Abstract
Strong associations exist between sleep disordered breathing (SDB) and both heart failure (HF) and atrial fibrillation (AF). Burgeoning epidemics of obesity, SDB, HF, and AF make these conditions priorities for health-care policymakers. Two observational studies now suggest outcome benefits from screening and treating for SDB in AF and HF.
Sleep disordered breathing (SDB) encompasses obstructive sleep apnoea (OSA), central sleep apnoea (CSA), obesity hypoventilation syndrome, and snoring. A diagnosis of SDB is usually made via the gold-standard test of attended polysomnography and quantified by the number of episodes of cessation (apnoea) or reduction (hypopnoeas) of airflow, lasting ≥10 s per hour of sleep.1 Investigators in numerous studies have reported an association between SDB and cardiovascular diseases, including atrial fibrillation (AF), coronary artery disease, heart failure (HF), hypertension, myocardial infarction, and sudden cardiac death. Direct causality is difficult to ascertain owing to confounders, of which the most important is obesity. Nevertheless, after adjusting for these confounders, an independent association between SDB and both AF and HF has been consistently reported. Data from two new observational studies now suggest that screening and treating for SDB might improve outcomes in patients with AF or HF.
AF is the most common cardiac arrhythmia, associated with substantial morbidity, mortality, and cost burdens. AF-related hospital admissions (the single most important determinant of cost) are twice as high as those in the general population.2 HF is also a substantial public-health problem with an increasing prevalence, in part because of reductions in mortality from coronary artery disease and myocardial infarction. Many patients with HF have debilitating symptoms, with high rates of hospital admission and mortality.3 An economic and public-health imperative for researchers and health governance bodies is to reduce morbidity, hospital readmissions, and mortality associated with AF and HF. The high prevalence of SDB in each of these conditions makes its diagnosis and treatment a potentially important strategy for improving outcomes and reducing cost of care.
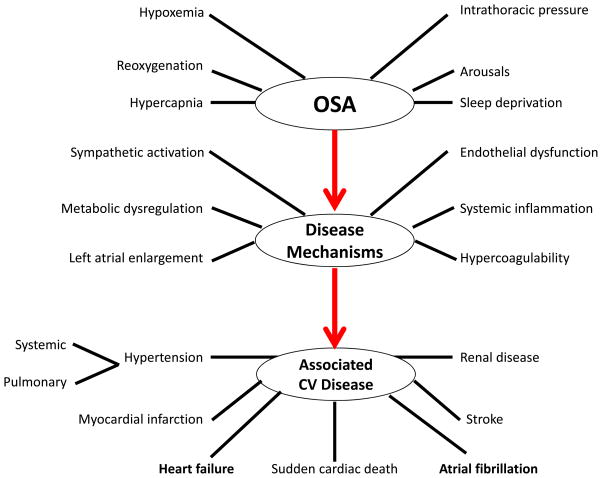
OSA is recognized as an associated factor in the pathogenesis of AF and features in both European and US guidelines. The main mechanisms by which AF might be initiated and maintained include autonomic responses to apnoea, hypoxaemia, hypercapnia, systemic inflammation, increased left ventricular afterload and atrial stretch from negative intrathoracic pressures (Figure 1).1 OSA might be a modifiable risk factor for attenuating paroxysms of AF, progression from paroxysmal AF (<7 days) to persistent AF (≥7 days, but <12 months) to long-standing persistent AF (≥12 months), recurrent AF after catheter ablation or cardioversion, the need for antiarrhythmic drugs, and stroke risk.1
Figure 1.
Schematic outlining proposed pathophysiological components of OSA, activation of cardiovascular disease mechanisms, and consequent development of established cardiovascular disease. Modified from Somers VK et al, with permission from Lippincott Williams & Wilkins. Copyright 2008, American Heart Association.1
1. Somers, V.K. et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118, 1080–111 (2008).
ORBIT-AF4 was established to assess outcomes in the management of AF at a national level in the USA. A total of 10,132 patients with documented AF were enrolled into this prospective, multicentre, outpatient-based registry between June 2010 and August 2011. A 2-year analysis of outcomes showed that 18.2% (n = 1,841) had AF and OSA.4 These patients had more severe or debilitating symptoms, higher risks of hospital admission, were more likely to have a history of cardioversion, more frequently took an antiarrhythmic drug, and were more likely to be receiving an anticoagulant drug despite having similar CHADS2 scores to patients with AF, but no OSA. No significant difference in major bleeding events was detected, nor was an increased risk of death, cardiovascular-related death, myocardial infarction, stroke or transient ischaemic attack, or AF progression reported. However, patients receiving continuous positive airway pressure (CPAP) therapy for OSA had a reduced likelihood of progressing to more permanent forms of AF.
This study provides important insights into the link between AF and OSA, but the lack of evidence for an increased risk of death is surprising. Whether a mortality effect might emerge in the longer term (5–10 years) remains to be determined. The investigators acknowledge the limitations of selection bias, reporting bias, and unmeasured confounding. Categorization of patients with OSA was performed using physician reports and medical records. The investigators did not have access to the sleep studies and, therefore, lacked information on types or severity of SDB. Treatment concordance of CPAP was based on self-reporting by patients, with no objective usage data available. Although the reported prevalence of OSA in the ORBIT-AF registry was high (18%), this percentage is considerably lower than that reported in other studies (30–80%).5 This disparity indicates an important possible confounder, namely the likelihood of undiagnosed SDB in those assumed to be free from OSA. Patients with AF and OSA do not have characteristic daytime somnolence.6 Furthermore, given that about one-third of the patients considered to be free from OSA also had HF, a sizable proportion of these patients is likely to have had undiagnosed OSA or CSA. This limitation would cause any differences between patients with and without OSA to be underestimated.
The high prevalence of undiagnosed SDB, even in an inpatient population of patients with HF in a tertiary-care referral centre, is highlighted by Khayat and colleagues.7 Owing to the overlap of symptoms (such as fatigue and paroxysmal nocturnal dyspnoea), SDB is often overlooked and sometimes mistaken as refractory HF. Recognising SDB in patients with HF is recommended in both ESC and ACCF/AHAUSA guidelines for HF, with the latter specifically making a class IIa recommendation for the use of CPAP to treat SDB in HF. Khayat et al. report on a prospective study in which screening polygraphy was used to detect SDB in all patients admitted with acute HF and reduced ejection fraction (<45%).7 The primary outcome was an in-hospital diagnosis of SDB and all-cause mortality after discharge from hospital. From 1,117 patients admitted over a 4-year period, 31% had CSA and 47% had OSA, with the remaining 22% having no or minimal SDB. Both OSA and CSA were independently associated with increased mortality (HR 1.61, 95% CI 1.1–2.4, P =0.02 and HR 1.53, 95% CI 1.1–2.2, P = 0.02, respectively).7 Despite the Kaplan–Meier survival curves indicating a reduction in mortality in patients who received treatment for OSA or CSA, this trend did not reach significance.
This prospective, observational study of patients with acute HF included all-comers with HF and reduced ejection fraction, minimizing the referral bias inherent to studies based on sleep clinic cohorts. Importantly, the study was designed to assess screening in acute HF and powered to detect a difference in mortality. The polygraphy device used (Stardust II; Philips, The Netherlands) has been validated and has a good positive predictive value, but is prone to recording failures and overestimation of the apnoea–hypopnoea index, which is a limitation.7 This group previously reported that SDB was linked to increased rates of rehospitalization in patients with HF after discharge.8 If treating SDB lowers rehospitalization, mortality, or both in patients with HF, this strategy would be valuable for lowering costs and improving outcomes in this patient population.
Both studies discussed in this News & Views article reinforce the already compelling retrospective and observational data implicating SDB in the pathophysiology, morbidity, and mortality associated with both AF and HF. However, definitive evidence of a reduction in hospitalizations, cardiovascular events, and mortality resulting from treatment of SDB in either of these conditions requires the completion of long-term, randomized interventional studies. No such data are currently available in patients with AF. The CANPAP trial,9 the only study of treating CSA (with CPAP) in patients with HF, showed no reduction in mortality. This result is perhaps because CPAP might not correct CSA, and adaptive servoventilation is currently favoured. Results from the SERVE-HF trial,10 a prospective, randomized trial of adaptive servoventilation in patients with CSA and HF with reduced ejection fraction are awaited. Proof of benefit, based on randomized controlled trials, are a prerequisite for diagnosis and treatment of OSA or CSA to become part of the standard of care for patients with AF, HF, or both.
Acknowledgments
V.K.S. is supported by NIH grant HL65176.
Footnotes
Competing interests
V.K.S. declares that he has received grant support from Philips Respironics Foundation (gift to Mayo Foundation); is a consultant for GlaxoSmithKline, ResMed, Respicardia, Rhonda Grey, PricewaterhouseCoopers, and U-Health; and is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. C.A.A.C. declares no competing interests.
References
- 1.Somers VK, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 2.Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace. 2011;13:1375–1385. doi: 10.1093/europace/eur194. [DOI] [PubMed] [Google Scholar]
- 3.Ziaeian B, Sharma PP, Yu TC, Johnson KW, Fonarow GC. Factors associated with variations in hospital expenditures for acute heart failure in the United States. Am Heart J. 2015;169:282–289.e15. doi: 10.1016/j.ahj.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmqvist F, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. doi: 10.1016/j.ahj.2014.12.024. http://dx.doi.org/10.1016/j.ahj.2014.12.024. [DOI] [PubMed]
- 5.Braga B, et al. Sleep-disordered breathing and chronic atrial fibrillation. Sleep Med. 2009;10:212–216. doi: 10.1016/j.sleep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Albuquerque FN, et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141:967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khayat R, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. doi: 10.1093/eurheartj/ehu522. http://dx.doi.org/10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed]
- 8.Khayat R, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18:534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley TD, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 10.Cowie MR, et al. Rationale and design of the SERVE-HF study: treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive servo-ventilation in patients with chronic heart failure. Eur J Heart Fail. 2013;15:937–943. doi: 10.1093/eurjhf/hft051. [DOI] [PMC free article] [PubMed] [Google Scholar]