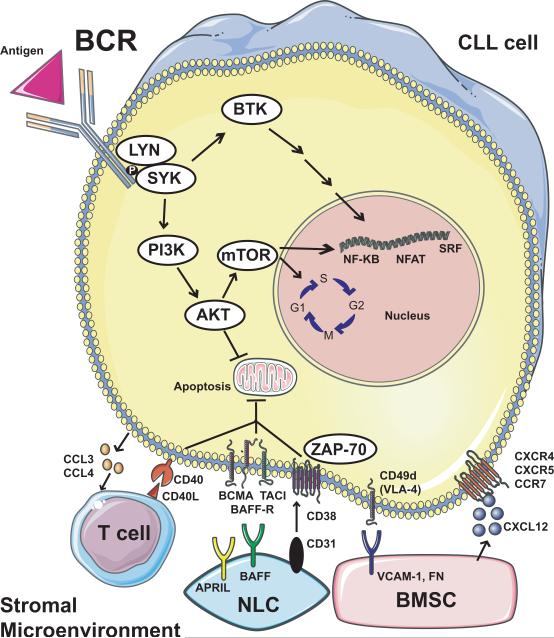
The BCR pathway is utilized by normal B cells to promote cell proliferation, differentiation, and function, including production of antibodies[11]. A simplified version of the BCR pathway and its molecular interactions with the CLL microenvironment is shown in Figure 1. Once stimulated by antigen, the activated BCR recruits other kinases such as spleen tyrosine kinase (SYK) and LYN kinase, which phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) on the cytoplasmic Ig domains of the receptor[12]. ITAM phosphorylation sets off a cascade of downstream events, including activation of Bruton's tyrosine kinase (BTK) and phosphoinositide 3-kinase (PI3K). Activated BTK and PI3K promote calcium mobilization and activation of downstream kinases such as PKC-β, AKT kinase, mammalian target of rapamycin (mTOR), and MAP kinase (ERK). These events promote increased survival and proliferation of B cells, largely mediated by the upregulation of transcription factors such as nuclear factor Κ-beta (NF-ΚB) and nuclear factor of activated T cells (NFAT)[13]. These activated kinases also have a profound influence on B cell trafficking by promoting B cell chemotaxis towards CXCL12/13, migration beneath stromal cells, and upregulation of CLL cell chemokine secretion[2]. It is likely that the prosurvival signals stimulated by both BCR activation and stroma are amplified by the convergence of these pathways on common downstream kinases.
Figure 1.
The B cell receptor (BCR) signaling pathway and molecular interactions in the CLL microenvironment. Upon engagement with antigen (and independent of antigen in some cases), the BCR activates LYN and SYK kinases, which stimulate several downstream mediators. BTK activation leads to a variety of downstream effects that eventually regulate key transcription factors for B cell survival and proliferation. PI3K stimulation leads to activation of mTOR and AKT. mTOR promotes cell cycle progression from G1 to S and activates important pro-survival transcription factors. AKT has an anti-apoptotic effect, the mechanism of which remains incompletely defined. The microenvironment promotes CLL survival in a variety of complex ways. CLL cells can produce chemokines such as CCL3 and CCL4, which recruit immune cells such as T cells, which exert pro-survival signals through CD40/CD40L interactions. Nurse-like cells (NLC) have anti-apoptotic effects on the CLL cell through a variety of mediators, including APRIL, BAFF, and CD31, the latter of which interacts with CD38 and ZAP-70 to drive CLL cell proliferation. Bone marrow stromal cells (BMSC) contribute to CLL survival both through direct cell-cell contact and by producing soluble factors. Ligands such as VCAM-1 and fibronectin (FN) on the BMSC cell surface interact directly with integrins such as CD49d (VLA-4) on the CLL cell. BMSCs also produce chemokines such as CXCL12, which recruit CLL cells into the microenvironment through interactions with receptors on the CLL cell such as CXCR4.
Although the BCR is usually activated by antigen in normal B cells, the receptor has also been found to undergo ligand-independent (tonic) signaling[14]. This tonic signaling is thought to contribute to the pathogenesis of CLL, as well as a variety of other B cell malignancies, including diffuse large B cell lymphoma[15] and mantle cell lymphoma[16]. Recently, it has been reported that one third of patients with CLL have stereotyped B cell receptors, which may respond differently to antigen than non-stereotyped B cell receptors[17]. A deeper understanding of these B cell receptor structures has the potential to enhance our biologic understanding of the disease and may eventually guide therapy, as different stereotyped subsets are associated with distinct clinical characteristics.
Given that several of the key mediators of the BCR pathway are kinases, the potential efficacy of small molecule kinase inhibitors has been widely recognized. A variety of different kinases in the BCR pathway have now been targeted, both in pre-clinical studies as well as in clinical trials. Other non-kinase targets that indirectly affect the BCR pathway, including inhibitors of NF-ΚB, BCL-2, and monoclonal antibodies to target surface proteins such as CD19 are in various stages of development, but are beyond the scope of this review.
B CELL RECEPTOR PATHWAY TARGETS
Spleen Tyrosine Kinase (SYK)
Biology
The spleen tyrosine kinase (SYK) is thought to be critical to normal B cell development, as mice deficient in SYK typically exhibit severe B cell lymphopenia[18,19]. Given its proximity to the BCR, SYK is critical for initiating downstream events and amplifying the BCR signal[11]. SYK also is thought to play a particularly important role in tonic BCR signaling in malignant lymphoid cells[15]. Compelling pre-clinical data in lymphoma patient samples demonstrated that inhibition of SYK could lead to proliferation arrest and apoptosis, both of which were associated with the degree of overexpression of SYK[20]. Inhibition of SYK decreases CLL cell migration toward chemokines such as CXCL12, thereby reducing the numbers of CLL cells that benefit from protective effects of stroma[21]. Furthermore, SYK inhibition leads to decreased BCR-dependent secretion of the chemokines CCL3 and CCL4 by CLL cells, CLL cell migration beneath stromal cells, and extracellular signal-regulated kinase (ERK) phosphorylation after BCR triggering[22]. These in vitro observations have also been confirmed in the Eu-TCL-1 transgenic mouse, an in vivo model of CLL[23]. In this model, SYK inhibition effectively inhibited BCR signaling, induced a transient early increase in circulating lymphocytes, reduced the proliferation and survival of the malignant B cells, and prolonged survival of the treated mice.
Therapy
Fostamatinib (R788)
Fostamatinib (R788) is an orally-bioavailable prodrug that gets converted in the body to an active metabolite, R406, which acts as a potent inhibitor of SYK, with an IC50 in vitro of 41 nM [24]. The compound is not entirely specific for SYK, and is thought to have several potential off target effects. Early trials of fostamatinib in patients with rheumatoid arthritis showed potent anti-inflammatory effects[25]. The largest study to date in CLL was a phase I/II study of single-agent fostamatinib in patients with relapsed refractory lymphoid malignancies[7]. In phase I, a dose of 200 mg twice daily was relatively well-tolerated, with dose-limiting toxicities of neutropenia, thrombocytopenia, and diarrhea. Six of the 11 CLL patients (55%) in the phase II portion of the study had an objective response according to lymphoma response criteria[26], the highest response rate of any histology on the study. An early lymphocytosis of greater than 50% of baseline was observed in 9 of the patients with CLL, initially prompting concern for possible progression of disease. However, given that these patients with lymphocytosis were also experiencing lymph node reduction, the authors speculated that inhibition of SYK may have disrupted the nodal microenvironment and led to increased trafficking of CLL cells out of nodal tissues and into the peripheral blood where they would then eventually die.
The company developing fostamatinib decided to pursue a registration strategy in rheumatoid arthritis, and therefore no follow-up studies in CLL have been performed to date. Clinical trials are proceeding with the drug in diffuse large B cell lymphoma, but it remains unclear whether fostamatinib will be available in the future for study in CLL. Other highly specific SYK inhibitors including PRT318 and P505-15 have since been developed by another company. Preliminary reports showed potent pre-clinical activity of these compounds in CLL[20], and follow-up studies confirmed that PRT318 and P505-15 are both highly effective at promoting CLL cell apoptosis and disrupting CLL cell tissue homing circuits, supporting the future clinical development of these drugs in CLL[22].
Mammalian Target of Rapamycin (mTOR)
Biology
The mammalian target of rapamycin (mTOR) is a highly conserved, ubiquitously expressed serine/threonine kinase with two major isoforms (mTOR1 and mTOR2) that serves as a key regulator of the initiation of translation and as a critical downstream mediator of BCR signaling[27]. mTOR also regulates the cell cycle by allowing progression from G1 to S phase through increasing cyclin E and cyclin A levels, thereby leading to upregulation of cyclin-dependent kinase 2 (CDK2) activity[28]. Rapamycin binds to mTOR, and has been found ex vivo to decrease CLL cell progression into S phase, leading to G1 arrest. The pro-survival factor survivin, a member of the inhibitors of apoptosis proteins (IAPs) family, has been previously shown to be expressed in CLL proliferation centers and is completely suppressed in the presence of rapamycin[29]. Interestingly, unlike most CLL cells, which undergo growth arrest in response to rapamycin, CLL cells deficient in the tumor suppressor TP53 undergo apoptosis[30], suggesting that mTOR inhibitors may be particularly active in CLL patients with the poor prognosis del(17p) genotype, which typically lacks TP53 function. mTOR inhibitors typically have differential specificity for mTOR1 or mTOR2, and a theoretical mechanism of drug resistance is the compensatory upregulation of the less well-inhibited isoform.
Therapy
Everolimus (RAD001) is a rapamycin analog FDA-approved for relapsed renal cell carcinoma that selectively inhibits mTOR and has been shown in vitro to induce cell cycle arrest in CLL cells without inducing apoptosis[31]. By blocking downstream in the BCR pathway, everolimus is also theoretically disruptive to the proliferative signals induced in CLL cells by stroma. A phase II pilot trial of everolimus (5 mg/day) in 7 patients with advanced CLL was stopped early due to toxicity concerns, which included four life-threatening immunosuppression-related infectious complications[32]. Some clinical activity was observed, with one patient achieving a partial response and 3 patients with stable disease. The authors suggested that the lack of mandated antimicrobial prophylaxis on this study contributed to these serious infectious complications.
Building on this early experience, the Mayo clinic group conducted a phase II study of oral single-agent everolimus (10 mg/day) for relapsed refractory indolent lymphoid malignancies[8]. Although only 4 of the 22 patients with CLL (18%) achieved a partial remission by NCI Working Group 1996 criteria, an additional 6 of these 22 patients (27%) achieved a nodal response with increased absolute lymphocyte count. The pattern of lymphocytosis with nodal response was similar to that observed with SYK inhibition, suggesting again that these patients were likely experiencing redistribution of lymphocytes rather than progressive disease. Including these patients with discordant blood and lymph node response, 45% of patients derived clinical benefit from everolimus monotherapy. Five serious infections (including 2 fatal infections) were observed on this study, again supporting the need for mandatory antimicrobial prophylaxis and growth factor support for future studies with this drug. The authors concluded that although the single-agent activity of everolimus was modest, the drug did lead to lymphocyte redistribution and therefore could be valuable in combination with other effective therapies for CLL. Second generation mTOR inhibitors, such as temsirolimus and deforolimus, have somewhat different specificity and toxicity profiles and are worthy of further investigation in CLL.
Phosphoinositide 3’-kinase (PI3K)
Biology
Phosphoinositide 3’-kinase (PI3K) is a particularly crucial downstream mediator of BCR signaling[33], and CLL cells generally express high levels of active PI3K[34]. In addition to promoting B cell survival and proliferation, PI3K also acts as a key intermediary signaling molecule between the microenvironment and the CLL cell, transmitting signals from membrane receptors such as the BCR, CXCR4, and CD40 to downstream pro-survival mediators such as AKT[35]. The delta-isoform of the p110 catalytic subunit of PI3K is the predominant form expressed in leukocytes, and PI3K-δ inhibition partially reverses the chemoresistance observed in stroma-exposed CLL cells and also reduces CLL cell chemotaxis into stroma[36]. In vitro, PI3K-δ inhibition leads to a modest degree of apoptosis, both in the presence or absence of stroma[37].
Pre-clinical in vitro studies are ongoing to determine which drugs might best complement the activity of a PI3K-δ inhibitor by blocking other pathways that contribute to CLL cell survival. One interesting in vitro study combined PI3K inhibition with lenalidomide, an immunomodulatory agent known to cause an immune-mediated tumor flare in CLL patients starting on the drug[38]. Pharmacologic inhibition or siRNA knockdown of PI3K-δ was found to abrogate CLL cell activation, costimulatory molecule expression, and vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) gene expression that would otherwise have been induced by lenalidomide. These findings suggest that PI3K might be an important mediator of the lenalidomide-induced tumor flare, and support the idea of studying the combination of PI3K inhibitors with lenalidomide clinically to mitigate this effect.
Therapy
CAL-101 (GS1101)
CAL-101 (GS1101) is a small molecule that potently and specifically inhibits the delta-isoform of PI3K[9]. The drug is thought to kill primarily through induction of apoptosis, and has been shown to kill primary CLL cells ex vivo in a dose- and time-dependent fashion[39]. Importantly, CAL-101 (GS1101) is able to kill CLL cells effectively in the presence of stroma.
A large phase I study of approximately 190 patients with relapsed refractory hematologic malignancies was conducted with CAL-101 (GS1101)[40]. Fifty-five CLL patients were included on this study, the majority of whom were high risk, with a median of 5 prior therapies and 82% with bulky lymphadenopathy. As with earlier studies of BCR pathway inhibitors such as fostamatinib (SYK) and everolimus (mTOR), the majority of CLL patients on the CAL-101 (GS1101) monotherapy study (58%) experienced transient lymphocytosis, again likely due to mobilization of CLL cells out of the lymph nodes and bone marrow. The lymphocytosis was clearly not related to disease progression, as eventually all evaluable CLL subjects had a decrease in lymph node size. The overall response rate (ORR = CR + PR) by IW-CLL criteria was a modest 24% due to the fact that the lymphocytosis induced by the drug again precluded counting many patients as responders. When including patients with discordant nodal and blood response, the rate of clinical benefit was greater than 90%, and even patients with poor prognostic factors such as del(17p) derived significant benefit. The median progression free survival (PFS) thus far is 15 months, and 21 patients remain on study after 1 year with continued benefit, suggesting that responses to CAL-101 (GS1101) can be quite durable. Importantly, in vivo correlative studies found that patients on CAL-101 (GS1101) experienced a rapid decline in plasma concentrations of the chemokines CCL3, CCL4, and CXCL13, and reduced constitutive expression of phospho-AKT, demonstrating pharmacodynamic inhibition of activated PI3K signaling.
Combination studies with CAL-101 (GS1101) in the relapsed/refractory setting are also now underway. A recent preliminary report was presented on a phase I study of CAL-101 (GS1101) in combination with rituximab or bendamustine in patients with relapsed refractory CLL[41]. Twenty-seven patients have been reported to date, and the combinations have had a favorable safety profile and > 80% ORR. Unlike with monotherapy, the transient lymphocytosis phenomenon has been significantly reduced, particularly with bendamustine, likely due to its rapid cytotoxic effects. Moving forward, registration studies are being initiated, and other studies of the activity of CAL-101 (GS1101) in combination with chemotherapy or immunotherapy will also be explored in the frontline and relapsed settings.
SAR245408 (S08)
Since PI3K-δ is highly expressed in leukocytes, it was logical that isoform-specific PI3K inhibition was the first strategy explored in CLL. However, given the ubiquitous nature of PI3K dysregulation in human cancers, pan-PI3K inhibition is also worthy of exploration in CLL. Alpha and gamma p110 isoforms of PI3K are expressed in CLL, and their upregulation in CLL cells would be a logical mechanism of resistance to a delta-isoform specific inhibitor. Furthermore, T cells are known to provide stromal support for CLL cells, and express the gamma isoform of PI3K[42]. Pan-PI3K inhibitors have already been studied in patients with solid tumors and have generally been found to be safe and well-tolerated[43], and the first efforts to evaluate pan-PI3K inhibitors in lymphoid malignancies are now underway.
SAR245408 (S08) is a class I pan-PI3K inhibitor that was previously found to be well-tolerated in patients with solid tumors[43]. A small pilot study in 5 patients with refractory CLL found that the drug was well-tolerated[44]. Although none of the 5 patients achieved an objective response, 3 patients (60%) benefited from a nodal response with transient lymphocytosis and remain on treatment. Though these early results in CLL have been promising, the company producing SAR245408 (S08) has decided to focus the development of the drug on solid tumors, and instead to study the activity of a dual pan-PI3K/mTOR inhibitor in lymphoid malignancies, including CLL (see below).
Dual PI3K/mTOR
Biology
Another theoretical resistance mechanism to PI3K inhibitor monotherapy is mTOR activation through alternative upstream pathways such as the RAS/MEK/ERK pathway. Resistance to mTOR inhibitor monotherapy could develop if PI3K were to preferentially activate alternative downstream messengers such as PKCβ and NF-ΚB. A drug able to inhibit upstream at PI3K and downstream at mTOR would have the potential to overcome both of these types of resistance mechanisms. PI3K and mTOR inhibitors also likely work by different mechanisms, with PI3K inhibitors directly inducing apoptosis in CLL cells, and mTOR inhibitors primarily causing induction of growth arrest. Targeting both PI3K and mTOR simultaneously would therefore potentially disrupt both CLL cell survival and proliferation, and has the potential to lead to a high level of clinical activity in CLL.
Therapy
SAR245409 (S09)
SAR245509 (S09) is a small molecule dual PI3K/mTOR inhibitor currently being evaluated in clinical trials. In a PTEN-deficient mantle cell lymphoma cell line, the drug inhibited PI3K and ERK, and led to a marked decrease in proliferation markers such as Ki-67 (unpublished data). SAR245509 (S09) was relatively well-tolerated in a phase I dose-escalation study of patients with advanced solid tumors[45], and is now being explored at the recommended phase 2 dose of 50 mg BID in a large, multicenter phase II study in patients with CLL, follicular, and mantle cell lymphomas. A phase I study of SAR245509 (S09) in combination with bendamustine and/or rituximab in the same patient population is also underway. Additional combination studies of SAR245509 (S09) are also in development.
Bruton's Tyrosine Kinase (BTK)
Biology
Bruton's tyrosine kinase (BTK) is a Tec family kinase member which lies near the B cell membrane and plays a key role in upstream BCR signaling[46]. Mutations in BTK were first discovered in patients with X-linked Bruton's agammaglobulinemia[47], a disorder that results in marked impairment of B cell function and number. BTK inhibition blocks BCR signaling in human peripheral B cells in vitro, and inhibits autoantibody production in a mouse model of autoimmune disease[10]. In CLL cells, inhibition of BTK decreases ERK1 and AKT phosphorylation, NF-ΚB DNA binding, and CpG mediated proliferation[48].
In addition to its role in CLL cell survival and proliferation, BTK also appears to be intricately intertwined in the biology of the CLL microenvironment. For example, BTK inhibition significantly decreases integrin-mediated adhesion and cell migration, response to chemotactic factors such as CXCL12 and 13, and CLL cell production of the chemokines CCL3 and CCL4[49,50]. In the TCL1 mouse model of CLL, BTK inhibition leads to a transient early lymphocytosis and profoundly inhibits CLL progression, as measured by weight, development, extent of hepatosplenomegaly, and survival[49]. Because BTK is expressed primarily in B cells (and not T cells or plasma cells)[51], BTK inhibition should theoretically lead to less immunosuppression than other agents that affect multiple types of immune cells.
Therapy
PCI-32765
PCI-32765 is a highly potent, orally bioavailable small molecule BTK inhibitor (in vitro IC50 0.5 nm) that forms a specific and irreversible bond to cysteine-481 in BTK[52]. An initial phase I study found that PCI-32765 monotherapy was well-tolerated and demonstrated a clear efficacy signal[53]. The follow-up study of PCI-32765 monotherapy is a large phase Ib/II study in patients with CLL which includes both relapsed refractory patients of all ages and treatment-naïve elderly patients in dosing cohorts of 420 mg/day and 840 mg/day continuous dosing. The updated data for the relapsed refractory cohorts were presented at the 2011 ASH annual meeting[54]. Sixty-one patients were enrolled (n=27 at 420 mg/day and n=34 at 840 mg/day), and the median follow-up for the two cohorts was 12.6 and 9.3 months, respectively. The drug has been well-tolerated, with grade 1/2 diarrhea, fatigue, nausea, and ecchymosis being the most frequently reported adverse events. The characteristic pattern of transient early lymphocytosis observed with other inhibitors of the BCR pathway was again observed in the majority of patients on this study. Early in therapy, the majority of patients showed nodal response with lymphocytosis, but over time, as lymphocytosis resolved, many of these patients converted to PR, such that objective responses by IW-CLL criteria were achieved in 67% of patients, with an additional 24% of patients achieving nodal response in the setting of transient lymphocytosis. Therefore, clinical benefit was achieved in over 90% of patients. The responses to PCI-32765 have thus far proven to be durable, with several patients still benefitting from the drug now for over two years. The median PFS thus far has been 86% at 12 months, and clinical activity was observed to be independent of poor-risk clinical or genetic factors.
Several combination studies of PCI-32765 with chemoimmunotherapy and antibodies in both the relapsed refractory and frontline settings are currently ongoing or in development. Registration trials are initiating this year. Like CAL-101 (GS1101), PCI-32765 would be expected to mitigate the tumor flare reaction observed in the majority of patients on lenalidomide, and therefore combination studies of PCI-32765 with lenalidomide in the relapsed/refractory setting are also being planned.
AVL-292
Another promising BTK inhibitor in development is AVL-292, which has a similar mechanism of action and higher binding affinity (in vitro IC50 <0.5nm) compared to PCI-32765. Detailed pharmacokinetic and pharmacodynamic studies were obtained in a preliminary study of AVL-292 in healthy human subjects [55]. The drug was found to be safe and well-tolerated following oral administration at dose levels ranging from 0.5-7.0 mg/kg. Based on these results, a phase I study of AVL-292 in patients with relapsed refractory CLL and lymphoma is now ongoing, and plans for future combination studies are also in development.
PREDICTING RESPONSE TO B CELL RECEPTOR INHIBITION
Although the overall response rates to BCR pathway inhibitors are high, a substantial minority of patients either do not respond or progress relatively shortly after starting on therapy. Therefore, identifying biomarkers that may predict for response to BCR pathway inhibitors would be valuable. Efforts are currently underway to study both conventional CLL prognostic markers and novel biomarkers as predictors of response to these agents. To date, the three markers that have been studied the most in preclinical models are ZAP-70, the mutation status of the immunoglobulin heavy chain (IGHV), and CCL3.
ZAP-70 is a cytoplasmic tyrosine kinase, normally associated with the T cell receptor, which is abnormally upregulated in the malignant B cells of a subset of CLL patients[56]. Increased ZAP-70 is associated with activation of the BCR pathway, and is often associated with unmutated IGHV status and more aggressive disease[57]. ZAP-70 may also promote increased CLL cell motility, as evidenced by its ability to increase CLL cell responsiveness to the chemokines CCL19, CCL21, and CXCL12[58]. Given these findings that increased ZAP-70 expression is associated with higher signaling through the BCR pathway, one would hypothesize that BCR antagonists would be more effective in patients with ZAP-70 positive CLL, though this has not yet been shown in clinical trials.
Unmutated IGHV status confers a poorer prognosis and predicts a shorter time to first treatment compared to mutated IGHV status[59]. Pre-clinical data predict that mutation status may serve as a marker of response to BCR pathway inhibition. When BCR ligation is induced by IgM stimulation in vitro, significant differences in gene expression occur between unmutated and mutated CLL cases[60]. For example, functional gene groups, including signal transduction, transcription, cell-cycle regulation, and cytoskeletal organization were all up-regulated in unmutated but not mutated CLL cases. Furthermore, IGHV unmutated CLL cells cultured ex vivo are more prone to spontaneous apoptosis and more dependent on stromal prosurvival signals from cell-to-cell contact and soluble factors compared to mutated CLL cells[61]. Ongoing investigation will determine whether patients with unmutated IGHV benefit differentially from BCR antagonists compared to mutated IGHV patients.
CCL3 is a chemokine of the CC subfamily that is produced by both normal and malignant lymphocytes and acts through the chemokine receptors CCR1 and CCR5 as a chemoattractant for other lymphocytes and adaptive immune cells[62]. CCL3 is secreted by CLL cells when they are activated via the BCR pathway[63]. Moreover, CCL3 is expressed in higher levels in CLL cells derived from the lymph node compared to those derived from the peripheral blood[5], suggesting that stroma can induce higher levels of CCL3 expression. Given these observations, one would hypothesize that agents that target the BCR pathway and disrupt stromal support signals would lead to decreased CCL3 levels. Indeed, both SYK[64] and PI3K-δ inhibition[37] have been shown to decrease CLL secretion of CCL3 in a nurse-like cell in vitro model. In addition, patients treated with the PI3K-δ inhibitor CAL-101 (GS1101) were found to normalize their CCL3 values in the peripheral blood after 28 days[37]. These findings support the hypothesis that elevated CCL3 levels may also serve as a predictive biomarker of lymphocytosis due to BCR pathway antagonism, though this hypothesis still needs to be formally evaluated in the clinic.
Although ZAP-70 and IGHV mutation status as well as CCL3 expression are all promising biomarkers for response to BCR pathway inhibition, thus far a definitive evaluation of their predictive value in vivo has not been possible due to the relatively small size of the clinical studies. Given the counterintuitive nature of poor risk markers such as ZAP-70 positivivity and unmutated IGHV predicting improved response to BCR pathway antagonists, it will be important to confirm these hypotheses in larger clinical trials. These larger studies are now underway, and in addition to looking at traditional prognostic markers, these trials should also incorporate novel biomarker evaluations, including genetic predictors of response, such as the novel mutations recently described in CLL such as SF3B1, NOTCH1, MYD88 and others[65-67]. These larger studies will hopefully provide useful information about how well these markers predict response to BCR pathway inhibitors in CLL. Given the preclinical data, one would theoretically expect that even patients with high-risk CLL who have short-lived responses to conventional therapy might have more durable responses to BCR pathway antagonists.
CONCLUSION
The explosion of knowledge over the last few years regarding the key role of the BCR pathway in the pathophysiology of CLL has fueled the development of a plethora of small molecule inhibitors of several key kinases in this pathway. The compounds that have moved the furthest along in the clinic thus far include inhibitors of SYK, mTOR, PI3K- δ, and BTK. Agents with broader activity, including dual PI3K-mTOR and pan-PI3K inhibitors are also now in clinical studies. Smaller studies are exploring inhibition of other kinases in the BCR pathway such as LYN kinase, which is inhibited by dasatinib[68], and AKT, which is inhibited by MK2206. The unique pattern of nodal response with redistribution lymphocytosis first observed with the SYK inhibitor fostamatinib is now clearly a class effect of BCR inhibitors, and may serve as a useful pharmacodynamic marker of pathway inhibition. Given that the IW CLL 2008 response criteria were devised prior to knowledge of the lymphocyte redistribution effect of BCR pathway antagonists, investigators are currently grappling with understanding the clinical utility of the established response criteria in the context of these new agents. A discussion is ongoing about modifying these criteria to acknowledge that an increasing lymphocyte count early in therapy with one of these agents should not be considered progressive disease in the absence of other evidence of disease progression.
As the CLL field tries to move away from effective but often toxic chemoimmunotherapy regimens, the BCR pathway inhibitors may form the backbone of a new therapeutic paradigm. The prospect of using small molecule BCR pathway inhibitors, either as monotherapy or in combination, to achieve long term disease remission without the toxicity and inconvenience of chemotherapy would potentially be quite beneficial for patients with CLL. But given the availability of numerous new agents and the complexity of the BCR pathway, several important questions need to be addressed in future studies.
For example, will BCR antagonists need to be combined with chemotherapy to achieve maximum effect, or will they be effective enough as monotherapy or in combination with antibodies to reduce the need for chemotherapy in this disease? What resistance mechanisms might arise with these new agents and how can they be addressed? Though overall response rates in the initial trials have been impressive, how durable will these responses ultimately be? What role might BCR pathway inhibitors have in the maintenance setting, either post chemoimmunotherapy or post allogeneic stem cell transplantation? Will BCR pathway inhibitors be effective enough to significantly reduce the need for allogeneic stem cell transplantation in relapsed refractory CLL?
Though clinical trials will help elucidate the answers to some of these questions, equally essential to the successful future development of these agents will be ongoing pre-clinical investigation to gain further insight into mechanisms of resistance and to help determine optimal drug combinations. Translating the findings of this pre-clinical work into the clinic will be critical to optimizing the therapeutic potential of inhibition of the BCR pathway in CLL.
REFERENCES
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004;103:4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 5.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger JA. Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. Seminars in cancer biology. 2010;20:424–430. doi: 10.1016/j.semcancer.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zent CS, LaPlant BR, Johnston PB, et al. The treatment of recurrent/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) with everolimus results in clinical responses and mobilization of CLL cells into the circulation. Cancer. 2010;116:2201–2207. doi: 10.1002/cncr.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsubata T, Wienands J. B cell signaling. Introduction. International reviews of immunology. 2001;20:675–678. doi: 10.3109/08830180109045584. [DOI] [PubMed] [Google Scholar]
- 12.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara H, Kurosaki T. Comprehending the complex connection between PKCbeta, TAK1, and IKK in BCR signaling. Immunological reviews. 2009;232:300–318. doi: 10.1111/j.1600-065X.2009.00836.x. [DOI] [PubMed] [Google Scholar]
- 14.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nature reviews. Immunology. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinaldi A, Kwee I, Taborelli M, et al. Genomic and expression profiling identifies the B-cell associated tyrosine kinase Syk as a possible therapeutic target in mantle cell lymphoma. British journal of haematology. 2006;132:303–316. doi: 10.1111/j.1365-2141.2005.05883.x. [DOI] [PubMed] [Google Scholar]
- 17.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one third of chronic lymphocytic leukemia: towards a molecular classification with implications for targeted therapeutic interventions. Blood. 2012 doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 19.Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Chen G, Fecteau J, Coffey G, Prussak C, et al. Highly Specific Inhibitor for Syk Induces Chronic Lymphocytic Leukemia Cell Apoptosis. ASH. 2011;641 [Google Scholar]
- 21.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115:4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 22.Hoellenriegel J, Coffey GP, Sinha U, et al. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2012. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suljagic M, Longo PG, Bennardo S, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 116:4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 24.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 25.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 27.Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Current molecular medicine. 2005;5:653–661. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- 28.Decker T, Hipp S, Ringshausen I, et al. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101:278–285. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 29.Granziero L, Ghia P, Circosta P, et al. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777–2783. doi: 10.1182/blood.v97.9.2777. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Molecular cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 31.Ringshausen I, Peschel C, Decker T. Mammalian target of rapamycin (mTOR) inhibition in chronic lymphocytic B-cell leukemia: a new therapeutic option. Leukemia & lymphoma. 2005;46:11–19. doi: 10.1080/10428190400005353. [DOI] [PubMed] [Google Scholar]
- 32.Decker T, Sandherr M, Goetze K, Oelsner M, Ringshausen I, Peschel C. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Annals of hematology. 2009;88:221–227. doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- 33.Ramadani F, Bolland DJ, Garcon F, et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Science signaling. 2010;3:ra60. doi: 10.1126/scisignal.2001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2004;18:1391–1400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 35.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–855. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 36.Niedermeier M, Hennessy BT, Knight ZA, et al. Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009;113:5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman SE, Lapalombella R, Gordon AL, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117:4323–4327. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110d Demonstrates Clinical Activity and Pharmacodynamic Effects in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. ASH. 2010;116 [Google Scholar]
- 41.Sharman J, de Vos S, Leonard J, Furman R, et al. A Phase 1 Study of the Selective PI3K Inhibitor CAL-101 (GS-1101) in Combination with Rituximab and/or Bendamustine in Patients with Relapsed or Refractory CLL. ASH. 2011;642 [Google Scholar]
- 42.Martin AL, Schwartz MD, Jameson SC, Shimizu Y. Selective regulation of CD8 effector T cell migration by the p110 gamma isoform of phosphatidylinositol 3-kinase. Journal of immunology. 2008;180:2081–2088. doi: 10.4049/jimmunol.180.4.2081. [DOI] [PubMed] [Google Scholar]
- 43.Edelman G, Bedell G, Shapiro SS, Pandya EL, Cwak C, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients with advanced malignancies. ASCO. 2010;28 [Google Scholar]
- 44.Brown JR, Davids MS, Rodon J, Abrisqueta P, DeCillis AP, et al. Phase I Trial of SAR245408 (S08), a Pan-PI3K Inhibitor, in Patients with CLL and Lymphoma. ASH. 2011;623 [Google Scholar]
- 45.Lorusso P, Markman J, Tabernero R, Shazer L, Heath E, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765, a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced solid tumors. ASCO. 2009;27:15s. [Google Scholar]
- 46.de Weers M, Brouns GS, Hinshelwood S, et al. B-cell antigen receptor stimulation activates the human Bruton's tyrosine kinase, which is deficient in X-linked agammaglobulinemia. The Journal of biological chemistry. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 47.de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW. Mutation analysis of the Bruton's tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Human molecular genetics. 1994;3:161–166. doi: 10.1093/hmg/3.1.161. [DOI] [PubMed] [Google Scholar]
- 48.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponader S, Chen SS, Buggy JJ, et al. Bruton's tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2011 doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012 doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 51.Genevier HC, Hinshelwood S, Gaspar HB, et al. Expression of Bruton's tyrosine kinase protein within the B cell lineage. European journal of immunology. 1994;24:3100–3105. doi: 10.1002/eji.1830241228. [DOI] [PubMed] [Google Scholar]
- 52.Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 53.Burger JA, O'Brien S, Fowler N, Advani R, Sharman J, et al. The Bruton's Tyrosine Kinase Inhibitor, PCI-32765, Is Well Tolerated and Demonstrates Promising Clinical Activity In Chronic Lymphocytic Leukemia (CLL) and Small Lymphocytic Lymphoma (SLL): An Update on Ongoing Phase 1 Studies. ASH. 2010 [Google Scholar]
- 54.O'Brien S, Burger JA, Blum KA, Furman RR, Coutre SE, et al. The Bruton's Tyrosine Kinase (BTK) Inhibitor PCI-32765 Induces Durable Responses in Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Follow-up of a Phase Ib/II Study. ASH. 2011;642 [Google Scholar]
- 55.Evans E, Tester P, Aslanian S, Chaturvedi P, Mazdiyasni H, et al. Clinical Development of AVL-292; A Potent, Selective Covalent Btk Inhibitor for the Treatment of B Cell Malignancies. ASH. 2011;604 [Google Scholar]
- 56.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100:4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 57.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 58.Richardson SJ, Matthews C, Catherwood MA, et al. ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL). Blood. 2006;107:3584–3592. doi: 10.1182/blood-2005-04-1718. [DOI] [PubMed] [Google Scholar]
- 59.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 60.Guarini A, Chiaretti S, Tavolaro S, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008;112:782–792. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 61.Coscia M, Pantaleoni F, Riganti C, et al. IGHV unmutated CLL B cells are more prone to spontaneous apoptosis and subject to environmental prosurvival signals than mutated CLL B cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2011;25:828–837. doi: 10.1038/leu.2011.12. [DOI] [PubMed] [Google Scholar]
- 62.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. The Journal of experimental medicine. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burger JA, Quiroga MP, Hartmann E, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quiroga MP, Balakrishnan K, Kurtova AV, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114:1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. The New England journal of medicine. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quesada V, Conde L, Villamor N, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nature genetics. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 68.Amrein PC, Attar EC, Takvorian T, et al. Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2977–2986. doi: 10.1158/1078-0432.CCR-10-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]