Abstract
Cystatin C is gaining acceptance as an endogenous filtration marker. Factors other than glomerular filtration rate (GFR) that affect the serum level have not been carefully studied. In a cross-sectional analysis of a pooled dataset of participants from clinical trials and a clinical population with chronic kidney disease (N=3418), we related serum levels of cystatin C and creatinine to clinical and biochemical variables after adjustment for GFR using errors-in-variables models to account for GFR measurement error. GFR was measured as urinary clearance of 125I-iothalamate and 15Cr-EDTA. Cystatin C was assayed at a single laboratory and creatinine was standardized to reference methods. Mean (SD) creatinine and cystatin C were 2.1 (1.1) mg/dL and 1.8 (0.8) mg/L, respectively. After adjustment for GFR, cystatin C was 4.3% lower for every 20 years of age, 9.2% lower for female sex but only 1.9% lower in blacks. Diabetes was associated with 8.5% higher levels of cystatin C and 3.9% lower levels of creatinine. Higher C-reactive protein and white blood cell count and lower serum albumin were associated with higher levels of cystatin C and lower levels of creatinine. Adjustment for age, sex and race had a greater effect on association of factors with creatinine than cystatin C. In conclusion, cystatin C is affected by factors other than GFR. Clinicians should consider these factors when interpreting the serum levels or GFR estimates from cystatin C.
Introduction
Estimates of glomerular filtration rate (GFR) are essential to the clinical assessment of kidney function and facilitate the detection, evaluation and management of chronic kidney disease (CKD) [1]. GFR estimating equations are based on serum levels of endogenous filtration markers in combination with other variables; however, serum levels of these markers are affected by factors other than glomerular filtration rate. GFR estimating equations based on serum creatinine, such as the Modification of Diet in Renal Disease (MDRD) Study equation, include the variables age, sex, and race as surrogates for creatinine generation by muscle [2, 3]. However, these variables do not account for variation in creatinine generation due to diet, physiologic, or clinical conditions that affect muscle mass. Consequently, GFR estimates based on serum creatinine may be inaccurate in healthy people with a high or low meat intake, building muscle, and in patients with illnesses complicated by malnutrition, inflammation, or deconditioning.
Cystatin C is an endogenous, 13 kilodalton protein which is filtered by the glomeruli and reabsorbed and catabolized by epithelial cells of the proximal tubule with only small amounts excreted in the urine. Cystatin C is being considered as a potential replacement for serum creatinine because it appears to less affected by muscle mass [4]. However, recent reports have shown substantial variability in the relationship between GFR and cystatin C among populations, suggesting that there may be differences in generation, tubular reabsorption, or extra-renal elimination [5]. Such differences would affect the interpretation of GFR estimates based on cystatin C.
Using a large, pooled database from three research studies and one clinical population, we have previously reported that a GFR estimating equation based on cystatin C was nearly as accurate as estimates based on creatinine, thus providing an alternative GFR estimate that is not linked to muscle mass. In this study, we examine the association of factors other than GFR to predict serum cystatin C and compare those associations to prediction of creatinine. Because GFR is measured with error, we used multivariable models that adjust for measured GFR and also incorporated estimates of GFR measurement error. These results will better inform us of the utility of cystatin C as an endogenous filtration marker.
Results
Table 1 summarizes the study characteristics and Table 2 details the clinical characteristics of participants in each study and overall. Mean measured GFR (5th -95th percentile) was 48 (15-95) mL/min/1.73m2 (0.80 [0.25-1.58] mL /s/1.73m2). The mean [standard deviation (SD)] of serum cystatin C and creatinine were 1.8 (0.8) mg/L (135 [60] nmol/L) and 2.1 (1.1) mg/dL (186 [97] umol/L), respectively. The mean age was 52 years. All patients were considered to have chronic kidney disease.
Table 1.
Study Characteristics
| Name | MDRD Study | AASK | CSG | NephroTest* |
|---|---|---|---|---|
| Type | RCT | RCT | RCT | CP |
| Location | U.S. | U.S. | U.S. | France |
| Center | MC | MC | MC | MC |
| N | 1085 | 1205 | 266 | 438 |
| Dates | 1989-1992 | 1995-1998 | 1987-1992 | 2000-2004 |
| Clearance method | Urinary | Urinary | Urinary | Urinary |
| Filtration marker | Iothalamate | Iothalamate | Iothalamate | EDTA |
MDRD Study, Modification of Diet in Renal Disease Study; AASK, African American Study of Kidney Diseases and Hypertension; CSG, Collaborative Study Group: Captopril in Diabetic Nephropathy Study. MC, multicenter; RCT, randomized clinical trial; CP, clinical population; EDTA, ethylenediaminetetraacetic acid.
The NephroTest initiative is a prospective hospital-based ongoing cohort that began in 2000, enrolling patients with all diagnoses of CKD stage 2 to 5 referred for extensive work-up by two nephrology departments. Data included in this study were collected between 2000 and 2004. These data are part of the dataset presented in [26]
Table 2.
Patient Characteristics: Overall and by Study
| Variable | Overall | MDRD | AASK | CSG | NephroTest a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| N | Mean/ % |
IQR | N | Mean/ % |
SD | N | Mean/ % |
SD | N | Mean/ % |
SD | N | Mean/ % |
SD | |
| Age (years) | 3418 | 53.2 | 19 | 1047 | 52 | 13 | 1647 | 54 | 10 | 287 | 34 | 8 | 438 | 59 | 15 |
| Female | 3418 | 32 | 1047 | 39.1 | 1647 | 35.9 | 287 | 46.9 | 438 | 28.8 | |||||
| Black | 3418 | 53.5 | 1047 | 9.7 | 1647 | 100 | 287 | 7.7 | 438 | 8.5 | |||||
| Diabetes | 3418 | 13.9 | 1047 | 5.7 | 1647 | 0 | 287 | 100 | 438 | 21.9 | |||||
| Hypertension | 3417 | 66.7 | 1047 | 34.9 | 1646 | 99.7 | 287 | 34.6 | 438 | 40 | |||||
| Systolic blood pressure (mm Hg) |
3404 | 139 | 28 | 1047 | 132 | 18 | 1647 | 150 | 23 | 287 | 135.6 | 17.2 | 424 | 138.3 | 20 |
| Diastolic blood pressure (mm Hg) |
3239 | 88 | 19 | 1047 | 81 | 10 | 1647 | 96 | 14 | 287 | 82.9 | 10.2 | 259 | 78 | 11.1 |
| BMI (kg/m2) | 3416 | 27.7 | 7 | 1046 | 27 | 4 | 1647 | 31 | 7 | 286 | 25.5 | 4.9 | 438 | 26 | 4.6 |
| GFR (ml/min per 1.73 m2) |
3418 | 44.4 | 34 | 1047 | 33 | 14 | 1647 | 57 | 23 | 287 | 74.8 | 32.5 | 438 | 33.6 | 16.8 |
| Serum creatinine (mg/100 ml) |
3418 | 1.8 | 1 | 1047 | 2.34 | 1.09 | 1647 | 1.71 | 0.82 | 286 | 1.33 | 0.56 | 438 | 2.54 | 1.22 |
| Blood urea nitrogen (mg/100 ml) |
3224 | 25 | 18 | 1047 | 36.3 | 14.5 | 1647 | 22.6 | 11.4 | 287 | 25.6 | 15.7 | 244 | 43.6 | 20.4 |
| Cystatin C (mg/l) |
3418 | 1.6 | 1 | 1047 | 2.3 | 0.8 | 1647 | 1.5 | 0.7 | 287 | 1.4 | 0.7 | 438 | 2.2 | 0.8 |
| Hemoglobin (g/100 ml) |
2856 | 13.3 | 2 | 1014 | 13 | 1.9 | 1557 | 13.3 | 1.7 | 286 | 13.2 | 2 | 0 | . | . |
| Potassium (mEq/l) |
2968 | 4.2 | 1 | 1035 | 4.3 | 0.6 | 1647 | 4.2 | 0.6 | 287 | 4.3 | 0.5 | 0 | . | . |
| Bicarbonate (mEq/l) |
3335 | 25 | 4 | 1036 | 23 | 4 | 1647 | 25 | 3 | 285 | 26.1 | 3.3 | 368 | 25.5 | 3.2 |
| Glucose (mg/100 ml) |
2969 | 92 | 21 | 1036 | 93 | 26 | 1647 | 95 | 18 | 287 | 234 | 125 | 0 | . | . |
| Calcium (mg/100 ml) |
2962 | 9.1 | 1 | 1029 | 9.1 | 0.5 | 1647 | 9.2 | 0.5 | 287 | 9 | 0.6 | 0 | . | . |
| Phosphate (mg/100 ml) |
2974 | 3.6 | 1 | 1042 | 3.9 | 0.8 | 1647 | 3.5 | 0.7 | 286 | 3.7 | 0.7 | 0 | ||
| Albumin (g/100 ml) |
3381 | 4.2 | 1 | 1047 | 4 | 0.4 | 1647 | 4.3 | 0.4 | 285 | 3.7 | 0.5 | 403 | 4.1 | 0.5 |
| Total protein (g/100 ml) |
2980 | 7 | 5 | 1047 | 2.1 | 0.8 | 1647 | 7.6 | 0.6 | 287 | 6.6 | 0.7 | 0 | . | . |
| Total cholesterol (mg/100 ml) |
2683 | 209 | 60 | 1041 | 216 | 46 | 1642 | 211 | 46 | 0 | . | . | 0 | . | . |
| C-reactive protein (g/100 ml) |
2997 | 0.4 | 1 | 1026 | 0.3 | 0.5 | 1643 | 0.4 | 0.6 | 0 | . | . | 328 | 2.3 | 5 |
| Hemoglobin A1c (g/100 ml) |
1032 | 5.6 | 0.9 | 1032 | 5.6 | 0.9 | 0 | . | . | 0 | . | . | 0 | . | . |
| Urine creatinine (mg/day) |
3008 | 1458 | 772 | 943 | 1406 | 420 | 1556 | 1679 | 659 | 247 | 1432 | 1262 | 262 | 1294 | 418 |
| Urine urea nitrogen (g/day) |
2896 | 8.7 | 5 | 943 | 9.4 | 2.9 | 1565 | 8.7 | 3.9 | 154 | 11 | 4.3 | 234 | 9.6 | 3.2 |
| Urine protein (mg/day) |
2755 | 165 | 986 | 943 | 330 | 1450 | 1565 | 71 | 353 | 247 | 1677 | 3133 | 0 | . | . |
MDRD Study, Modification of Diet in Renal Disease Study; AASK, African American Study of Kidney Diseases and Hypertension; BMI, body mass index; CSG, Collaborative Study Group: Captopril in Diabetic Nephropathy Study; GFR, glomerular filtration rate; IQR, interquartile range; MDRD Study, Modification of Diet in Renal Disease Study; SD, standard deviation.
To convert GFR from ml/min per 1.73 m2 to ml/s per 1.73 m2, multiply by 0.01667; to convert serum creatinine from mg/100 ml to mol/l, multiply by 88.4; to convert blood urea nitrogen from mg/100 ml to mmol/l, multiply by 0.357; to convert cystatin C from mg/l to nmol/l, multiply by 74.9; to convert hemoglobin from g/100 ml to g/l, multiply by 10; to convert potassium from mEq/l to mmol/l, multiply by 1; to convert bicarbonate from mEq/l to mmol/l, multiply by 1; to convert glucose from mg/100 ml to mmol/l, multiply by 0.05551; to convert calcium from mg/100 ml to mmol/l, multiply by 0.2495; to convert phosphate from mg/100 ml to mmol/l, multiply by 0.3229; to convert albumin from g/100 ml to g/l, multiply by 10; to convert total protein from g/100 ml to g/l, multiply by 10; to convert total cholesterol from mg/100 ml to mmol/l, multiply by 0.02586; to convert C-reactive protein from g/100 ml to nmol/l, multiply by 95,240; to convert hemoglobin A1C from g/100 ml to g/l, multiply by 10; to convert urine creatinine from mg/day to g/day, divide by 1000; to convert urine protein from mg/day to g/day, divide by 1000.
In separate errors-in-variables models relating either log cystatin C or log serum creatinine to log GFR after adjusting for age, race, sex, and study, the coefficients for log GFR were −67.0% [95% confidence intervals (CI) −66.3, −67.7] and −70.5% [−69.8, −71.2], respectively. A coefficient of less than 100% signifies that a percent change in GFR is associated with smaller percent change in the serum levels of cystatin C and creatinine, indicating an association of the serum levels with factors other than GFR. A lower absolute level for the association with GFR of cystatin C than creatinine suggests factors other than GFR are more strongly associated with cystatin C than with creatinine.
Tables 3 and 4 shows the regression coefficients and 95% confidence intervals relating serum cystatin C and creatinine to potential predictor variables, after controlling for GFR and study in models that adjust for measurement error in GFR and after adjustment for age, sex and race. The coefficient represents the average percent difference in cystatin C or creatinine level for a difference between the 75th and 25th percentile (the interquartile range) in the continuous variables (age, body mass index, and blood and urine levels) and for a difference between categories for dichotomous predictor variables (sex, race and diabetes). The smaller the interquartile range, the larger the effect of a small change in the variable on serum levels of the markers. Within each column, the coefficients show the relative strength of association among variables.
Table3.
Percent change in level of cystatin C
| Variable | IQR | Not Adjusted | Adjusted for GFR | Adjusted for GFR Measurement Error (0.015) |
Adjusted for GFR Measurement Error (0.015), age, sex and race |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | L | H | Coeff | L | H | Coeff | L | H | Coeff | L | H | ||
| Age*(years) | 19.38 | 5.5 | 2.0 | 9.2 | −3.8 | −5.2 | −2.5 | −4.3 | −5.7 | −2.9 | |||
| Female* | 1 | −0.7 | −5.2 | 3.9 | −8.8 | −10.6 | −7 | −9.2 | −11.0 | −7.4 | |||
| Black | 1 | −36.1 | −38.7 | −33.4 | −4.1 | −6.1 | −2 | −1.9 | −4 | 0.3 | |||
| Hypertension | 1 | −23.8 | −27.2 | −20.3 | −1.2 | −3.2 | 0.8 | 0.1 | 2.2 | −2. | 2.6 | −0.2 | 5.5 |
| Height*(cm) | 14.4 | −2.1 | −5.3 | 1.2 | 4.5 | 3.0 | 6.0 | 4.8 | 3.3 | 6.3 | −0.5 | −2.4 | 1.4 |
| Weight*(kg) | 25.2 | −7.6 | −10.2 | −5 | 5.4 | 4.0 | 6.7 | 6.0 | 4.7 | 7.4 | 5.2 | 3.7 | 6.6 |
| BMI*(kg/m2) | 7.29 | −7 | −9.3 | −4.5 | 3.6 | 2.4 | 4.9 | 4.2 | 2.9 | 5.5 | 5.2 | 3.9 | 6.6 |
| Systolic Blood Pressure* (mmHg) | 28 | −2.5 | −5.1 | 0.2 | 1.3 | 0.0 | 2.6 | 1.5 | 0.2 | 2.8 | 2.6 | 1.2 | 4.0 |
| Diastolic Blood Pressure (mmHg) | 19 | −15.4 | −17.9 | −12.8 | 0.0 | −1.3 | 1.5 | 0.9 | −0.5 | 2.3 | 0.4 | −1.2 | 1.9 |
| Log Serum Creatinine* (mg/dL) | 0.65 | 123.5 | 120.2 | 126.8 | 35.0 | 31.0 | 39.1 | 20.4 | 15.8 | 25.2 | 15.9 | 14.2 | 17.6 |
| Log SUN* (mg/dL) | 0.69 | 95.3 | 91.0 | 99.7 | 14.8 | 12.0 | 17.5 | 7.7 | 4.9 | 10.5 | 6.5 | −0.1 | 13.5 |
| Hemoglobin*(g/dL) | 2.4 | −22.3 | −24.7 | −19.9 | 3.1 | 1.6 | 4.7 | 4.9 | 3.3 | 6.6 | 3.2 | 0.5 | 5.9 |
| Log WBC*(cells/uL) | 0.4 | 8.0 | 4.5 | 11.5 | 3.3 | 1.7 | 5.0 | 3.1 | 1.5 | 4.8 | 3.0 | 1.4 | 4.5 |
| Sodium (Meq/L) | 4 | −1.1 | −3.6 | 1.4 | 0.4 | −0.6 | 1.5 | 0.5 | −0.5 | 1.6 | 0.8 | −0.8 | 2.4 |
| Potassium (Meq/L) | 0.7 | 17.1 | 13.0 | 21.3 | 1.3 | −0.1 | 2.8 | 0.5 | −0.9 | 2.0 | 0.0 | −1.1 | 1.0 |
| Bicarbonate (Meq/L) | 4 | −26.4 | −28 | −4.7 | −2.8 | −4 | −1.5 | −1.2 | −2.5 | 0.2 | −0.9 | −2.2 | 0.5 |
| Log Glucose (mg/dL) | 0.22 | −7.0 | −8.4 | −5.7 | 0.9 | 0.1 | 1.7 | 1.4 | 0.6 | 2.2 | 1.1 | 0.3 | 1.9 |
| Albumin* (g/dL) | 0.5 | −13.3 | −15.6 | −10.9 | −2.5 | −3.7 | −1.3 | −1.9 | −3.1 | −0.7 | −2.2 | −3.5 | −0.9 |
| Calcium (mg/dL) | 0.6 | −9.9 | −12.5 | −7.2 | 0.1 | −1 | 1.3 | 0.7 | −0.5 | 1.9 | 1.2 | 0.0 | 2.4 |
| Phosphate (mg/dL) | 0.8 | 32.9 | 27.9 | 38.1 | 1.6 | 0.3 | 2.9 | −0.1 | −1.4 | 1.3 | −0.1 | −1.4 | 1.3 |
| Total Cholesterol (mg/dL) | 60 | −0.3 | −3.5 | 2.9 | −1.5 | −2.9 | −0.1 | −1.6 | −2.9 | −0.2 | −0.4 | −1.7 | 1.0 |
| Log CRP* (g/dL) | 1.75 | 10.1 | 7 | 13.3 | 2.6 | 1.3 | 4 | 2.3 | 0.9 | 3.7 | 3.4 | 1.9 | 4.9 |
| Urine Creatinine* (mg/day) | 0.77 | −13.8 | −18.5 | −8.9 | 3.1 | 1.6 | 4.6 | 4.1 | 2.4 | 5.8 | 1.7 | 0.4 | 3.0 |
| Urine Phosphate* (mg/day) | 0.38 | −14.6 | −18.4 | −10.7 | 6.4 | 4.1 | 8.7 | 7.7 | 5.4 | 10.1 | 5.8 | 3.4 | 8.3 |
| Urine Urea Nitrogen* (g/day) | 4.69 | −11.6 | −14.2 | −9.0 | 4.4 | 2.9 | 5.8 | 5.3 | 3.9 | 6.8 | 4 | 2.5 | 5.5 |
| Log Urine Protein*(mg/day) | 3.15 | 63.1 | 57 | 69.5 | 14.5 | 12.1 | 17 | 12.1 | 9.7 | 14.6 | 10.8 | 8.3 | 13.4 |
BMI, body mass index; Coeff, coefficient; CRP, C-reactive protein; GFR, glomerular filtration rate; H, higher confidence limit; IQR, interquartile range; L, lower confidence limit; WBC, white blood cell.
Each row shows different models based on the variable. Continuous variables are expressed as interquartile range, which is the difference between the 25th and 75th percentile. The model in column 1 includes the variable adjusted for study. Column 2 includes the model adjusted for variable, study terms, GFR, and the interaction of GFR and study. Column 3 includes the model adjusted for variable, study terms, GFR, GFR measurement error, and the interaction of GFR and study. Column 4 includes the model adjusted for variable, study terms, GFR, GFR measurement error, the interaction of GFR and study, age, sex, and race. The coefficient is expressed as 100*(ecoeff−1), which can be interpreted as a geometric mean percent change in the filtration marker for a change of two quartiles in the variable.
P-value < 0.0001 for the interaction of study*variable in a model that includes variable, study terms, GFR, GFR measurement error, age, sex ,and race, and interaction of study by GFR and by variable.
To convert serum creatinine from mg/dL to umol/L, multiply by 88.4. To convert SUN mg/dL to mmol/L, multiply by 0.357. To convert hemoglobin from g/dL to g/L, multiply by 10. To convert WBC from 103/uL to 109/L, multiply by 1. To convert sodium from mEq/L to mmol/L, multiply by 1. To convert potassium from mEq/L to mmol/L, multiply by 1. To convert bicarbonate from mEq/L to mmol/L, multiply by 1. To convert glucose from mg/dL to mmol/L, multiply by 0.05551. To convert albumin from g/dL to g/L, multiply by 10. To convert calcium from mg/dL to mmol/L, multiply by 0.2495. To convert phosphate from mg/dL to mmol/L, multiply by 0.3229. To convert total cholesterol from mg/dL to mmol/L, multiply by 0.02586. To convert C-reactive protein from g/dL to g/L, multiply by 10. To convert urine creatinine from mg/day to g/day, divide by 1000. To convert urine phosphate from mg/day to g/day, divide by 1000. To convert urine protein from mg/day to g/day, divide by 1000.
Table 4.
Percent change in serum creatinine
| Variable | IQR | Not Adjusted | Adjusted for GFR | Adjusted for GFR Measurement Error (0.015) |
Adjusted for GFR Measurement Error (0.015), age, sex and race |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | L | H | Coeff | L | H | Coeff | L | H | Coeff | L | H | ||
| Age * (years) | 19.38 | 0.5 | −3.2 | 4.3 | −9.0 | −10.3 | −7.2 | −9.2 | −10.7 | −7.7 | |||
| Female * | 1 | −25 | −28.6 | −21.2 | −31.6 | −32.8 | −29.9 | −31.7 | −33.1 | −30.2 | |||
| Black | 1 | −28.3 | −31.5 | −24.8 | 12.5 | 8.1 | 13.8 | 13.6 | 10.7 | 16.7 | |||
| Diabetes | 1 | −24.7 | −30 | −18.9 | −4.4 | −8.3 | −1.6 | −3.9 | −7.3 | −0.4 | 2 | −2 | 6.2 |
| Hypertension | 1 | −18.7 | −22.7 | −14.5 | 7.3 | 3.8 | 9.2 | 7.9 | 5.2 | 10.7 | 0.4 | −2.3 | 3.1 |
| Height * (cm) | 14.4 | 13.4 | 9.5 | 17.4 | 21.6 | 19.4 | 23.4 | 21.8 | 19.8 | 23.8 | 0.5 | −1.5 | 2.5 |
| Weight* (kg) | 25.2 | −2.3 | −5.1 | 0.6 | 12.6 | 10.4 | 13.8 | 12.9 | 11.1 | 14.6 | 2.7 | 1.3 | 4.2 |
| BMI* (kg/m2) | 7.29 | −7.8 | −10.3 | −5.2 | 3.3 | 1.4 | 4.5 | 3.5 | 2 | 5.1 | 2.5 | 1.2 | 3.9 |
| Systolic Blood Pressure* (mmHg) | 28 | −3.2 | −6 | −0.4 | 0.8 | −0.9 | 2.3 | 0.9 | −0.7 | 2.5 | −0.8 | −2.1 | 0.6 |
| Diastolic Blood Pressure (mmgHg) | 19 | −10.7 | −13.5 | −7.8 | 6.9 | 4.5 | 8.1 | 7.2 | 5.4 | 9.1 | −1.1 | −2.7 | 0.6 |
| Log Serum Cystatin C(mg/l) | 0.65 | 151.3 | 146.2 | 156.5 | 45.9 | 51 | 66.3 | 36.2 | 27.7 | 45.1 | 16.1 | 8.7 | 24.1 |
| Log SUN* (mg/dl) | 0.69 | 104.1 | 99.1 | 109.3 | 18.6 | 19.4 | 26.5 | 15.7 | 12.1 | 19.3 | 16.1 | 12.8 | 19.4 |
| Hemoglobin* (g/dl) | 2.4 | −20.3 | −22.9 | −17.5 | 7.9 | 4.9 | 8.6 | 8.6 | 6.7 | 10.6 | 0.6 | −1.1 | 2.3 |
| Log WBC* (cells/mcl) | 0.4 | 1.4 | −1.9 | 4.8 | −3.1 | −4.7 | −1.2 | −3.2 | −5 | −1.4 | −1.8 | −3.3 | −0.2 |
| Sodium (mg/dl) | 4 | 0.8 | −2 | 3.6 | 2.4 | 1.1 | 3.6 | 2.5 | 1.2 | 3.7 | 0.7 | −0.4 | 1.8 |
| Potassium (Meq/L) | 0.7 | 19.4 | 14.9 | 24 | 2.8 | 1.4 | 5.1 | 2.5 | 0.7 | 4.3 | 0.9 | −0.5 | 2.3 |
| Bicarbonate (Meq/L) | 4 | −26.7 | −28.6 | −24.8 | −1.6 | −4.2 | −1 | −0.9 | −2.6 | 0.8 | −0.7 | −2.1 | 0.8 |
| Log Glucose (mg/dl) | 0.22 | −8.7 | −10 | −7.4 | −0.7 | −1.7 | −0.2 | −0.5 | −1.3 | 0.3 | 0.2 | −0.6 | 1.1 |
| Albumin* (g/dl) | 0.5 | −6.9 | −9.5 | −4.2 | 5.6 | 3.8 | 6.7 | 5.9 | 4.4 | 7.4 | 2.3 | 1 | 3.6 |
| Calcium (mg/dl) | 0.6 | −11.9 | −14.6 | −9 | −1.7 | −3.5 | −0.5 | −1.5 | −3 | 0 | −0.2 | −1.4 | 1.1 |
| Phosphate (mg/dl) | 0.8 | 35 | 30.1 | 40 | 2.2 | 1.6 | 4.9 | 1.5 | −0.2 | 3.3 | 3.2 | 1.9 | 4.7 |
| Total Cholesterol (mg/dl) | 60 | −3.4 | −6.7 | 0.1 | −4.5 | −6.2 | −2.8 | −4.6 | −6.3 | −2.8 | 0.4 | −1 | 1.8 |
| Log CRP* (g/dl) | 1.75 | 4.3 | 1.2 | 7.6 | −3.1 | −4.4 | −1.4 | −3.3 | −4.8 | −1.7 | 0.4 | −0.9 | 1.8 |
| Urine Creatinine* (mg/day) | 0.77 | −2.7 | −5.1 | −0.3 | 18.7 | 11.1 | 25.3 | 19.2 | 11.8 | 27.1 | 7.8 | 4.4 | 11.3 |
| Urine Phosphate* (mg/day) | 0.38 | −14.3 | −18.6 | −9.8 | 11.9 | 8 | 13.9 | 12.6 | 9.5 | 15.7 | 5.9 | 3.1 | 8.7 |
| Urine Urea Nitrogen* (g/day) | 4.69 | −8.1 | −10.8 | −5.2 | 9.7 | 7.4 | 10.9 | 10.1 | 8.4 | 11.9 | 6.1 | 4.5 | 7.7 |
| Log Urine Protein* (mg/day) | 3.15 | 61 | 54.4 | 67.9 | 10.9 | 9.6 | 15.2 | 10 | 7.2 | 12.8 | 5.1 | 2.7 | 7.5 |
BMI, body mass index; Coeff, coefficient; CRP, C-reactive protein; GFR, glomerular filtration rate; H, higher confidence limit; IQR, interquartile range; L, lower confidence limit; WBC, white blood cell.
Each row shows different models based on the variable. Continuous variables are expressed as interquartile range, which is the difference between the 25th and 75th percentile. The model in column 1 includes the variable adjusted for study. Column 2 includes the model adjusted for variable, study terms, GFR, and the interaction of GFR and study. Column 3 includes the model adjusted for variable, study terms, GFR, GFR measurement error, and the interaction of GFR and study. Column 4 includes the model adjusted for variable, study terms, GFR, GFR measurement error, the interaction of GFR and study, age, sex, and race. The coefficient is expressed as 100*( ecoeff−1), which can be interpreted as a geometric mean percent change in the filtration marker for a change of two quartiles in the variable.
P-value < 0.0001 for the interaction of study*variable in a model that includes variable, study terms, GFR, GFR measurement error, age, sex, and race, and interaction of study by GFR and by variable.
To convert serum creatinine from mg/dL to umol/L, multiply by 88.4. To convert SUN mg/dL to mmol/L, multiply by 0.357. To convert hemoglobin from g/dL to g/L, multiply by 10. To convert WBC from 103/uL to 109/L, multiply by 1. To convert sodium from mEq/L to mmol/L, multiply by 1. To convert potassium from mEq/L to mmol/L, multiply by 1. To convert bicarbonate from mEq/L to mmol/L, multiply by 1. To convert glucose from mg/dL to mmol/L, multiply by 0.05551. To convert albumin from g/dL to g/L, multiply by 10. To convert calcium from mg/dL to mmol/L, multiply by 0.2495. To convert phosphate from mg/dL to mmol/L, multiply by 0.3229. To convert total cholesterol from mg/dL to mmol/L, multiply by 0.02586. To convert C-reactive protein from g/dL to g/L, multiply by 10. To convert urine creatinine from mg/day to g/day, divide by 1000. To convert urine phosphate from mg/day to g/day, divide by 1000. To convert urine protein from mg/day to g/day, divide by 1000.
Figure 1 compares the regression coefficients for cystatin C and creatinine that are displayed in tables 3 and 4. Each plot character represents a predictor variable. Distance of the plot character from the zero on the horizontal and vertical axis indicates the strength of association of the predictor variable with level of cystatin C and creatinine, respectively. Panel A shows that after adjustment for GFR and GFR measurement error (but not age, race and sex), older age and female sex were associated with lower cystatin C (by 4.3% and 9.2% respectively) and lower creatinine (by 9.2% and 31.7% respectively). Black race was not significantly associated with cystatin C (−1.9%) but was associated with higher creatinine levels (13.6%). Higher levels of height (4.8 and 21.8) , weight (6.0 and 12.9) , body mass index (4.2 and 3.5), and urine creatinine (4.1 and 19.2) were associated with higher levels of cystatin C and creatinine, respectively, and, except for body mass index, the magnitude of association was greater with creatinine than with cystatin C. A higher level of urine protein (on the log scale) was associated with a higher level of cystatin C (12.1%) and creatinine (10.0%). Diabetes was associated with higher levels of cystatin C (8.5%) and lower levels of creatinine (−3.0%). Similarly, higher C-reactive protein and white blood cell count and lower serum albumin were associated with higher levels of cystatin C (2.3, 3.1, and −1.9%, respectively) and lower levels of creatinine (−3.3, −3.2, and 5.9%, respectively). Higher urine urea nitrogen and urine phosphorus were associated with higher cystatin C (5.3 and 7.7%, respectively) and creatinine (10.1 and 12.6%, respectively).
Figure 1. Comparison of coefficients of variables predicting log cystatin and log creatinine.
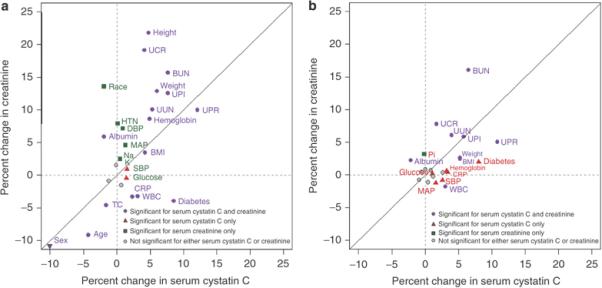
Solid diagonal line is the line of identity. For continuous predictor variables the coefficients are expressed as the percent differences in cystatin or creatinine associated with a difference of one interquartile range in the predictor variable (i.e., a change from the 25th to 75th percentile) after adjusting for GFR in models that incorporate measurement error in the GFR assay. For dichotomous predictor variables the coefficients indicate the percent differences in cystatin or creatinine associated with the presence vs. the absence of the factor. Variables that fall along the line of identity have a similar relationship to serum creatinine and cystatin C. Points away from the line of identity represent variables with a different magnitude of association with cystatin C and creatinine. Variables near the origin have a weak relationship with the filtration marker.
The plot character colors indicate significance of the relationships between the predictor variable to cystatin C, creatinine, neither or both. Grey dots indicate variables that were not significantly associated with either cystatin C or creatinine. For all variables, the coefficients for cystatin C and creatinine were significantly different from one other (P<0.001).
HTN, hypertension; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; WBC, white blood cell count; Na, sodium; K, potassium; Pi, phosphate; Ca, calcium; HCO3, bicarbonate; TC, total cholesterol; alb, albumin; gluc, glucose; UUN, urine urea nitrogen; UCR, urine creatinine; UPI, urine phosphate; UPR, urine protein
Panel a: After adjustment for GFR and GFR measurement error
Variables that were not significantly associated with either variable (indicated by gray dots) include serum bicarbonate, total calcium and phosphate. Sex is indicated on the margins of the figure as a downward arrow, as the coefficients are bigger than the scale.
Panel b: After adjustment for GFR and GFR measurement error, age, sex and race
Variables that were not significantly associated with either cystatin C or creatinine (indicated by gray dots) include hypertension, height, diastolic blood pressure, sodium, bicarbonate, total calcium, and total cholesterol.
Panel B of figure 1 shows the same associations after adjustment for age, sex and race. Adjustment for age, sex and race markedly decreased the association of factors with serum creatinine. In contrast, this adjustment had little effect on the associations with serum cystatin C. After adjustment for age, sex and race, the strongest associations with cystatin C were for proteinuria (10.8% on the log scale) and diabetes (8.0%). Stronger associations were seen for cystatin C than for serum creatinine for many variables [diabetes (8.0 vs. 2.0%), systolic blood pressure (2.6 vs. 0.8%), weight (5.2 vs. 2.7%). body mass index (5.2 vs. 2.5%), white blood cell count (3.0 vs. 1.8%), hemoglobin (3.2 vs 0.6%), C-reactive protein (3.4 vs. 0.9%), and urine protein (10.8 vs. 5.1%)].
After accounting for GFR, its measurement error, as well as age, sex, and race, the percent change for log creatinine in predicting log cystatin C was 15.9% and the percent change for log cystatin C in predicting log creatinine was 16.1%. Serum urea nitrogen was also significantly associated with both creatinine and cystatin C after adjustment for the same factors.
Discussion
Cystatin has been proposed as an alternative filtration marker to serum creatinine, and it clearly has promise to be so. The main findings of this study are that many factors other than GFR are associated with serum cystatin C, including key variables such as diabetes, measures of body size, and inflammation. These associations would lead to systematic bias of GFR estimates based on cystatin C in selected populations or clinical conditions as well as imprecision of GFR estimates in all populations. Clinicians can use these findings to aid in interpretation of serum levels and GFR estimates based on cystatin C.
Physiological processes other than glomerular filtration, such as tubular reabsorption or secretion, generation, and extra-renal elimination can affect the serum levels of endogenous filtration markers. Urinary excretion of the marker facilitates study of these processes. For example, the effect of medications on tubular secretion of creatinine was verified by comparing creatinine clearance to GFR measured using exogenous markers, and the relationship of muscle mass and diet to creatinine generation was established from studies of urinary excretion of creatinine. In addition, in clinical practice, clinicians can measure urinary creatinine excretion to assist in interpretation of unexpected values for GFR estimates based on creatinine. In contrast, the absence of urinary excretion of cystatin C makes it difficult to measure these physiological processes and to interpret GFR estimates from cystatin C in clinical practice. Instead, understanding of the determinants of cystatin C other than GFR in humans relies on epidemiologic associations. Our study provides the first comprehensive investigation of associations with cystatin C to factors other than GFR.
We found a stronger association of serum creatinine than cystatin C with surrogates of muscle mass, including age, sex, race, and urine creatinine. This likely reflects smaller contribution of muscle to generation of cystatin C mass than creatinine. It is possible that GFR estimates based on cystatin C may be more accurate than estimates based on creatinine in patients with variation in creatinine generation due to diet or clinical conditions that affect muscle mass. This hypothesis has not been explicitly tested as such patients have not been systematically included in research studies.
The relationship of race with cystatin C levels, independent of GFR has not, to our knowledge, been previously noted by others. Similar to the findings here, when we included age, sex, and race as coefficients in an equation to estimate GFR from serum cystatin C, the coefficents for these factors were significant but substantially smaller than in equations to estimate GFR from serum creatinine [4]. The association with race varies by modeling strategy and weakened by adjustment for measurement error in GFR in this paper compared to the GFR estimating equation. In contrast, in analyses from the National Health and Nutrition Examination Survey (NHANES), we previously reported differences in serum levels of cystatin C among races, even among young healthy individuals in whom GFR is presumably normal [6]. Possibly, this difference could reflect true differences in GFR among race groups, such as hyperfiltration among African-Americans compared to whites, but also could reflect variation in other characteristics among the race groups in NHANES. GFR measurements in a representative multi-ethnic population will be necessary to determine whether the cause of variation in cystatin C levels reflects variation in measured GFR or in factors affecting cystatin C other than GFR.
We also observed stronger magnitude of associations of body mass index and weight with cystatin C than with creatinine, which may indicate an association of cystatin C with fat mass. In this context, the association of higher cystatin C with diabetes may, in part, also reflect the association with fat mass. These are important considerations for use of cystatin C in clinical practice, given the high and increasing prevalence of obesity and diabetes [7]. The association of proteinuria with higher cystatin C may reflect the association of diabetes with proteinuria in our dataset but could also reflect tubular damage. The association of higher urine urea nitrogen and urine phosphate with higher serum levels of both markers after adjustment for GFR suggests that diet may also be a determinant of cystatin C.
Our findings of associations of cystatin C with body mass index, as well as inflammation, and proteinuria are consistent with previous reports [8-13]. Recent studies have shown that in studies of pre-adipocyte cell cultures, there is increased cystatin C production during pre-adipocyte differentiation [14]. Since obesity is now recognized as an inflammatory state, the findings of both inflammation and obesity are informative. Other studies have also demonstrated the association of cystatin C with thyroid hormone levels [15]. We were not able to verify these data as thyroid hormone levels were not measured in the current studies.
Many studies have shown stronger association of serum cystatin C with mortality and cardiovascular disease than serum creatinine, particularly in studies of older adults and we previously demonstrated higher levels of cystatin C in older adults in NHANES [6, 16-19]. In part, these findings may reflect greater accuracy of cystatin C than creatinine as a filtration marker in this population. Another possible explanation, as is suggested by this study, is differential effects of factors other than GFR on levels of serum cystatin C and creatinine that are more prevalent in older adults [20]. In this study, diabetes, higher C-reactive protein, higher white blood cell count, and lower serum albumin (all risk factors for mortality) were associated with a higher serum cystatin C and lower serum creatinine. The opposite direction of the relationships of these factors to the filtration markers would confound the comparison of the filtration markers in their prediction of risk. These studies adjusted for many of the factors that we identified, and therefore the findings in these studies may reflect residual confounding due to errors in measurements of these factors or confounding by other unmeasured and unknown factors.”
The associations of cystatin C with non-GFR determinants that we report in this study, although are significant, are relatively small. The observed effect sizes reflects the average levels within the current study population and are likely to be larger in individual patients or in populations with selected clinical conditions, such as the obese, the chronically ill, or those with high levels of inflammation. Larger-than-average effects of non-GFR determinants could lead to important errors in GFR estimation from serum cystatin C in individual patients and systematic bias in selected populations. Rule and colleagues showed that bias of cystatin C based estimating equation differed among patients with native kidney disease, kidney transplant recipients, and potential kidney donors, consistent with systematic differences in non-GFR determinants of cystatin C among these populations [21]. Similarly, variation in non-GFR determinants would explain the observed imprecision of cystatin C based estimating equations even in relatively homogenous populations with known CKD [4].
Clinicians can use knowledge of non-GFR determinants of cystatin C to assist in interpretation of serum levels and GFR estimates based on cystatin C. This is analogous to the interpretation of GFR estimates based on knowledge of non-GFR determinants of serum creatinine. For example, because the relationship between serum creatinine and muscle mass is understood, an attentive clinician can interpret the level of the serum creatinine or the estimated GFR differently in a patient with vs. without muscle wasting, even though there is no term for muscle wasting in the GFR estimating equation. As such, our data suggest that the value of a cystatin C level should be interpreted with knowledge of several factors, such as obesity, inflammation, and diabetes.
Strengths of the study include the large study population composed of 3,418 patients with CKD in three research studies and one clinical population; measurement of cystatin C in a single laboratory; calibration of the creatinine assays in each study to standardized values; careful measurement of GFR using urinary clearances of exogenous filtration markers; use of analytical techniques that incorporated measurement error in GFR; and the large number of potential predictors available.
There are also several limitations. First, we have shown results of associations of single variables, adjusted only for GFR, age, sex and race, rather than a full multivariable adjustment. Second, incomplete adjustment for measurement error and biological variation in GFR may lead to residual confounding between variables associated with GFR and cystatin C, which could explain the association of serum cystatin C and creatinine with each other and with serum urea nitrogen even after adjustment for GFR. We estimated the level of measurement error in only two of the studies: MDRD Study and the African American Study of Kidney Disease (AASK). Possibly, the measurement error may be different in the other two studies included in the pooled database. Third, the study population was restricted to patients with native kidney disease and without serious co-morbid conditions that would exclude them from participating in clinical trials. Fourth, study participants were likely selected in part on the basis of previous creatinine values, which can lead to a bias in the estimated regression coefficients for creatinine and cystatin, since cystatin C remained associated with creatinine after controlling for GFR. Nevertheless, all studies were of CKD populations and previous studies have suggested that for creatinine based estimating equations differences among subgroups based on demographic characteristics are minimal for populations with native kidney disease [1, 22]. Finally, the data are pooled from multiple studies and there is variation among studies in some of the observed relationships. This variation may due to population differences or to differences in how the covariates were ascertained or measured.
In summary, while cystatin has promise as an alternative filtration marker to creatinine, like creatinine, cystatin C is affected by factors other than GFR that must be considered in interpretation of its serum level in clinical practice. The best GFR estimate may be the combination or sequential use of both filtration markers, with the expectation that the use of both markers minimizes the impact of physiological processes other than GFR that affect each marker. Further research is required to better understand the non-GFR determinants of cystatin C across a broader range of populations and to define the use of both creatinine and cystatin C in GFR estimation.
Methods
Sources of Data
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) is a research group formed to develop and validate improved estimating equations for GFR by pooling data from research studies and clinical populations (hereafter referred to as “studies”) [4]. The current analysis is based on a pooled dataset of individual patient data from four studies where frozen samples were available for assay of cystatin C: Modification of Diet in Renal Disease (MDRD) Study, African American Study of Kidney Disease and Hypertension (AASK), Collaborative Study Group (CSG) Study [2, 3, 5, 23-25] and the NephroTest cohort, a clinical population in Paris, France [26] (Table 1). Data from the baseline examination for these studies was used.
Measurements
GFR was measured as four period urinary clearance of 125I-iothalamate in the MDRD Study, AASK and CSG and as five period urinary clearance of 51Cr-EDTA in NephroTest and is reported adjusted for body surface area (Table 1). Comparisons of 125I-iothalamate and 51Cr-EDTA clearances to urinary clearance of inulin, the reference standard for GFR measurements, demonstrated high correlation [26-28]. Samples were assayed for cystatin C with a particle-enhanced immuno-nephelometric assay (N Latex Cystatin C, Dade Behring, IL) in samples stored at −80oC. The inter- and intra-assay coefficients of variation (CV) for cystatin C were 3.2¬4.4%, and 2.0-3.0%, respectively. Stability in serum stored at −80oC has been demonstrated[29]. Serum creatinine assays were calibrated to standardized serum creatinine values at the Cleveland Clinic Research Laboratory (CCRL) [1, 22]. The results of the calibration procedures have been previously described [4, 30].
Variables
A potential list of variables that are hypothesized to affect serum cystatin C or creatinine by mechanisms other than GFR (non-GFR determinants) was developed from review of the literature and from physiologic and clinical considerations. Variables included in the analysis included measures of muscle mass and body size (age, sex, race, height, weight, body mass index, urine creatinine), cardiovascular disease risk factors (hypertension, diabetes, systolic blood pressure, diastolic blood pressure, glucose, total cholesterol), measures related to severity of kidney disease (hemoglobin, serum levels of sodium, potassium, bicarbonate, calcium, phosphate and urine protein), measures of inflammation (albumin, C-reactive protein, white blood cell count), and measures of dietary intake (urine phosphate, urine urea nitrogen). Other endogenous filtration markers were also considered as covariates (serum cystatin C, creatinine and urea nitrogen). Measurement methods and definitions for each of the categorical variables have been described in the individual reports of these studies [2, 3, 23-26, 31].
Statistical analyses
Summary statistics and scatter plots were used in initial exploratory analyses to investigate the relationships between candidate variables and the levels of serum cystatin C and serum creatinine in the overall dataset. Continuous variables were transformed so as to create a linear relationship with log-transformed cystatin C and creatinine in bivariate analyses. Sex and race were expressed as binary factors indicating presence or absence of female sex and black race, respectively. Diabetes and hypertension were expressed as present or absent.
The relationships of cystatin C and creatinine with the predictor variables were investigated by first performing separate linear regressions to relate log transformed cystatin C and creatinine to each individual predictor variable after controlling for log transformed GFR, study, and the interaction between GFR and study. We repeated these analyses using errors-in¬variables regression analysis to incorporate measurement in GFR into these models[32]. A measurement error variance of 0.015 was assumed for log transformed GFR based on analyses of the longitudinal variability in log transformed baseline GFR measurements spaced an average of approximately 3 months apart in the MDRD Study and 0.6 months apart in the AASK Study[33]. Sensitivity analyses were performed with different levels of measurement error ranging from 0 to 0.020. Results were consistent for measurement error variance ranging from 0.010 to 0.020, which covers the plausible range. This errors-in-variables regression was repeated after adding terms for age, female sex, and black race to the model for each predictor variable.
The relative strengths of relationships of the predictor variables with log cystatin C and log creatinine were compared and graphically displayed in scatter plots. For continuous variables, regression coefficients were standardized to indicate the geometric mean percent difference in either serum cystatin C or creatinine associated with a 1.0 interquartile range (IQR) higher value for the predictor variable; for dichotomous predictor variables the regression coefficients are expressed as the geometric mean percent difference in the response variable associated with presence vs. absence of the predictor. The statistical significance of the difference between the coefficients for the predictor variables with log cystatin C and log serum creatinine was determined by applying the sign test to compare 800 bootstrap samples with p-value of <0.001 indicating a significant difference between the coefficients.
Analyses were computed using R (Version 2, Free Software Foundation, Inc., Boston, MA) and SAS software (version, 9.1, Cary, NC).
Acknowledgements
We would also like to acknowledge Michael Dowd for his contributions in development of the initial analytical approach described here.
This research was supported by the following grants:
UO1 DK 053869, UO1 DK 067651 and UO1 DK 35073.
Role of the Funding Source
CKD-EPI is funded by grants from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) as part of a cooperative agreement in which the NIDDK has substantial involvement in the design of the study and the collection, analysis, and interpretation of the data. The NIDDK was not required to approve publication of the finished manuscript. The institutional review boards of all participating institutions approved the study.
Footnotes
Disclosure
The authors of this manuscript have no potential conflicts of interest to disclose.
Additional investigators and research staff of CKD-EPI include:
Tufts Medical Center: Robert D. Bruce III, BA; Cleveland Clinic: Frederick Van Lente, PhD; Johns Hopkins University: Jane Manzi, PhD, Brad Astor, PhD, MPH, Elizabeth Selvin, PhD, MPH; University of Pennsylvania: Harold I. Feldman, MD, MSCE, J. Richard Landis, PhD; National Institute of Diabetes and Digestive and Kidney Diseases: Paul W. Eggers, PhD, Robert Star, MD.
Collaborators contributing data for this study:
Modification of Diet in Renal Disease Study: Gerald Beck, PhD
Collaborative Study Group: Captopril in Diabetic Nephropathy Study: Roger Rodby, MD, Richard Rohde, BS
African American Study of Kidney Disease and Hypertension: Gabriel Contreras, MD, MPH, Julia B. Lewis, MD
NephroTest: Jerôme Rossert, MD, PhD; Marc Froissart, MD, PhD
Statistical evaluation was performed by the following authors:
Christopher H. Schmid, PhD, Tom Greene, PhD, Liang Li, PhD, Marshall Joffe, PhD
The results of this research were presented in abstract form at the Annual Meeting of the American Society of Nephrology in San Francisco, CA, November 3, 2007.
References
- 1.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function - measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madero M, Sarnak MJ, Stevens LA. Serum cystain C as a marker of glomerular filtration rate. Curr Opin Neph Hyper. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 6.Kottgen A, Selvin E, Stevens LA, et al. Serum cystatin C in the U.S.: The third National Health and Nutrition Examination Survey (NHANES III) Am J Kid Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 8.Knight E, Verhave J, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald J, Marcora S, Jibani M, et al. A GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;45:712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Risch L, Herklotz R, Blumberg A, et al. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059. [PubMed] [Google Scholar]
- 11.Grubb AO. Cystatin C-properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasen E, Isoaho R, Mattila K, et al. Serum cystatin C in the aged: relationships with health status. Am J Kidney Dis. 2003;42:36–43. doi: 10.1016/s0272-6386(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 13.Thaczyk M, Nowicki M, Lukamowicz J. Increased cystatin C concentration in urine of nephrotic children. Pediatr Nephrol. 2004;19:1278–1280. doi: 10.1007/s00467-004-1566-1. [DOI] [PubMed] [Google Scholar]
- 14.Taleb S, Cancello R, Clément K, et al. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology. 2006;147:4940–4945. doi: 10.1210/en.2006-0386. [DOI] [PubMed] [Google Scholar]
- 15.Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–349. doi: 10.1007/BF03347201. [DOI] [PubMed] [Google Scholar]
- 16.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 19.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Levey AS. Chronic kidney disease in the elderly --How to assess risk? [Editorial] N Engl J Med. 2005;352:2122–2124. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 21.Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtraton rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Wright JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK Trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JB, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African-Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38:744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 25.Lewis EJ, Kunsicker LG, Bain RP, et al. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 26.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 27.Israelit AH, Long DL, White MG, et al. Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 28.Perrone R, Steinman T, Beck G, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-Iothalamate, 169Yb¬DTPA, 99mTc-DTPA, and inulin. Am J Kidney Dis. 1990;26:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 29.Erlandsen E, Randers E, Kristensen J. Evaluation of the Dade Behring N latex Cystatin C assay on the Dade Behring nephelometer II system. Scand J Clin Lab Invest. 1999;59:1–9. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, et al. Expressing the MDRD study equation for estimating GFR with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Greene T, Beck G, et al. Dietary Protein Restriction and the Progression of Chronic Renal Disease: What Have All of the Results of the MDRD Study Shown? J Am Soc Nephrol. 1999;10:2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 32.Fuller W. Measurement Error Models. Wiley; New York: 1984. Vector Explanatory Variables; pp. 100–184. [Google Scholar]
- 33.Li L, Greene T. Varying coefficients model with measurement error. Biometrics. 2008;64:519–526. doi: 10.1111/j.1541-0420.2007.00921.x. [DOI] [PubMed] [Google Scholar]


