Abstract
The PI3K/Akt signaling pathway is constitutively activated in some pancreatic cancers; when activated, it inhibits chemotherapy-mediated apoptosis. We examined whether Akt activity correlates with apoptotic resistance to chemotherapy in pancreatic cancer.
Methods
A panel of human pancreatic cancer cells was evaluated for basal AKT activity as well as response to three chemotherapies. Chemotherapy-induced cell death was evaluated following either up- or down-regulation of AKT activity. Evaluation of phosphorylation of p21Cip/Waf1, a downstream target of AKT, was also evaluated.
Results
There was a broad distribution among pancreatic cancer cell lines by AKT activity as well as sensitivity to the three chemotherapeutic agents with no apparent correlation. Phosphorylation of p21Cip/Waf1, but not change in total levels, correlated with the chemosensitizing effect of AKT inhibition to paclitaxel.
Conclusions
Basal AKT activity does not appear to be a useful predictor for selection of pancreatic cancers in targeting AKT to broadly induce chemosensitivity.
Keywords: Pancreatic cancer, Akt, apoptosis
Significant advances in cancer biology have uncovered several potential targets for molecularly-based therapy. The phosphatidylinositol-3 kinase (PI3K)/Akt is a fundamental signaling pathway that mediates several cellular processes, including cell proliferation, growth, survival, and motility (1–3). Increased activation, deregulation, and mutation of the components in the PI3K/Akt pathway have been implicated in driving tumorigenesis and conferring resistance to chemotherapy (4–6). Akt (protein kinase B) is a well-characterized serine/threonine kinase that is the central protein in the PI3K/Akt signaling pathway. Increased Akt activity has been demonstrated in many types of cancer, where it transmits a potent survival/anti-apoptotic signal7. Akt promotes cell survival through effects on numerous downstream targets, including the inactivation of pro-apoptotic proteins such as BAD and caspase-9, the activation of NF-κB resulting in transcription of anti-apoptotic genes, and the progression of the cell cycle through the cytoplasmic sequestration of p21 and the stabilization of cyclin D (8–13).
Akt activation is a frequent event in pancreatic cancer and correlates with outcome; immunohistochemical presence of phosphorylyated Akt (pAkt) has been associated with worse prognostic variables and outcome (14–16). It has been demonstrated previously that inhibition of the PI3K/Akt pathway sensitizes pancreatic cancer cells to the apoptotic effect of chemotherapy (10) in vitro and in vivo(17–26). However, the relative degree of Akt activation is quite variable across pancreatic cancer cell lines; kinase activation is rarely an on/off type of dichotomous variable. The activation of the HER-2/neu kinase in breast cancer has been divided into four categories (0 to 3+ based on immunohistochemical staining) with only the highest expressing group demonstrated to be sensitivity to targeted anti-HER-2/neu therapy (27). It is currently unknown whether the degree of Akt activity may be useful in identifying pancreatic cancers that are more sensitive to chemotherapy in the context of Akt inhibition. Furthermore, current research has primarily focused on the chemotherapy gemcitabine, as it is the only approved chemotherapy in pancreatic cancer. Yet, in vitro studies targeting HER-2/neu in breast cancer have demonstrated synergy with chemotherapeutic agents that have not been traditionally considered standard in that disease (28). This may well be due to the interaction of the targeted signaling pathway and the biochemical events involved in the apoptotic effect of the specific chemotherapeutic agent.
In this study, it was sought to determine the relationship between the degree of Akt activation and the response to diverse chemotherapeutic agents in pancreatic cancer. Furthermore, the level of Akt activity was modulated in different cell lines in an effort to determine whether basal level of Akt activation predicted the apoptotic response to chemotherapeutic agents. These studies will provide the necessary rationale for further development of Akt inhibition in pancreatic cancer by allowing appropriate tumor and chemotherapy selection.
Materials and Methods
Materials
All chemical reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) unless otherwise specified. Cell culture supplies and media were purchased from Becton Dickinson (San Diego, CA, USA) and Gibco/BRL Life Technologies (Gaithersburg, MD, USA), respectively. Paclitaxel (TaxolR) was purchased from Sigma-Aldrich, reconstituted in DMSO, and stored at −20 °C. Gemcitabine (GemzarR; Eli Lilly; Indianapolis, IN) was reconstituted in sterile PBS, and stored at −20 °C. Monoclonal antibodies to Akt, phospho-Akt, p21Cip/Waf1 and phospho-p21Cip/Waf1 and phospho-GSK were purchased from Cell Signaling (Beverly, MA, USA); a polyclonal antibody to actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Plasmids for transfection experiments were purified using Qiagen’s maxi kit.
Cell culture
The human pancreatic cancer cell lines MiaPaCa-2, Panc-1, BxPC-3, AsPC-1, Capan-1 and Capan-2 were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in recommended medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, L-glutamine, vitamins, penicillin, and streptomycin. Cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Western blotting
Following treatments, cells were harvested by trypsinization (trypsin 0.25% w/v, 1 mM ethylenediaminetetraacetic acid), washed with PBS, and lysed overnight at −20 °C in a lysis buffer purchased from Cell Signaling (Beverly, MA, USA) containing 20mM Tris (pH 7.5), 150 mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM β-Glycerolphosphate, 1mM Na3VO4, 1µg/ml Leupeptin, and 1mM PMSF. Debris was sedimented by centrifugation for 10 min at 14,000 g, and the protein concentration of the supernatant was determined using a Bio-rad protein detection assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Protein (75–100 µg) was solubilized at 100 °C in Laemmlis sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 15% 2-mercaptoethanol. Each sample was separated on a 10% SDS-PAGE gel by electrophoresis at 150V for 90 minutes. Separated polypeptides were then electrophoretically transferred to 0.2 mm nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA) for 90 minutes at 100V. Membranes were blocked for 1 h in Tris-buffered saline-Tween (TBS-T; 25 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween-20) containing 5% (w/v) nonfat dried milk. Blots were then probed overnight with primary antibodies and developed using species-specific secondary antibodies. Immunoreactive material was detected by enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ, USA).
Modulation of Akt activity
In order to demonstrate the role of Akt in response to chemotherapy, modulation of basal Akt activity was performed. Akt expression was augmented through the transient transfection of constitutively active myristylated Akt1 (myr-Akt) cDNA in pUSEamp (Upstate, Charlottesville, VA, USA). In brief, cells were plated to a density of 5 × 104 cells/ml. After allowing 24 hours for cellular recovery and adherence, cells were transfected with 1 mcg of the myr-Akt plasmid in association with 5 µl of Lipofectin reagent in serum deprived media. Approximately 12–16 hours following transfection, serum containing media (DMEM + 10% FBS) was reintroduced and cells were incubated for an additional 48 hours. Increases in Akt expression were demonstrated via western blotting as previously described. Treatment with Paclitaxel or Gemcitabine was initiated at this 48 hour time point when Akt activity was known to be at its peak.
Conversely, anti-sense RNA was used to reduce the level of Akt activity in the pancreatic cancer cell lines. Knockdown of Akt protein expression was accomplished through the transient transfection of SMARTpool Akt siRNA (Upstate, Charlottesville, VA, USA). In brief, cells were plated to a density of 2.5 × 104 cells/ml. After allowing 24 hours for cellular recovery and adherence, cells were transfected with 100 nM SMARTpool Akt siRNA in serum deprived media using the siImporter reagent system obtained from Upstate (Charlottesville, VA, USA). Approximately 12–16 hours following transfection, serum-containing media was reintroduced and cells were incubated for an additional 72 hours. Knockdown of Akt expression was demonstrated by western blotting. Treatment was initiated at 72 hr post siRNA transfection. In addition, A-443654, a small molecule pseudosubstrate peptide, was used to inhibit Akt (generous gift from Vincent Giranda, Abbott Laboratories, Inc.) (18).
Akt kinase assay
To demonstrate proof of principle, Akt kinase assays were performed following modulation of Akt activity. A nonradioactive Akt kinase assay kit (Cell Signaling, Beverly, MA, USA) was used. In brief, cells were plated to a density of 5 × 104 cells/ml in P-100 dishes. Transfection with either myr-Akt or Akt siRNA was performed as described previously. Following appropriate incubation time, cells were harvested under nondenaturing conditions. This includes washing with ice-cold PBS, followed by incubation with 1ml of 1× ice-cold Cell Lysis Buffer (20mM Tris (pH 7.5), 150 mM NaCl, 1mM EDTA, 1mM EGTA, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM β-Glycerolphosphate, 1mM Na3VO4, 1µg/ml Leupeptin, and 1mM PMSF) on ice for 10 minutes. Cells were scraped off the dish and debris was sedimented by centrifugation for 10 min at 14,000 g at 4 °C. Next, Akt was immunoprecipitated from 200 µg of cell lysate using resuspended Immobilized Akt Antibody slurry with gentle rocking at 4 °C overnight. The pellets were washed twice with 500 µl of 1× Cell Lysis Buffer and 500 µl of kinase b and then incubated with 200 µM ATP and 1 µg GSK-3 fusion protein for 30 minutes at 30 °C. The reaction was terminated with 14 µl of 4× SDS sample buffer, vortexed, and centrifuged for 2 minutes. Each sample was separated on a 12% SDS-PAGE gel by electrophoresis, transferred to a nitrocellulose membrane, blocked, probed, and developed as per the previously detailed western blot protocol.
FACS analysis
To identify and quantitate changes in the cell cycle distribution and the induction of apoptosis, treated cells underwent propidium iodide (PI) staining and fluorescence-activated cell sorting (FACS) as previously described (26). In brief, cells were plated at a density of 1×105 cells/ml. After allowing 24 hours for cell adherence, cells were transfected and/or treated. Cells were collected by gentle trypsinization, washed in phosphate-buffered saline (PBS), pelleted by centrifugation and fixed in 70% ethanol. Immediately prior to staining, cells were washed twice in PBS and resuspended in PBS containing RNAse A (20 µg/ml). Cells were stained with propidium iodide (final concentration 10 mcg/ml) for 10 min at room temperature. Samples were analyzed by FACS (FL-3 channel) using a Beckman Coulter Counter Epics XL flow cytometer (Beckman Coulter, Miami, FL, USA). For each sample, 50,000 events were collected and stored for subsequent analysis using EXPO software (version 2.0; Applied Cytometry Systems, Sheffield, UK). The percentage of cells in the sub-G0 phase was quantitated as an estimate of cells undergoing apoptosis.
Active Caspase-3 immunoassay
Active caspase-3 levels were quantitated using the human Active Caspase-3 Immunoassay kit as suggested by the manufacturer (R&D Systems, Inc., Minneapolis, MN, USA). MiaPaCa-2 and PANC-1 cells were plated in 6 well plates at 1 × 105 cells/well. After allowing 24 hours for cell adherence, cells were treated. Cell treatments were performed in triplicate. After treatment, caspase-3 was labeled by addition of 2 µl of 5 mM biotin-ZVKD-fmk per 1 ml of culture medium and cells were incubated in a humidified incubator containing 10% CO2 at 37° C for 1 hour. Cells were rinsed with phosphate buffered saline and 110 µL of extraction buffer (1X) containing 7M urea (Fisher Scientific, Pittsburgh, PA, USA) and protease inhibitors was added to each well and the plates were incubated overnight at 4 °C. The cell lysates were diluted 5-fold by addition of 400 µl of Diluent RD5-20 (1X). Caspase 3-standards supplied by the manufacturer and 100 µl of the diluted cell lysates were added to the active-caspase-3 microplate in duplicate and incubated for 2 h at room temperature. The microplate was washed five times with 400 µl of wash buffer per well for 10 s. The active caspase-3 conjugate (strepavidin–HRP) was added to each well and incubated for 1 hr at room temperature. After substrate addition and quenching per the manufacturer’s instructions, plates were read at 450 nm with wavelength correction at 540 nm. A standard curve was constructed and the amount of active caspase-3 in the treated samples was calculated.
Immunofluorescence
For fluorescence microscopy, cells were cultured on glass coverslips and fixed in 3.7% paraformaldehyde (5 minutes at room temperature). Cells were washed in PBS and then solubilized by treatment with 0.5% Triton X-100 for 20 minutes at room temperature. Blocking of nonspecific binding was achieved by incubation in 3% milk for 15 minutes at room temperature. Coverslips were incubated with monoclonal antibodies to p21Cip/Waf1 and phospho-p21Cip/Waf1 for 2 hours at 37°C, washed with PBS, and then incubated (4°C, overnight) with species-specific secondary antibodies (Alexa FluorR 647; Invitrogen; Carlsbad, CA). Cells were then counterstained for nuclear visualization with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; 300 nM; Molecular Probes, Eugene, OR, USA).
Results
Basal expression of AKT differs among pancreatic cancer cell lines
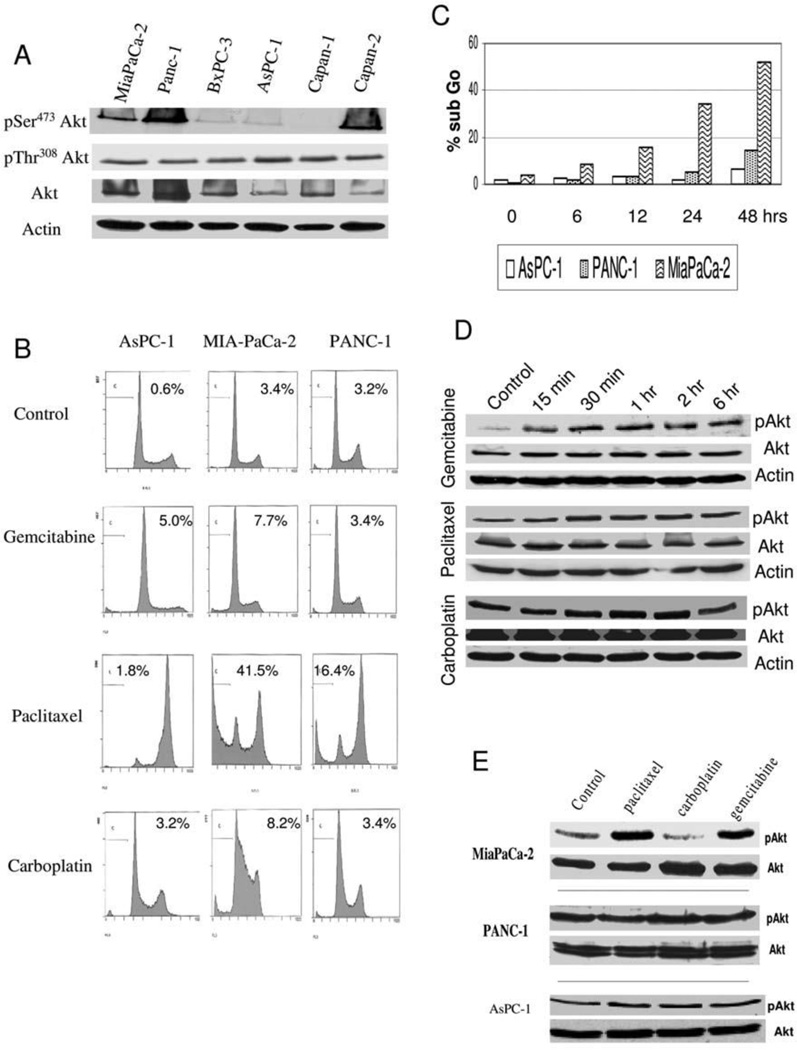
Constitutive activation of Akt has been reported in many cancers, including breast, ovarian, and prostate7. The first step in this investigation was to analyze the basal expression of Akt in pancreatic cancer. Cell lysates from six pancreatic cancer cell lines were screened by western blotting for the level of pSer473Akt, pThr308Akt and total Akt. Three of the seven cell lines (MIA-PaCa-2, PANC-1, and Capan-2) demonstrated moderate to high levels of Akt activation as determined by pSer473Akt levels, while three cell lines (BXPC-3, ASPC-1, and Capan-1) showed low levels of Akt activation (Figure 1A). Of note, pThr308Akt level did not vary among the six cell lines. Total Akt level was variable and did not necessarily correlate with abundance of pSer473Akt. For further studies of apoptotic induction following exposure to diverse chemotherapies, the following cell lines were used: AsPC-1 for low Akt activation, MIA-PaCa-2 for moderate Akt activation, and PANC-1 for high Akt activation.
Figure 1.
A) Western blot for basal levels of Akt and phospho-Akt in six pancreatic cancer cell lines with loading equivalency confirmed by immunoblotting for actin, B) FACS analysis following treatment with gemcitabine (100 µM), paclitaxel (100 nM) or carboplatin (270 µM) for 24 hours in AsPC-1, MiaPaCa-2 and PANC-1 with measurement of the sub-G0 peak is used as a marker of apoptosis, C) Induction of apoptosis following paclitaxel treatment (100 nM) over 48 hours in AsPC-1, PANC-1 and MIA-PaCa-2, D) Western blot following the indicated times of treatment following gemcitabine (100 µM), paclitaxel (100 nM) or carboplatin (270 µM) in MiaPaCa-2 cells for total Akt, phospho-Akt (pSer473) or actin to show for equivalency of loading, E) Western blot following the 6 hrs treatment with gemcitabine (100 µM), paclitaxel (100 nM) or carboplatin (270 µM) in MiaPaCa-2, PANC-1 or AsPC-1 cells for total Akt or phospho-Akt (pSer473).
Differential response to chemotherapy among cell lines with varying levels of Akt activation
Many studies in pancreatic cancer have implicated activation of the PI3K/Akt pathway in conferring resistance to chemotherapy-induced apoptosis in pancreatic cancer, though the majority of studies have only utilized the chemotherapy gemcitabine (17, 23–26). The response in three pancreatic cancer cell lines was compared with differential activation of Akt to three chemotherapy agents with diverse cellular function. The chemotherapies examined were: gemcitabine, a nucleoside analog that blocks DNA replication; paclitaxel, which inhibits microtubule depolymerization and carboplatin which causes DNA adducts. Gemcitabine induced only a small amount of increased cell death in AsPC-1 and MIA-PaCa-2 cells but had no effect on PANC-1, consistent with the recent report by Pan et al.29 (Figure 1B). Paclitaxel treatment increased the fraction of cells in G2/M phase of the cell cycle in all cell lines, though differential induction of apoptosis was observed. AsPC-1 cells underwent very little apoptosis (1.8%), PANC-1 sustained a modest induction of apoptosis (16.4%), and MIA-PaCa-2 underwent significant cell death (41.5%). Following carboplatin treatment, only MIA-PaCa-2 was noted to increase the cell population in the S phase of the cell cycle, consistent with its mechanism of inducing DNA adducts and halting DNA synthesis. To further elaborate on the differential effect of paclitaxel, a time course study was conducted in these three cell lines; MIA-PaCa-2 was the most sensitive to the apoptotic effect of paclitaxel with apoptosis observed as early as 6 hours of paclitaxel treatment (Figure 1C). At 48 hours, only a minimal increase in apoptotic fraction was observed in the other two cell lines (Figure 1C). If basal level of Akt activation predicted apoptotic response, AsPC-1 would have been uniformly sensitive to the tested agents and PANC-1 resistant, though the data does not demonstrate that correlation.
Effect of chemotherapy exposure on activation of Akt
Following exposure to apoptotic stimuli, cells may engage survival mechanisms to subvert the induction of cell death. Activation of the Akt signaling pathway has been observed following exposure of diverse cancer cell types to various chemotherapeutic agents. Given the varying degree of basal activation of Akt in pancreatic cancer, it was examined whether there was further activation upon exposure to these three chemotherapeutic agents. MIA-PaCa-2 cells were treated with gemcitabine, paclitaxel or carboplatin and alterations in pAkt level examined over a time frame of 15 minutes to 6 hrs. Gemcitabine treatment was observed to induce a rapid increase in pSer473Akt levels; paclitaxel treatment led to a similar though less dramatic increase in pAkt levels while carboplatin had no effect (Figure 1D). The evaluation was then expanded to the other cell lines with examination at 2 hrs of treatment, given the reproducible activation of Akt in Mia-PaCa-2 following paclitaxel and gemcitabine at this time point. No significant activation of Akt in PANC-1 or AsPC-1 was observed following any of the treatments (Figure 1E). Therefore, treatment-mediated activation does not appear to be a common response among these pancreatic cell lines, nor specifically correlated with chemotherapy-induced apoptosis.
Effect of increasing Akt activity on chemotherapy-induced apoptosis
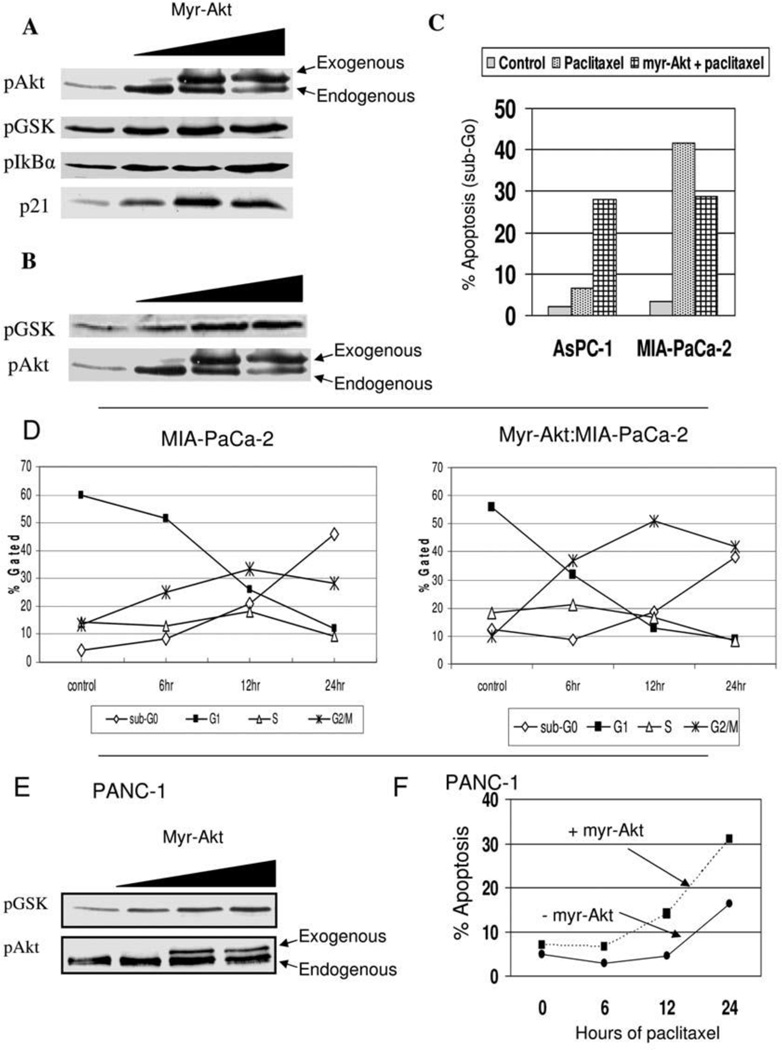
It was sought to increase Akt expression in both the AsPC-1 cells (low Akt activation) and the MiaPaCa-2 cells (moderate Akt activation) to determine if the chemotherapy response would be more like that of PANC-1 (high Akt activation). The cells were transfected with a plasmid that encodes a constitutively active Akt (myr-Akt) and cells were analyzed by Western blot for pAkt levels or downstream signaling events of phosphorylation of GSK and I-κB, or stabilization of p21Cip1 (Figure 2A; data shown only for MIA-PaCa-2; data not shown for AsPC-1). In addition, Akt kinase activity was confirmed to be increased following transfection of the myr-Akt (Figure 2B). Increasing Akt activity in either AsPC-1 or MIA-PaCa-2 had no effect on basal levels of cell death or cell cycle distribution (data not shown). In AsPC-1, when Akt activity was increased by the myr-Akt transfection, paclitaxel treatment led to increased cell death, though in MIA-PaCa-2 the increased Akt activity led to decreased cell death (Figure 2C). Following paclitaxel treatment, apoptotic fraction increased from 6.6% in AsPC-1 to 27.9% in the setting of increased Akt activation; however in MIA-PaCa-2 the effect of exogenous Akt activation was to decrease the apoptotic fraction from 41.5% to 28.8%. In association with this decrease in paclitaxel-induced cell death in MIA-PaCa-2 following myr-Akt transfection was a more rapid and heightened G2/M cell cycle arrest (Figure 2D). The effect of exogenous Akt activation increased the G2/M fraction following paclitaxel treatment, most notably at 12 hours of treatment, with a decrease in the induction of apoptosis. However, increasing AKT activity did not alter the cellular response to gemcitabine in these cells (data not shown). Therefore, while increasing Akt activity in MIA-PaCa-2 made the cellular response of MIA-PaCa-2 more similar to the high-Akt PANC-1 cell line (i.e. reduced cell death though preserved G2/M cell cycle arrest), apoptosis actually increased in the low-Akt AsPC-1 cell line in response to paclitaxel.
Figure 2.
A) Western blot illustrating a dose-dependent increase in phospho-Akt levels following transfection of myr-Akt in MiaPaCa-2 cells with effect on phosphorylation of downstream targets (GSK and IκBα) as well as, p21Cip/Waf1, B) Akt kinase assay following myr-Akt transfection in MIA-PaCa-2 cells demonstrates increasing phosphorylation of the target protein GSK with immunoblotting demonstrating a corresponding increase in phospho-Akt expression, C) Apoptotic fraction of AsPC-1 or MIA-PaCa-2 cells following treatment with paclitaxel (100 nM, 24 hrs) in the absence or presence of myr-Akt transfection, D) Time course of cell cycle fractions of MIA-PaCa-2 without (left) or with (right) myr-Akt following the indicated times of treatment with paclitaxel (100 nM), E) Akt kinase assay following myr-Akt transfection in PANC-1 cells demonstrates increasing phosphorylation of the target protein GSK with immunoblotting demonstrating a corresponding increase in phospho-Akt expression, F) Apoptotic fraction of PANC-1 cells following treatment with paclitaxel (100 nM, 24) over time in the absence or presence of myr-Akt transfection.
Although PANC-1 demonstrated the highest level of Akt activation of the seven cell lines, whether further increase in activity has cellular consequences was evaluated. Using the same myr-Akt transfection, the Akt activity was capable of further upregulation (Figure 2E). Interestingly, the increase in Akt activity following myr-Akt transfection increased the apoptotic response in PANC-1 cells to paclitaxel (Figure 2F), without significantly impacting G2/M arrest (data not shown). Therefore, an enforced increase in Akt activity increased paclitaxel-induced cell death in AsPc-1 and PANC-1 cells, but actually decreased the apoptotic response in MIA-PaCa-2 cells.
Effect of decreasing Akt activity on chemotherapy-induced apoptosis
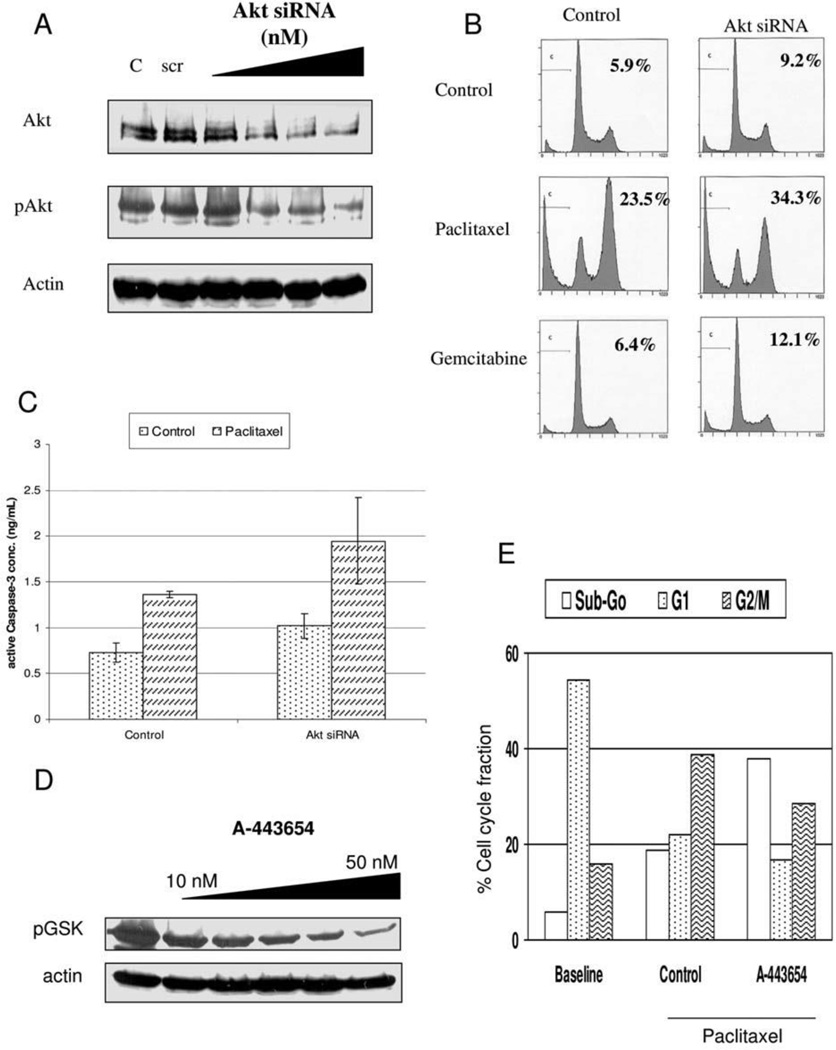
Next, it was sought to examine if there was a consistent response to reducing the level of Akt expression, anticipating that this would increase cellular susceptibility to the apoptosis-inducing effects of chemotherapy. An siRNA approach was initially used to decrease Akt levels, with confirmation of decreased Akt activity. PANC-1 cells were transfected with Akt siRNA to achieve a dose-dependent knockdown of total Akt, which did correlate with a decrease in pAKT (Figure 3A). The cellular response to paclitaxel and gemcitabine was then examined in the absence or presence of Akt siRNA (100 nM). In the presence of Akt knockdown, both chemotherapeutic agents induced a greater degree of apoptosis; notable was the baseline increase in apoptosis in the absence of any chemotherapy treatment (Figure 3B). While Akt knockdown increased paclitaxel-induced apoptosis, there was a decrease in paclitaxel-induced G2/M cell fraction. The flow cytometry results were supported by a caspase-3 ELISA in which the Akt knockdown increased the baseline level of caspase-3 activation as well as following paclitaxel treatment (Figure 3C). To corroborate these findings, it was evaluated whether small molecule inhibition of Akt altered the cellular response to paclitaxel in PANC-1 cells. A-443654 is a specific indole-pyridine inhibitor of Akt (18). Following 6 hr of treatment with A-443654, PANC-1 cells demonstrated a dose-dependent inhibition of Akt demonstrated by reduced phosphorylation of the downstream substrate GSK (Figure 3D). Similar to the findings of siRNA-mediated AKT knockdown, A-443654 (50 nM) blunted the G2/M cell cycle arrest following 24 hrs of paclitaxel treatment but increased apoptosis in PANC-1 (Figure 3E).
Figure 3.
A) Akt siRNA mediated knockdown of Akt/ with effect on phosphorylated Akt (pSer473) or actin to show for equivalency of loading in PANC-1. Controls include a mock transfection (C) and nonspecific scramble siRNA (scr), B) FACS analysis of PANC-1 in the absence or presence of Akt siRNA transfection and subsequent treatment with paclitaxel or gemcitabine (apoptotic fraction is denoted), C) Caspase-3 activation of PANC-1 in the absence or presence of Akt siRNA transfection and subsequent treatment with paclitaxel, D) Akt kinase assay following various doses of treatment with A-443654 in PANC-1 cells demonstrates decreased phosphorylation of the target protein GSK, E) Cell cycle fractions of PANC-1 cells at baseline or following paclitaxel treatment (100 nM, 24 hrs) in the absence or presence of A-443654.
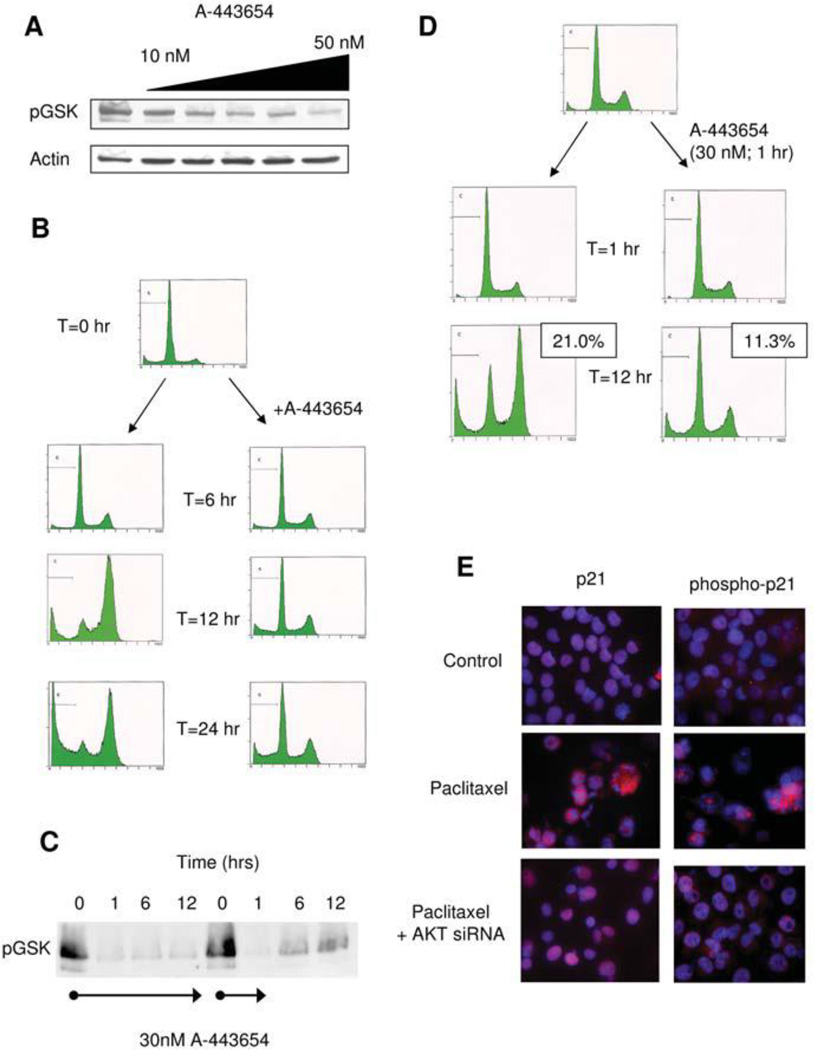
Next, the cellular response to paclitaxel in MIA-PaCa-2 cells following AKT inhibition with A-443654 was examined. Although these cells have an overall lower level of AKT activation compared to PANC-1, A-443654 was still able to effectively inhibit AKT activity (Figure 4A). Similar to PANC-1 cells, A-443654 decreased the G2/M cell cycle fraction following paclitaxel treatment (Figure 4B). However, unlike PANC-1 cells, cell death was notably decreased following AKT inhibition with A-443654 in combination with paclitaxel treatment. These data suggest that AKT plays separate roles in the G2/M cell cycle arrest and the cell death following paclitaxel therapy as observed by the differential response of these cell lines to either AKT activation or inhibition (Table I). Furthermore, we it was not possible to predict the effect of AKT modulation on paclitaxel-induced cell death based on basal level of AKT activation, AKT response to chemotherapy, or type of chemotherapy used. also It was also noted that the effect of AKT inhibition on G2/M arrest did not parallel the alteration of paclitaxel-induced cell death: AKT inhibition reduced the paclitaxel-induced G2/M fraction in both MIA-PaCa-2 and PANC-1 yet these changes were associated with an increase in cell death in PANC-1 but a decrease in cell death in MIA-PaCa-2.
Figure 4.
A) Akt kinase assay following various doses of treatment with A-443654 in MIA-PaCa-2 cells demonstrates decreased phosphorylation of the target protein GSK, B) FACS analysis of MIA-PaCa-2 after the indicated times of treatment with paclitaxel (100 nM) in the absence or presence of the Akt inhibitor A-443654 (30 nM), C) Akt kinase assay following various times of treatment with A-443654 (continuous treatment on left; one hour treatment and media change on right) demonstrate mild recovery of Akt activity after 1 hr of Akt inhibition, D) FACS analysis of MIA-PaCa-2 cells in the absence (left) or presence (right) of the Akt inhibitor (30 nM) for 1 hour and then ongoing paclitaxel treatment (100 nM) with apoptotic fraction noted, E) Immunofluorescence for p21Cip/Waf1 and phospho-p21Cip/Waf1 in MIA-PaCa-2 cells following paclitaxel therapy in the absence (middle panel) or presence of Akt siRNa (bottom panel)
Table 1.
Summary of Akt and chemotherapy response
| Basal AKT activation |
AKT response to paclitaxel |
Paclitaxel response following AKT activation (myr- AKT transfection) |
Paclitaxel response following AKT inhibition (siRNA or A- 443654) |
|
|---|---|---|---|---|
| AsPC-1 | Low | No change | ↓G2/M ↑cell death |
|
| MIA-PaCa-2 | Moderate | Dramatic activation | ↑G2/M ↓cell death |
↓G2/M ↓cell death |
| PANC-1 | High | No change |
↑cell death |
↓G2/M ↑cell death |
Using A-443654, a reversible small molecule inhibitor of AKT, the time-dependent role of paclitaxel-induced AKT activation in the G2/M cell cycle arrest as well as cell death was examined. Following just 1 hr exposure, AKT activity is dramatically reduced in MIA-PaCa-2 cells, though after removal of the inhibitor, AKT activity increases toward baseline levels of activation (Figure 4C). The cellular response to paclitaxel following just a 1 hr treatment with the AKT inhibitor A-443654 to block the initial basal and paclitaxel-mediated induction of AKT was then examined. Despite some recovery of AKT activity by twelve hours as noted in Figure 4C, paclitaxel was ineffective at initiating either G2/M arrest or cell death (Figure 4D). These data indicate that AKT activity is essential early in both the cell cycle arrest and subsequent cell death induced by paclitaxel. The role of p21Cip/Waf1 in these events was further examined, given its role in the G2/M arrest following paclitaxel by direct phosphorylation by AKT. Following paclitaxel treatment in MIA-PaCa-2 cells, which has been previously shown to induce AKT activation, total and phosphorylated levels of p21Cip/Waf1 are increased (Figure 4E). In the setting of AKT inhibition by siRNA transfection, paclitaxel still increased levels of p21Cip/Waf1 though the phosphorylation of p21Cip/Waf1 was nearly abrogated (Figure 4E). These data suggest that paclitaxel induces an AKT-dependent phosphorylation of p21Cip/Waf1, which may be coupled with the G2/M cell cycle arrest and cell death, though the increased levels of p21Cip/Waf1 do not seem to be associated with the AKT-mediated events following paclitaxel therapy.
Discussion
AKT plays an important role in cancer therapy by promoting resistance to the apoptosis-inducing effects of chemotherapy (30, 31). Thus, AKT-targeted molecular therapy has become an intense area of research in pancreatic cancer given the dismal results of targeting other common genetic events (e.g. K-ras mutation, erbB2 activation). Enthusiasm for this approach has been based on results obtained from pharmacologic inhibition of PI3K, the upstream activator of AKT (32, 36). Only recently were specific small molecule inhibitors of AKT developed in an attempt to avoid the toxicity of PI3K inhibitors (i.e. LY294002 or wortmannin). Yet the background identification of tumor or chemotherapy selection in pancreatic cancer has not been reported to provide the essential background for further preclinical development of AKT inhibitors. The results of the present study illustrate the variable expression and activity of AKT across a panel of pancreatic cancer cell lines, though basal level of activation could not be used to predict sensitivity to three diverse chemotherapeutic agents. Furthermore, exogenously increasing AKT activity did not uniformly confer resistance to chemotherapy-induced cell death, nor did inhibition of AKT activity uniformly sensitize cells to chemotherapy-induced cell death. Lastly, chemotherapy-mediated activation of AKT was cell-specific, and may in part mediate the G2/M cell cycle arrest and subsequent cell death induced by paclitaxel.
The disparate results between the high-AKT (ie PANC-1) and low-AKT (ie MIA-PaCa-2) cell lines related to cellular effects of paclitaxel in combination with AKT inhibition warrant critical interpretation. In PANC-1, AKT inhibition increased basal and paclitaxel-mediated cell death while the reverse was observed in MIA-PaCa-2. These data would suggest that high levels of AKT activity may be a useful predictive marker for targeted AKT inhibition in combination with paclitaxel. However, broad application of AKT inhibitor therapy to those with low basal levels of activation may abrogate the effectiveness of other chemotherapies. Furthermore, it was also demonstrated that exogenous increase in activation of AKT in both PANC-1 and AsPC-1 increased paclitaxel-mediated cell death, while this same increase in AKT activation decreased paclitaxel-mediated cell death in MIA-PaCa-2. These observations may well be attributed to the mechanism of AKT activation in pancreatic cancer. Asano et al demonstrated silencing of the PTEN promoter contributed to AKT activation in MIA-PaCa-2, but not AsPC-1 or PANC-1 (37). Wendel et al. demonstrated that sensitivity to mTOR inhibition (one signaling event downstream of AKT) in lymphoma was best predicted by loss of PTEN (38). Thus, the sensitivity of MIA-PaCa-2 to AKT-mediated sensitization to paclitaxel may be explained by the observation that only this cell line has a defect in PTEN signaling. Other events responsible for activation of AKT in pancreatic cancer include K-ras mutational activation as well as erbB2 activation. Ihle et al. examined predictors of response to PI3K inhibitors in a variety of human tumor xenografts. Mutational activation of Ras, present in all cell lines that were examined, conferred resistance to PI3K inhibitors; mutation of PTEN or dependence of AKT activity on the activation of an upstream growth factor receptor conferred sensitivity to PI3K inhibitors (39). While EGF-R may be mutated or amplified in other cancers, these events are uncommon in pancreatic cancer (40, 41). It has previously been shown that activation of erbB2 contributes to increased AKT activity in MIA-PaCa-2 cells, though PANC-1 cells do not demonstrate erbB2 activation (14). Therefore, these data suggest that rather than the basal level of AKT activation, the best predictive markers for targeted AKT inhibition to mediate chemosensitization in pancreatic cancer would appear to loss of PTEN or erbB2 activation.
In the development of targeted therapy in oncology, surrogate biomarkers of targeted inhibition are useful to demonstrate efficiency of kinase silencing as well as evaluation for ongoing response to therapy. While AKT inhibition decreases total p21Cip/Waf1 levels, this did not seem to correlate with AKT-mediated paclitaxel response. Instead, phosphorylated p21Cip/Waf1 appeared to be a more reliable marker of AKT inhibition and predicting response to paclitaxel-mediated cell death. However the clear coupling of this to the sensitizing effect of AKT inhibition in the setting of paclitaxel therapy is unclear. In ovarian cancer, Mitsuuschi et al. demonstrated that the phosphorylation of p21Cip/Waf1 following paclitaxel treatment was dependent on AKT, yet the cell death induced by paclitaxel proceeded independent of AKT (42). This is in contrast to the current data in PANC-1, where AKT inhibition prevented paclitaxel-mediated p21Cip/Waf1 phosphorylation, G2/M cell cycle arrest and cell death. These findings may possibly be explained by the pharmacokinetics of paclitaxel as Heliez et al. reported that low doses of paclitaxel (10 nM) trigger p21Cip/Waf1 phosphorylation but are ineffective at inducing G2/M arrest while higher doses (100 nM) are required to induce the G2/M arrest (43). So while not mechanistically involved, p21Cip/Waf1 phosphorylation may be sufficient as a surrogate marker of AKT inhibition in the setting of paclitaxel therapy.
In summary, the level of AKT activation is not likely to be useful in selecting individual pancreatic tumors for AKT inhibition in combination with chemotherapy nor is a specific chemotherapy likely to be more effective in combination with AKT inhibition. However, phosphorylation of p21Cip/Waf1 may be an appropriate surrogate biomarker for AKT inhibition and sensitization to paclitaxel-mediated cell death in pancreatic cancer. Further studies to identify whether loss of PTEN or alterations in erbB1/erbB2 are appropriate selection markers for AKT inhibition in pancreatic cancer seem to be justified.
Acknowledgements
This work was supported by NIH 1RO3 CA123004 and the Isabelle J. McDonald Endowment (to RJB). We are grateful to the support and the reagents provided by Vincent Giranda (Abbott Oncology Laboratories).
References
- 1.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 2.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4(4):257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 4.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14(5):381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 6.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159(2):431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 9.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem. 2000;275(11):8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 10.Fahy BN, Schlieman MG, Virudachalam S, Bold RJ. Inhibition of AKT abrogates chemotherapy-induced NF-kappaB survival mechanisms: implications for therapy in pancreatic cancer. J Am Coll Surg. 2004;198(4):591–599. doi: 10.1016/j.jamcollsurg.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 12.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3(3):245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 14.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89(11):2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10(8):2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 16.Chadha KS, Khoury T, Yu J, Black JD, Gibbs JF, Kuvshinoff BW, Tan D, Brattain MG, Javle MM. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13(7):933–939. doi: 10.1245/ASO.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60(19):5451–5455. [PubMed] [Google Scholar]
- 18.Luo Y, Smith RA, Guan R, Liu X, Klinghofer V, Shen J, Hutchins C, Richardson P, Holzman T, Rosenberg SH, Giranda VL. Pseudosubstrate peptides inhibit Akt and induce cell growth inhibition. Biochemistry. 2004;43(5):1254–1263. doi: 10.1021/bi034515p. [DOI] [PubMed] [Google Scholar]
- 19.Perugini RA, McDade TP, Jr, Vittimberga FJ, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-Kinase dependent. J Surg Res. 2004;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 20.Shah SA, Potter MW, Hedeshian MH, Kim RD, Chari RS, Callery MP. PI-3' kinase and NF-kappaB cross-signaling in human pancreatic cancer cells. J Gastrointest Surg. 2001;5:603–612. doi: 10.1016/s1091-255x(01)80102-5. [DOI] [PubMed] [Google Scholar]
- 21.Arlt A, Gehrz A, Muerkoster S, Vorndamm J, Kruse ML, Folsch UR, Schafer H. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22(21):3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 22.Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3'-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28(3):353–358. doi: 10.1097/00006676-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7(10):3269–3275. [PubMed] [Google Scholar]
- 24.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1(12):989–997. [PubMed] [Google Scholar]
- 25.Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98(18):10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahy BN, Schlieman M, Virudachalam S, Bold RJ. AKT inhibition is associated with chemosensitization in the pancreatic cancer cell line MIA-PaCa-2. Br J Cancer. 2003;89(2):391–397. doi: 10.1038/sj.bjc.6601037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornier M, Risio M, Van Poznak C, Seidman A. HER2 testing and correlation with efficacy of trastuzumab therapy. Oncology. 2002;16(10):1340–1348. [PubMed] [Google Scholar]
- 28.Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18(13):2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 29.Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, Lopez-Berestein G, Vivas-Mejia PE, Sood AK, McConkey DJ, Logsdon CD. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14(24):8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1(9):707–717. [PubMed] [Google Scholar]
- 31.Page C, Lin HJ, Jin Y, Castle VP, Nunez G, Huang M, Lin J. Overexpression of Akt/AKT can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000;20(1A):407–416. [PubMed] [Google Scholar]
- 32.Perugini RA, McDade TP, Vittimberga FJ, Jr, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90(1):39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 33.Ng SSW, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000;60(19):5451–5455. [PubMed] [Google Scholar]
- 34.Shah SA, Potter MW, Hedeshian MH, Kim RD, Chari RS, Callery MP. PI-3' kinase and NF-kappaB cross-signaling in human pancreatic cancer cells. J Gastrointest Surg. 2001;5(6):603–612. doi: 10.1016/s1091-255x(01)80102-5. [DOI] [PubMed] [Google Scholar]
- 35.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3'-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1(12):989–997. [PubMed] [Google Scholar]
- 36.Ng SS, Tsao MS, Nicklee T, Hedley DW. Wortmannin inhibits pkb/akt phosphorylation and promotes gemcitabine antitumor activity in orthotopic human pancreatic cancer xenografts in immunodeficient mice. Clin Cancer Res. 2001;7(10):3269–3275. [PubMed] [Google Scholar]
- 37.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23(53):8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 38.Wendel HG, Malina A, Zhao Z, Zender L, Kogan SC, Cordon-Cardo C, Pelletier J, Lowe SW. Determinants of sensitivity and resistance to rapamycin-chemotherapy drug combinations in vivo. Cancer Res. 2006;66(15):7639–7646. doi: 10.1158/0008-5472.CAN-06-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ihle NT, Lemos R, Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009;69(1):143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buck E, Eyzaguirre A, Haley JD, Gibson NW, Cagnoni P, Iwata KK. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther. 2006;5(8):2051–2059. doi: 10.1158/1535-7163.MCT-06-0007. [DOI] [PubMed] [Google Scholar]
- 41.Tzeng CW, Frolov A, Frolova N, Jhala NC, Howard JH, Vickers SM, Buchsbaum DJ, Heslin MJ, Arnoletti JP. EGFR genomic gain and aberrant pathway signaling in pancreatic cancer patients. J Surg Res. 2007;143(1):20–26. doi: 10.1016/j.jss.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 42.Mitsuuchi Y, Johnson SW, Selvakumaran M, Williams SJ, Hamilton TC, Testa JR. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 2000;60(19):5390–5394. [PubMed] [Google Scholar]
- 43.Héliez C, Baricault L, Barboule N, Valette A. Paclitaxel increases p21 synthesis and accumulation of its AKT-phosphorylated form in the cytoplasm of cancer cells. Oncogene. 2003;22(21):3260–3268. doi: 10.1038/sj.onc.1206409. [DOI] [PubMed] [Google Scholar]