Abstract
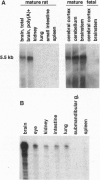
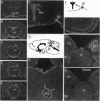
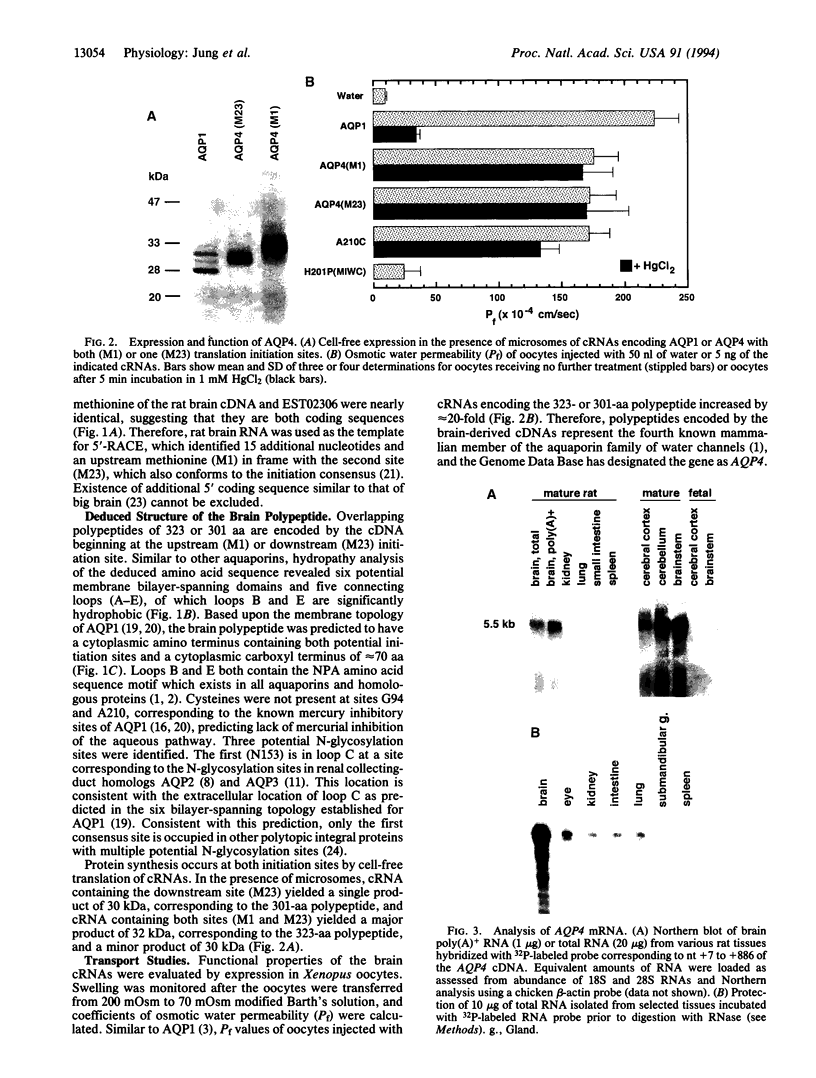
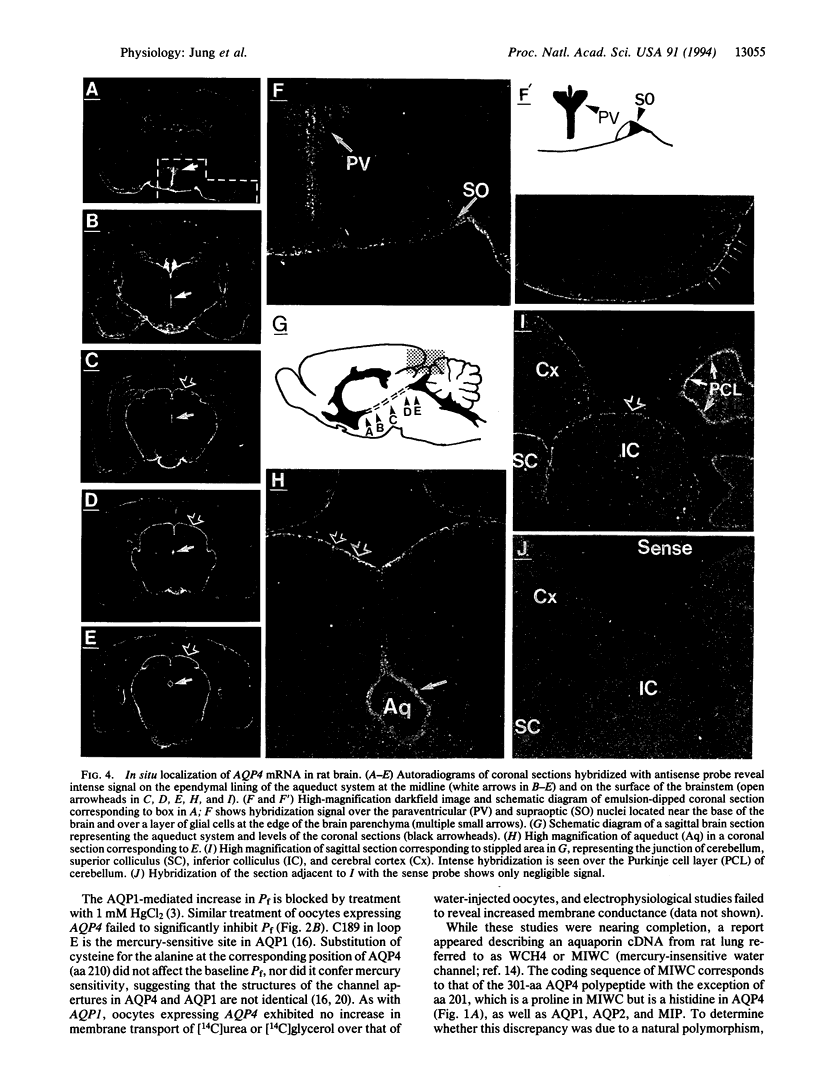
The aquaporins transport water through membranes of numerous tissues, but the molecular mechanisms for sensing changes in extracellular osmolality and regulating water balance in brain are unknown. We have isolated a brain aquaporin by homology cloning. Like aquaporin 1 (AQP1, also known as CHIP, channel-forming integral membrane protein of 28 kDa), the deduced polypeptide has six putative transmembrane domains but lacks cysteines at the known mercury-sensitive sites. Two initiation sites were identified encoding polypeptides of 301 and 323 amino acids; expression of each in Xenopus oocytes conferred a 20-fold increase in osmotic water permeability not blocked by 1 mM HgCl2, even after substitution of cysteine at the predicted mercury-sensitive site. Northern analysis and RNase protection demonstrated the mRNA to be abundant in mature rat brain but only weakly detectable in eye, kidney, intestine, and lung. In situ hybridization of brain localized the mRNA to ependymal cells lining the aqueduct, glial cells forming the edge of the cerebral cortex and brainstem, vasopressin-secretory neurons in supraoptic and paraventricular nuclei of hypothalamus, and Purkinje cells of cerebellum. Its distinctive expression pattern implicates this fourth mammalian member of the aquaporin water channel family (designated gene symbol, AQP4) as the osmoreceptor which regulates body water balance and mediates water flow within the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Bhat R. V., Baraban J. M., Johnson R. C., Eipper B. A., Mains R. E. High levels of expression of the tumor suppressor gene APC during development of the rat central nervous system. J Neurosci. 1994 May;14(5 Pt 2):3059–3071. doi: 10.1523/JNEUROSCI.14-05-03059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Denker B. M., Smith B. L., Kuhajda F. P., Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988 Oct 25;263(30):15634–15642. [PubMed] [Google Scholar]
- Dunn F. L., Brennan T. J., Nelson A. E., Robertson G. L. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973 Dec;52(12):3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Ma T., Skach W., Matthay M. A., Verkman A. S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994 Feb 25;269(8):5497–5500. [PubMed] [Google Scholar]
- Ishibashi K., Sasaki S., Fushimi K., Uchida S., Kuwahara M., Saito H., Furukawa T., Nakajima K., Yamaguchi Y., Gojobori T. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J. S., Preston G. M., Smith B. L., Guggino W. B., Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994 May 20;269(20):14648–14654. [PubMed] [Google Scholar]
- Knepper M. A. The aquaporin family of molecular water channels. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6255–6258. doi: 10.1073/pnas.91.14.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Landolt-Marticorena C., Reithmeier R. A. Asparagine-linked oligosaccharides are localized to single extracytosolic segments in multi-span membrane glycoproteins. Biochem J. 1994 Aug 15;302(Pt 1):253–260. doi: 10.1042/bj3020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., DiGiovanni S. R., Christensen E. I., Knepper M. A., Harris H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S., Smith B. L., Christensen E. I., Knepper M. A., Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993 Jan;120(2):371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993 Jul 22;364(6435):341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. J Biol Chem. 1994 Jan 21;269(3):1668–1673. [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Rao Y., Jan L. Y., Jan Y. N. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990 May 10;345(6271):163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Valenti G., Verbavatz J. M., Van Hoek A. N., Verkman A. S., Ausiello D. A., Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 1992 Dec;263(6 Pt 1):C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992 Jul;3(1):12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]