Abstract
The impact of retinoic acid (All Trans Retinoic Acid; ATRA) and Mold spores (MLD) in the development of lung pathology and in vivo tissue remodeling have not been well established in the literature. In addition, the role of citral (inhibitor of retinoid function) in the improvement of lung pathology has not been ascertained in animal studies. Therefore, it is hypothesized that ATRA and Mold (MLD) exposure will sensitize lung tissues leading to lung tissue pathology and that Citrals (C1 and C2) will reverse, ameliorate or improve the associated pathological damage to lung tissues. The study used an IACUC approved between-subject in vivo randomized split plot factorial design (F344 rat model; N=40). Animals were exposed to eight different treatments including vehicle, MLD, ATRA, Citrals (C1 and C2) and their MLD combinations (MLD+ ATRA, MLD+ C1, and MLD+ C2) by intra-peritoneal route. Rat weight and blood data were collected on Days 1 and 21, all animals were sacrificed on day 21, and lung tissues were processed for histopathology. Results from weight and blood data (ANOVA and Duncan) as well as from histopathological analyses supported the findings that exposure of F344 rats to MLD combinations with ATRA and Citrals showed various levels of lung tissue damage that were impacted by either C1 or C2. This promising study showed impressive responses on the interaction of MLD, Citrals, and ATRA as related to their impact on associated lung tissue pathologies
Keywords: ATRA, Citral, F344, Ovalbumin, Chronic Lung Pathology, Hpervitaminosis A, Mold Spores
INTRODUCTION
The current known clinical uses of some selected retinoids include the treatment of dermatologic diseases such as acne, psoriasis and eczema, photo-damaged skin, and specific forms of cancer [1, 2]. Biological effects of retinoids are generally exerted through a series of nuclear receptors that are ligand-inducible transcription factors belonging to the steroid/thyroid receptor superfamily such as RAR and RXR retinoid nuclear receptors. The impact of retinoic acid (All Trans Retinoic Acid; ATRA) and MLD exposure in the development of lung pathology and tissue remodeling were not well established in the literature. Equally, the role of citral (inhibitor of retinoid function) in the improvement of lung pathology was not ascertained under an in vivo setting. Retinoids represent the chemical derivatives of vitamin A or all-trans retinol and include retinol, retinaldehyde, and all forms of the final oxidized product retinoic acid (RA). Vitamin A is generally obtained from the diet in the form of retinyl esters that are linked to fatty acids such as palmitic acid (retinylpalmitate; RP) or in the form of carotenoids which are dimers of retinal; the oxidative aldehyde form of retinoid isomers [3, 4 and 5].
A recent challenge in the treatment of chronic lung disease includes restoration of alveolar surface area, respiratory and mechanical function of the lung parenchyma has led to a focus on retinoids as therapeutic agents [6, 7, and 8]. It has been well known that alveolar architecture depends on the anatomy of prenatal airways in mice, rats and humans. Airway branching, elongation, and cellular differentiation are influenced by retinoids, however, one of the important component of the alveolus is the septum which is composed of epithelial and endothelial cells, fibroblasts and some immune and neuro-endocrine cells. Retinoids are known as alveolar morphogens based on the fact that RA was shown to ameliorate emphysema in rats after the intra-tracheal instillation of elastase [9, 10, and 11].
Circulating retinoids as well as the endogenous stores furnish the required amounts of retinoids to body cells through the hydrolysis of RP [12, 13]. Retinoids are pleiotropic regulatory compounds that are capable of modulating the structure and function of a wide range of inflammatory, immune and structural body cells. They possess a hormone-like function that regulate epithelial cell proliferation, pattern formation in developing tissues, morphogenesis in the lung, and cellular differentiation [14].
Citral has been reported to exhibit activity as a Vitamin A antagonist by inhibiting the oxidation of retinal to retinoic acid. This suggests that Citral is able to block the endogenous RA signaling pathway [15]. Hypervitaminosis A is a condition representing retinoid toxicity, which may reflect its effects on the lungs as a damaging agent. To this end and in reference to the literature, the findings on the responses to ATRA both in vitro and in vivo appeared to be contadory [2, 3 and 16]. This study was undertaken to explore this contraversy.
This paradox in the function of retinoids [17–24] as curing or damaging agents has prompted the execution of our study with the hypothesis that application of supraphysiologic levels of retinoids ATRA will cause lung pathologic damage similar to MLD exposure. The objective of the study, was to assess the role of exposing the F344 rat model to supraphysiologic levels of MLD+ATRA, MLD+C1, MLD+C2, and their comparison to untreated control and single treatments including MLD, ATRA, C1 and C2 as an attempt to provide insights into the role of citral in ameliorating such pathology with regards to the treatment of chronic lung disease in an in vivo setting.
METHODS
High purity All Trans Retinoic Acid (ATRA), Citral 1 (C1; diethyl acetal and Citral 2 (C2; cis and Trans dimethyl), DMSO, Isoflurane and PBS were purchased from Sigma Aldrich Company, St. Louis, MO. The spores from four MLD strains including were purchased from ATCC, USA.
Animals and housing
Fisher rats (F344; 260–324 g) were obtained from Harlan Laboratories (Frederic, MD). The animals were housed at the Jackson State University (JSU) Animal Core Facilities (Olaw class 2 level). Animals were acclimatized for a week and all protocols including handling, husbandry, anesthesia, euthanasia, and experimental protocols were approved by JSU-IACUC (protocol # 08-13-08) and were performed according to Olaw recommendations. Animals were kept under controlled environment at 12/12 light/dark cycles and were allowed unrestricted access to water and rodent chow. Each animal was kept in a separate rat cage that is well maintained by a technical staff supervised by a veterinarian.
Experimental design
A total of 40 F344 rats were used in this study. Five animals were not treated and designated as negative control. The remaining 35 animals were divided into seven different groups of 5 animals each and were exposed as treatment group through intra-peritoneal (iP) and intra-tracheal (iT) routes on day 1 using all trans retinoic acid (ATRA; 80 mg/Kg), citral 1 (C1; 50 mg/Kg), Citral 2 (C2; 50 mg/Kg) by the intra-peritoneal route and mold (MLD; (Aspergillus, Penicillum, Stachybotrys and Cladosporium); sores were collected, mixed and treated with equal volumes of 95% ethanol and 5% vinegar for inactivation to sterility by subculture, centrifuged and re-suspended in PBS, a dose of 100 μL of 5.0X1012 by iT route was used. All animals were weighed on days 1 and 21 and were sacrificed (euthanized by inhalation of CO2 in special chamber) on day 21 following approved protocols and blood was collected for parameter analysis, which was done by the Mississippi State Veterinary lab; using an automated cell sorter. Dead animals were subjected to in-house post-mortem (Necropsy) procedures and entire lungs from all animals were collected in 10% formalin for histopathological analysis.
Tissue processing, slide preparation and digitizing
Formalized lung tissue was processed for histopathology following standard procedures. Tissues were embedded in paraffin blocks and were then sectioned using a Leica Microtome to produce 5μm sections. Sections were floated in a water bath, adhered to standard glass slides and allowed to dry. Slides were then stained using standard Hematoxylin and Eosin staining procedure for further analysis. Slides were examined under the microscope and digitized into photos to document pathological changes from the impact of their exposure to these chemicals.
Statistical analysis
As based on the experimental design of the study, factorial analysis statistics for F-ratios associated with ANOVA was employed to support significance and interpretation of data using the standard software packages SPSS. Variance in mean differences (p<0.05) was determined by ANOVA and the Duncan ranking statistics and presented as mean ± SD.
RESULTS
Data on the comparison of weight gain and survival for the F344 rat model exposed to 8 different treatments is presented in table 1. Animal weights ranged between 229.7±9.5 and 297.4±10.3 g. As can be seen comparison to the ve control; MLD treatment boosted weight gain (150.21* %); and ATRA control (91.55%) showed no significant effects on weight gain, however, the combination of MLD and ATRA is highly synergistic (146.84* %). The combinations of MLD with either C1 or C2 showed antagonism with respect to weight gain by this model (150.21, 31.22* and 13.90* %). All animals in this group survived to term.
Table 1.
Comparison of weight data and survival of F344 rats exposed to ATRA, Mold+ATRA, Mold+C1, Mold+C2, C1, C2 and Mold (n=40)
| # | Treatment Type | N | Initial Weight (g) | Final Weight (g) | Weight Difference (g) | Standard Difference (g) | Weight Gain/Av Control % | Survival to term |
|---|---|---|---|---|---|---|---|---|
| 1 | − Control | 5 | 264.0±8.0 | 287.7±2.6 | 23.7 | 0.00 | 100.00 | +++++ |
| 2 | + ATRA Control | 5 | 229.7±9.5 | 251.3±17.6 | 21.7 | −2.00 | 91.55 | +++++ |
| 6 | MLD + ATRA | 5 | 262.6±13.8 | 297.4±10.3 | 34.8* | +11.10 | 146.84 | +++++ |
| 3 | + C1 Control | 5 | 244.6±10.3 | 255.0±4.6 | 10.4* | −13.30 | 44.00 | +++++ |
| 7 | MLD + C1 | 5 | 260.1±10.9 | 267.5±11.1 | 7.4* | −16.3 | 31.22 | +++++ |
| 4 | + C2 Control | 5 | 261.4±8.4 | 267.9±9.8 | 6.5* | −17.12 | 27.50 | +++++ |
| 8 | MLD + C2 | 5 | 257.7±10.4 | 261.0±11.5 | 3.3* | −20.4 | 13.90 | +++++ |
| 5 | + MLD Control | 5 | 256.9±11.5 | 292.5±3.09 | 35.6* | +11.90 | 150.21 | +++++ |
Statistically significant at p<0.05.
Data on the comparison of RBC parameters for the F344 rat model exposed to 8 different treatments is presented in table 2. As can be seen only MLD+ATRA (−2.29±0.40* M/μL) and C1 (−2.2±1.90* M/μL) showed negative significant differences for RBCs. HCT showed negative significance for ATRA, MLD+ATRA, and MLD+C1 (−4.35±10.50*, −4.30±2.19*, and −8.80±2.23 %, respectively). The MCV parameter showed positive significance for MLD+C1 and MLD Control (3.5±1.04* and (3.72±2.4* fl). The Hb parameter showed negative significance for MLD+ATRA (−7.74±0.38* g/dl), C1 control (−5.10±2.72* g/dl), MLD+C2 (−4.02±1.19* g/dl), and positive significance for MLD control (3.27±2.14* g/dl). MCH showed negative significance for MLD+ATRA (−3.54±0.63* pg), MLD+C1 (−1.93±1.97* pg), MLD+C2 (−2.20±0.22* pg), and MLD control (−2.35±2.14* pg) and the MCHC parameter showed negative significance for all treatments. Means for these parameters were within the normal mean range for this model. All three MLD combinations caused synergistic negative impact on RBC parameters as compared to individual controls of the combination.
Table 2.
Comparison of RBC parameters of F344 rats exposed to ATRA, MLD+ATRA, MLD+C1, MLD+C2, C1, C2 and MLD (n=40)
| # | Treatment Type | N | RBCs (M/μL) | HCT (%) | MCV (fl) | Hb (g/dl) | MCH (pg) | MCHC (g/dl) |
|---|---|---|---|---|---|---|---|---|
| 1 | −Control | 5 | 1.11±0.69 | 12.84±3.92 | 2.48±0.95 | 0.72±0.97 | −1.46±0.38 | −5.20±1.07 |
| 2 | + ATRA Control | 5 | −0.96±0.50 | −4.35±10.54* | −2.80±1.00 | −2.03±0.85 | −0.43±0.21 | −4.03±2.43* |
| 6 | MLD + ATRA | 5 | −2.29±0.4* | −4.30±2.19* | 0.38±1.03 | −7.74±0.38* | −3.54±0.63* | −4.93±1.17* |
| 3 | + C1 Control | 5 | −2.20±1.9* | −0.63±2.02 | 1.68±0.43 | −5.10±2.72* | −1.10±1.21 | −3.98±0.61* |
| 7 | MLD + C1 | 5 | −0.57±0.39 | −8.80±2.23* | 3.50±1.04* | −2.80±0.95 | −1.93±1.97* | −8.82±1.39* |
| 4 | + C2 Control | 5 | −0.39±0.37 | −2.57±2.72 | 0.50±0.40 | −2.18±0.54 | −1.63±0.29 | −3.93±3.53* |
| 8 | MLD + C2 | 5 | 1.11±0.77 | 1.81±1.53 | 1.14±0.80 | −4.02±1.19* | −2.20±0.22* | −5.28±0.67* |
| 5 | + MLD Control | 5 | −073±1.81 | −0.67±10.60 | 3.72±2.04* | −3.27±2.14* | −2.35±2.14* | −6.75±3.13* |
| Reference Mean** | 8.95±1.69 | 52.24±3.90 | 54.48±2.95 | 15.72±2.80 | 18.36±1.38 | 35.20±1.07 |
Statistically significant at p<0.05. Data was centered on untreated group mean (standardized).
Data on total white blood cells WBC) and percent of Lymphocyte (Lymph), Monocyte, Eosinophil and segmented neutrophils (Segs) parameters upon exposure to 7 different treatments on the F344 rat model is presented in table 3. As can be seen, Lymph and Monocytes did not show any significant changes for all treatments. Eosinophils showed positive significance for C1 control and WBCs showed negative significance for MLD control (3.00±2.00 and −3.63±3.80 %). The Segs parameter showed negative significance for ATRA control (-9.00±6.53* %), MLD+C2 (−8.40±2.68* %) and MLD control (−21.00±7.53* %). Means for these parameters were within the normal cited reference mean ranges for this model**.
Table 3.
Comparison of WBC parameters of F344 rats exposed to ATRA, MLD+ATRA, MLD+C1, MLD+C2, C1, C2 and MLD (n=40)
| # | Treatment Type | N | Lymph (%) | WBC (k/μL) | Monocytes (%) | Eosinophils (%) | Segs (%) |
|---|---|---|---|---|---|---|---|
| 1 | −Control | 5 | 0.00±0.00 | 2.01±3.70 | 0.70±1.79 | −1.00±0.00 | −16.15±3.85 |
| 2 | + ATRA Control | 5 | 0.00±0.00 | −0.33±2.80 | 0.00±0.00 | −0.50±1.00 | −9.00±6.53* |
| 6 | MLD + ATRA | 5 | 0.00±0.00 | 0.20±2.53 | 0.60±1.34 | −0.40±0.00 | 4.20±9.02 |
| 3 | + C1 Control | 5 | 0.00±0.00 | −1.58±2.85 | 0.00±0.00 | 3.00±2.00* | −0.25±10.84 |
| 7 | MLD + C1 | 5 | −1.00±1.68 | 1.20±6.73 | 0.00±0.00 | 0.75±2.50 | −2.00±12.53 |
| 4 | + C2 Control | 5 | 0.00±0.00 | 0.35±1.08 | 0.00±0.00 | −1.50±1.00 | −1.50±7.33 |
| 8 | MLD + C2 | 5 | 0.00±0.00 | 1.42±2.49 | 0.00±0.00 | 0.00±1.10 | −8.40±2.68* |
| 5 | + MLD Control | 5 | 0.00±0.00 | −3.63±3.8* | 0.00±0.00 | 0.17±1.60 | −21.00±7.53* |
| Reference Mean** | 82.2±5.12 | 8.80±1.97 | 2.00±0.67 | 0.00±0.00 | 33.1±2.34 |
Statistically significant at p<0.05. Data was centered on untreated group mean (standardized).
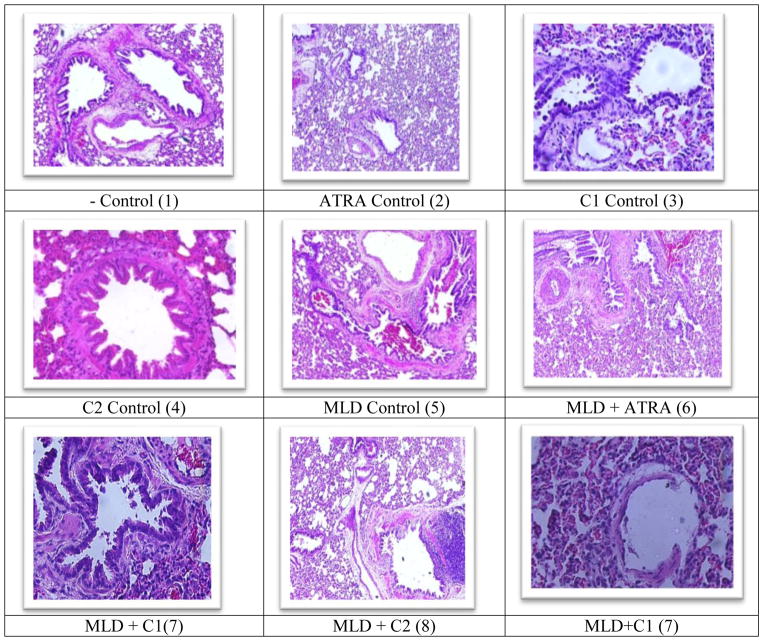
As seen in Figure 1: Panel (1) depicts negative or no treatment control of the F344 rat lung illustrating normal lung tissue of with bronchial walls as well as variable sized bronchioles made up of ciliated pseudostratified columnar epithelium and smooth muscles. Panel (5) Showing MLD treatment of the F344 rat lung and illustrating hypersensitivity, constricted bronchi, congestion of vessels as well as damaged lung tissues with the appearance of hyperplasic distended and widely open arteries. Panel (2) Depicts the ATRA control treatment of the F344 rat lung showing thickening and constriction of alveoli, congestion, constricted vessels and appearance of papillary projections into lumen bronchioles. The C1 control; Panel (3) treatment of the F344 rat lung illustrating an abundance of papillary projections, very thick alveolar walls, more basophilic condition, normal columnar epithelium, evident congestion and constricted vessels and alveoli. Panel (4) The C2 Control treatment of the F344 rat lung illustrating thick alveolar walls but no inflammation, eosinophilic appearance, thick walled and dilated blood vessels with numerous red blood cells, vessel congestion and major constriction of the alveoli.
Fig 1.
Comparison of lung pathology of F344 rats exposed to MLD + C1, MLD + ATRA and MLD + C2. In comparison to relative controls, MLD + C1 show loss of alveolar boarders as well as loss of alveolar projections. MLD + ATRA show constriction of alveoli and loss of lung space and Mold + C2 showed eosinophilia changes and lung structure destruction.
Figures 1 also shows MLD+ATRA (6) histopathology display in response to treatment of the F344 rat lung illustrating vessels that appear to be narrow and closed. MLD+C1 (7) treatment illustrating overall inflammation and apparent congestion and constriction, and MLD+C2 (8) treatment illustrating an abundance of red blood cells and apparent congestion.
Figure 2 compares lung histopathology panels for the F344 rat model exposed to MLD + C1 (7), MLD + ATRA (6) and MLD + C2 (8). In comparison to relative controls, MLD + C1 (13) showed loss of alveolar boarders as well as loss of alveolar projections. The panel for MLD + ATRA showed constriction of alveoli and loss of lung space and MLD + C2 showed eosinophilia changes and lung structure destruction. Figure 2: depicts MLD-hypersensitized lung histopathological display of Fischer 344 rat model. Figure 2: (6A) panel shows MLD+ATRA treatment illustrating vessels that appear to be narrow and closed. The (6B) panel for MLD+ATRA treatment illustrating overall inflammation and apparent congestion and constriction, and the (6C) panel for MLD+ATRA treatment illustrating an abundance of red blood cells and apparent congestion. The (6D) panel for MLD+ATRA treatment illustrates overall inflammation and the abundance of papillary projections.
Figure 2.
Mold hypersensitized lung histopathological display of Fischer 344 rat model. As seen in Figure 2: (6A) Mold+ATRA treatment to F344 rat lung illustrating vessels that appear to be narrow and closed. (6B) Mold+ATRA treatment to F344 rat lung illustrating overall inflammation and apparent congestion and constriction, and (6C) Mold+ATRA treatment to F344 rat lung illustrating an abundance of red blood cells and apparent congestion; 400 x. Figure 3.4.4: Mold hypersensitized lung histopathological display of Fischer 344 rat model. As seen in Figure 2: The (6D) Mold+ATRA treatment of the F344 rat lung illustrates overall inflammation and the abundance of papillary projections; 400 x. (7A) shows Mold+C1 treatment of the F344 rat lung illustrating appearance of constricted bronchi and very thick alveolar walls. (7B) shows Mold+C1 treatment of the F344 rat lung illustrating constricted arteries and vessels. (7H) shows Mold+C1 treatment of the F344 rat lung illustrating bronchial damage. (7I) shows Mold+C1 treatment of the F344 rat lung illustrating dilatation of alveoli, congestion and abundance of red blood cells within the vessels. (8) Mold+C2 show that Alveolar walls are thickened; papillary projections are prevalent; epithelium is columnar and massive inflammation projecting into the alveoli space: Somewhat dilated alveoli and vessels.
As seen in Figures 2: Panel (7A) shows MLD+C1 illustrating appearance of constricted bronchi and very thick alveolar walls. Panel (7B) shows MLD+C1 treatment illustrating constricted arteries and vessels. Panel (7H) shows MLD+C1 treatment illustrating bronchial damage. Panel (7I) shows MLD+C1 treatment illustrating dilatation of alveoli, congestion and abundance of red blood cells within the vessels. Panel (8) MLD+C2 show that Alveolar walls are thickened; papillary projections are prevalent; epithelium is columnar and massive inflammation projecting into the alveoli space: Somewhat dilated alveoli and vessels.
In summary, except for MLD + C1, the addition of ATRA and C2 as a combination with MLD improved the pathology of the lung and produced a display closer to the control. The combination with C1, however, did not improve lung pathology and produced more damage to the exposed lung (Fig 2).
DISCUSSION
In reference to weight gain, MLD + ATRA proved to be synergistic in weight gain and t hat the addition of ATRA to MLD (150.21%) boosted the ATRA (no effect) weight impact level significantly (91.55 vs. 146.84%). MLD failed to improve the loss of weight due to exposure to both C1or C2 (150.21 vs. 31.22 and 13.90%; table 1). MLD treatment has failed to protect from the negative effects of both C1 and C2 on weight gain because there is no specific chemical interaction between MLD and C1 or C2 to reverse their negative impact on weight gain by the model. It is apparent that MLD had a neutral impact on the effects of C1 and C2 but has a positive synergistic effect on this model when combined with ATRA; an expected outcome. In support of our findings with ATRA, Hangfang and Wang [24] showed that animals losing weight have implications for the presence of ATRA in airway inflammation in asthmatic rats. The study shows that total cell count and proportion of inflammatory cells in BALF in two treatment groups were significantly reduced.
In regards to the RBC parameters, all three MLD double combinations with ATRA, C1 and C2 caused synergistic negative impact on the RBC parameters as compared to individual treatment of their components (Table 2). The MLD double combinations with ATRA, C1 and C2 improved the negative impacts of individual treatments on the WBC parameters (Table 3). The literature supports the assumptions made by the present study that ATRA, MLD, C1 and C2 would have a negative weight effect on the treated animals. Treatment of animals with ATRA triggers increase in eosinophils, neutrophils, lymphocytes, and monocytes [25], while decreasing them in a MLD setting; being in contrast to our findings [26].
In reference to lung histopathology, except for MLD+ C1, the addition of ATRA and C2 as a combination with mold improved the pathology of the lung and produced a display closer to the control. The combination with C1, however, did not improve lung pathology and produced more damage to the exposed lung (Fig 2). This finding is in contrast to the findings with OVA combinations (improvement of C1 not C2 impact; Farah et. al. [27] with regards to lung pathology confirming that C2 impact but not C1 was reversed in a MLD setting. This shows that MLD did not influence the impact of ATRA on C1 but was able to support the interaction of ATRA and C2 and reversed their negative effects on lung pathology. Menegola et al. [28] demonstrated the inhibitory effects of citral in retinoic acid synthesis have resulted in reduction in damage to lung tissues. DiRenzo et al. [29] demonstrated that the combination of citral and MLD treatment decreased the effects on the synthesis of retinoic acid (ATRA) that consequently participated in alveolar inflammation, bronchoconstriction and other lung complications.
CONCLUSIONS
The objective of the study was to assess the impact of exposing the F344 rat model to supraphysiologic levels of MLD+ATRA, MLD+C1, MLD+C2, and their comparison to untreated control and single treatments including MLD, ATRA, C1 and C2. In reference to weight gain, MLD + ATRA proved to be synergistic in weight gain and that the addition of ATRA to MLD boosted the ATRA (no effect) weight impact level significantly. However, MLD failed to improve the loss of weight due to exposure to either C1or C2. It is apparent that MLD has failed to protect from the negative effects of both C1 and C2 on weight gain because there is no specific chemical interaction between mold and C1 or C2 to reverse their negative impact on weight gain by the model. The findings of this study states that MLD had a neutral impact on the effects of C1 and C2 but has a positive synergistic effect on this model by ATRA. In regards to the RBC parameters, all three MLD double combinations with ATRA, C1 and C2 caused synergistic negative impact on the RBC parameters as compared to individual treatments of their components. The MLD double combinations with ATRA, C1 and C2 improved the negative effects of individual treatments on the WBC parameters. In reference to lung pathology, except for Mold + C1, the addition of ATRA or C2 as a combination with MLD improved the pathology of the lung and produced a display closer to the control. The combination with C1, however, did not improve lung pathology and produced more damage to the exposed lung. Confirming that C2 impact but not C1 was reversed in a MLD setting. These findings show that mold did not influence the impact of ATRA on C1 but was able to support the interaction of ATRA and C2 and reversed their negative effects on lung pathology. The findings of this study call for further investigation of these relationships to confirm the anti-inflammatory benefits of Citrals.
Acknowledgments
This research is supported by NIH/NCRR RCMI grant # G12-MD007581.
References
- 1.McGowan SE. Contributions of Retinoids to the Generation and Repair of the pulmonary alveolus. Chest. 2002;121:206S–208S. doi: 10.1378/chest.121.5_suppl.206s. [DOI] [PubMed] [Google Scholar]
- 2.Belloni PN, Garvin L, Mao C-Ping, Bailey-Healy I, Leaffer D. Effects of All-Trans-Retinoic Acid in Promoting Alveolar Repair. Chest. 2000;117:235S–241S. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- 3.Massaro GD, Massaro D. Retinoic Acid Treatment Abrogates Elastase-induced Pulmonary Emphysema. Nature Med. 1997;3:675–703. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 4.Chambone PA. A Decade of Molecular Biology of Retinoic Acid Receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 5.Chytil F. Retinoids in Lung Development. FASEB J. 1996;10:986–992. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- 6.McGowan SE, Doro MM, Jackson SK. Endogenous Retinoids Increase Perinatal Elastin Gene Expression in Rat Lung Fibroblasts and Fetal Explants. Am J Physiol Lung Cell Mol Physiol. 1997;273:L410–L416. doi: 10.1152/ajplung.1997.273.2.L410. [DOI] [PubMed] [Google Scholar]
- 7.Massaro GD, Massaro D. Retinoic Acid Treatment Partially reverses Failed Septation in Rats and Mice. AM J Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 8.Morabia A, Menkes M, Comstock G, et al. Serum Retinol and Airway Obstruction. AM J Epidemiol. 1990;132:77–82. doi: 10.1093/oxfordjournals.aje.a115645. [DOI] [PubMed] [Google Scholar]
- 9.Paiva S, Goday I, Vannucchi H, et al. Assessment of Vitamin A Status in Chronic Obstructive Pulmonary Disease Patients and Healty Smokers. Am J Clin Nutr. 1996;64:929–934. doi: 10.1093/ajcn/64.6.928. [DOI] [PubMed] [Google Scholar]
- 10.Napoli J. Retinoic Acid Biosynthesis and Metabolism. FASEB J. 1996;10:993–1001. doi: 10.1096/fasebj.10.9.8801182. [DOI] [PubMed] [Google Scholar]
- 11.Haq R, Pfahl M, Chytil F. Retinoic Acid Affects the Expression of Nuclear Retinoic Acid Receptors in Tissues of Retinol Deficient Rats. Proc Natl Acad Sci USA. 1991;88:8272–8276. doi: 10.1073/pnas.88.18.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zachman R. Role of Vitamin A in Lung Development. J Nut. 1995;125(Suppl):1934 S–1638S. doi: 10.1093/jn/125.suppl_6.1634S. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Harvey C, McGowan S. Retinoic Acid increase Elastin in Rat Lung Fibroblasts Cultures. Am J Physiol. 1993;265:L430–L437. doi: 10.1152/ajplung.1993.265.5.L430. [DOI] [PubMed] [Google Scholar]
- 14.Okabe T, Yorifuji H, Yamada E, et al. Isolation and characterization of Vitamin A Storing Lung Cells. Exp Cell Res. 1984;154:125–135. doi: 10.1016/0014-4827(84)90673-6. [DOI] [PubMed] [Google Scholar]
- 15.Schuh TJ, Kraft J, Hall BL. V-erbA and Citral Reduce the Teratogenic Effects of all-trans Retinoic Acid and Retinol, Respectively, in Xenopus Embryogenesis. Development. 1993;119:785–798. doi: 10.1242/dev.119.3.785. [DOI] [PubMed] [Google Scholar]
- 16.Tanumihardjo SA, Penniston KL. The Acute and Chronic Toxic Effects of Vitamin A. American Journal of Clinical Nutrition. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 17.Massaro GD, Massaro D. Postnatal Treatment with Retinoic Acid Increases the Number of Pulmonary Alveoli in Rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 18.Swee M, Parks W, Pierce R. Developmental regulation of Elastin Production. J Biol Chem. 1995;270:14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- 19.Austead G. Steroids, Retinoids and Wound Healing. Adv Wound Care. 1998;11:277–285. [PubMed] [Google Scholar]
- 20.Hunt T. Vitamin A and Wound Healing. J Am Acad Dermatol. 1986;15:817–821. doi: 10.1016/s0190-9622(86)70238-7. [DOI] [PubMed] [Google Scholar]
- 21.Tepper J, Pfeiffer J, Aldrich M, et al. Can Retinoic Acid Ameliorate the Physiologic and Morphologic effects of elastase instillation in the Rat. Chest. 117(suppl 1):242S–244S. doi: 10.1378/chest.117.5_suppl_1.242s. [DOI] [PubMed] [Google Scholar]
- 22.Maple S, Mendelssohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- 23.Mao JT, Goldin JG, Ermand J, et al. A pilot study of all-trans retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- 24.Fan H, Hongfang J, Wang H. Effects of all-trans retinoic acids on airway inflammation in asthmatic rats and mechanism. Journal of Huazhong University of Science and Technology. 1997;24(3):229–232. doi: 10.1007/BF02831997. [DOI] [PubMed] [Google Scholar]
- 25.Wynn EA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 26.Beeman CS, Berndt W, Kronmillera JE, Nguyen T. Blockade of the initiation of murine odontogeness in vitro by citral, an inhibitor of endogenous retinoic acid synthesis. Archives of Oral Biology. 1995;4:7. doi: 10.1016/0003-9969(95)00015-h. [DOI] [PubMed] [Google Scholar]
- 27.Farah IO, Holt-Gray C, Cameron JA, Tucci M, Cason Z, Benghuzzi HA. Impact of Paired Combinations of Retinoic Acid (ATRA) and Ovalbumin on F344 Rat Lung Tissues and Improvement of Related Pathology by Citral. Biomed Sci Instrum. 2014;50:423–30. [PMC free article] [PubMed] [Google Scholar]
- 28.Nenegola E, Giavini E, DiRenzo FY, Broccia MI. Citral, an inhibitor of retinoic acid synthesis, attenuates the frequency and severity of bronchial arch abnormalities induced by triazole-derivative fluconazole in rat’s embryos culture in vitro. Reproductive Toxicology. 2007;24(3):326–332. doi: 10.1016/j.reprotox.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 29.DiRenzo F, Broccia ML, Giavini E, Menegola E. Citral, an inhibitor of retinoic acid synthesis, attenuates the frequency and severity of bronchial arch abnormalities induced by triazole-derivative fluconazole in rat embryos cultured in vitro. Reproductive Toxicology. 2007;24(3–4):326–32. doi: 10.1016/j.reprotox.2007.07.012. [DOI] [PubMed] [Google Scholar]