Abstract
Cystatin C is being considered as a replacement for serum creatinine for estimating glomerular filtration rate (GFR). Data are limited on the non-GFR determinants of cystatin C and their relationship with protein intake. We compared creatinine and cystatin C levels at baseline (N=741) and at 24 months post-randomization (N=426) for participants in the Modification of Diet in Renal Disease Study. Participants in Study A (GFR 25-55 ml/min/1.73 m2) were assigned a low (0.58 g/kg/day) vs. usual (1.3 g/kg/day) protein intake, and in Study B (GFR 13-24 ml/min/1.73 m2), a very low (0.28 g/kg/day) vs. low protein intake. We associated creatinine, cystatin C and estimated protein intake at baseline and compared randomized groups to examine the effect of protein intake on creatinine and cystatin C independent of GFR. The mean (SD) measured GFR, creatinine and cystatin C at baseline was 38.6 (8.9) ml/min/1.73 m2, 1.9 (0.5) mg/dl, 1.9 (0.4) mg/l in Study A and 18.5 (3.4) ml/min/1.73 m2, 3.4 (0.9) mg/dl, 3.0 (0.5) mg/l in Study B. Lower dietary protein intake reduced change in creatinine, but did not affect change in cystatin C [Δ(CI) creatinine vs. cystatin C, −0.22 (−0.36, −0.08) mg/dl vs. 0.02 (−0.08, 0.13) mg/l in Study A, −0.28 mg/dl (−0.82, 0.21) vs. 0.10 (−0.15, 0.26) mg/l in Study B]. Creatinine, but not cystatin C, is affected by dietary protein intake independent of GFR. Cystatin C may allow more accurate GFR estimates than creatinine for patients with reduced protein intake.
Introduction
Accurate estimation of the glomerular filtration rate (GFR) is essential for the diagnosis, staging and management of chronic kidney disease (CKD).(1, 2) Serum creatinine is most commonly used to estimate GFR, with current estimating equations taking into account age, sex, race and weight as non GFR determinants of creatinine.(3, 4)
Cystatin C is being proposed as potentially superior biomarker for GFR estimation. (5). Cystatin C is an endogenous 13 kDa protein that is freely filtered at the glomerulus, and then nearly completely reabsorbed and catabolized by proximal tubular epithelial cells with only small amounts excreted in the urine. Cystatin C generation is felt to be constant, thus serum levels are not affected by variables other than kidney function.(6) Therefore, cystatin C is felt to be a promising candidate for replacing creatinine as a biomarker for estimating GFR.
More recent studies, however, have found variability in the relationship between cystatin C and measured GFR suggesting the potential for non GFR determinants of cystatin C.(7¬9) Understanding these potential determinants would be important to develop and evaluate GFR estimating equations based on cystatin C and to better understand the relationship between cystatin C and adverse outcomes.
In order to address this question, we analyzed data from the Modification in Diet and Renal Disease (MDRD) study, which was a randomized controlled trial of protein restriction and blood pressure control in patients with CKD stages 3 and 4.(7) We examined the relationship between dietary protein intake and creatinine and cystatin C levels at baseline after adjustment for measured GFR and GFR measurement error. In addition, we tested the effect of a dietary protein intake prescription on creatinine and cystatin C independent of GFR in a longitudinal analysis comparing randomized groups.
Results
Study Population
Baseline characteristics of the study population are presented in Table 1. Cystatin C measurements were available in 574 out of 585 patients in Study A and 251 out of 275 patients in study B at the time of randomization. The mean measured GFR at time of randomization was 38.6 ml/min/1.73 m2 in Study A and 18.5 ml/min/1.73 m2 in Study B. The etiology of kidney disease included polycystic kidney disease (22%), glomerular disease (27%), hypertensive nephrosclerosis (17%), tubulointerstitial diseases (7%) and other or unknown (14%). Only 3.5 % of the patients had diabetic nephropathy, because patients with diabetes requiring insulin were excluded from the MDRD study. Estimated protein intake was slightly higher in Study A compared with Study B.
Table 1.
Baseline characteristics of the study population
| Study A (574) | Study B (251) | |||
|---|---|---|---|---|
| Mean/N | SD/% | Mean | SD/% | |
| Age (years) | 52 | 12.2 | 50.9 | 12.9 |
| Female | 224 | 61 | 102 | 40.6 |
| White | 52 | 9.1 | 13 | 5.2 |
|
Smoking Status | ||||
| Regularly | 55 | 10.7 | 28 | 12.2 |
| Occasionally | 281 | 54.5 | 111 | 48.3 |
| Never | 139 | 24.2 | 59 | 23.5 |
| Systolic Blood Pressure (mm Hg) | 131.4 | 17.5 | 133.1 | 17.7 |
| Diastolic Blood Pressure (mm Hg) | 81.0 | 10.0 | 80.9 | 10.3 |
|
Etiology of Kidney Disease | ||||
| Polycystic Kidney Disease | 139 | 24.2 | 59 | 23.5 |
| Hereditary Nephritis and Tubulointerstitial | 175 | 30.5 | 74 | 29.5 |
| Disease | ||||
| Hypertensive Kidney Disease | 100 | 17.4 | 39 | 15.5 |
| Diabetic Nephropathy | 17 | 3 | 9 | 3.6 |
| Glomerular Disease | 143 | 24.9 | 70 | 27.9 |
|
Laboratory Data | ||||
| Serum Creatinine | 1.9 | 0.5 | 3.4 | 0.9 |
| (mg/dl) Serum Cystatin C (mg/l) | 1.9 | 0.4 | 3.0 | 0.5 |
| Measured GFR (ml/min/1.73 m2) | 38.6 | 8.9 | 18.5 | 3.4 |
| Proteinuria (g/day) | 1.0 | 1.6 | 1.4 | 1.7 |
| Serum Albumin (g/dl) | 4.0 | 0.3 | 4.0 | 0.4 |
| Serum CRP (mg/l) | 0.5 | 0.6 | 0.4 | 0.6 |
| Estimated Protein | 1.1 | 0.2 | 0.9 | 0.2 |
| Intake (g/kg/day)** | ||||
* Data are presented as mean +/−SD for continuous variables and N/percentage for categorical variables
Protein intake estimated from urine urea nitrogen excretion rate was available for 741/825 participants
Eighty four patients (10 %) did not have 24 hour urine collections available at the final baseline visit for determination of estimated protein intake, and were therefore excluded from the cross-sectional analysis. A higher proportion of men and patients from Study A were missing estimated protein intake at baseline. Patients with missing estimated protein intake also had slightly lower values for creatinine and cystatin C and higher measured GFR (Appendix A, Table 1). Over the follow up period of 2 years, 23 patients died and 86 went on to develop kidney failure. Cystatin C measurements were available in 426 patients at 2 years, with 290 patients missing cystatin C measurements at followup. These patients had no statistically significant differences in age, etiology of kidney disease and proteinuria, from the group with available cystatin C measurements. Small differences in measured GFR and estimated protein intake were again observed. (Appendix A, Table 2)
Cross Sectional Analysis
The cross sectional associations relating estimated protein intake to serum creatinine and cystatin C are presented in Table 2. Estimated protein intake was more strongly associated with serum creatinine than cystatin C at baseline after adjustment for GFR, GFR measurement error, age, sex and race; a 0.2 g/kg/day higher estimated protein intake was associated with a 2.4 (0.6) % higher serum creatinine and a 0.9 (0.6)% higher cystatin C. The association for creatinine, but not cystatin C, was statistically significant.
Table 2.
Association of estimated protein intake with creatinine and cystatin C at baseline*
| N | Not adjusted | Adjusted for GFR | Adjusted for GFR Measurement Error (0.015) | Adjusted for GFR Measurement Error (0.015), age, sex and race** | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff (SD) | p-value | Coeff (SD) | p-value | Coeff (SD) | p-value | Coeff (SD) | p-value | ||
| Serum Creatinine (%) | 741 | −0.141 (0.010) | <0.001 | −0.006 (0.008) | 0.45 | 0.012 (0.008) | 0.145 | 0.024 (0.006) | <0.001 |
| Serum Cystatin C (%) | 741 | −0.11 (0.009) | <0.001 | −0.007 (0.006) | 0.26 | 0.022 (0.006) | <0.001 | 0.009 (0.006) | 0.13 |
Protein intake is estimated from urine urea nitrogen, cystatin and creatinine are log transformed.
Interpretation: After adjustment for GFR, age, race and sex, and GFR measurement error, a 0.2 g/kg/day increase in baseline protein intake is associated with a 2.4 (0.6) % higher baseline serum creatinine and a 0.9 (0.6) % higher baseline serum cystatin C.
Longitudinal Analysis
A longitudinal analysis of randomized groups in presented in Table 3 and Figure 1. The change in measured GFR from baseline to year 2 was identical in the usual and low protein diet groups (−0.3 ml/min per 1.73 m2 in Study A and −0.2 ml/min per 1.73 m2 in Study B). In Study A, the change in serum creatinine was lower [−0.22 (−0.36, −0.08) mg/dl] in the low protein intake arm compared with the usual protein intake arm. Consequently, the change in the creatinine based GFR estimate (eGFRcr) was higher [2.2 (0.6, 3.9) ml/min per 1.73 m2] in the low protein intake arm compared with the usual protein intake arm. In Study B, the changes in serum creatinine levels and eGFRcr did not differ significantly between the low and very low protein diet [−0.28 (−0.82, 0.21) mg/dl and 0.8 (−1.0, 2.6) ml/min/1.73 m2, respectively]. Changes in the serum cystatin C concentration and the cystatin based GFR estimate (eGFRcys) did not differ between randomized groups in either study.
Table 3.
Effect of prescribed dietary protein on change in measured GFR, creatinine and cystatin C independent of GFR, and estimated GFR using creatinine and cystatin C.*
| Study A (n=302) | Study B (n=124) | |||
|---|---|---|---|---|
| ΔGFR (ml/min/1.73 m2) | −0.3 | [−2.1, 1.6] | −0.2 | [−1.9, 1.4] |
| Δ Serum Creatinine (mg/dl) | −0.22 | [−0.36, −0.08] | −0.28 | [−0.82, 0.21] |
| Δ eGFRcr (ml/min/1.73 m2) | +2.2 | [0.6, 3.9] | +0.8 | [−1.0, 2.6] |
| Δ Serum Cystatin C (mg/l) | 0.02 | [−0.08, 0.13] | 0.10 | [−0.15, 0.26] |
| Δ eGFRcys (ml/min/1.73 m2) | −0.4 | [−2.1, 0.9] | −0.3 | −1.5, 2.2] |
Differences between randomized groups (change in low protein diet group minus change in usual protein diet group in Study A; very low minus low protein diet group).
Change is defined as 24 mo-baseline. Changes in creatinine and cystatin C and eGFR measurements are adjusted for change in measured GFR.
All values are reported as mean and 95% CI. Values highlighted in boldface font are statistically significant with p<0.01
Figure 1.
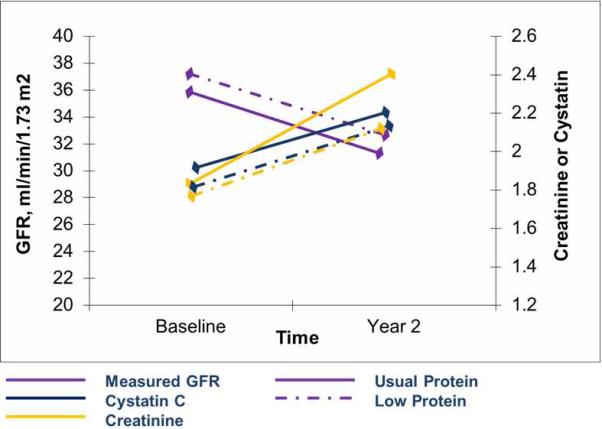
Change in creatinine, cystatin C and measured GFR in Study A over the follow up period. * *Absolute values for creatinine, cystatin C and GFR are shown
Performance of Estimating Equations
A comparison of the performance of the MDRD Study equation and the CKD-EPI cystatin C 2008 equation at baseline and at 2 years for the treatment groups is presented in Table 4. When computing eGFR using serum creatinine, in Study A, the difference in bias between the low and usual protein diet groups was not significantly different at baseline, [0.23 (−1.61, 1.15) ml/min/1.73 m2] but was significantly greater in the low protein diet group at follow-up [−2.77 (−4.08, −1.48) ml/min/1.73 m2], reflecting a greater overestimation of measured GFR in the low protein diet group. In Study B the results were qualitatively similar, although the difference at follow-up was not statistically significant. The relative change in bias, compared to mean baseline mGFR, was 8.01 % in Study A and 7.44 % in Study B. In contrast, when computing eGFR using serum cystatin C, there was no difference in bias between randomized groups at either baseline or follow up in Study A or Study B, and no change over time (<1 ml/min/1.73 m2 and <2.5%).
Table 4.
Effect of prescribed dietary protein on bias in GFR estimation using the MDRD study equation and the CKD-EPI Cystatin C 2008 study equation.*
| Equation Performance | Timing | Study A (n=302) | Study B (n=124) | ||
|---|---|---|---|---|---|
| Δ Bias eGFRcr (ml/min/1.73 m2) | Baseline | −0.23 | [−1.61, 1.15] | −0.35 | [−1.75, 1.10] |
| Δ Bias eGFRcr (ml/min/1.73 m2) | Follow up | −2.77 | [−4.08, −1.48] | −1.37 | [−2.88, 0.15] |
| Δ Bias eGFRcys (ml/min/1.73 m2) | Baseline | −0.60 | [−2.20, 0.99] | −0.50 | [−0.75, 1.76] |
| Δ Bias eGFRcys (ml/min/1.73 m2) | Follow up | −0.31 | [−1.75, 1.13] | −0.02 | [−1.70, 1.75] |
Differences in mean bias between randomized groups (bias in low protein diet group minus bias in usual protein diet group in Study A; very low minus low protein diet group in Study B). Bias is defined as measured-estimated GFR.
All values are reported as mean and 95 % CI. Values highlighted in boldface are statistically significant with p<0.01. p for eGFRcr at follow up in Study B is 0.08.
Discussion
In this study of a dietary protein intake intervention in patients with moderate to severe chronic kidney disease, we found that serum cystatin C, unlike serum creatinine is not affected by dietary protein intake independent of changes in GFR. Consequently, eGFRcr but not eGFRcys is affected by protein intake, and an estimating equation based on cystatin C is more accurate than an estimating equation based on serum creatinine in patients ingesting a low protein diet. Our findings suggest that cystatin C may be a better filtration marker in patients with CKD and decreased protein intake.
Cystatin C is being increasingly proposed as a replacement for serum creatinine as an endogenous marker of GFR.(5, 6) However, prior to the widespread adoption of cystatin C measurements in clinical practice, it is necessary to understand its non-GFR determinants. Non-GFR determinants of endogenous filtration markers include generation, renal tubular reabsorption and secretion, and extra-renal elimination.(8) Knowledge of these determinants can aid the interpretation of cystatin C levels, and facilitate development of GFR estimating equations based on cystatin C.
The non-GFR determinants of serum creatinine have been well studied in diverse patient populations.(9) Studies examining creatinine generation and excretion have shown that these parameters can vary with dietary protein intake and blood pressure interventions. These studies have also demonstrated that creatinine based outcomes may be misleading in interpretation of randomized controlled trials of dietary protein intake restriction.(10)
However, unlike creatinine, cystatin C is not excreted in the urine, making it difficult to study differences in its non-GFR determinants, leaving epidemiological studies as the usual method to infer the contribution of non GFR determinants to variation in cystatin C. These epidemiological studies have demonstrated that the previous assumptions regarding the constant rate of generation of cystatin C may not be valid. Small studies showing associations of cystatin C with inflammatory markers and markers of metabolism (thyroid hormone levels) also suggest that cystatin C generation may not be constant.(11) Furthermore, studies in non-renal inflammatory diseases such as asthma have demonstrated changes in cystatin C with disease activity and with immunosuppressive medications such as steroids and calcineurin inhibitors.(12, 13) On a population scale, data from NHANES have shown variability in cystatin C levels with age and race, reflecting the role of possible non-GFR determinants and potential confounders in the relationship between cystatin C and GFR. Other epidemiologic studies, including studies of the PREVEND cohort, have also found associations between greater height and weight and cystatin C levels.(14, 15) These studies however, used serum creatinine or measured creatinine clearance, rather than measured GFR, to adjust for GFR, and therefore, may have been susceptible to confounding from non-GFR determinants of creatinine and creatinine clearance.
In contrast, our findings regarding the lack of association of estimated protein intake with cystatin C differ from the previous literature on cystatin C and nutritional markers. In our own previous study using a cross-sectional analysis of the pooled dataset from the CKD-EPI collaboration, which included the MDRD Study, after adjustment for GFR, there was a 6.5% higher serum creatinine and a 4 % higher serum cystatin C for each 4.7 g/day higher urine urea nitrogen, equivalent to approximately 1.5 % and 1 % higher serum levels per 0.2 g/kg/d higher estimated protein intake.(16) In the current study, after adjustment for GFR and GFR measurement error, we similarly found a positive association with cystatin C in our cross sectional analysis, which replicates the findings from the prior study, however in longitudinal analysis, dietary protein prescription did not affect cystatin C. This suggests that the positive associations with cystatin C and protein intake observed in the cross sectional analyses likely reflect associations with unmeasured confounders, which were balanced in the randomized design, highlighting the strengths of our longitudinal analysis.
Our findings have implications in the clinical care and in research of patients with protein energy malnutrition and chronic kidney disease. Protein energy malnutrition, due to underlying uremia, has been well documented in the advanced stages of CKD.(17-19) The effect of decreased protein intake to lower creatinine generation, independent of GFR, can lead to falsely low GFR estimates using creatinine in patients with advanced CKD and protein energy malnutrition. In the presence of a true decline in GFR and underlying uremia leading to diminished protein intake, using serum creatinine to estimate GFR in these patients can lead to a false assumption of stable disease and may delay appropriate preparation for or initiation of renal replacement therapy. Cystatin C, on the other hand, does not appear to be affected by dietary protein intake and therefore, may be a better endogenous filtration marker in these patients.(20) On average, we found a differential bias between the creatinine and cystatin based GFR estimates of approximately 7-8%. In clinical practice, with larger reductions in protein intake, the differential bias in GFR estimates is likely to be greater. In principle, if the clinician suspects that a GFR estimate is unreliable, then it is important to have a confirmatory test. At this time, the only confirmatory test is for eGFR based on serum creatinine is measured GFR, using either exogenous filtration markers, or a timed urine collection; both have limitations. Our results identify cystatin C as a potential filtration marker to as a confirmatory test for GFR estimates based on serum creatinine in patients ingesting a low protein diet. In addition, using the change in eGFRcys instead of eGFRcr, as an outcome measure in a clinical trial of a protein intake intervention can avoid potentially misleading conclusions.
The strengths of our study are that we used measured GFR as a covariate to examine the true independent effect of a dietary protein intervention on cystatin C in a well characterized, randomized controlled trial of patients with moderate to severe CKD. We were able to adjust for measured GFR and GFR measurement error, further adding to the precision of our findings. Finally, we also examined the effect of the dietary protein intake intervention on the performance of the MDRD Study equation and the CKD-EPI cystatin C 2008 equation.
Our analysis has some limitations: The MDRD Study included patients with moderate to severe CKD, and as a result, a significant number of patients progressed to kidney failure or were censored due to death at our followup time of 2 years. Our findings, therefore, may not be generalizable to those who died or those who progressed rapidly to kidney failure. Secondly, a significant proportion of our baseline cohort (N=290) did not have cystatin C levels available at the 2 year follow up.(Appendix A, Table 1) These patients, were therefore, only analyzed in the cross sectional arm of the study and did not participate in the longitudinal analysis of randomized groups. Although, we did not observe clinically significant differences in age, etiology of kidney disease, proteinuria and measured GFR in this group compared with our longitudinal study sample (N=426), differences in unmeasured confounders may exist. Finally, the MDRD Study had a specific dietary protein intervention that is well described, extending the conclusions of this study to other dietary interventions including low fat and low carbohydrate diets warrants caution.
In conclusion, we find that serum cystatin C, unlike serum creatinine, is not affected by a low protein intake intervention, and may be better filtration marker than creatinine in patients with decreased protein intake. Further research on other non-GFR determinants of cystatin C is needed prior to widespread adoption.
Methods
MDRD Study
The details of the entry criteria, design, and results of the MDRD Study have been published previously.(7) Briefly, 1782 men and women aged 18 to 70 yr with CKD entered a baseline period to determine eligibility for the trial. On the basis of measured GFR, the participants were enrolled in either study A or study B. Participants in study A (n = 585) had entry GFR of 25 to 55 ml/min per 1.73 m2; participants in study B (n = 255) had entry GFR of 13 to 24 ml/min per 1.73 m2. Participants in study A were randomly assigned to either a usual-protein diet or a low-protein diet. The usual-protein diet contained 1.3 g/kg body weight per d protein and 16 to 20 mg/kg body weight per day phosphorus. The low-protein diet contained 0.575 g/kg body weight per d protein (with 65% of protein from high biologic value sources) and 5 to 10 mg/kg body weight per day phosphorus. In study B, the patients were randomly assigned either to the low-protein diet described above or to a very low–protein diet (0.28 g/kg body weight per d) supplemented with a mixed salt preparation made up of basic amino acids (tyrosine and threonine) and ketoacid analogs of other essential amino acids (totaling 0.28 g/kg body weight per day). Estimated protein intake (EPI) was computed from urine urea nitrogen measurements.(21) Patients in both studies were also randomized to usual (≤107 mm Hg for age ≤60 years, and ≤113 mm Hg for age >61 years) versus low (≤92 mm Hg for age ≤60 years, and ≤98 mm Hg for age >61 years) blood pressure goals in a 2 × 2 factorial design. (7). For this report, participants in both blood pressure groups are combined for all analyses.
Measurement of GFR, Cystatin C and Creatinine and Estimation of GFR
GFR was measured as four period urinary clearance of 125I-iothalamate. Samples were assayed for cystatin C with a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring, IL, USA) in samples stored at −80 C. The inter-and intraassay coefficients of variation for cystatin C were 3.2–4.4 and 2.0–3.0%, respectively. Stability in serum stored at −80 C has been demonstrated.(22, 23) Serum creatinine assays were calibrated to standardized serum creatinine values at the Cleveland Clinic Research Laboratory. The results of the calibration procedures have been previously described. GFR estimates using serum creatinine and cystatin C were calculated using the MDRD Study equation and the CKD-EPI cystatin equation 2008 respectively. (24, 25)
Descriptive Analysis
Descriptive statistics are reported as percentages for categorical data, and mean and standard deviation for normally distributed continuous data. Continuous variables were transformed so as to create a linear relationship with log-transformed cystatin C and creatinine in bivariate analyses. Sex and race were expressed as binary factors indicating presence or absence of female sex and black race, respectively. Differences between groups were tested using the chi-square test, Student t test, and the Mann-Whitney test as appropriate.
Cross Sectional Analysis
The relationships of cystatin C and creatinine with the predictor variables were investigated by first performing separate linear regressions to relate log-transformed cystatin C and creatinine to estimated protein intake at the final baseline visit after controlling for age, sex and log-transformed GFR. An increment of 0.2 g/kg/day for protein intake was used for its clinical applicability (14 g/day for a 70 kg person) and to maintain consistency with previous work.(10) We repeated these analyses using errors¬invariables regression analysis to incorporate measurement error in GFR into these models. A measurement error variance of 0.015 was assumed for log-transformed GFR based on analyses of the longitudinal variability in log-transformed baseline GFR.(26) GFR measurements were spaced an average of approximately 3 months apart in the MDRD Study.
Longitudinal Analysis
The effects of the dietary protein intervention on the change in measured and estimated GFR, and serum levels of creatinine and cystatin C from baseline to 2 years was examined in Study A and Study B. For the serum levels of creatinine and cystatin C, the change was estimated using analysis of covariance, with the model adjusting for baseline serum levels of the filtration markers, baseline and follow up GFR; and indicator variables for randomized diet group respectively. In this analysis, patients were analyzed according to their randomized group assignment, irrespective of achieved protein intake during follow-up.
Performance of GFR Estimating Equations
The performance of the MDRD Study and CKD-EPI cystatin C 2008 equations was evaluated at baseline and after the two year follow up in both the usual and low protein diet groups in Study A and low and very low protein diet groups in Study B. The mean difference between measured and estimated GFR is defined as bias. The mean difference in bias between randomized groups was compared for both studies at baseline and after two years. The mean change over time in the mean difference in bias for the two equations was compared using unpaired t-tests.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the participants of the MDRD Study, and collaborators in the CKD-EPI collaboration.
Support: NT is supported by the KRESCENT post-doctoral fellowship award, a joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research and the Canadian Society of Nephrology.
This work is also supported by NIDDK grants UO1 DK 053869, UO1 DK 067651 and UO1 DK 35073.
Footnotes
Disclosures: None to declare. The preliminary findings from this paper were presented at Renal Week 2009 in San Diego, California.
References
- 1.Levey AS, Coresh J, Balk E, Kausz AT, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Annals of internal medicine. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. The New England journal of medicine. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 3.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Schmid CH, Zhang YL, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 6.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Current opinion in nephrology and hypertension. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 7.Klahr S, Levey AS, Beck GJ, Caggiula AW, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. The New England journal of medicine. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Levey AS. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20:2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 9.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clinical chemistry. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 10.Effects of diet and antihypertensive therapy on creatinine clearance and serum creatinine concentration in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol. 1996;7:556–566. doi: 10.1681/ASN.V74556. [DOI] [PubMed] [Google Scholar]
- 11.Fricker M, Wiesli P, Brandle M, Schwegler B, et al. Impact of thyroid dysfunction on serum cystatin C. Kidney international. 2003;63:1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 12.Bokenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B. Effect of corticosteroid therapy on serum cystatin C and beta2-microglobulin concentrations. Clinical chemistry. 2002;48:1123–1126. [PubMed] [Google Scholar]
- 13.Cimerman N, Brguljan PM, Krasovec M, Suskovic S, et al. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clinica chimica acta; international journal of clinical chemistry. 2000;300:83–95. doi: 10.1016/s0009-8981(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 14.Knight EL, Verhave JC, Spiegelman D, Hillege HL, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney international. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 15.Kottgen A, Selvin E, Stevens LA, Levey AS, et al. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Schmid CH, Greene T, Li L, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney international. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. The Journal of nutrition. 1999;129:247S–251S. doi: 10.1093/jn/129.1.247S. [DOI] [PubMed] [Google Scholar]
- 18.Kopple JD, Berg R, Houser H, Steinman TI, et al. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl. 1989;27:S184–194. [PubMed] [Google Scholar]
- 19.Kopple JD, Greene T, Chumlea WC, Hollinger D, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney international. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 20.Hari P, Bagga A, Mahajan P, Lakshmy R. Effect of malnutrition on serum creatinine and cystatin C levels. Pediatric nephrology (Berlin, Germany) 2007;22:1757–1761. doi: 10.1007/s00467-007-0535-x. [DOI] [PubMed] [Google Scholar]
- 21.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney international. 1985;27:58–65. doi: 10.1038/ki.1985.10. [DOI] [PubMed] [Google Scholar]
- 22.Erlandsen EJ, Randers E, Kristensen JH. Reference intervals for serum cystatin C and serum creatinine in adults. Clin Chem Lab Med. 1998;36:393–397. doi: 10.1515/CCLM.1998.067. [DOI] [PubMed] [Google Scholar]
- 23.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scandinavian journal of clinical and laboratory investigation. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Manzi J, Levey AS, Chen J, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Greene T. Varying coefficients model with measurement error. Biometrics. 2008;64:519–526. doi: 10.1111/j.1541-0420.2007.00921.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


