Abstract
Objective
We aimed to measure separately the contributions of heat and humidity to changes in levels of 34 markers of inborn disorders in dried-blood-spot (DBS) samples.
Design and Methods
Paired sets of DBSs were stored at 37°C for predetermined intervals in low-humidity and high-humidity environments. Marker levels of all samples in each complete sample set were measured in a single analytic run.
Results
During the 30±5 day study, galactose-1-phosphate uridyltransferase and biotinidase lost almost 65% of initial activities in low-humidity storage; most of the degradation in 27 other markers was attributable to adverse effects of high humidity storage; seven markers in DBSs stored at high humidity lost more than 90% of initial levels by the end of the study and four of the seven lost more than 50% of initial levels within the first week of storage.
Conclusions
Minimizing both humidity and temperature in the DBS transportation and storage environments is essential to maintaining sample integrity.
Keywords: Newborn screening, Disorder markers, Dried-blood spots, Storage conditions, Temperature effects, Humidity effects, Sample stability
Introduction
Blood spot samples, collected from blood obtained by heel pricks, applied to filter paper and dried, are used to screen newborns for more than 50 treatable inborn disorders [1,2]. For more than 30 years, investigators have studied the relationship between temperature and the stabilities of markers for many of these disorders in dried-blood-spot (DBS) samples [3,4,5,6,7,8,9,10,11,12]. These fragmentary retrospective and prospective studies of DBS samples illustrate the adverse effects of uncontrolled or elevated temperatures and/or humidities on marker stabilities, but differences in markers studied, study durations, DBS storage conditions, and data analysis methods make comparison of results among studies difficult. Published guidelines [13] for optimal long-term storage of residual DBSs after newborn screening analysis only address some of the markers that are presently recommended.
The examination of the stabilities of markers in the United States’ core panel of 29 recommended inborn disorders and 25 targeted disorders that are detectable in the course of screening for the recommended panel [1,2] has not taken place in a single normalized study which included temperature, humidity and elution variables. Understanding and controlling for these variables are critical to sustaining effective operations of newborn screening and associated external quality assurance programs that store and transport DBS samples in a wide range of environments.
As part of routine studies to evaluate DBS materials prepared by the Newborn Screening Quality Assurance Program (NSQAP) of the Centers for Disease Control and Prevention (CDC) to assist laboratories with monitoring the performance of their newborn screening tests [14], we performed accelerated degradation studies of 34 markers. This report summarizes results from those studies and covers markers for 25 recommended core disorders and 22 targeted disorders [1,2]. Hemoglobin S, the marker for the recommended sickle cell diseases, was excluded from the accelerated degradation studies because it is usually measured in a qualitative manner (no calibration), and hearing loss was excluded because accelerated degradation studies are not applicable. Targeted panel markers for various other hemoglobinopathies, galactoepimerase deficiency, and disorders measured by DNA testing –the recently added core panel severe combined immune deficiency [15] and targeted T-cell-related lymphocyte deficiencies—were also excluded from our studies. These studies were initiated to compare the stabilities of the markers in DBSs stored at elevated temperature (37°C) and at low (below 30%) or high (above 50%) relative humidities for predetermined intervals. The objectives of the studies were to measure separately, under normalized conditions for all markers tested, the contributions of heat and humidity to the degradation of markers of inborn disorders in DBSs and to evaluate the effectiveness of extending DBS elution times to improve marker recoveries from the treated samples.
2. Materials and Methods
2.1. Preparation of DBS materials
Sets of DBS materials for 34 inborn disorder markers were prepared from human blood adjusted to a hematocrit of 50±1% to simulate the hematocrit of newborns and dispensed in 75 μL aliquots onto Whatman Grade 903 filter paper. The blood enriched with amino acids and some acylcarnitines was prepared from saline-washed packed red cells and clarified serum to create a normalized base matrix for these markers. Other hematocrit adjustments were made by plasma removal except for the thyroid-stimulating hormone (TSH) enriched blood which was adjusted to 50% hematocrit by adding thyroxine (T4) -depleted serum to packed red cells.
Most of the disorder markers in normal blood occur at very low levels. Therefore, samples were enriched to achieve marker levels that allowed measurement of degradation with accuracy. Purified standards were used for all blood enrichments except for TSH enrichment which was made with the Third International Reference Preparation (81/565). Non-enriched natural donor blood was used to prepare the DBS materials for measurements of galactose-1-phosphate uridyltransferase (GALT) and biotinidase (BIOT) activities.
2.2. Accelerated degradation study design
Paired sets of each DBS material were stored at 37°C in Bitran Series S liquid-tight zip-closure specimen bags (Com-Pac International) containing cards (Desiccare, Inc.) that indicate by color-change humidities below 30% (blue) and above 50% (pink). The paired sets of DBSs for the immunoreactive trypsinogen (IRT) studies contained 8 samples each; all other sets contained 10 samples each. One set of each pair was stored with humidity controlled to below 30% by enclosing desiccant packets (Poly Lam Products, Corp.) with the DBSs and zip-sealing the bags before storage. The other set was stored without desiccant in open Bitran bags in a high-humidity chamber which was monitored by periodic hygrometer readings to maintain relative humidity above 90%. At predetermined intervals throughout the 30±5 day accelerated degradation studies, DBSs from paired sample sets were removed from 37°C storage and transferred to optimal storage at −70°C. Immediately before transfer to −70°C, desiccant packets were added to the bags containing DBSs that had been stored at high humidity, and those bags were zip-sealed to ensure that they were stored at optimal humidity. The marker levels of all samples in each complete sample set were measured in triplicate in a single analytical run. Complete data from 10 storage intervals were collected from all markers except T4 (9 intervals) and IRT (8 intervals), and all collected data were included in the data set.
2.3. Blood spot analysis methods
Each analytic run of amino acids, acylcarnitines, succinylacetone (SUAC), total galactose (T-GAL), and GALT also contained triplicate analyses of sample eluates taken after the elution time had been extended to twice the routinely used elution interval to account for any recovery loss attributable to higher retention of the markers in treated DBSs. The routine elutions of DBSs for measurements of the hormones, IRT, and BIOT are overnight, so samples analyzed for those markers were not subjected to extended elution intervals.
Delfia neonatal kits (PerkinElmer Life and Analytical Sciences) were used for measurements of T4, TSH, 17 α-hydroxyprogesterone (17-OHP), and IRT, and a neonatal kit from PerkinElmer Life and Analytical Sciences was used to measure GALT activity. Conversion of the synthetic BIOT substrate, N-biotinyl-p-aminobenzoate, to its purple product, p-aminobenzoate, [16] was used to measure BIOT activity. Enzymatic conversions of galactose-1-phosphate to simple galactose and NAD to NADH [17] were used in coupled reactions to measure T-GAL concentrations. Amino acids and acylcarnitines were converted to their butyl esters [18] and analyzed by our routinely used in-house tandem mass spectrometry method. SUAC was converted to its hydrazine derivative and measured by tandem mass spectrometry [19].
The coefficients of variation (CVs) of analytic tests were: T4—18%; TSH—7%; 17-OHP—13%; IRT—6%; GALT—15%; T-GAL—5%; and BIOT—11%. The CVs of tests for the eight aminoacidopathy markers ranged from 9% (SUAC) to 15% (arginine [ARG]). CVs of tests for free carnitine (C0) and most acylcarnitines ranged from 8% (palmitoylcarnitine [C16]) to 15% (isovalerylcarnitine [C5] and 3-hydroxypalmitoylcarnitine [C16OH]), but CVs of the dicarboxylic acylcarnitine tests were higher: malonylcarnitine (C3DC)—20% and glutarylcarnitine (C5DC)—22%.
2.4. Statistical analysis methods
The geometric means of triplicate measurements of initial (Day 0) marker levels and marker levels remaining on the last day of each accelerated degradation study were used to determine the percentage of each marker’s initial levels that were lost during the study. We attributed marker losses from samples stored at low humidity to the effects of the elevated storage temperature. We subtracted the percentage loss of each marker from sample sets stored at low humidity from the percentage loss sustained by paired sample sets stored at high humidity and attributed the difference to the effects of elevated storage humidity.
The effect of storage interval (days) was explored separately for DBSs stored in low and high humidity environments using separate one-way analysis of variance (ANOVA) models. This was followed by comparing the baseline (Day 0) storage interval to each of the other storage intervals using Dunnett’s multiple comparison [20] with a significance level of 0.05. Table 1 shows the estimated losses at high and low humidity from baseline to the last storage interval and estimates that are significantly different from zero, based on the Dunnett’s method, are footnoted (e).
Table 1.
Percentages of 33 inborn-disorder markers lost from dried-blood-spot samples stored at 37°C in low-humidity and high-humidity environmentsa for 30 ± 5 days and the corresponding 95% confidence intervals.
| Inborn-disorder marker (enrichment level)b | Study Length (Days) | Loss during storage at high humidity (%) | Loss during storage at low humidity (%)c | Loss attributable to adverse effects of high humidity (%)c,d,f |
|---|---|---|---|---|
| Thyroid-Stimulating Hormone (70 μIU/mL) | 31 | 63.7e | 18.9e | 44.8f |
| Thyroxine (10 μg/dL) | 35 | 86.4e | 8.4 | 78.0f |
| 17 α-Hydroxyprogesterone (100 ng/mL) | 31 | 41.9e | 21.8e | 20.1f |
| Immunoreactive Trypsinogen (160 ng/mL) | 25 | 53.3e | 16.8e | 36.5f |
| Total Galactose (30 μmol/L) | 35 | 39.6e | 17.5e | 22.1f |
| Galactose-1-phosphate uridyl-transferase (nonenriched) | 32 | 70.0e | 62.6e | 7.4 |
| Biotinidase (nonenriched) | 32 | 97.7e | 63.9e | 33.8f |
| Succinylacetone (50 μmol/L) | 31 | 96.4e | 4.3 | 92.1f |
| Arginine (300 μmol/L) | 35 | 94.8e | 11.7e | 83.1f |
| Citrulline (300 μmol/L) | 35 | 32.3e | 14.4e | 17.9f |
| Leucine (800 μmol/L) | 35 | 23.0e | 6.1 | 16.9f |
| Methionine (400 μmol/L) | 35 | 29.7 | 11.6 | 18.1f |
| Phenylalanine (600 μmol/L) | 35 | 33.5e | 6.9 | 26.6f |
| Tyrosine (750 μmol/L) | 35 | 56.3e | 9.1 | 47.2f |
| Valine (1000 μmol/L) | 35 | 5.9 | 9.4 | −3.6 |
| Acetylcarnitine (50 μmol/L) | 35 | 95.2e | 4.7 | 90.5f |
| Propionylcarnitine (10 μmol/L) | 35 | 75.6e | −6.7 | 82.3f |
| Butyrylcarnitine (5.0 μmol/L) | 35 | 55.3e | −3.4 | 58.7f |
| Isovalerylcarnitine (2.5 μmol/L) | 35 | 1.1 | −2.3 | 3.4 |
| Hexanoylcarnitine (2.5 μmol/L) | 35 | 37.6e | −0.5 | 38.1f |
| Octanoylcarnitine (2.5 μmol/L) | 35 | 36.4e | 7.0 | 29.4f |
| Decanoylcarnitine (2.5 μmol/L) | 35 | 29.5e | 4.7 | 24.8f |
| Tetradecanoylcarnitine (2.5 μmol/L) | 35 | 15.0 | 1.1 | 13.9f |
| Palmitoylcarnitine (10 μmol/L) | 35 | 17.6e | 0.2 | 17.4f |
| Stearoylcarnitine (5.0 μmol/L) | 35 | 19.4e | 5.7 | 13.7f |
| Tiglylcarnitine (2.0 μmol/L) | 31 | 58.9e | −2.6 | 61.5f |
| Decenoylcarnitine (1.3 μmol/L) | 31 | 92.5e | 28.5e | 64.0f |
| Tetradecenoylcarnitine (2.0 μmol/L) | 32 | 98.6e | 5.8 | 92.8f |
| 3-Hydroxybutyrylcarnitine (2.0 μmol/L) | 31 | 48.0e | 17.7 | 30.3f |
| 3-Hydroxyisovalerylcarnitine (2.5 μmol/L) | 35 | 11.7 | 1.6 | 10.1f |
| 3-Hydroxypalmitoylcarnitine (2.0 μmol/L) | 31 | 29.7e | 20.4e | 9.3f |
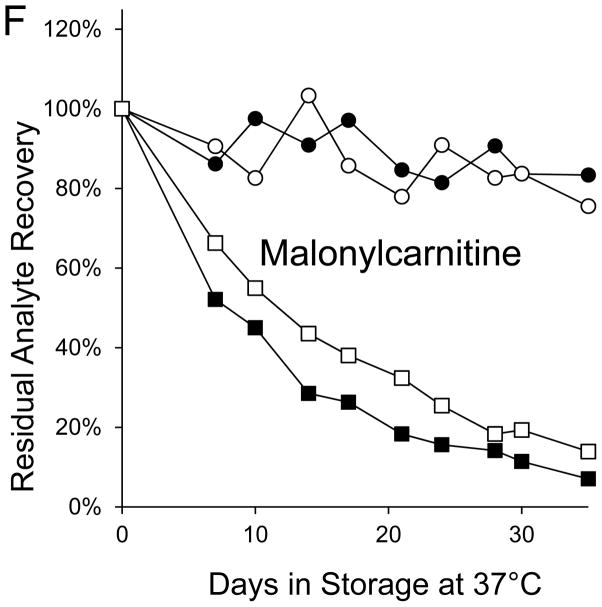
| Malonylcarnitine (2.5 μmol/L) | 35 | 92.9e | 16.6 | 76.3f |
| Glutarylcarnitine (2.5 μmol/L) | 35 | 67.7e | −2.7 | 70.4f |
Percentages shown were derived from geometric means of triplicate determinations.
Low relative humidity < 30%; high relative humidity > 50%
TSH, T4, and 17-OHP concentrations are expressed in serum units; all other concentrations are expressed in whole blood
Negative values are used to show gains (higher values after storage than initially).
Derived by subtracting loss during storage at low humidity from loss during storage at high humidity (adjusted least square
A statistically significant marker loss (significance level = 0.05) between initial level and level on last day of study based on Dunnett’s multiple comparison procedure (significance level 0.05).
A statistically significant difference (significance level 0.05) between change in samples stored at low humidity and change in samples stored at high humidity.
To determine whether the change in samples stored at low humidity was significantly different from the change in samples stored at high humidity, we constructed an F-test to compare the geometric means of initial levels at both humidities with the geometric means of the last storage interval at both humidities. This F-test used the least square means from a two-way ANOVA model including the effect of humidity (high/low), storage interval, and a storage interval by humidity comparison. These results are reported in the last column of Table 1 and estimates that are significantly different from zero based on the F-test are footnoted (f).
Results
3.1. Accelerated degradation study outcomes
The degradation of 33 of the 34 disorder markers during storage are summarized in Table 1. Recovery of C0 increased during storage because C0 is liberated when acylcarnitines degrade. The increase in C0 concentration of paired sets of DBSs enriched with an array of acylcarnitines and stored for 35 days at 37°C was 6-fold greater in DBSs stored at high humidity than in paired DBSs stored at low humidity.
Results from the comparison of marker levels on the initial and final days of the 37°C accelerated stability studies (Table 1) indicate that 10 of 33 disorder markers in DBSs stored at low humidity and 28 of 33 markers in DBSs stored at high humidity experienced statistically significant degradation during the 30±5 day storage periods.
Most of the degradation of 27 of the 33 markers was attributable to the adverse effects of high humidity; most of the degradation of 17-OHP, BIOT and C16OH was attributable to the adverse effects of the 37°C storage temperature, and degradations of GALT, valine (VAL) and C5 were not significantly different in DBSs stored at high and low humidities for more than 30 days indicating that these markers were not affected by elevated humidity.
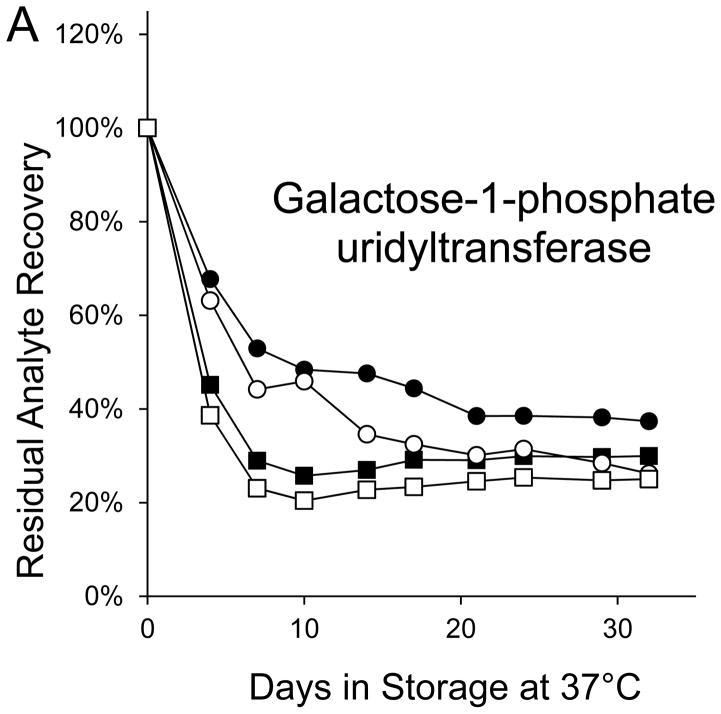
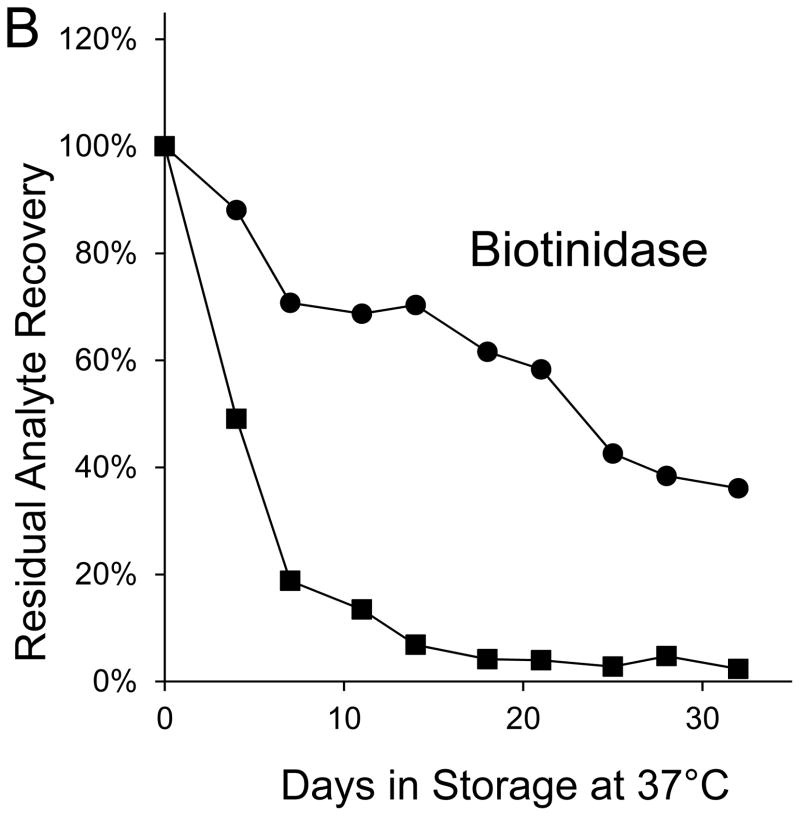
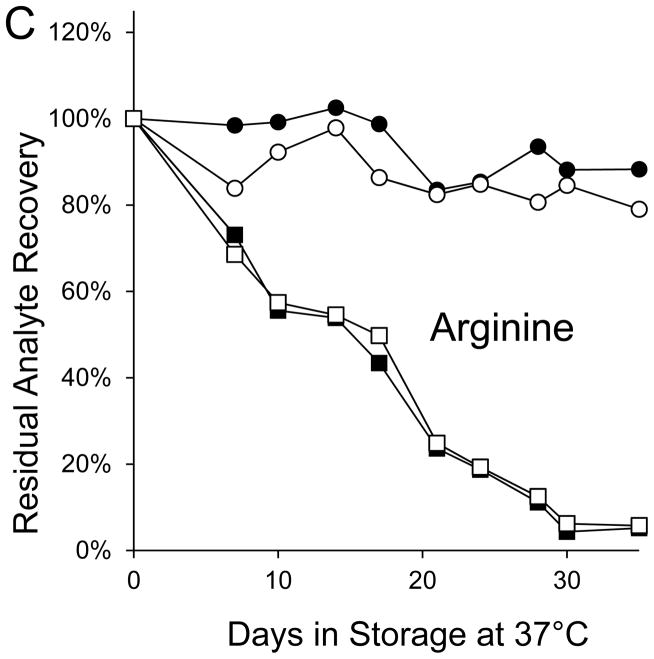
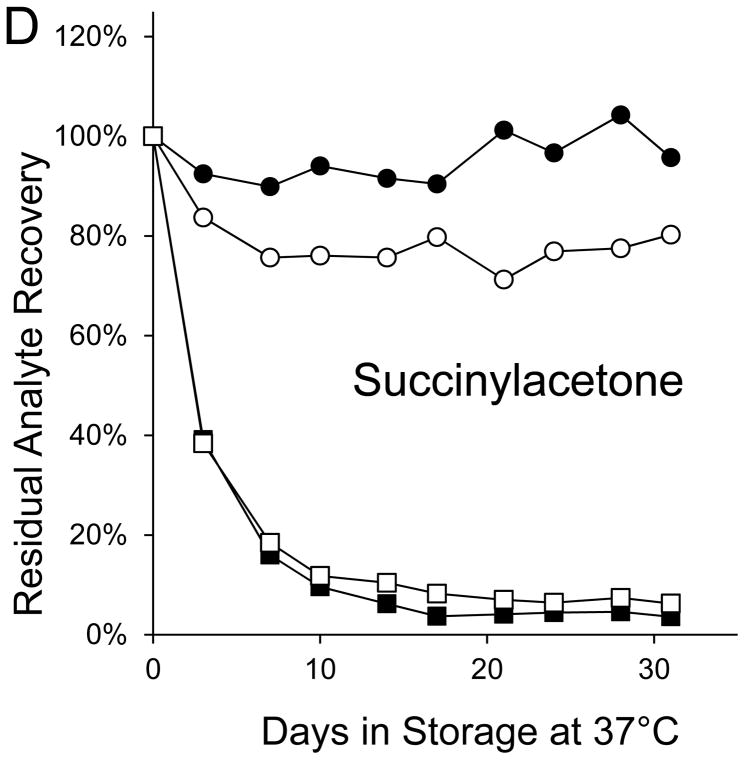
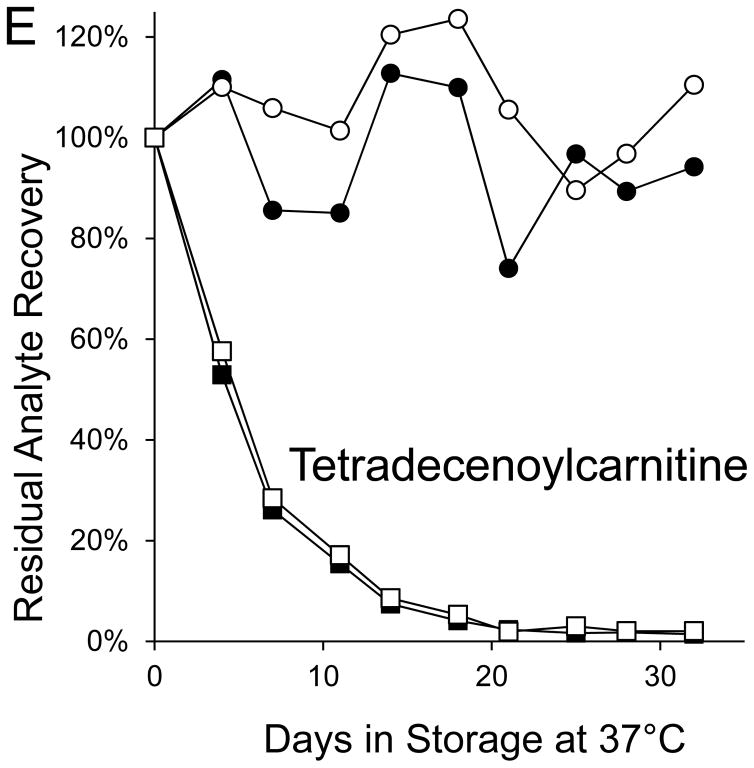
Seven markers—BIOT, SUAC, ARG, C2, C3DC, decenoylcarnitine (C10:1), and tetradecenoylcarnitine (C14:1)—lost more than 90% of their initial levels by the end of the month-long accelerated degradation studies (Table 1), and four of these—BIOT, SUAC, (C10:1), and (C14:1)—lost more than half of their initial levels during the first week of high humidity storage. As examples, Figure 1 shows the degradation curves for GALT, BIOT, SUAC, ARG, C14:1, and C3DC.
Figure 1. Recoveries of inborn disorder markers from dried-blood spots stored for predetermined intervals at 37°C in low-humidity and high-humidity environments.
● = low humidity, routine elution
■ = high humidity, routine elution
○ = low humidity, extended elution
□ = high humidity, extended elution
Biotinidase was not included in the extended elution studies because its routine elution is overnight.
Both GALT and BIOT lost > 60% of their initial levels in DBSs stored for 32 days at 37°C in the low humidity environment; whereas, all other markers lost < 30% of initial levels in DBSs stored for 30±5 days under those conditions (Table 1). GALT activity in DBSs stored at 37°C in high humidity declined by more than 50% during the first 4 days of storage and by about 70% during the first week, but it experienced no additional activity loss for the remainder of the 32-day study (Figure 1A). BIOT activity in DBSs stored at 37°C in the high humidity environment declined by more than 50% during the first 4 days of storage, by about 80% within the first week, and by more than 95% by the end of the 32-day study (Figure 1B).
SUAC lost less than 5% of its initial concentration from DBSs stored for a month in the low-humidity environment; however, SUAC loss from identical DBSs stored in the high-humidity environment was about 60% after 3 days of storage, about 85% after 7 days of storage, and greater than 95% after 17 days of storage (Figure 1C). Degradations of the seven amino acids in DBSs stored in the low humidity environment were less than 15%; whereas, amino acid degradations in identical DBSs stored in the high humidity environment ranged from about 5% (VAL) to 95% (ARG).
Losses of the 10 saturated acylcarnitines were less than 10% in DBSs stored for 35 days in the low-humidity environment. In identical DBSs stored at high humidity, losses of the 9 saturated acylcarnitines with straight carbon chains ranged from 18% of C16 to 95% of acetylcarnitine (C2), and loss of the branched-carbon-chain acylcarnitine, C5, was 1%.
All unsaturated, hydroxylated, and dicarboxylated acylcarnitines in DBSs stored at low humidity retained more than 70% of their initial concentrations after the accelerated degradation studies. During high humidity storage, losses of unsaturated acylcarnitines ranged from 59% to 99%; losses of hydroxylated acylcarnitines ranged from 12% to 48%; and losses of dicarboxylic acylcarnitines were 68% and 93%. With the exception of C16OH, losses of these markers were primarily attributable to high humidity during storage.
3.2. Extended elution outcomes
Recoveries of five of the 29 markers measured after both routine and extended elution times were increased by extending the elution times of samples that had been stored at low humidity. The recovery increases of only two of these were >10% (tetradecenoylcarnitine [C14]—15% and C14:1—17%). Recoveries of 20 markers were increased by extending elution times of DBS samples that had been stored at high humidity; however, the recovery increases of only three of these were >10% (C5DC—29%, C8—16%, and decanoylcarnitine [C10]—14%) (data not shown).
Discussion
The accelerated degradation studies were carried out at 37°C at low and high relative humidities for 30±5 days for study convenience. (Elevated storage temperature [3, 7, 9] or elevated humidity [5, 6, 8] accelerates the degradation process thus enabling measurement of changes in marker stability or degradation in a short study period.) Additionally, the study conditions can mimic those encountered during some transportation environments, such as summer transit in hot courier trucks, where the DBS samples may be exposed to elevated temperatures before arrival at testing laboratories.
In DBS sample sets packaged with desiccant to minimize humidity below 30%, only five markers—17-OHP, GALT, BIOT, C10:1, and C16OH—lost 20% or more of their initial levels during a month of storage at 37°C. In the high humidity environment, only six markers—Val, C5, C14, C16, stearoylcarnitine, and C5OH—lost less than 20% of initial concentrations after a month of DBS storage, and seven markers—BIOT, SUAC, ARG, C2, C3DC, C10:1 and C14:1—lost more than 90% of their initial levels. In terms of statistical significance, 10 markers—TSH, 17-OHP, IRT, T-GAL GALT, BIOT, ARG, citrulline, C10:1, and C16OH—experienced significant degradation at 37°C and low humidity, and 5 markers—methionine, VAL, C5, C14, and C16OH—did not experience significant degradation at 37°C and high humidity.
Accelerated degradation study results for the two markers of congenital hypothyroidism illustrate the importance of minimizing the storage humidity of DBSs. After a month of DBS storage at 37°C, TSH degradation at high humidity was more than three times greater than its degradation at low humidity, and T4 degradation at high humidity was more than 10 times greater than its degradation at low humidity.
Most of the GALT and BIOT activity losses were attributable to the 37°C storage temperature exposure. The high-humidity environment accelerated the loss of GALT, but by the end of the 32-day storage period, DBSs stored in the high-humidity and low-humidity environments both retained about 1/3 of initial activity. BIOT gradually lost about 2/3 of its initial activity in DBSs stored for 32 days at low humidity, but it lost almost all activity during the first two weeks of DBS storage at high humidity. Our finding that both enzymes lost substantial activity during the first week of storage at elevated temperature and low or high humidity is consistent with the observation that population means of GALT and BIOT activities in newborn screening samples were more than 20% lower in summer than in winter [8].
SUAC in DBSs stored at low humidity retained almost all of its initial concentration throughout its 31 days of exposure, but it lost about 60% during the first 3 days of storage at elevated temperature and high humidity. Losses of the amino acids attributable to adverse effects of high humidity were greater than the losses caused by effects of high temperature. ARG, which lost 95% of its concentration during high humidity storage, was the most sensitive to the effects of high humidity; VAL, which lost 6% of its initial concentration during high humidity storage, was the least sensitive.
C0 is both a marker of carnitine transporter deficiency (which is characterized by C0 concentrations below the reference range) and an acylcarnitine degradation product. C0 concentration increases observed during our 35-day accelerated degradation studies reflect levels of the acylcarnitines in the DBSs used in our studies. They are mentioned to illustrate the separate contributions of heat and humidity to acylcarnitine degradation and to sensitize the reader to the effect of acylcarnitine degradation on detection of carnitine transporter deficiency.
The saturated acylcarnitines were stable for more than a month at 37°C in a low humidity environment, but their degradations in DBSs stored at 37°C in a high humidity environment confirm a previous report [12] that acylcarnitine stabilities increase as carbon chain length increases. Most of the losses of the unsaturated, hydroxylated, and dicarboxylic acylcarnitines were attributable to the adverse effects of high humidity during DBS storage. Three of these markers (C10:1, C14:1, and C3DC) lost more than 90% of their initial levels during 35 days in the high-humidity environment.
The goal of these studies was to measure the separate contributions of elevated temperature and elevated humidity exposure to changes in levels of 34 markers of inborn disorders in DBS samples. Results from our accelerated degradation studies showed that degradation of 27 of the markers was primarily caused by the adverse effects of high humidity in the DBS storage environment; whereas, the degradation of only 4 markers was primarily caused by DBS exposure to the 37°C storage temperature. The markers varied widely in the degree and rapidity of degradation that occurred during 37°C storage at low and high humidities. For example, the markers of galactosemia, biotinidase deficiency, and tyrosinemia Type I (GALT, BIOT, and SUAC) lost more than ½ of initial levels in the first three or four days of storage; 7 markers lost more than 90% of initial levels during 30±5 day studies; and two markers (VAL and C5) were resistant to degradation from either elevated temperature or elevated humidity. Only small amounts of the marker losses that occurred during the degradation studies could be recovered by extending the pre-analysis elution times of the treated DBS samples.
Understanding the susceptibilities of markers in DBS samples to heat-related and moisture-related degradation is important for maintaining sample integrity for high quality measurements. We have shown that minimizing both the humidity and the temperature of the DBS transport and storage environment is essential for ensuring sample integrity especially when transportation times exceed a few days. The NSQAP DBS samples are transported worldwide in closed containers with desiccant to assure that quality DBSs arrive for assessment of laboratory performance. Of course, assay calibrators and controls for all the markers must receive maximum protection from potential degradation caused by their storage and transport environments.
Acknowledgments
We thank Hien Nguyen for performing the tandem mass spectrometry amino acid analyses for the studies reported here. Ms. Nguyen and co-author David Simms were funded by the Research Participation Program at the Centers for Disease Control and Prevention, an interagency agreement with the U.S. Department of Energy administered by the Oak Ridge Institute for Science and Education.
Abbreviations
- DBS
Dried-blood spot
- NSQAP
Newborn Screening Quality Assurance Program
- TSH
thyroid-stimulating hormone
- T4
thyroxine
- GALT
galactose-1-phosphate uridyltransferase
- BIOT
biotinidase
- IRT
immunoreactive trypsinogen
- SUAC
succinylacetone
- T-GAL
total galactose
- 17-OHP
17 α-hydroxyprogesterone
- ARG
arginine
- C0
free carnitine
- C16
palmitoylcarnitine
- C5
isovalerylcarnitine
- C16OH
3-hydroxypalmitoylcarnitine
- C3DC
malonylcarnitine
- C5DC
glutarylcarnitine
- ANOVA
analysis of variance
- VAL
valine
- C2
acetylcarnitine
- C14
1, tetradecenoylcarnitine
- C14
tetradecanoylcarnitine
- C8
octanoylcarnitine
- C10
decanoylcarnitine
References
- 1.Newborn screening: toward a uniform screening panel and system. Genet Med. 2006;8(5) Suppl:S12–S252. doi: 10.1097/01.gim.0000223891.82390.ad. as authored by the American College of Medical Genetics (ACMG) and commissioned by the Health Resources and Services Administration (HRSA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn screening: toward a uniform screening panel and system—executive summary. Pediatrics. 2006;117(Suppl):296–307. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- 3.Adam BW, Hubert IL, Hannon WH, Bayse DD. Quality control program for neonatal hypothyroidism. Atlanta (GA): Centers for Disease Control, Bureau of Laboratories; 1980. Summary Report I. [Google Scholar]
- 4.Levy HL, Simmons JR, MacReady RA. Stability of amino acids and galactose in the newborn screening filter paper blood specimen. J Pediatr. 1985;107:757–60. doi: 10.1016/s0022-3476(85)80411-x. [DOI] [PubMed] [Google Scholar]
- 5.Frazier DM, Clemons EH, Kirkman HN. Minimizing false positive diagnoses in newborn screening for galactosemia. Biochem Med Metab Biol. 1992;48:177–8. doi: 10.1016/0885-4505(92)90066-8. [DOI] [PubMed] [Google Scholar]
- 6.Zytkovicz TH, Eaton RB, Foley TP, Lister BC, Rojas DA, Schwerier ME. In: Therrell BL, Aldis BG, editors. Instability of enzymes, antibodies, and other analytes in dried blood spots – is the major problem heat or humidity; Proceeding of the Eleventh National Symposium on Neonatal Screening; Sept. 12–16, 1995; Corpus Christi, Texas. Washington, DC: ASTPHLD; 1995. pp. 293–295. [Google Scholar]
- 7.Chace DH, Adam BW, Smith SJ, Alexander JR, Hillman SL, Hannon WH. Validation of accuracy-based amino acid reference materials in dried-blood spots by tandem mass spectrometry for newborn screening assays. Clin Chem. 1999;45:1269:77. [PubMed] [Google Scholar]
- 8.Freer DE. Observations on heat/humidity denaturation of enzymes in filter-paper blood spots from newborns. Clin Chem. 2005;51:1060–62. doi: 10.1373/clinchem.2005.049270. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Zhou Y, Bell CJ, Earley MC, Hannon WH, Mei JV. Development and characterization of dried blood spot materials for the measurement of immunoreactive trypsinogen. J Med Screen. 2006;13:79–84. doi: 10.1258/096914106777589623. [DOI] [PubMed] [Google Scholar]
- 10.Strnadova KA, Holub M, Muhl A, Heinze G, Ratschmann R, Mascher H, Stockler-Ipsiroglu S, Waldhauser F, et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin Chem. 2007;53:717–22. doi: 10.1373/clinchem.2006.076679. [DOI] [PubMed] [Google Scholar]
- 11.Lando VS, Batista MC, Nakamura IT, Mazi CR, Mendonca BB, Brito VN. Effects of long-term storage of filter paper blood samples on neonatal thyroid stimulating hormone, thyroxin, and 17-alpha-hydroxyprogesterone measurements. J Med Screen. 2008;15:109–11. doi: 10.1258/jms.2008.007086. [DOI] [PubMed] [Google Scholar]
- 12.Fingerhut R, Ensenauer R, Roschinger W, Arnecke R, Olgemuller B, Roscher AA. Stabilities of acylcarnitines and free carnitine in dried blood samples: implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal Chem. 2009;81:3571–5. doi: 10.1021/ac8022235. [DOI] [PubMed] [Google Scholar]
- 13.Therrell BL, Hannon WH, Pass KA, Lorey F, Brokopp C, Eckman J, Glass M, Heidenreich R, et al. Guidelines for the retention, storage, and use of residual dried blood spot samples after newborn screening analysis: statement of the Council of Regional Networks for Genetic Services. Biochemical and Molecular Medicine. 1996;57:116–24. doi: 10.1006/bmme.1996.0017. [DOI] [PubMed] [Google Scholar]
- 14.Bell CJ, editor. Newborn screening quality assurance program: 2010 annual summary report. Vol. 28. CDC; Atlanta: 2011. [Google Scholar]
- 15.Sebelius K. [accessed June 2011];Letter from the Secretary of Health and Human Services. 2010 May 21; http://www.hrsa.gov/heritabledisorderscommittee/correspondence/response5_21_2010.pdf.
- 16.Heard GS, Secor McVoy JR, Wolf B. A screening method for biotinidase deficiency in newborns. Clin Chem. 1984;30:125–7. [PubMed] [Google Scholar]
- 17.Orfanos AP, Jinks DC, Guthrie R. Microassay for estimation of galactose and galactose-1-phosphate in dried blood specimens. Clin Biochem. 1986;19:225–8. doi: 10.1016/s0009-9120(86)80031-5. [DOI] [PubMed] [Google Scholar]
- 18.Chace DH, DiPerna JC, Mitchell BL, Sgroi B, Hofman LF, Naylor EW. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin Chem. 2001;47:1166–82. [PubMed] [Google Scholar]
- 19.Chace DH, Lim T, Hansen CR, De Jesus VR, Hannon WH. Improved MS/MS analysis of succinylacetone extracted from dried blood spots when combined with amino acids and acylcarnitine butyl esters. Clin Chim Acta. 2009;407:6–9. doi: 10.1016/j.cca.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. Journal of the American Statistical Association. 1955;50:1096–1121. [Google Scholar]