Abstract
Ruta, which belongs to tribe Ruteae, is the type genus of the subfamily Rutoideae and the family Rutaceae. Molecular systematic studies have shown that the genera in Ruteae are closer related to Aurantioideae than to most other genera of Rutoideae, some of the genera traditionally placed in Ruteae have been shown to be nested within the Aurantioideae clade, but the diagnostic characters for determining new patterns in the relationship are poor. In this study, we investigated the floral development of Boenninghausenia in Ruteae (sensu stricto), Haplophyllum in the basal position of Aurantioideae and Murraya in traditional Aurantioideae using scanning electron microscopy. The androecium of Boenninghausenia is obdiplostemony. As androecia in other genera within Ruteae (s.s.) are also obdiplostemonous, reconstruction of the ancestral state indicates that obdiplostemony is an ancestral character in this clade. Because the androecia of Haplophyllum and Murraya are also obdiplostemonous, obdiplostemony is also an ancestral character in Aurantioideae clade. The ancestral state reconstruction indicates this character can serve as a synapomorphy of the Ruteae-Aurantioideae clade. The results of our work also shed light on the evolution of the androecium in Rutaceae, as the obdiplostemony of this group is clearly derived from haplostemony in the ancestral genera in Rutaceae and has develop into polyandry by increasing antepetalous stamens.
Introduction
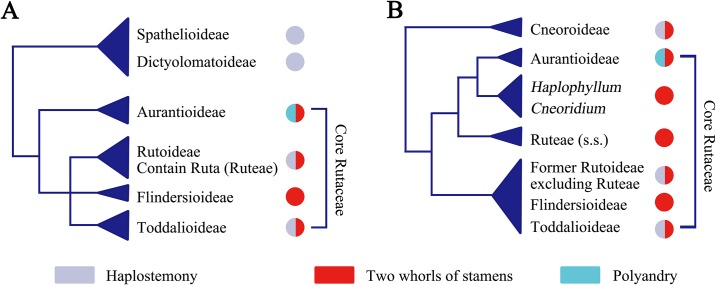
The Rutaceae family, which is well known due to its economic importance (Citrus), is a large family including 154 genera and approximately 2100 species [1]. The distribution of this family is nearly cosmopolitan, with species in both the Old World and New World, but it is mainly tropical and subtropical [2–5]. An important systematic treatment of the group was conducted by Engler [6,7], who recognized seven subfamilies. Along with the modern systematics of this family, many of the subfamilies are controversial [8,9]. Recently, great progress has been made in the infra-familial phylogeny through molecular systematic studies [2–5,10–14]. The consensus consists of traditional subfamilies that are not natural groups, and Rutoideae, Toddalioideae, Flindersioideae and Aurantioideae form a ‘core Rutaceae’ clade [1]. At the base of ‘core Rutaceae’, former member of Spathelioideae and Dictyolomatoideae formed a clade under the name of Cneoroideae (subfamily Cneoroideae), sister to ‘core Rutaceae’. This clade is clearly the most early-diverging within Rutaceae [15,16]. In the core Rutaceae, Rutoideae and Toddalioideae are merged together, whereas the genera within Flindersioideae are dispersed in both of the clades. Under this systematic framework, the position of Ruta seems unusual (Fig 1). As the type genus of Rutoideae, Ruta is closely linked to Aurantioideae rather than Rutoideae [2,4,13,17].
Fig 1. Comparing the traditional system of Rutaceae with current molecular phylogeny with general androecium characters mapping in the tree: A: Traditional system of Rutaceae (mostly based on Engler’s treatment [6,7]). B: Summary of the major clades of Rutaceae based on published molecular phylogenies [2,4,11,14,17].
Ruta and related genera are grouped in the tribe Ruteae. The Englerian tribe Ruteae was mainly defined based on genera with the same life form, habits and distributions. Recent molecular phylogenetic studies have shown that Ruteae has a closer relationship with Aurantioideae than with other Rutoideae groups [13,14,17]. If this relationship is accurate, it may result in important changes in subfamily nomenclature. Despite the definitive molecular systematics results, the morphological foundation for new patterns of relationships is currently poor. Although Ruteae is noteworthy for the different growth habits of its members compared with the other genera within Rutoideae, diagnostic characters are lacking regarding its affinity with Aurantioideae.
It is no exaggeration to state that morphological data, based on the external form of organisms, have been and still are the most commonly used type of data in plant classification [18]. Among such data, the androecium of flowers is obviously of high taxonomic value within angiosperms, and the anthers exhibit many different sizes, shapes, and numbers [19]. The family Rutaceae bears varied types of androecia, with haplostemonous, diplostemonous, obdiplostemonous and polyandrous androecia present in different genera [1,20]. The androecia of Ruteae are heterogeneous among the Old World Rutoideae, exhibiting two whorls of stamens. However, the existence of two whorls of stamens is not rare in Rutoideae, whose members are mostly distributed in Africa, Australia and South America. All except one genus (Harrisonia) of the early-diverging subfamily Cneoroideae are haplostemonous.[1]. Meanwhile, in the genera of Old World Rutoideae, there are some other genera with haplostemonous androecia, such as Zanthoxylum, Phellodendron, Tetradium and Toddalia. These genera represent the extant genera in ‘core Rutaceae’ with the earliest fossil record [21]. Therefore, determining the evolutionary trends involving these androecia will be useful for clarifying the relationships among the different genera.
In past decades, developmental data have been used taxonomically to some extent as a corollary to comparisons of mature floral structures [22–25]. This method applies to revealing homologies among mature structures [26]. Stebbins [27,28] and Endress [23] have rightly noted the great value of developmental data for interpreting evolutionary relationships and trends at higher levels of the hierarchy in angiosperms. Many studies have examined floral development and make contributions of systematic relevance for different taxa [29–34]. Within Ruteae, the floral development of Psilopeganum sinense and Ruta graveolens has been studied in detail [35]. Both genera possess an obdiplostemonous androecium. Compared with the haplostemonous androecium found in Phellodendron [36] and Tetradium [37], obdiplostemony in these genera is a derived character [33]. Aurantioideae also includes genera with two whorls of stamens, such as Clausena and Murraya. According to current molecular systematics, these genera represent the ancestral clade in Aurantioideae [38]. Therefore, determining the mode of androecium development in these genera is important for understanding the relationship between Ruteae and Aurantioideae.
There are seven genera within Ruteae: Ruta, Psilopeganum, Haplophyllum, Boenninghausenia, Dictamnus, Thamnosma and Cneoridium. Recent phylogenetic studies showed that Ruteae is not monophyletic [39,40]. Ruta, Psilopeganum, Thamnosma and Boenninghausenia form a clade [17] that we name Ruteae sensu stricto here. The other two genera, Haplophyllum and Cneoridium, are placed in the basal position of the Aurantioideae clade [39,40]. Recently, Dictamnus was excluded from Ruteae based on chemosystematics, anatomy and molecular systematics [13,39–41], and the floral development of Ruta and Psilopeganum has been studied. Therefore, we chose Boenninghausenia albiflora to represent the Ruteae (s.s.); Haplophyllum dauricum to represent the basal Aurantioideae group and Murraya exotica to represent the genera in traditional Aurantioideae with two whorls of stamens. The floral developments of these genera were studied. The aims of this study were to clarify the mode of androecium development in these genera through floral ontogeny; to reconstruct the ancestral character of the androecium in Ruteae and discuss the evolutionary trend of the androecium in Rutaceae; and finally, to clarify the relationships between Ruteae and Aurantioideae.
Materials and Methods
Taxon Sampling
Haplophyllum dauricum plants were collected from the Inner Mongolia Grassland Ecosystem Research Station (Wei 10117) of the Chinese Academy of Sciences. Boenninghausenia albiflora plants were collected on West Mountain in Kunming (Wei 12097). Murraya paniculata plants were collected from the Beijing Botanic Garden (Wei 10023), Chinese Academy of Sciences. Voucher specimens were deposited in the Chinese National Herbarium (PE).
Floral Development Analysis
The plant material was fixed in formalin—acetic acid—alcohol (FAA). The FAA-fixed materials were dehydrated in an ethanol series. Flower buds were dissected and examined in 95% ethanol using a dissecting microscope, then transferred through an ethanol-iso-amyl acetate series, critical-point dried, mounted on a metal stub and sputter-coated with gold/palladium. The flower buds were observed, and micrographs taken with a Hitachi S-4800 scanning electron microscope (SEM) (Hitachi, Tokyo, Japan) at 10 kV.
Ancestral State Reconstruction
To trace the evolutionary history of the androecium, we conducted character-state reconstruction in Mesquite v2.75 (http://mesquiteproject.org) [42]. The topology of the sampled species was determined according to Groppo et al. [4], Salvo et al. [13], Poon et al. [11], Morton et al. [17], and Manafzadeh et al. [43]. According to androecium state of species, we coded four states: 1 for haplostemony, 2 for diplostemony, 3 for obdiplostemony and 4 for polyandry. Because of no androecium data of Thamnosma and Cneoridium, we coded them as missing. The evolution of the androecium was reconstructed using a maximum likelihood approach with the Markov k-state one-parameter (MK1) model.
Results
Floral Initiation and Development in Haplophyllum dauricum
The actinomorphic flowers are grouped in loose cymes. The flowers are usually pentamerous, with ten stamens arranged in two whorls. In the mature flower, the 10 stamens surround the gynoecium (Fig 2B). The gynoecium is usually 3-carpellate, but the merosity ranges from two to five.
Fig 2. Haplophyllum dauricum (A, B), Boenninghausenia albiflora (C) and Murraya exotica (D).
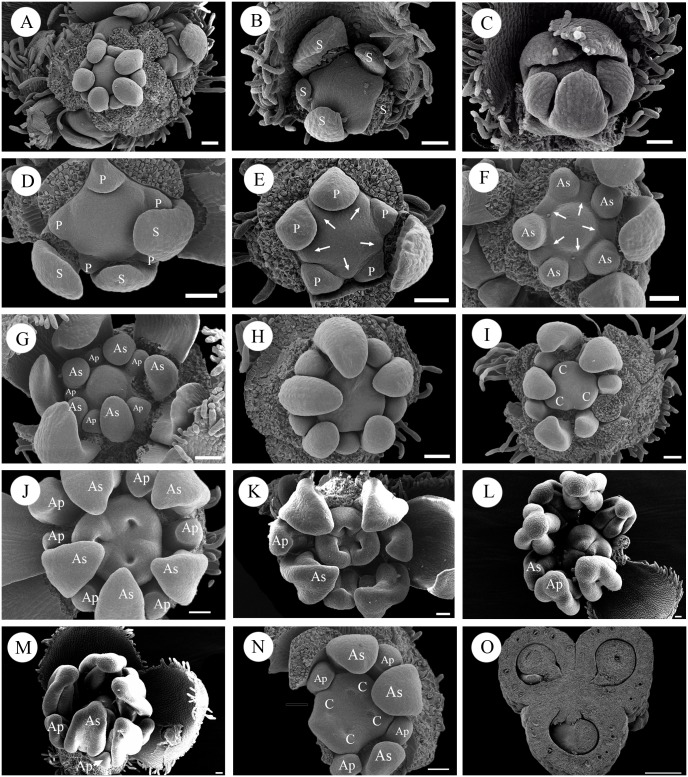
The flower primordia and related bracts and bracteoles arise helically and acropetally on the main inflorescence branch (Fig 3A). The bract and bracteoles are easily distinguished from the sepals and petals by the trichomes along their margins (Fig 3B). The first sepal primordium emerges on the abaxial side of the flower after the two lateral bracteoles become visible, followed by others in rapid sequence (Fig 3B). The calyx is initiated in a classical quincuncial spiral sequence (Fig 3C). During the development of the calyx, the floral apex increases in height and flattens, and the five petal primordia appear in the antesepalous position in very close succession (Fig 3D). Initiation may begin on the abaxial side, as the primordia are slightly larger in the abaxial area.
Fig 3. Floral development in Haplophyllum dauricum.
A: Apical view of a young inflorescence with lateral subunits. B, C: Apical view of a young floral bud showing the initiation sequence of the sepals. D: Initiation of petals. E: Initiation of antesepalous stamens. Arrows indicate the primordia. F: Initiation of antepetalous stamens. Arrows indicate the primordia. G: Fully initiated androecium showing the different sizes of antesepalous and antepetalous stamens. H: Apical view of a floral bud in the middle stage. I: Initiation of carpels. J: Development of the three carpels (note: in rare cases, as shown here, an additional stamen may be formed). K: Later stage of carpel development. L: Post-genital union of three carpels. M: Later stage of the floral bud, showing the peripheral position of the antepetalous stamens. N: Apical view of a floral bud with a tetramerous mutation showing the antepetalous position of the carpels. O: Transection of the mature ovary. Abbreviations: S, sepal; P, petal; As, antesepalous stamen; Ap, antepetalous stamen; C, carpel. Scale bars = 40 μm.
Immediately after initiation of the petals, the stamen primordia begin to emerge. The first whorl of the stamen primordia arises inside the petal whorl in an antesepalous position (arrows in Fig 3E). The antepetalous stamens arise acropetally after the antesepalous stamens (arrows in Fig 3F). At this stage, the development of the androecium is diplostemonous (Fig 3G). The antesepalous stamens grow rapidly, whereas the growth of the antepetalous stamens is retarded. As a result, the two whorls of stamens are clearly different in size (Fig 3H). In late development, each stamen enlarges laterally and differentiates into an anther and filament and the median and transverse furrows divide the anther into two thecae and four pollen sacs. After stamen initiation, the floral apex becomes flat and the carpel primordia emerge around its margin (Fig 3H). As the antesepalous stamens grow rapidly and occupy more space on the floral apex, the three carpel primordia appear in an antepetalous position (Fig 3I). Three furrows become visible, and the carpels soon rise above the floral apex (Fig 3J). Along with the development of the carpels, the antepetalous stamens shift to the periphery (Fig 3K–3M). In the mature flower, the carpels become united (Fig 3L) and further differentiate to form the style and stigma. In some rare flower buds with mutations, while all of the floral organs are isomerous and tetramerous (flowers with four carpels and eight stamens in two whorls), the developmental succession is mostly the same, with the carpels clearly arising in the antepetalous position (Fig 3N).
Floral Initiation and Development in Boenninghausenia albiflora
The actinomorphic flowers of Boenninghausenia albiflora are grouped in monochasium. The floral merism is strictly tetramerous (Fig 2C). The eight stamens are grouped into two whorls, and the antesepalous stamens are longer than the antepetalous stamens.
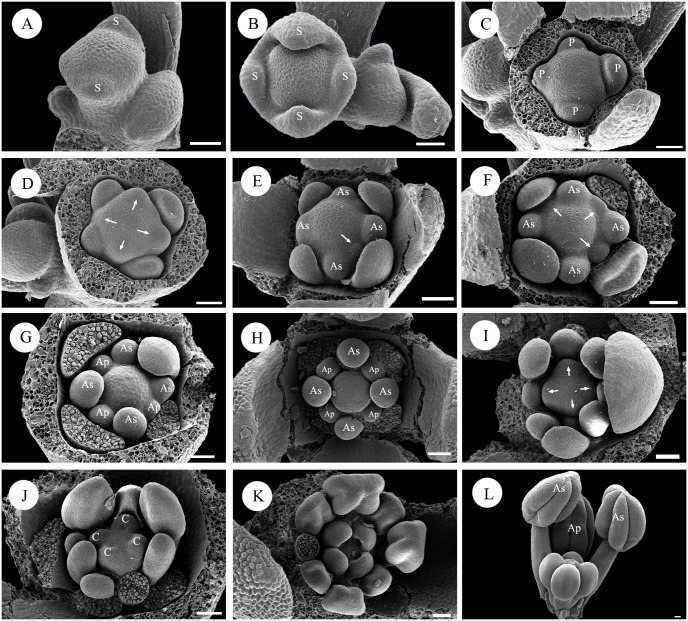
The inflorescence apex becomes a floral primordium, and two bracteoles arise on the lateral side (Fig 4A). The secondary floral primordium usually arises on the axil of the bracteoles (Fig 4B). The sepal primordia occur in a ring, consisting of the four sepal lobes. The four sepal lobes arise out of the ring in a later stage, and the adaxial and abaxial lobes are slightly larger than the lateral lobes (Fig 4B). After the appearance of the sepals, the petal primordia begin to arise. The first petal primordium usually occurs on the abaxial side, and the other three petal primordia arise in very close succession either clockwise or counter-clockwise (Fig 4C).
Fig 4. Floral development in Boenninghausenia albiflora.
A: Apical view of a young flower located terminally on the inflorescence apex. B: Initiation of sepals. C: Initiation of petals. The different petal sizes indicate the initiation sequence. D: Initiation of antesepalous stamens. Arrows indicate the primordia. E, F: Sequential initiation of antepetalous stamens. Arrows indicate the primordia. G: Fully initiated androecium showing different sizes between antesepalous and antepetalous stamens. H: Apical view of a floral bud in the middle stage. I: Initiation of carpels. Arrows indicate the primordia. J: Development of the four carpels at the antepetalous position. K: Later stage of carpel development. L: Part of a nearly mature flower showing the position and different lengths between antesepalous and antepetalous stamens. Abbreviations: S, sepal; P, petal; As, antesepalous stamen; Ap, antepetalous stamen; C, carpel. Scale bars = 40μm.
The development of the androecium is nearly the same as in Haplophyllum dauricum. Two whorls of stamens arise in a regular order, with the antesepalous stamens arising first (arrows in Fig 4D). The antepetalous stamens arise at the same level as the antesepalous stamens (arrows in Fig 4E and 4F). The antesepalous stamens are larger than the antepetalous stamens throughout development (Fig 4G). In late development, each stamen differentiates into an anther and filament. After stamen initiation, the floral apex becomes flat, with four corners (Fig 4H). Four independent carpel primordia emerge simultaneously at the antepetalous positions (Fig 4I and 4J). The four carpels become post-genitally united and further differentiate to form the style and stigma (Fig 4K and 4L).
Floral Initiation and Development in Murraya exotica
The actinomorphic flowers of Murraya exotica are grouped in dichasial cymes. The flowers are pentamerous, and the gynoecium is composed of two carpels. The ten stamens, of unequal length, are arranged in two whorls and surround the gynoecium (Fig 2D).
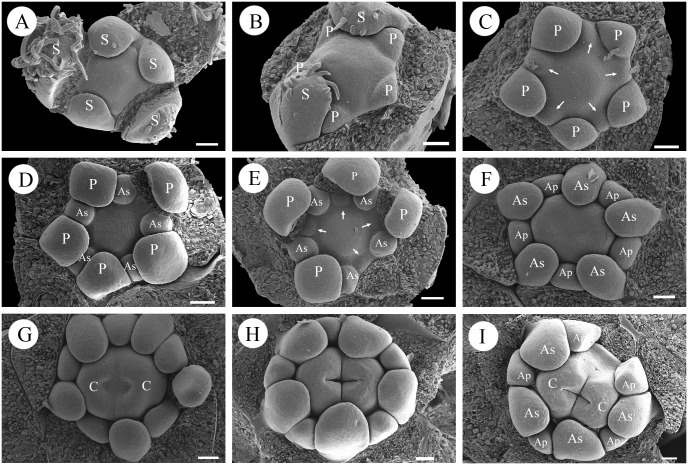
Floral development is similar to that of Haplophyllum dauricum and Boenninghausenia albiflora. The floral primordia are subtended by their respective bracts. The sepal primordia begin to emerge after two bracteoles become visible. The first sepal primordium emerges on the abaxial side, followed by the adaxial and lateral sepals (Fig 5A). The initiation of the calyx follows a classical 2/5 quincuncial spiral sequence. The surface of the sepals is covered by trichomes (Fig 5A). The development of the petals begins on the abaxial side in very close succession immediately after sepal initiation (Fig 5B).
Fig 5. Floral development in Murraya exotica.
A: Apical view of a young floral bud showing the initiation sequence of the sepals. B: Initiation of petals. C: Initiation of antesepalous stamens. Arrows indicate the primordia. D: Fully initiated antesepalous stamens. E: Initiation of antepetalous stamens. Arrows indicate the primordia. F: Fully initiated androecium showing the different sizes of antesepalous and antepetalous stamens. G: Initiation of carpels. H: Development of the two carpels. I: Apical view of a floral bud in the late stage. Abbreviations: S, sepal; P, petal; As, antesepalous stamen; Ap, antepetalous stamen; C, carpel. Scale bars = 40 μm.
The development of the androecium is nearly the same as in Haplophyllum dauricum and Boenninghausenia albiflora. The ten stamens divide into two whorls, and the antesepalous stamens appear first (arrows in Fig 5C), followed by the antepetalous stamens (arrows in Fig 5E). The two whorls of stamens emerge acropetally and appear diplostemonous (Fig 5F). The two whorls of stamens are different in size, reflecting their different growth rates (Fig 5F). In late development, each stamen differentiates into an anther and filament. After stamen initiation, the floral apex begins to elongate and two furrows, representing two carpels, emerge at the lateral position (Fig 5G). The carpels become basally fused during enlargement, forming a continuous structure (Fig 5H). At a later stage, the carpellary locules begin to inflate, and as the space is occupied by the antesepalous stamens, this inflation causes the antepetalous stamens to shift to the periphery (Fig 5I), such that in the mature flower, the antepetalous stamens are outside the antesepalous stamens.
Ancestral State Reconstruction
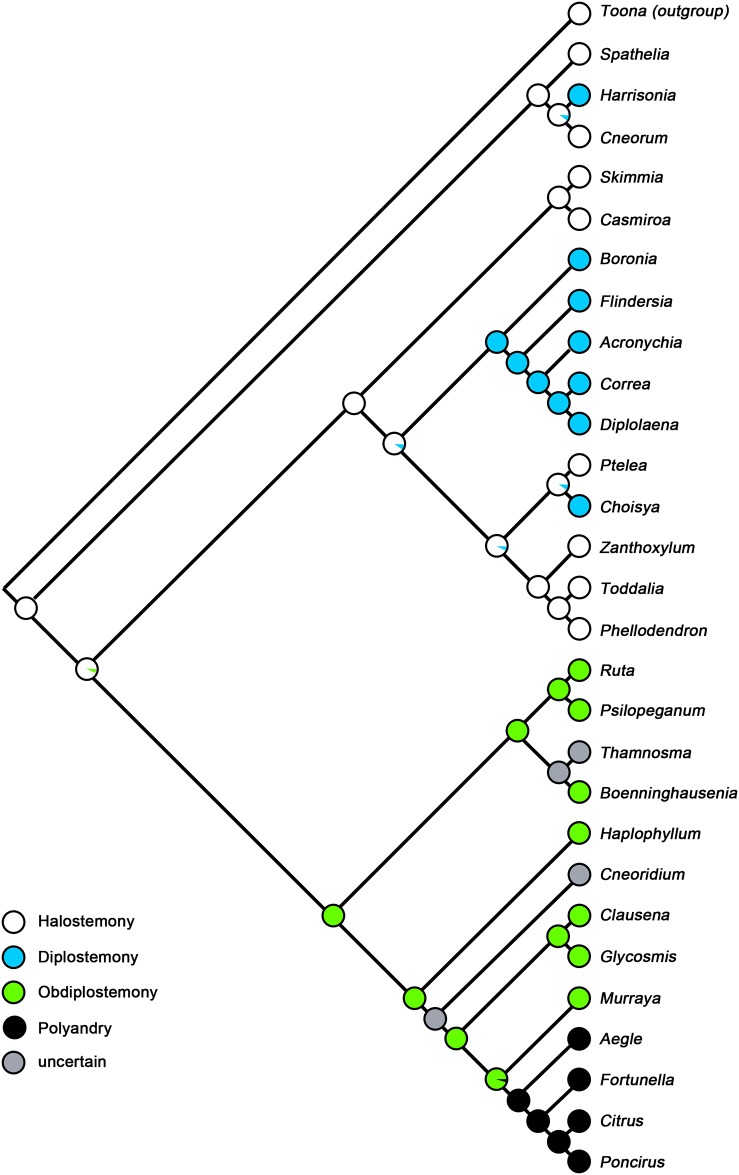
The ancestral state reconstruction (Fig 6), based on 28 out of 154 genera of Rutaceae, but sampling all major lineages, clearly showed that haplostemony is ancestral in Rutaceae as the genera in early-diverging clade Cneoroideae mostly have haplostemonous androecia except Harrisonia [1].
Fig 6. Ancestral state reconstruction for the androecium in the major clade Rutaceae.
The topology of the sampled species was determined according to Groppo et al. [4], Salvo et al. [13], Poon et al. [11], Morton et al. [17], and Manafzadeh et al. [43]. The different colors represent the different status of androecium.
The core Rutaceae is divided into two main clades: the Ruteae-Aurantioideae clade and a clade consisting of the remaining genera belonging to the former Flindersioideae, Toddalioideae and Rutoideae (= Amyridoideae sensu Morton & Telmer [17]). In the Toddalioideae-Rutoideae clade, the ancestral state is most likely haplostemony with diplostemony having evolved several times separately, especially in the genera distributed in the New World, such as Correa, Acronychia and Choisya.
In the Ruteae-Aurantioideae clade, the ancestral state is obdiplostemony. The genera in Ruteae and the ancestral genera in Aurantioideae, such as Clausena, Glycosmis and Murraya all have obdiplostemonous androecia. Comparing to these genera, the remaining genera in the clade such as Citrus, Poncirus and Fortunella all have polyandrous androecia, making polyandry secondary in this clade.
Discussion
The mode of androecium development in Boenninghausenia albiflora is clearly obdiplostemony. Although the two whorls of stamens arise in a regular order and are approximately inserted in one whorl, the carpels arise at the antepetalous position. This pattern may correspond to the maximum use of space and is a clearly an indication of obdiplostemony [44,45].
In the early stage of the floral ontogeny of Haplophyllum dauricum, the arrangement of the stamen primordia is in accord with Hofmeister’s rule as well as the inhibitory field theory. That means the new lay-out should arise with the least possible disturbance of neighboring structures [46]. In later stages, because the antesepalous stamens grow rapidly and occupy more space on the floral apex, the carpel primordia are initiated at the antepetalous position. Along with the inflation of the carpellary locules, the antepetalous stamens shift externally. It is worth noting that in some rare buds with a tetramerous mutation, while the flower is isomerous, the carpel arises at the antepetalous position. Therefore, the androecium in H. dauricum is also obdiplostemonous.
In the typical developmental pattern of obdiplostemony, the abnormal placement of two whorls of stamens is mainly caused by the irregular inception of certain floral organs, e.g., the centrifugal inception of the antepetalous stamens or the early inception of the carpels [20,47]. Compared with obdiplostemony, the developmental sequences of Boenninghausenia albiflora and Haplophyllum dauricum are regular. Only after inception in the acropetal sequence are the antepetalous stamens pushed outward by the growing carpels. This pattern corresponds to secondary obdiplostemony according to Ronse De Craene [20], indicating that obdiplostemony was a late phenomenon in these taxa [48].
Knowledge of ontogenetic patterns generally contributes to systematics and phylogenetic considerations [48], but previous studies have indicated that obdiplostemony is not relevant for systematics [49] because this phenomenon can be found in most members of the Rosids with pentamerous isomerous flowers, such as Zygophyllaceae, Oxalidaceae, Onagraceae and Tiliaceae [20,44,45,50]. As these families belong to different clades of Rosids, obdiplostemony is thought to have evolved separately. However, at the infrafamilial level, the situation may be different. In Rutaceae, the most early-diverging lineage Cneoroideae is represented by haplostemony [1]. Most genera in this subfamily have small haplostemonous flowers like Cneorum [51]. The ancestral state reconstruction also showed that haplostemony is the ancestral character in Rutaceae. The Toddalioideae-Rutoideae clade also contains some genera that are haplostemonous, such as Zanthoxylum, Toddalia and Phellodendron. Intrestingly, these genera represent the earliest fossil record of the extant genera in Rutaceae [21]. So, compared with haplostemony, two whorls of stamens is a derived character. There are two forms in which two whorls of stamens can be arranged in Rutaceae: diplostemony and obdiplostemony. According to Kubitzki [1], most genera in Toddalioideae-Rutoideae clade are diplostemonous, such as Correa, Acronychia and Choisya. The ancestral state reconstruction showed the diplostemony evolved several times independently. In the present study, the mature androecium of Boenninghausenia was found to be obdiplostemonous, and the ontogenetic pattern shows that obdiplostemony is caused by the shift of the antepetalous stamens. The same situation exists in Ruta and Psilopeganum [35], and this pattern is thought to be an evolutionarily late phenomenon. As the character state reconstruction showed that obdiplostemony is the ancestral condition in Ruteae (s.s.), it can serve as a morphological synapomorphy for Ruteae and provides good evidence that obdiplostemony was evolved from the haplostemonous androecium.
Polyandry is undoubtedly an advanced character in Rutaceae. The genera of this group that possess polyandrous androecia all belong to Aurantioideae. However, within Aurantioideae, two whorls of stamens also exist in the ancestral genera, e.g. Bergera, Clausena, Micromelum, Glycosmis. According to our findings, the development of the androecium of Murraya exotica is obdiplostemonous. Based on our observation of the mature flowers, the carpels of Clausena and Glycosmis are in the antepetalous position. This is a clear indication of obdiplostemony for a pentacyclic flower. Considering the close relationship between these genera and Murraya, we assume the developmental mode of androecia is in agreement with Murraya. In Murraya, the two whorls of stamens arise in a centripetal order, but at later stages, the antepetalous stamens shift externally because of the inflation of the carpellary locules. Current phylogenetic studies showed that Haplophyllum is sister to the Aurantioideae clade [39,40]. Based on our observation, the androecium is obdiplostemonous in Haplophyllum and the developmental mode is nearly the same with Murraya, as well as the genera in Ruteae (s.s.). As Haplophyllum, Murraya, Clausena and Glycosmis all possess obdiplostemonous androecia, the ancestral state reconstruction clear shows this is an ancestral character in the Aurantioideae clade.
The varied mode of development of the polyandrous androecium often provides a highly informative systematic character for families and taxa of higher orders. Compared with the polyandry observed in Aurantioideae, obdiplostemony explains the evolutionary mode of the androecium in Aurantioideae. In the floral ontogeny of Citrus, a genus with a polyandrous androecium, there is a clear time lag in the initiation of the stamen primordia. The antesepalous stamens are initiated first, and additional stamen primordia are subsequently initiated between these primordia, forming a single whorl [52]. This pattern is reminiscent of the mode of androecium development observed in Haplophyllum, Murraya and Ruteae (s.s.). The only difference is that instead of the single antepetalous stamen found in those species, there are many stamen primordia at the antepetalous position in Citrus. Based on this comparison, we can infer that the numerous stamens observed in Aurantioideae evolved from the obdiplostemonous androecium found in Murraya-like genera via increasing the number of antepetalous stamens. Among the Rosids, there are other families that appear to have undergone a secondary increase in their stamen number in the same manner, such as Zygophyllaceae [53]. In Peganum, Ronse De Craene reported that antepetalous stamen pairs occur in the obdiplostemonous androecium and interpreted the stamen pairs as a result of a secondary increase. Although in the early stage of the androecium in Murraya, antepetalous stamen pairs did not exist, in the floral development of Haplophyllum, there are some rare floral buds with antepetalous stamen pairs (Fig 3J). The same situation exists in Ruta, a genus in Ruteae (s.s.) clade [35] The antepetalous stamen pairs in Haplophyllum and Ruta indicate although the odds are low, the situation is quite common in Ruteae-Aurantioideae clade. Why is secondary obdiplostemony often related to an increase in stamen number? The answer is that in this type of obdiplostemony, the antepetalous stamens shift externally because of the maximum use of the floral apex, leaving more space in the antepetalous zone for the increase to occur.
In the current molecular systematics, the genera in traditional tribe Ruteae shows a closer relationship to Aurantioideae than to the traditional subfamily Rutoideae [17]. As the traditionally used fruit type and carpel characters are different between Ruteae and Aurantioideae, obtaining compelling morphological data to support this relationship is important. In the present study, the mode of androecium development was found to be the same in Ruteae (s.s.), Haplophyllum and the ancestral genus Murraya within Aurantioideae, and the ancestral state reconstruction showed that obdiplostemony can serve as a synapomorphy of the clade. More importantly, the secondary obdiplostemony observed in Ruteae and Murraya is both a derived stage from the ancestral haplostemony and a pre-stage for the secondary increase in stamens in Aurantioideae.
Based on our findings, the pattern of androecium development clearly indicates a close relationship between Ruteae and Aurantioideae, and Ruteae is an important clade in the evolution of the androecium in Rutaceae.
Acknowledgments
We thank Y. H. Xiao for technical assistance with SEM and Dr. Y. Niu for help in collecting plant materials. We thank Dr. David E. Boufford (Harvard University) for his careful reviewing of earlier versions of this manuscript. We are grateful to Cynthia Morton and another anonymous reviewer for their vital and constructive comments that greatly improved the quality of this work.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was financially supported by the National Natural Science Foundation of China (Grant no. 31200149). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kubitzki K, Kallunki JA, Duretto M, Wilson PG (2011) Rutaceae In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer; pp. 276–356. [Google Scholar]
- 2. Chase MW, Morton CM, Kallunki JA (1999) Phylogenetic relationships of Rutaceae: A cladistic analysis of the subfamilies using evidence from rbcL and atpB sequence variation. American Journal Of Botany 86: 1191–1199. [PubMed] [Google Scholar]
- 3. Scott KD, McIntyre CL, Playford J (2000) Molecular analyses suggest a need for a significant rearrangement of Rutaceae subfamilies and a minor reassessment of species relationships within Flindersia. Plant Systematics And Evolution 223: 15–27. [Google Scholar]
- 4. Groppo M, Pirani JR, Salatino MLF, Blanco SR, Kallunki JA (2008) Phylogeny of Rutaceae based on two noncoding regions from cpDNA. American Journal Of Botany 95: 985–1005. 10.3732/ajb.2007313 [DOI] [PubMed] [Google Scholar]
- 5. Bayer RJ, Mabberley DJ, Morton C, Miller CH, Sharma IK, Pfeil PE, et al. (2009) A Molecular Phylogeny Of the Orange Subfamily (Rutaceae: Aurantioideae) Using Nine Cpdna Sequences. American Journal Of Botany 96: 668–685. 10.3732/ajb.0800341 [DOI] [PubMed] [Google Scholar]
- 6. Engler A (1896) Rutaceae In: Engler A, Prantl K, editors. Dia natürlichen pflanzenfamilien. 1st ed Leipzig, Germany. [Google Scholar]
- 7. Engler A (1931) Rutaceae In: Engler A, Prantl K, editors. Dia natürlichen pflanzenfamilien. 2nd ed Leipzig, Germany: pp. 187–359. [Google Scholar]
- 8. Cronquist A (1993) An integrate system of classification of flowering plants. New York: Columbia university press. [Google Scholar]
- 9. Thorne RF (1992) Classification And Geography Of the Flowering Plants. Botanical Review 58: 225–348. [Google Scholar]
- 10. Samuel R, Ehrendorfer F, Chase MW, Greger H (2001) Phylogenetic analyses of Aurantioideae (Rutaceae) based on non-coding plastid DNA sequences and phytochemical features. Plant Biology 3: 77–87. [Google Scholar]
- 11. Poon WS, Shaw PC, Simmons MP, But PPH (2007) Congruence of molecular, morphological, and biochemical profiles in rutaceae: a cladistic analysis of the subfamilies rutoideae and toddalioideae. Systematic Botany 32: 837–846. [Google Scholar]
- 12. Morton CM, Grant M, Blackmore S (2003) Phylogenetic relationships of the Aurantioideae inferred from chloroplast DNA sequence data. American Journal Of Botany 90: 1463–1469. 10.3732/ajb.90.10.1463 [DOI] [PubMed] [Google Scholar]
- 13. Salvo G, Bacchetta G, Ghahremaninejad F, Conti E (2008) Phylogenetic relationships of Ruteae (Rutaceae): New evidence from the chloroplast genome and comparisons with non-molecular data. Molecular Phylogenetics And Evolution 49: 736–748. 10.1016/j.ympev.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 14. Bayly MJ, Holmes GD, Forster PI, Cantrill DJ, Ladiges PY (2013) Major Clades of Australasian Rutoideae (Rutaceae) Based on rbcL and atpB Sequences. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Appelhans MS, Kessler PJA, Smets E, Razafimandimbison SG, Janssens SB (2012) Age and historical biogeography of the pantropically distributed Spathelioideae (Rutaceae, Sapindales). Journal Of Biogeography 39: 1235–1250. [Google Scholar]
- 16. Appelhans MS, Smets E, Razafimandimbison SG, Haevermans T, van Marle EJ, Couloux A, et al. (2011) Phylogeny, evolutionary trends and classification of the Spathelia-Ptaeroxylon clade: morphological and molecular insights. Annals Of Botany 107: 1259–1277. 10.1093/aob/mcr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morton CM, Telmer C (2014) New Subfamily Classification for the Rutaceae. Annals Of the Missouri Botanical Garden 99: 620–641. [Google Scholar]
- 18. Stuessy TF (2009) Plant taxonomy: the systematic evaluation of comparative data. New York: Columbia University Press. [Google Scholar]
- 19. D'Arcy WG, Keating RC (1996) The Anther: Form, Function, and Phylogeny. New York: Cambridge University Press. [Google Scholar]
- 20. Decraene LPR, Smets EF (1995) The Distribution And Systematic Relevance Of the Androecial Character Oligomery. Botanical Journal Of the Linnean Society 118: 193–247. [Google Scholar]
- 21. Gregor HJ (1989) Aspects Of the Fossil Record And Phylogeny Of the Family Rutaceae (Zanthoxyleae, Toddalioideae). Plant Systematics And Evolution 162: 251–265. [Google Scholar]
- 22. Gonzalez FA, Rudall PJ (2010) Flower and fruit characters in the early-divergent lamiid family Metteniusaceae, with particular reference to the evolution of pseudomonomery. American Journal Of Botany 97: 191–U125. 10.3732/ajb.0900194 [DOI] [PubMed] [Google Scholar]
- 23. Endress PK (2006) Angiosperm floral evolution: Morphological developmental framework. Advances In Botanical Research: Incorporating Advances In Plant Pathology, Vol 44 44: 1–61. [Google Scholar]
- 24. Xu FX, De Craene LR (2010) Floral ontogeny of Annonaceae: evidence for high variability in floral form. Annals Of Botany 106: 591–605. 10.1093/aob/mcq158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sehr EM, Weber A (2009) Floral Ontogeny Of Oleaceae And Its Systematic Implications. International Journal Of Plant Sciences 170: 845–859. [Google Scholar]
- 26. Kaplan DR (1984) The concept of homology and its central role in the elucidation of plant systematic relationships In: Duncan T, Stuessy TF, editors. Cladistics: Perspectives on the reconstruction of evolutionary history. New York: Columbia University Press; pp. 51–70. [Google Scholar]
- 27. Stebbins GL (1973) Morphogenesis, vascularization and phylogeny in angiosperm. Breviora 418: 1–19. [Google Scholar]
- 28. Stebbins GL (1974) Flowering plants: Evolution above the spevies level. Cambridge: Belknap Press of Harvad University Press. [Google Scholar]
- 29. Tucker SC (2000) Floral development and homeosis in Saraca (Leguminosae: Caesalpinioideae: Detarieae). International Journal Of Plant Sciences 161: 537–549. [Google Scholar]
- 30. Tucker SC, Hodges SA (2005) Floral ontogeny of Aquilegia, Semiaquilegia, and Enemion (Ranunculaceae). International Journal Of Plant Sciences 166: 557–574. [Google Scholar]
- 31. Bello MA, Hawkins JA, Rudall PJ (2008) Floral morphology and development in Quillajaceae and Surianaceae (Fabales), the species-poor relatives of Leguminosae and Polygalaceae. Annals Of Botany 101: 1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Endress PK, Igersheim A (2000) Reproductive structures of the basal angiosperm Amborella trichopoda (Amborellaceae). International Journal Of Plant Sciences 161: S237–S248. [Google Scholar]
- 33. Matthews ML, Amaral MDE, Endress PK (2012) Comparative floral structure and systematics in Ochnaceae s.l. (Ochnaceae, Quiinaceae and Medusagynaceae; Malpighiales). Botanical Journal Of the Linnean Society 170: 299–392. [Google Scholar]
- 34. Matthews ML, Endress PK (2005) Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae). Botanical Journal Of the Linnean Society 149: 129–194. [Google Scholar]
- 35. Wei L, Wang YZ, Li ZY (2012) Floral ontogeny of Ruteae (Rutaceae) and its systematic implications. Plant Biology 14: 190–197. 10.1111/j.1438-8677.2011.00475.x [DOI] [PubMed] [Google Scholar]
- 36. Zhou QY, Wang YZ, Jin XB (2002) Ontogeny of floral organs and morphology of floral apex in Phellodendron amurense (Rutaceae). Australian Journal Of Botany 50: 633–644. [Google Scholar]
- 37. Zhou QY, Bertin RI, Fu DZ (2006) Gender dimorphism in Tetradium daniellii (Rutaceae): Floral biology, gametogenesis, and sexual system evolution. International Journal Of Plant Sciences 167: 201–212. [Google Scholar]
- 38. Mou FJ, Zhang DX (2012) Chromosome studies in the tribe Clauseneae and the cytological homogeneity in the orange subfamily (Aurantioideae, Rutaceae). Journal Of Systematics And Evolution 50: 460–466. [Google Scholar]
- 39. Moore JA (1936) Floral anatomy and phylogeny in Rutaceae. New Phytologist 35: 318–322. [Google Scholar]
- 40. Corner EJH (1976) The seeds of dicotyledons. Cambridge: Cambridge University Press. [Google Scholar]
- 41. Dasilva MFDF, Gottlieb OR, Ehrendorfer F (1988) Chemosystematics Of the Rutaceae—Suggestions for a More Natural Taxonomy And Evolutionary Interpretation Of the Family. Plant Systematics And Evolution 161: 97–134. [Google Scholar]
- 42.Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis, version 2.72. Available: http://mesquiteprojectorg.
- 43. Manafzadeh S, Salvo G, Conti E (2014) A tale of migrations from east to west: the Irano-Turanian floristic region as a source of Mediterranean xerophytes. Journal Of Biogeography 41: 366–379. [Google Scholar]
- 44. Endress PK (2010) Synorganisation without organ fusion in the flowers of Geranium robertianum (Geraniaceae) and its not so trivial obdiplostemony. Annals Of Botany 106: 687–695. 10.1093/aob/mcq171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matthews ML, Endress PK, Schonenberger J, Friis EM (2001) A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the problem of their systematic position. Annals Of Botany 88: 439–455. [Google Scholar]
- 46. Kirchoff BK (2003) Shape matters: Hofmeister's rule, primordium shape, and flower orientation. International Journal Of Plant Sciences 164: 505–517. [Google Scholar]
- 47. Dickson A (1865) On diplostemonous flowers, with some remarks upon the position of the carpels in Malvaceae. Transactions of the Botanical Society of Edinburgh 8: 86–107. [Google Scholar]
- 48. Erbar C, Leins P (1997) Different patterns of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. International Journal Of Plant Sciences 158: S49–S64. [Google Scholar]
- 49. Leins P, Erbar C (2010) Flower and fruit: morphology, ontogeny, phylogeny, function and ecology: Schweizerbart; Stuttgart. [Google Scholar]
- 50. De Craene LPR (2010) Floral diagrams: an aid to understanding flower morphology and evolution: Cambridge University Press. [Google Scholar]
- 51. Caris P, Smets E, Coster K, De Craene LPR (2006) Floral ontogeny of Cneorum tricoccon L. (Rutaceae). Plant Systematics And Evolution 257: 223–232. [Google Scholar]
- 52. Lord EM, Eckard KJ (1985) Shoot Development In Citrus-Sinensis L (Washington Navel Orange) .1. Floral And Inforescence Ontogeny. Botanical Gazette 146: 320–326. [Google Scholar]
- 53. Decraene LPR, DeLaet J, Smets EF (1996) Morphological studies in Zygophyllaceae .2. The floral development and vascular anatomy of Peganum harmala. American Journal Of Botany 83: 201–215. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.