Abstract
A method was developed that utilizes a platinum trap for mercury from mainstream tobacco smoke which represents an improvement over traditional approaches that require impingers and long sample preparation procedures. In this approach, the trapped mercury is directly released for analysis by heating the trap in a direct mercury analyzer.
The method was applied to the analysis of mercury in the mainstream smoke of little cigars. The mercury levels in little cigar smoke obtained under Health Canada Intense smoking machine conditions ranged from 7.1 × 10−3 mg/m3 to 1.2 × 10−2 mg/m3. These air mercury levels exceed the chronic inhalation Minimal Risk Level corrected for intermittent exposure to metallic mercury (e.g., 1 or 2 hours per day, 5 days per week) determined by the Agency for Toxic Substances and Disease Registry.
Multivariate statistical analysis was used to assess associations between mercury levels and little cigar physical design properties. Filter ventilation was identified as the principal physical parameter influencing mercury concentrations in mainstream little cigar smoke generated under ISO machine smoking conditions. With filter ventilation blocked under Health Canada Intense smoking conditions, mercury concentrations in tobacco and puff number (smoke volume) were the primary physical parameters that influenced mainstream smoke mercury concentrations.
1. Introduction
The extensive toxicological and pathological consequences of mercury exposure have been well documented (1). Health concerns regarding the neurological and systemic toxicity of mercury following acute or chronic exposures have made monitoring this toxic metal in exposed individuals an important public health endeavor (2–5). Because of its toxicity, mercury was included in an EPA list of 33 substances of greatest concern for airborne environmental exposures, and was described as a respiratory tract irritant that may exacerbate asthma (6,7).
The Agency for Toxic Substances and Diseases Registry (ATSDR) has calculated a chronic inhalation minimum risk level (MRL) of 2×10−4 mg/m3 for metallic mercury vapor (corrected for intermittent exposure) based on adverse neurological effects (1). In addition to neurotoxicity, there is evidence to suggest that chronic exposure to low mercury concentrations is linked with altered immune response (8).
We have previously reported mercury concentrations in filler tobacco from 50 cigarette varieties available in the U.S. (9). Limited reports of mercury concentrations in cigarette smoke are available (10). The ISO smoking regimen (11) has historically been used for standard reporting of harmful constituents of cigarette smoke, but is not considered representative of human smoking behavior (12–15). While no person smokes like a machine, the more intense conditions of the Health Canada smoking regimen provides a closer approximation of the upper range of smoke toxicant deliveries (12,14,15). Very little information on how little cigars are smoked is available. Hence, data from ISO and the Health Canada Intense smoking regimens provide a “lower and upper” bracketing of smoke constituent deliveries from below the normal range to near the upper range. The similar size and physical properties of cigarettes and little cigars and reports that little cigars can be smoked like cigarettes (16,17) supported the utilization of standard smoking regimens. The principal visible distinction between little cigar design and cigarette design is that cigarettes have wrappers made from paper, whereas little cigar wrappers are made from tobacco. Typically, the little cigars contain a higher mass of tobacco compared to conventional cigarettes.
We report mercury concentrations in the gas phase of little cigar mainstream smoke which is often inhaled in a manner similar to mainstream cigarette smoke. We also analyzed mercury concentrations in little cigar filler to assess mercury transfer from filler to mainstream smoke during combustion.
Established methods for determination of cigarette mainstream smoke mercury take into account that mercury is in elemental form under smoking conditions, similarly to that observed during the combustion of other forms of biomass (18). It has been reported that mercury resides in the vapor phase of mainstream smoke (19). The concentration of mercury in the particulate phase is negligible (19). This follows logically since mercury (II) oxide, if formed, decomposes to Hg0 and O2 at approximately 500°C, well below the temperature of the burning coal of a cigarette. Well-validated traditional methods for trapping volatile elemental mercury from the gas phase of mainstream smoke include use of strongly oxidizing and acidic solutions such as permanganate and sulfuric acid in impingers (19,20), or bromine monochloride prepared in situ from potassium bromate in hydrochloric and hydrobromic acid (21) to rapidly oxidize the elemental mercury to the less volatile ionic form. The highly oxidizing and acidic solutions require further microwave digestion (21), neutralization of excess oxidizing equivalents (19–21), concentration, reduction (19), purging with inert gas (19), concentration by amalgamation with gold or gold-platinum alloy (19–21), and desorption from the amalgamation medium prior to analysis using cold vapor atomic absorption or atomic fluorescence (19–21). Such traditional procedures were well-validated, analytically sound, and appropriate for the instrumentation; however, they were also labor intensive, required significant sample preparation, and were not amenable to high throughput analyses. The reagent masses required for traditional procedures result in considerable amounts of chemical waste and present a risk of contamination, leading to the need for extensive background correction (19). The method reported here for analysis of mercury in the vapor phase of mainstream smoke requires substantially simpler sample preparation than previously reported smoke-based methods.
Sales of little cigars, often flavored, filtered, and approximately the same size range as cigarettes, increased in the United States when they were taxed at a lower rate than cigarettes (22). While little cigar taxes are now more equivalent to cigarette taxes, little cigars remain popular especially among adolescents, young adults, and minorities (23,24). At present, little cigars do not fall under FDA’s regulatory jurisdiction and are often highly flavored or could contain undisclosed additives. For this reason, we chose this understudied class of tobacco products for an initial application of this new high throughput analytical method which allows mercury quantitation in mainstream tobacco smoke with a significantly faster and simpler sample preparation schema.
Experimental
Samples
Little cigar brands were purchased from online retail outlets. The samples were assigned unique identification numbers and logged into a database. Only authorized lab personnel had access to the samples.
Physical Parameters
Physical parameters were determined using a C2 instrument (Cerulean, Milton Keynes, United Kingdom). Some parameters such as tobacco rod length and mass were determined manually.
Tobacco sample preparation for analysis
Little cigars were cut open and dried in perfluoroalkoxy (PFA) containers for a minimum of 1 hour at 90°C. Tobacco sheet wrappers were discarded. Only the filler tobacco was retained, to enable more direct comparisons with cigarette filler tobacco. Dried tobacco was rendered more homogeneous by grinding for 20 seconds with a Smart Grind coffee grinder (Black and Decker, Middleton, WI, USA). Samples were tightly sealed until weighed for analysis. Well homogenized tobacco samples (0.050 ± 0.010 g) were used for analysis. This method modification achieved higher accuracy with lower sample mass than the 0.100 to 0.150 g sample previously required (9) possibly due to reduction of a uv-absorbing interferent when utilizing the lower tobacco mass. The only additional update of the previous method was expansion of the calibration range to span from 0.100 ng to 10.0 ng mercury. Correlation coefficients for calibration using these standards were ≥ 0.9999. Drying and weighing of the tobacco were the only sample preparation steps prior to mercury analysis in tobacco.
Tobacco samples were analyzed with a Nippon North America MA3000 combustion mercury analyzer using the “Organism” program in the manufacturer’s software (Nippon, College Station, TX, USA). Mercury was quantitated by UV absorption at 253.7 nm in the Nippon system. The temperature program has been previously described (9). Mercury mass determined in each sample was divided by the respective tobacco mass used in the analysis to convert the units to ng/g tobacco.
Smoking conditions
Prior to smoking, the little cigars were conditioned according to ISO method 3402 (25). Smoking parameters were established using Borgwaldt KC RM20H rotary smoking machine software (Richmond, VA, USA) for the ISO 3308 conditions (11) or according to Hammond et al. (15), for Health Canada Intense smoking conditions. Air flow, leak tests, and puff volume tests were performed daily; and adjustments were made as necessary to ensure compliance with the respective smoking protocols. When the Intense smoking regimen was used, the standard cigarette holders were replaced with filter ventilation-blocking cigarette holders. A 92 mm “Cambridge” glass fiber filter pad (CFP) (Borgwaldt, Richmond, VA, USA) was placed in the filter module of the rotary smoking machine prior to smoking each little cigar.
Platinum traps for mercury
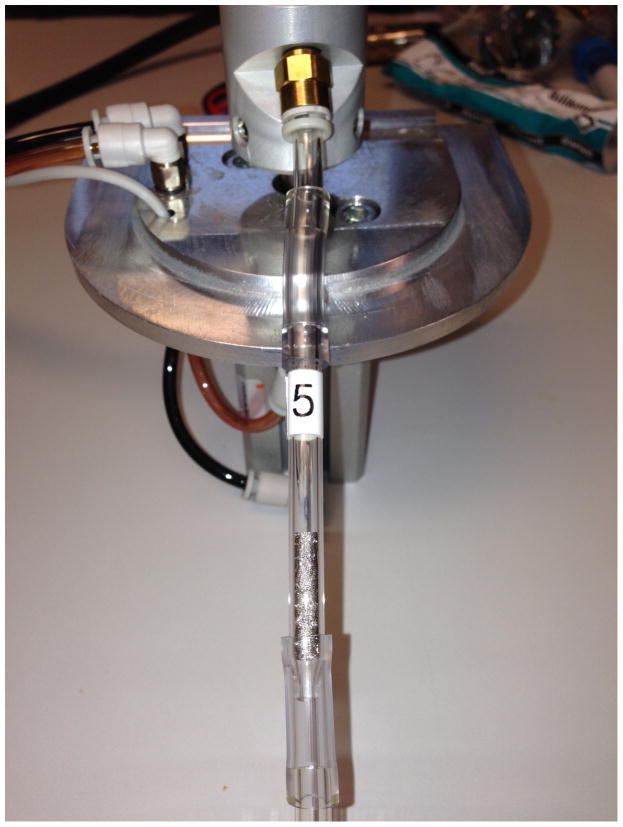
Traps for mercury were prepared from 1.0 g coarse platinum (Pt) powder (Sigma-Aldrich, St. Louis, MO, USA) inside 100 mesh Pt gauze (5.0 cm × 2.5 cm, Sigma-Aldrich, St. Louis, MO, USA). The powder was rolled inside the Pt gauze to fit snugly inside 2.0 mm i.d. quartz injector tubes. Prior to analysis, the Pt gauze and powder were repeatedly heated in ceramic boats to 900°C in the mercury analyzer until mercury backgrounds were < LOD. The quartz tubes were then inserted inline downstream from the Borgwaldt RM20H rotary smoking 92 mm CFP (Figure 1). During method development, two Pt traps were placed in tandem in order to determine whether the first trap quantitatively trapped mercury from the smoke gas phase. After mercury breakthrough from the first trap was determined to be less than 2% (concentration < MDL), only one trap was used in subsequent smoking sessions. A Borgwaldt KC V10 ventilation tester was used to verify that pressure drops remained within specifications when platinum traps were placed in tandem with filter pads.11 A Borgwaldt KC service representative verified that puff profiles, and pressure drops remained within specifications11 with platinum traps in place. One little cigar was smoked per analysis.
Figure 1.
A Pt trap used for quantitative determination of Hg in mainstream cigarette smoke is shown placed in tandem with a glass fiber filter pad attachment on a rotary smoking machine.
sample preparation and analysis
Mercury from the gas phase of little cigar mainstream smoke was adsorbed on the Pt traps. The traps were transferred from the quartz tubes to ceramic boats. The Hg was desorbed from the Pt traps and analyzed with a Nippon North America MA3000 combustion mercury analyzer. The temperature program to desorb mercury from the Pt traps was a modified “Purge” program where the Pt traps were heated to 900°C for 240 seconds at 100% duty cycle. Mercury was quantitated by UV absorption at 253.7 nm. Blanks were prepared using Pt traps which had not been exposed to little cigar smoke. Calibration was established using mercury standards diluted to provide a standard range of 0.100 ng to 10.0 ng mercury pipetted in 100 μL mercury standard solutions in 0.2% nitric acid (GFS, Columbus, OH, USA) with 0.01% L-cysteine (Bioultra, Sigma-Aldrich, St. Louis Mo, USA) standard solution. Correlation coefficients for calibration using these standards were considered acceptable if ≥ 0.999.
Limit of Detection, Lowest Reportable Level
The Procedural Detection Limits (statistically adjusted LODs) were determined as follows:
| ( 26) |
Meanprocedural blank and Sprocedural blank were determined as the mean and standard deviation from analyses of 30 procedural blanks (−0.037 ± 0.089). Factors A (slope) and B (intercept) for tobacco mercury and smoke analyses were determined separately following the methodology prescribed by Taylor (27).
Tobacco mercury LODs were determined by plotting between run standard deviations for the procedural blank, Smokeless Tobacco Reference Product 1S3 (North Carolina State University, Raleigh, NC, USA), and CRM INCT-PVTL-6 (INCT, Warsaw, Poland) versus their mean concentrations over 30 analytical runs.
Smoke mercury LODs were determined by plotting between run standard deviations versus mean concentrations in both ISO and Intense smoking regimens for the procedural blank, 3R4F Reference cigarette (University of Kentucky, Lexington, KY, USA) and Coresta Monitor 6 cigarettes (CM6, Coresta, Paris, France) over 20 analytical runs.
The Lowest Reportable Concentration Levels (LRLs) were chosen from the higher of the adjusted LODs, or the mercury mass in the lowest calibration standard, whichever was higher. The smoke LOD and LRL were expressed in terms of ng/cigarette since one cigarette was smoked per analysis.
Quality Control
Quality control was maintained for tobacco by analysis of Smokeless Reference Tobacco Product (STRP) 1S3 and Certified Reference Material (CRM) INCT-PVTL-6 before and after each group of samples. Quality control was maintained for smoke by analysis of mercury trapped from 3R4F and CM6 mainstream smoke before and after each group of samples. QC results were monitored using SAS software (Cary, NC, USA.) The analytical QC samples were evaluated using a modified Westgard evaluation approach (28). When a QC was determined to be out of control according to the modified Westgard criteria, results in the respective batch were not used and analyses were repeated.
Statistical Analysis of Data
Multivariate and bivariate statistical analysis of data was performed using JMP software (SAS, Cary, NC, USA).
Results and Discussion
The cycle time for tobacco mercury analysis is limited only by the mercury analyzer itself, approximately 5 minutes per sample.
The method reported here for analysis of mercury in the gas phase of mainstream smoke requires substantially simpler sample preparation than previously reported smoke-based methods (19–21). Our new method eliminates use of impingers and their associated strongly oxidizing and acidic reagents. The cycle time for analysis of mercury from mainstream smoke on Pt traps is limited by the time required for a smoking run on the rotary smoking machine, approximately 10 minutes, plus five minutes analysis time on the mercury analyzer, for a total of 15 minutes per sample. The cycle time required for previous methods cited earlier would include the time to smoke cigarettes through impingers, including impinger cleaning for sequential analyses. Subsequently, the cycle time would differ depending on the specific method, which oxidizing medium were used, and whether cold vapor atomic absorption or atomic fluorescence were utilized. Steps required for each of these methods were described in the introduction. Minimum cycle time for the multi-step methods cited would be conservatively estimated as several hours. It is possibly for this reason that data on mercury concentrations in smoke are seldom reported.
We have previously reported the use of a trap made from platinum gauze for trapping cadmium breakthrough with traditional CFPs in a commercial smoking machine (29). Though the cadmium trap design did not work as well for mercury it led to the development of an alternate design optimized for trapping mercury in mainstream smoke when placed in tandem with a CFP. Vapor phase mercury from the smoke passes through the CFP while particulate phase constituents are filtered out. The vapor phase mercury passing through the CFP is trapped on platinum powder and gauze surfaces by amalgamation. The platinum trap is then placed in a ceramic sample boat for analysis with a commercial mercury analyzer, where the mercury desorbs from the platinum surfaces by rapid heating to 900°C. Thermal desorption of mercury from the platinum trap simplifies and reduces sample preparation time, permitting much higher throughput for mainstream smoke mercury analyses.
Mainstream smoke mercury trapping efficiency with new Pt trap
Mercury trapping efficiency was assessed based on breakthrough measured in the second of two tandem Pt traps. Trapped mercury in the second trap was compared with the first trap over ten runs using the Intense smoking regimen with CM6 and 3R4F cigarettes.
Mercury collected from 3R4F and CM6 cigarettes in the first traps ranged from 4.246 to 7.251 ng/cigarette. Mercury collected in the second traps ranged from 0.025 to 0.097 ng/cigarette. Therefore, the mean mercury mass not trapped in the first Pt trap was 0.98% (range 0.34% to 1.9%). Since the trapping efficiency was ≥ 98.1%, mercury trapping was considered quantitative with a single Pt trap, ensuring puff profiles and pressure drop on the smoking machine were within manufacturer and ISO specifications (11).
Limits of Detection and Lowest Reportable Levels
The adjusted LOD determined for tobacco analyses was 0.27 ng/g mercury. Normalization of the lowest standard (0.1 ng Mercury) to 0.050 g tobacco yielded a tobacco equivalent of 2.0 ng/g. Therefore, 2.0 ng/g, the higher of the two values, was the LRL for tobacco samples.
The adjusted LOD determined for mainstream smoke analyses was 0.097 ng Mercury/cigarette. Since 0.10 ng Mercury was the lowest standard, 0.10 ng/cigarette, the higher of the two values, was the LRL for mainstream smoke samples.
Accuracy and precision
Method accuracy for tobacco mercury analysis was assessed by comparison of data collected from analyses of STRP 1S3 compared to previously reported results and to CRM INCT-PVTL-6 (Table 1). Our results for INCT-PVTL-6 show good agreement with certified values. Our results for 1S3 show good agreement with previously reported results (9).
Table 1.
Comparison of results with certified or previously reported values for reference or quality control tobacco and cigarettes.
| Reference Tobacco Mercury Concentrations (ng/g) | |||||||
|---|---|---|---|---|---|---|---|
| STRP 1S3CDC | STRP 1S39 | CRM INCT-PVTL-6CDC | INCT-PVTL-6 (Cert.) | ||||
| 22.3 ± 0.6 | 23 ± 2 | 21.8 ± 0.6 | 23.2 ± 1.6 | ||||
| Mainstream Smoke Mercury Concentrations, Intense Smoking Regimen (ng/Cigarette) | |||||||
| 3R4FCDC | 3R4F | 2R4FCDC | 2R4F | CM6CDC | CM6 | CM7CDC | CM7 |
| 4.4 ± 0.4 | NDA | 4.8 ± 0.4 | NDA | 6.8 ± 0.5 | NDA | 7.0 ± 0.7 | NDA |
| Mainstream Smoke Mercury Concentrations, ISO Smoking Regimen (ng/Cigarette) | |||||||
| 3R4FCDC | 3R4F30 | 2R4FCDC | 2R4F31 | CM6CDC | CM6 | CM7CDC | CM7 |
| 2.0 ± 0.2 | 2.43 ± 0.06 | 2.1 ± 0.1 | 2.18 ± 0.11 | 4.1 ± 0.3 | NDA | 4.2 ± 0.3 | NDA |
NDA: No Data Available for comparison with results reported here. CDC data represents 33 replicate analyses.
Method accuracy for mainstream smoke mercury analysis could not be assessed by comparison of data with certified reference values, since no certified smoke concentrations exist. Therefore, we compared our data from analyses of mainstream smoke obtained from smoking Kentucky Reference cigarettes 3R4F and 2R4F using the ISO smoking regimen with previously reported results obtained using traditional methods (Table 1). Our results and the results reported by Kuroki et al. for 3R4F compared favorably (30) as did our results and the results previously reported by Counts et al. for 2R4F (31). No previously reported mainstream smoke mercury results were found for 3R4F or 2R4F using the Intense smoking regimen. No previously reported mainstream smoke mercury results were found for CORESTA Monitor No. 6 or No. 7 using either smoking regimen. Precision at the LRL (2 × S/n1/2, standard 1, 0.1 ng Hg, n=30) was 0.0026 (0.26%).
Analytical Results
Results from pentuplicate analyses of tobacco and mainstream smoke gas phase (ISO and Intense smoking regimens) from little cigars were obtained (Table 2). Vaquero Natural had the lowest mean tobacco mercury concentration; and Hav-A-Tampa Natural the highest. Smokers Best Lights and Menthols (ventilated filters) had the lowest mean ISO regimen smoke mercury deliveries per little cigar, and Al Capone the highest. Al Capone brand also had the highest Intense regimen smoke mercury delivery, whereas Winchester Classics had the lowest. The tobacco mass and number of puffs required to consume the product varied between little cigar brands and these variables were taken into account along with other physical variables when comparing smoke deliveries. For example, while Hav-A-Tampa had the highest mean tobacco mercury concentration, it had the third lowest mean tobacco mass, and required an intermediate number of puffs to consume the product. These and other variables contributed to total mercury delivery in smoke.
Table 2.
Concentrations of mercury in little cigar tobacco and mainstream smoke.*
| Tobacco Mercury Concentration (ng/g ± standard deviation) | ISO Mainstream Smoke Mercury Concentration (ng/cigar ± standard deviation) | Intense Mainstream Smoke Mercury Concentration (ng/cigar ± standard deviation) | Tobacco Mass (g)/Mean ISO Puffs per Little Cigar/Mean Intense Puffs per Little Cigar | |
|---|---|---|---|---|
| Al Capone | 21.3 ± 0.6 | 7.5 ± 1.1 | 9.6 ± 0.5 | 0.943 / 17.36 / 18.16 |
| Captain Black | 22.8 ± 0.8 | 5.3 ± 0.2 | 7.1 ± 0.6 | 1.015 / 9.964 / 10.82 |
| Cheyenne Full Flavor | 20.5 ± 1.0 | 4.4 ± 0.2 | 6.5 ± 1.0 | 0.976 / 12.48 / 16.66 |
| Clipper Black Red | 21.8 ± 0.7 | * | * | 1.024 / * / * |
| Hav-A-Tampa Natural | 24.9 ± 0.7 | 4.6 ± 0.3 | 8.1 ± 0.6 | 0.877 / 12.46 / 14.66 |
| Murano Regular | 21.3 ± 0.4 | 5.4 ± 0.2 | 7.7 ± 0.1 | 1.033 / 11.06 / 14.44 |
| Muriel Sweets | 22.9 ± 0.6 | 6.9 ± 1.2 | 8.3 ± 0.4 | 0.839 / 14.96 / 14.44 |
| Phillies | 24.2 ± 0.7 | 6.0 ± 0.3 | 8.5 ± 0.8 | 0.939 / 13.00 / 17.58 |
| Prime Time Blueberry | 19.4 ± 0.9 | 4.7 ± 0.4 | 6.6 ± 0.4 | 1.125 / 9.70 / 12.34 |
| Remington Full Flavor | 18.1 ± 0.8 | 5.1 ± 0.4 | 6.8 ± 0.3 | 0.961 / 11.18 / 13.34 |
| Santa Fe Original | 22.5 ± 0.6 | 3.0 ± 0.4 | 7.4 ± 0.7 | 1.054 / 18.28 / 18.96 |
| Smokers Best Lights | 22.1 ± 0.5 | 2.6 ± 0.3 | 7.1 ± 0.5 | 1.004 / 14.62 / 16.32 |
| Smokers Best Menthol | 20.9 ± 0.5 | 2.6 ± 0.4 | 6.6 ± 0.6 | 0.966 / 14.24 / 15.74 |
| Swisher Sweets | 19.1 ± 0.8 | 4.2 ± 0.3 | 6.5 ± 0.6 | 0.899 / 9.18 / 10.64 |
| Vaquero Natural | 17.9 ± 0.8 | 2.8 ± 0.2 | 5.5 ± 0.4 | 0.940 / 11.28 / 12.8 |
| Vendetta | 18.1 ± 0.7 | 5.1 ± 0.5 | 7.0 ± 0.5 | 1.094 / 10.98 / 13.86 |
| Winchester Classic | 18.3 ± 0.9 | 3.1 ± 0.2 | 5.2 ± 0.4 | 0.789 / 9.20 / 10.96 |
Insufficient stock for these determinations. All brand names are trademarks of the respective manufacturers.
Correlations between Physical Parameters and Mercury Delivery in Smoke
Fewer physical parameters were statistically correlated with mercury delivery from little cigars than previously reported for other metals in cigarette tobacco (Table 3) (32), perhaps due to the fact that mercury is present in the vapor phase of the smoke rather than in the particulate phase like many other metals in tobacco smoke.
Table 3.
Multivariate statistical correlations between mercury delivery in mainstream smoke and physical parameters.
| Parameter | t Ratio (ISO) | p (ISO) | t Ratio (Intense) | p (Intense) |
|---|---|---|---|---|
| Diameter | −1.11 | 0.30 | 0.01 | 0.99 |
| Pressure Drop (Vents Shut) | −0.34 | 0.74 | 0.54 | 0.59 |
| Total Ventilation | −3.89 | 0.0046 | −1.78 | 0.11 |
| Filter Length | 0.46 | 0.65 | −0.75 | 0.47 |
| Tobacco Length | 0.51 | 0.62 | 0.84 | 0.43 |
| Tobacco Mass | −0.76 | 0.47 | 0.04 | 0.97 |
| Number of Puffs | 2.00 | 0.080 | 2.37 | 0.046 |
| Tobacco Mercury Concentration | 1.18 | 0.27 | 2.53 | 0.036 |
When little cigars were smoked using the ISO smoking regimen, the only parameter that had a statistically significant correlation with mercury concentration in mainstream smoke was total ventilation. This negative correlation was not unexpected due to the effect of smoke dilution with air when the little cigars filter ventilation holes are unblocked. When the little cigar ventilation data was further analyzed using bivariate statistical analysis, filter ventilation was significantly correlated with ISO regimen mercury concentration in the mainstream smoke (p = 0.0092), whereas wrapper ventilation (porosity) was not significantly correlated with mercury concentration in smoke (p = 0.62).
When little cigars were smoked using the Intense smoking regimen, number of puffs and tobacco mercury concentration were the parameters which were significantly positively correlated with mercury concentration in mainstream smoke. When further analyzed using bivariate statistical analysis, the correlations between number of puffs (p=0.013) and tobacco mercury concentration (p=0.0033) were even more strongly correlated with mercury concentration in mainstream smoke.
Conclusions
Though no machine smoking regimen accurately reflects the smoking habits of all smokers, the Health Canada Intense regimen reportedly reflects the higher smoke yields from modern cigarettes better than the ISO regimen (14,15). When a cigarette or a little cigar, is smoked using the Intense smoking regimen, the cigar is smoked to the ISO-specified filter plus 8 mm or overwrap plus 3mm limit (33). The Intense Regimen requires a 55 mL puff volume. On the basis of this per puff volume, Cheyenne Full Flavor (16.66 puff mean) delivered almost a liter of smoke (total puff volume of 916.3 mL per little cigar). The mercury levels per little cigar and the total smoke volume can be used to calculate a mean smoke concentration (mg/m3). For example, Cheyenne Full Flavor little cigars delivered 6.5 ng mercury to mainstream smoke with an average puff number of 16.66 under Canadian Intense smoking conditions (55 mL puff) resulting in a mercury concentration of 7.1 × 10−3 mg/m3. This concentration is more than 30 times greater than the 2 × 10−4 mg/m3 MRL calculated by ATSDR for chronic inhalation of metallic mercury vapor corrected for intermittent exposure (e.g., 1 or 2 hours per day, 5 days per week).1 At 18.96 mean puffs per little cigar, and 7.4 ng mercury per little cigar delivered to mainstream smoke, the mercury concentration in the mainstream smoke of Santa Fe Originals also resulted in a mercury concentration of 7.1 × 10−3 mg/m3. The other little cigar brands had yet higher mainstream smoke mercury concentrations. Captain Black brand required only 10.8 puffs. With a mean mercury delivery of 7.1 ng mercury, the mainstream smoke mercury concentration is calculated to be 1.2 × 10−2 mg/m3, the highest total concentration delivered and 60 times greater than ATSDR’s 2 × 10−4 mg/m3 MRL. Chronic inhalation exposure to mercury concentrations as low as the 1.4 × 10−2 mg/m3 LOAEL reportedly led to impaired behavior on neurobehavioral tests.1 Therefore, mainstream smoke mercury levels from little cigars raise important questions of their potential to increase avoidable exposures to mercury since smoking little cigars is not the only source of inhalation exposure to mercury.
Acknowledgments
This study was funded by an interagency agreement by the U.S. Food and Drug Administration Center for Tobacco Products.
Abbreviations
- ATSDR
Agency for Toxic Substances and Disease Registry
- CDC
Centers for Disease Control and Prevention
- CFP
Cambridge Filter Pad
- CRM
Certified Reference Material
- EPA
U.S. Environmental Protection Agency
- LOAEL
Lowest Observed Adverse Effect Level
- MRL
Minimum Risk Level
- NOAEL
No Observed Adverse Effect Level
- SRM
Standard Reference Material
- STRP
Smokeless Tobacco Reference Product
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.ATSDR. Toxicological Profile for Mercury. 1999:29–357. http://www.atsdr.cdc.gov/ToxProfiles/tp46.pdf.
- 2.McShane WJ, Pappas RS, Wilson-McElprang V, Paschal D. A rugged and transferable method for determining blood cadmium, mercury, and lead with inductively coupled plasma-mass spectrometry. Spectrochim Acta B. 2008;63:638–644. [Google Scholar]
- 3.Nixon DE, Neubauer KR, Eckdahl SJ, Butz JA, Burritt MF. Comparison of tunable bandpass reaction cell inductively coupled plasma mass spectrometry with conventional inductively coupled plasma mass spectrometry for the determination of heavy metals in whole blood and urine. Spectrochim Acta B. 2004;59:1377–1387. [Google Scholar]
- 4.Lauwerys R, Buchet J-P, Roels H, Berlin A, Smeets J. Intercomparison program of lead, mercury, and cadmium analysis in blood, urine, and aqueous solutions. Clin Chem. 1975;21:551–557. [PubMed] [Google Scholar]
- 5.Bautista LE, Stein JH, Morgan BJ, Stanton N, Young T, Nieto FJ. Association of blood and hair mercury with blood pressure and vascular reactivity. Wis Med J. 2009;108:250–252. [PMC free article] [PubMed] [Google Scholar]
- 6.Leikauf GD, Kline S, Albert RE, Baxter CS, Bernstein D, Bernstein J, Buncher CR. Evaluation of a possible association of urban air toxics and asthma. Environ Health Perspect. 1995;103(Supplement 6):253–271. doi: 10.1289/ehp.95103s6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leikauff GD. Hazardous air pollutants and asthma. Environ Health Perspec. 2002;110(Supplement 4):505–526. doi: 10.1289/ehp.02110s4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet LI, Zelikoff JT. Toxicology and immunotoxicology of mercury: A comparative review in fish and humans. J Toxicol Environ Health B. 2001;4:161–205. doi: 10.1080/109374001300339809. [DOI] [PubMed] [Google Scholar]
- 9.Fresquez MR, Pappas RS, Watson CH. Establishment of Toxic Metal Reference Range in Tobacco from U.S. Cigarettes. J Anal Toxicol. 2013;37:298–304. doi: 10.1093/jat/bkt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul Toxicol Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.International Organization for Standardization. Routine analytical cigarette-smoking machine - Definitions and standard conditions. ISO 3308; 2000. pp. 1–23. [Google Scholar]
- 12.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12:424–430. doi: 10.1136/tc.12.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlowski LT, Pillitteri JL. Monograph 7: The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of US cigarettes. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services; Washington (DC): 1996. [accessed 21 February 2014]. Compensation for nicotine by smokers of lower yield cigarettes; pp. 161–172. http://cancercontrol.cancer.gov/tcrb/monographs/7/index.html. [Google Scholar]
- 14.Burns DM, Dybing E, Gray N, Hecht S, Anderson C, Sanner T, O’Connor R, Djordjevic M, Dresler C, Hainaut P, Jarvis M, Opperhuizen A, Straif K. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob Control. 2008;17:132–141. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond D, Fong GT, Cummings KM, O’Connor RJ, Giovino GA, McNeill A. Cigarette yields and human exposure: A comparison of alternative testing regimens. Cancer Epidem Biomar. 2006;15:1495–1501. doi: 10.1158/1055-9965.EPI-06-0047. [DOI] [PubMed] [Google Scholar]
- 16.Delnevo CD, Hrywna M. “A Whole ‘Nother Smoke” or a cigarette in disguise: how RJ Reynolds reframed the image of little cigars. Am J” Publ Health. 2007;97:1368–1375. doi: 10.2105/AJPH.2006.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolly DH. Exploring the use of little cigars by students at a historically black university. [Accessed 29 October 2014];Prev Chronic Dis. 2008 5(3) http://www.cdc.gov/pcd/issues/2008/jul/07_0157.htm. [PMC free article] [PubMed] [Google Scholar]
- 18.Obrist D, Moosmüller H, Schürmann R, Chen L-WA, Kreidenweis SM. Particulate-phase and gaseous elemental mercury emissions during biomass combustion: controlling factors and correlation with particulate matter emissions. Environ Sci Technol. 2008;42:721–727. doi: 10.1021/es071279n. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel RL, Torrence KM, Self DA, Chang MJ. Determination of mercury in mainstream smoke by conventional and amalgamation cold vapor atomic absorption spectrometry. Beitr zur Tabakforsch. 2001;19:267–276. [Google Scholar]
- 20.Health Canada. Determination of mercury in mainstream tobacco smoke. Ottawa, Canada: 1999. pp. 1–12. [Google Scholar]
- 21.U.S. Environmental Protection Agency. Method 1631, Revision E: Mercury in water by oxidation, purge and trap, and cold vapor atomic fluorescence spectrometry. Washington D.C., USA: 2002. pp. 1–38. [Google Scholar]
- 22.Tumulty B. Tobacco tax increase expected to reduce smoking. [last accessed 29 April 2014];USA Today. 2009 http://usatoday30.usatoday.com/news/health/2009-03-27-tobacco-tax_N.htm.
- 23.Nasim A, Blank MD, Berry BM, Eissenberg T. Cigar use misreporting among youth: data from the 2009 Youth Tobacco Survey. Prev Chronic Dis. 2012;9:110084. doi: 10.5888/pcd9.110084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King BA, Tynan MA, Dube SR, Arrazola R. Flavored-little-cigar and flavored-cigarette use among U.S. middle and high school students. J Adolesc Health. 2014;54:40–46. doi: 10.1016/j.jadohealth.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Organization for Standardization. Tobacco and tobacco products - Atmosphere for conditioning and testing. ISO 3402; 1999. pp. 1–4. [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards (NCCLS) Protocols for Determination of Limits of Detection and Limits of Quantitation. 2004;24:9–35. [Google Scholar]
- 27.Taylor JK. Quality Assurance of Chemical Measurements. 1. Boca Raton, FL: CRC Press; 1987. pp. 79–81.pp. 194 [Google Scholar]
- 28.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 29.Pappas RS, Fresquez MR, Watson CH. Cigarette smoke cadmium breakthrough from traditional filters: implications for exposure. J Anal Toxicol. 2015;39 doi: 10.1093/jat/bku115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuroki Y, Yokohama S, Takahashi H, Fujiwara M. Development of an analytical method for the simultaneous determination of trace metals and mercury in mainstream cigarette smoke by icp-ms. 63rd Tobacco Science Research Conference; 2009; Poster 53. [Google Scholar]
- 31.Counts ME, Hsu FS, Tewes FJ. Development of a commercial cigarette “market map” comparison methodology for evaluating new or non-conventional cigarettes. Regul Toxicol Pharmacol. 2006;46:225–242. doi: 10.1016/j.yrtph.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic Metal Concentrations in Mainstream Smoke from Cigarettes Available in the U.S. J Anal Toxicol. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Organization for Standardization. Cigarettes – determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine. ISO 4387; 2000. p. 5. [Google Scholar]