Abstract
Cytomegalovirus (CMV) reactivates in >30% of CMV seropositive patients after allogeneic hematopoietic cell transplantation (HCT). Previously, we reported an increase of NK cells expressing NKG2C, CD57 and inhibitory killer-cell immunoglobulin-like receptors (KIRs) in response to CMV reactivation post-HCT. These NK cells persist after the resolution of infection and display ‘adaptive’ or memory properties. Despite these findings, the differential impact of persistent/inactive vs. reactivated CMV on NK vs. T cell maturation following HCT from different graft sources has not been defined. We compared the phenotype of NK and T cells from 292 recipients of allogeneic sibling (n = 118) or umbilical cord blood (UCB; n = 174) grafts based on recipient pre-transplant CMV serostatus and post-HCT CMV reactivation. This cohort was utilized to evaluate CMV-dependent increases in KIR-expressing NK cells exhibiting an ‘adaptive’ phenotype (NKG2C+CD57+). Compared to CMV seronegative recipients, those who reactivated CMV (React+) had the highest adaptive cell frequencies, while intermediate frequencies were observed in CMV seropositive recipients harboring persistent/non-replicating CMV. The same effect was observed in T cells and CD56+ T cells. These adaptive lymphocyte subsets were increased in CMV seropositive recipients of sibling, but not UCB grafts, and correlated with lower rates of CMV reactivation (sibling 33% vs. UCB 51%; p<0.01). These data suggest that persistent/non-replicating recipient CMV induces rapid production of adaptive NK and T cells from mature cells from sibling, but not UCB grafts. These adaptive lymphocytes are associated with protection from CMV reactivation.
Introduction
Natural killer (NK) cells are an important component of the innate immune response against both tumors and virally-infected cells. NK cells mediate an anti-viral response through the direct killing of infected cells and through secretion of cytokines and chemokines (e.g. IFN-γ, TNF, MIP-1β) that recruit or modulate the adaptive immune response. NK cell function is not triggered directly through recognition of pathogen-associated antigens. Instead, the NK cell response is regulated by various inhibitory NK cell receptors (iNKR) and activating NK cell receptors (aNKR) that recognize ligands on target cells(1-3). The best defined iNKRs are the killer-cell immunoglobulin-like receptors (KIRs) that recognize polymorphic epitopes on histocompatibility leukocyte antigen (HLA) molecules (mainly, HLA-B and –C) and the C-type lectin heterodimer, NKG2A/CD94, that recognizes the non-classical HLA molecule, HLA-E. When triggered by self-HLA, iNKRs induce an inhibitory signal cascade that prevents NK cell activation. Inhibitory signals can be overridden by aNKRs (e.g. NKG2D, NKG2C), also expressed on NK cells. The net balance between inhibitory and activating signals determines whether NK cells kill transformed or virally infected targets(2). In the presence of targets with surface expression of aNKR ligands and down-modulation of self-HLA, the balance of signaling in NK cells is skewed towards activation.
Human cytomegalovirus (CMV) is a common β-herpesvirus which infects more than 60% of the US population(4). In healthy, immunocompetent individuals the immune response quickly suppresses CMV replication resulting in asymptomatic or mild illness(5) leaving residual persistent CMV where only a few CMV genes are undergoing transcription(6). While healthy individuals rarely reactivate CMV causing symptomatic infection, viral reactivation which can occur during immunosuppression can lead to severe, life threatening complications(7, 8). Upon primary infection, viremia results in activation of the innate and adaptive arms of the immune system leading to control of the virus. This culminates in a polyclonal T cell response to viral epitopes displayed in the context of HLA class I and II(9-11) as well as neutralizing antibodies(12). Interestingly, some studies have demonstrated that responding T cells can acquire properties of NK cells, including the expression of CD56 and associated cytotoxicity and cytokine production(13). Others have shown that CMV-reactive T cell clones can also mediate anticancer activity(14), suggesting a profound and potentially unique impact of CMV on the adaptive immune system. An increase of the CD56+ T cell subset has been observed in elderly CMV seropositive (Sero+) individuals(15) and in healthy CMV Sero+ individuals whose NK cells express high levels of NKG2C and CD57 and produce IFN-γ and TNFα after exposure to CMV antigens(13).
Recently, a subset of murine NK cells was identified that expanded following productive murine CMV (MCMV) infection. This subset expresses the aNKR Ly49H and expands after interaction between Ly49H and the MCMV-encoded protein m157(16). Upon rechallenge with MCMV, this NK subset exhibits a memory-like recall response. A similar increase of NKG2C-expressing NK cells expands in humans after co-culture with CMV-infected fibroblasts and is highly enriched in CMV Sero+ individuals(17, 18). NK cells expressing NKG2C along with CD57 (NKG2C+CD57+) are considered to be “adaptive”, exhibiting memory-like responses with specific function against CMV-infected cells. This population is also increased in patients infected with Hantavirus(19) and HIV(20), but only in those who are co-infected with CMV. In CMV seronegative (Sero-) patients with other viral infections, the frequency of this NK population is low(17). NKG2C is an aNKR that recognizes HLA-E, but with a lower affinity than the iNKR NKG2A(21). It is not known whether the increase of NKG2C-expressing cells is mediated though interaction with HLA-E, viral peptide loaded HLA-E or an unknown ligand of viral or host origin.
In this study we evaluated whether the source of the graft used for HCT affects CMV-induced cellular immune responses. We hypothesized that adaptive NK and T lymphocyte populations would be enhanced after allogeneic sibling grafts from adult donors compared to UCB grafts, which are of fetal origin. We believe that mature CMV-specific lymphocytes reconstituting from sibling donor grafts have the potential to recognize recipient CMV, whereas UCB grafts have few, if any, mature NK and T cells. We compared the reconstitution kinetics of CD56+CD3- NK cells, CD56+CD3+ T cells and CD56-CD3+ T cells in CMV Sero- or Sero+ recipients stratified based on post-HCT CMV reactivation (React+) vs. non-reactivation (React-) to evaluate the impact of CMV infection in shaping this adaptive immune response.
Materials and Methods
Patients and Samples
Our cohort included 292 HCT recipients of allogeneic sibling (n = 118) or UCB (n = 174) grafts. These recipients were approximately half CMV sero- (n = 140) and half CMV sero+ (n = 152), of whom 83 did not reactivate (React-) and 69 did reactivate (React+). Peripheral blood mononuclear cells (PBMC) were collected, stained and analyzed fresh from recipients post-HCT at days 28, 60, 100, 180 and 365. Patients were monitored weekly for CMV reactivation by a quantitative polymerase chain reaction (PCR) assay for viral DNA. A second cohort of 394 HCT seropositive recipients of UCB (n= 270) or allogeneic sibling (n= 124) from seronegative donors (n = 61) or seropositive donors (n = 63) was used to evaluate CMV reactivation post-transplant. CMV reactivation was defined as viremia with >100 viral DNA copies per mL of blood from the University of Minnesota Medical Center clinical virology laboratory. Samples from transplanted patients were acquired after obtaining informed consent and approval from the University of Minnesota Institutional Review Board and in accordance with the declaration of Helsinki.
Staining, acquisition and analysis
The fluorochrome-conjugated antibodies used to assess phenotypes were: PE-Texas Red-conjugated anti-CD3 (clone S4.1, Life Technologies), PE-Cy7-conjugated anti-CD56 (clone NCAM16.2, BD Biosciences), FITC-conjugated anti-CD158b (clone CH-L, BD Pharmingen), FITC-conjugated anti-CD158a (clone HP-3E4, BD Pharmingen), FITC-conjugated or PE-conjugated anti-NKB1 (clone DX9, BD Biosciences), APC-conjugated anti-NKG2A (clone 13144, R&D Systems), PE-conjugated anti-NKG2C (clone 134591, R&D Systems) and Pacific Blue-conjugated anti-CD57 (clone HCD57, Biolegend). Data was collected using an LSRII flow cytometer (BD) and analysis was performed using FlowJo 9.3.2 software (TreeStar).
Statistical Analysis
The percentages of NK cells for different patient groups at different time points were presented with mean ± standard error of the mean (SEM). For comparisons between groups with different pre-HCT CMV serostatus or post-HCT CMV reactivation, t test, with equal or unequal variance (as appropriate) was used. The cumulative incidences of CMV reactivation up to 100 days post-HCT were estimated for the allogeneic sibling and UCB HCT groups. Fine and Gray regression was employed to assess the independent effect of donor type treating non-reactivation mortality as a competing risk. Other factors considered in the regression models were diagnosis, year of transplant, conditioning, GVHD prophylaxis, gender, disease risk, acute GVHD, age and prior autologous transplant. Adjusted cumulative incidence curves were used to estimate CMV reactivation adjusting for risk factors from the Fine and Gray regression model. All tests were two-sided. Statistical analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and the R package cmprsk.
Results
CMV seropositive recipients and CMV reactivators reconstitute with increased frequencies of NK and T cells bearing an adaptive phenotype in the first year after HCT
To monitor immune reconstitution in HCT recipients, peripheral blood was drawn from 292 patients receiving sibling (n = 118) or UCB (n = 174) grafts at various time points post-HCT and evaluated for maturation and known functional subsets of NK cells(22, 23). Patients were stratified based on recipient CMV serostatus prior to HCT and CMV reactivation after transplantation to identify the impact of these two factors on immune reconstitution. We compared 3 groups; CMV seronegative recipients (without CMV reactivation; Sero-/React-), CMV seropositive recipients without reactivation but with persistent/non-replicating CMV (Sero+/React-) and CMV reactivators (React+) as determined by weekly clinical PCR for Cytomegalovirus DNA.
We first measured expression of NKG2C and CD57 as markers for CMV-induced adaptive cells. When compared to recipients seronegative for CMV (Sero-/React-), we measured increased expression of NKG2C (including the NKG2C+CD57- subset) on NK cells from seropositive recipients (Sero+/React-) throughout the first year post-HCT. The largest increases were found in the subset of seropositive recipients who reactivated CMV (React+) (Figure 1A and B). Increased NKG2C+CD57+ NK cells were found in seropositive versus seronegative recipients at all time points evaluated, but the difference was most prominent at 6 months (Figure 1B). Consistent with the ontogeny of NKG2A expression preceding KIR, decreased NKG2A expression was observed in CMV seropositive recipients, with the most notable difference at 1 year post-HCT (56% ± 4.2 [Sero+/React-] vs. 73% ± 1.3 [Sero-/React-]; p=0.0004]. A similar decrease was observed in CMV seropositive recipients who reactivated (React+) (Figure 1C).
Figure 1.
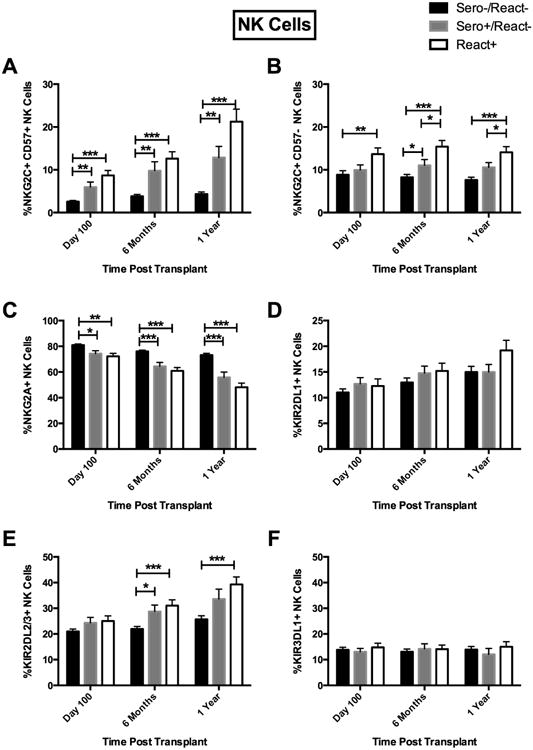
Recipient CMV Sero+/React- and React+ results in expansion of KIR2DL2/3+ and NKG2C+CD57+ NK cells post transplant. (A) NKG2C+CD57+ cells were evaluated from Sero-/React-(black bars, n=91), Sero+/React- (light gray bars, n=53) and CMV React+ (white bars, n=59), (B) NKG2C+CD57-, (C) NKG2A+, (D) KIR2DL1+, (E) KIR2DL2/3+ and (F) KIR3DL1+ expression was evaluated on CD56+CD3- NK cells post transplant from CMV Sero-/React- (black bars, n=116), Sero+/React- (light gray bars, n=54) and CMV React+ (white bars, n=57). Recipient samples were analyzed at 100 days, 6 months and 1 year post transplant. Bars represent the mean percentage of lymphocytes expressing each phenotype ± SEM. T test (equal-variance or unequal-variance t test, based on variance test result) was used for the pairwise comparisons between groups at each time point. *p<0.05, **p<0.01, ***p<0.001.
We also evaluated KIR expression as a marker of NK maturation and enhanced function(24). While the percentage of NK cells expressing the HLA-C2 specific KIR2DL1 (CD158a) was not different in each group (Figure 1D), the CMV seropositive recipients did have a significantly increased frequency of NK cells that recognize HLA-C1 (expressing KIR2DL2/3 stained with CD158b) (29% ± 2.5 [Sero+/React-] vs. 22% ± 1.0 [Sero-/React-]; p=0.02) (Figure 1E). The difference was more pronounced when comparing the subset from those who reactivated CMV (React+) with CMV seronegative recipients. No significant differences were observed in expression of KIR3DL1 (stained by CD158e), which recognizes HLA-Bw4 (Figure 1F).
Persistent, non-reactivated CMV and CMV reactivation were associated with similar changes to the phenotype of NKG2C+ T cell subsets (Supplemental Figure 1). Significant increases in the percentage of NKG2C+CD57+ CD56+CD3+ T cells were found in CMV seropositive recipients, especially in CMV reactivators (15% ± 2.5 [React+]) vs. CMV seronegative recipients (2.1% ± 0.5) [Sero-/React-]; p<0.0001) for up to 1 year (Figure 2A). Similarly, NKG2C+CD57- CD56+CD3+ T cells were increased (7.3% ± 1.2 [React+] vs. 1.6% ± 0.2 [Sero-/React-]; p<0.0001) (Figure 2B). The same NKG2C+CD57+ subset was increased in CD56-CD3+ T cells (0.7% ± 0.2 [React+] vs. 0.1% ± 0.02 [Sero-/React-]; p=0.0003) (Figure 3A) as was the NKG2C+CD57- subset (0.6% ± 0.2 [React+] vs. 0.1% ± 0.02 [Sero-/React-]; p=0.0345) (Figure 3B) throughout the first year. CMV reactivation was also associated with a concurrent decrease in NKG2A expression on CD56+CD3+ T cells (Figure 2C); however no significant decreases were observed in NKG2A expression on CD56-CD3+ T cells (Figure 3C). In contrast to NK cells, where persistent/non-replicating or reactivated CMV in recipients was not associated with increases in KIR2DL1+, CD56+CD3+ and CD56-CD3+ T cells from the React+ groups expressed significantly more KIR2DL1+ from day 100 and persisting to 1 year compared to CMV seronegative recipients (Figures 2D and 3D). Corresponding increases in KIR2DL2/3-expressing CD56+CD3+ and CD56-CD3+ T cells were observed over the first year post-HCT in CMV reactivators vs. CMV seronegative recipients (Figures 2E and 3E). Interestingly, CMV was not associated with changes in the expression of KIR3DL1 on NK cells while both CD56+CD3+ and CD56-CD3+ T cells exhibited increased expression of this inhibitory receptor after CMV reactivation, but not in CMV seropositive non-reactivating recipients (Figures 2F and 3F).
Figure 2.
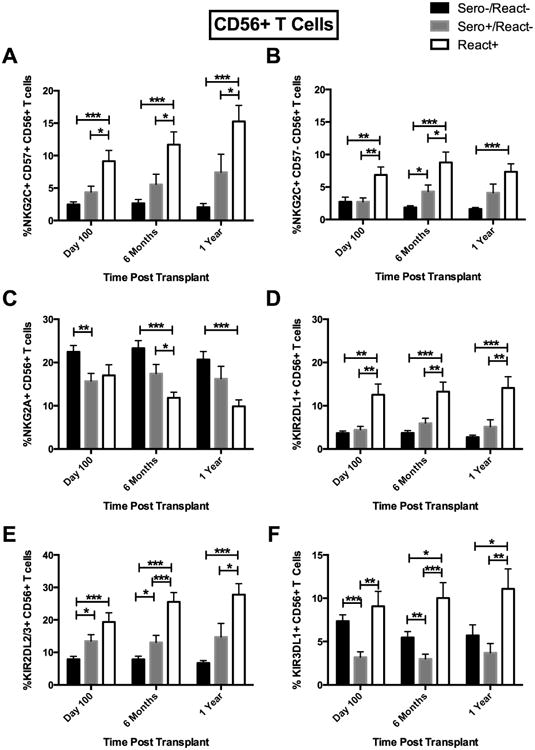
Recipient CMV Sero+/React- and React+ results in expansion of KIR2DL2/3+ and NKG2C+CD57+ CD56+CD3+ T cells post-HCT. (A) NKG2C+CD57+ CD56+CD3+ T cells in CMV Sero-/React- (black bars, n=116), Sero+/React- (light gray bars, n=53) and CMV React+ (white bars, n=57), (B) NKG2C+CD57-, (C) NKG2A+, (D) KIR2DL1+, (E) KIR2DL2/3+ and (F) KIR3DL1+ expression was evaluated on CD56+CD3+ T cells from CMV Sero-/React- (black bars, n=115), Sero+/React- (light gray bars, n=54) and CMV React+ (white bars, n=57). Analysis as in Fig 1. *p<0.05, **p<0.01, ***p<0.001.
Figure 3.
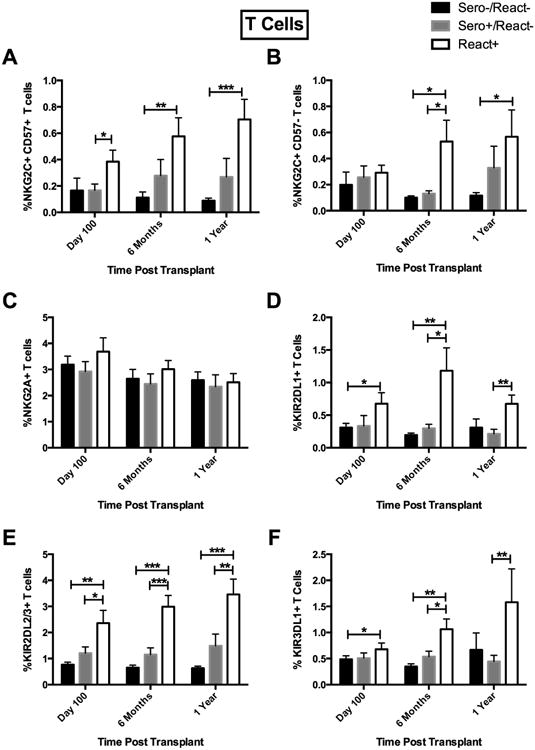
CMV Sero+/React- and React+ results in expansion of KIR2DL2/3+ and NKG2C+CD57+ CD56-CD3+ T cells post-HCT. (A) NKG2C+CD57+ T cells in Sero-/React- (black bars, n=116), Sero+/React- (light gray bars, n=54) and CMV React+ (white bars, n=57), (B) NKG2C+CD57-, (C) NKG2A+, (D) KIR2DL1+, (E) KIR2DL2/3+ and (F) KIR3DL1+ CD56-CD3+ T cells were evaluated from Sero-/React- (black bars, n=116), Sero+/React- (light gray bars, n=54) and CMV React+ (white bars, n=57). Analysis as in Fig 1. *p<0.05, **p<0.01, ***p<0.001.
It should be noted that the NK cell and T cell increases with CMV reactivation were similar in recipients of sibling and UCB grafts (data not shown), whereas the increases to non-reactivated persistent CMV in the seropositive non-reactivating recipients differed based on graft type. While no increases were observed in seropositive recipients of UCB grafts, the increases in adaptive cells were specific to the CMV seropositive recipients who received sibling grafts. Thus CMV, persistent or non-replicating in seropositive recipients or replicating in recipients who reactivate, imparts a global impact on reconstituting NK cells and T cells after HCT. Increased frequencies of cells primed for adaptive function are marked by NKG2C, and this imprint is not exclusive to CD57+ cells. In most cases the data demonstrate a stepwise increase in the frequency of the CMV adaptive lymphocyte subsets with significant, but smaller adaptive responses in persistent CMV seropositive recipients and the largest effect in recipients who reactivate CMV. These findings suggest a dose response to CMV exposure in mediating these cellular responses.
Persistent recipient CMV drives the increase of adaptive NK and T cell subsets in sibling but not UCB graft recipients
To further evaluate the impact of persistent CMV infection on NK and T cell reconstitution after HCT we compared CMV seropositive and seronegative recipients who did not reactivate (Sero-/React- and Sero+/React-), stratifying further on the graft source (sibling vs. UCB). After allogeneic sibling transplantation, Sero+/React- recipients, who express persistent/non-replicating CMV, exhibited an increase in CMV adaptive KIR+ (KIR2DL2+/3+) NK cells [32% ± 3.9] by 6 months post HCT compared to Sero-/React- recipients [21% ± 1.3; p=0.01] (Figure 4A). Similarly, decreased expression of NKG2A was measured on seropositive vs. seronegative recipients beginning 60 days post-HCT and continuing to the 6 month time point (57% ± 4.1 [Sero+/React-] vs. 77% ± 1.6 [Sero-/React-]; p=0.0002] (Figure 4B). This was also associated with increased NKG2C+ NK cells with (Figure 4C) or without (Figure 4D) CD57 for up to 6 months (12% ± 2.7 [Sero+/React-] vs. 6% ± 0.7 [Sero-/React-]; p=0.05; and 10% ± 2.3 [Sero+/React-] vs. 3% ± 0.6 [Sero-/React-]; p=0.0095, respectively). Importantly, the effect of persistent/non-replicating recipient CMV expression on NK and T cell reconstitution was not seen in recipients of UCB grafts (Figures 4E-4H). Unlike UCB grafts, which are CMV naïve, sibling donors are often exposed to CMV (39% in our cohort) and produce grafts which may contain lymphocytes which immunologically recognize CMV. Thus the differential response of CMV seropositive recipients to grafts from sibling vs. UCB grafts may reflect the influence of donor exposure to CMV on the reconstitution of graft cells or alternatively, their level of maturation.
Figure 4.
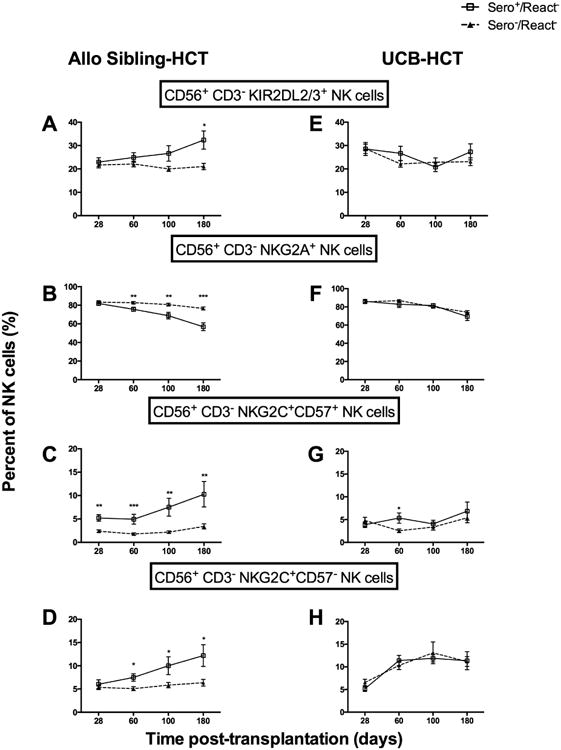
Recipient CMV Sero+ is associated with increased KIR2DL2/3 and NKG2C expressing adaptive CD56+CD3- NK cells in React- recipients of sibling but not UCB grafts. CD56+CD3- NK cells from CMV Sero-/React- (▲, n=77) or CMV Sero+/React- (□, n=50). Shown are the percentage of cells expressing (A) KIR2DL2/3+, (B) NKG2A+, (C) NKG2C+CD57+ or (D) NKG2C+CD57-. The same subsets are shown for React- UCB HCT who were either CMV Sero-/React- (▲, n=61) or CMV Sero+/React- (□, n=47) (E-H). Analysis as in Fig 1-3 *p<0.05, **p<0.01, ***p<0.001.
Evaluation of the T cell subsets demonstrated the same response in CMV seropositive recipients, again specific to those receiving sibling and not UCB grafts (Figure 5). For example, at day 28 post-HCT, CMV seropositive recipients expressed significantly higher percentages of KIR2DL2/3+ T cells (20% ± 1.6 [Sero+/React-] vs. 9.5% ± 1.6 [Sero-/React-]; p<0.0001) (Figure 5A). The NKG2A-expressing T cells were decreased (19% ± 2.1 [Sero+/React-] vs. 29% ± 2.6 [Sero-/React-]; p=0.003] by 6 months (Figure 5B) and we observed increased T cells bearing the adaptive phenotypes NKG2C+CD57+ (8.5% ± 2.3 [Sero+/React-] vs. 3.3% ± 1.1 [Sero-/React-]; p=0.04] and NKG2C+CD57-(4.2% ± 1.0 [Sero+/React-] vs. 1.5% ± 0.4 [Sero-/React-]; p=0.01) (Figures 5C and D). Recipient CMV serostatus was not associated with any changes in T lymphocyte reconstitution in the recipients of UCB grafts (Figure 5E-H).
Figure 5.
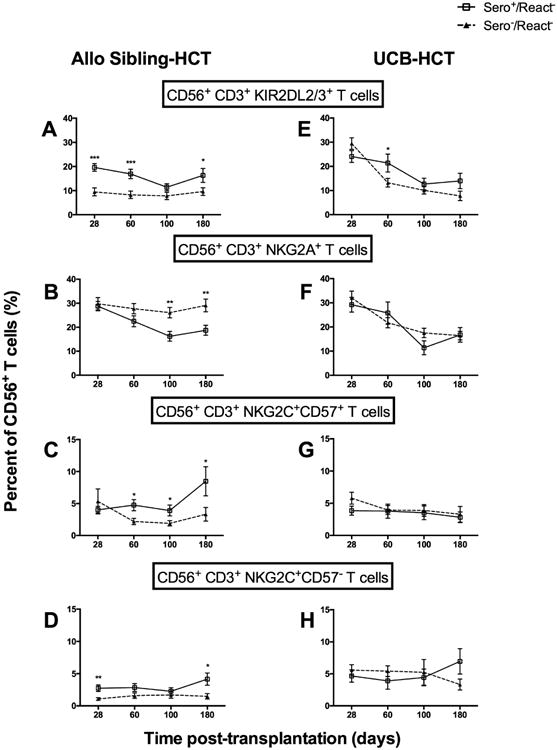
Recipient CMV sero+ is associated with increased KIR2DL2/3 and NKG2C expressing adaptive CD56+CD3+ T cells in React- recipients of sibling but not UCB grafts. CD56+CD3+ T cells CMV Sero-/React- (▲, n=73) or CMV Sero+/React- (□, n=106). Shown are the percentage of cells expressing (A) KIR2DL2/3+, (B) NKG2A+, (C) NKG2C+CD57+ or (D) NKG2C+CD57-. The same subsets are shown for React- UCB HCT who were either CMV Sero-/React- (▲, n=59) or CMV Sero+/React- (□, n=47) (E-H). Analysis as in Fig 1-3 *p<0.05, **p<0.01, ***p<0.001.
CMV reactivation is reduced in recipients of allogeneic sibling vs. UCB grafts
We evaluated the incidence of CMV reactivation in a larger cohort of 394 CMV seropositive recipients of sibling (N=124) and UCB (N=270) grafts (see demographics in Supplemental Table 1) treated at the University of Minnesota between 2001-2013. They all received similar CMV prophylaxis with acyclovir and underwent routine monitoring for CMV reactivation. CMV reactivation was more frequent in UCB vs. sibling HCT in adjusted univariate and regression models (51% (95% CI, 45-57%) vs. 33% (95% CI, 24-41%); RR=1.6 [95% CI, 1.1-2.3], p<0.01). While UCB units are CMV naïve, sibling donors may be CMV seropositive or seronegative. Therefore CMV reactivation rates of allogeneic sibling transplant recipients were further stratified based on the serostatus of the stem cell donor to evaluate if pre-exposure of the donor to CMV antigens impacted the incidence of CMV-reactivation in HCT recipients. We found no difference in CMV reactivation between the CMV seropositive (n = 63) and CMV seronegative (n = 61) donors (37% vs. 33% respectively, p = NS) indicating that donor CMV history has little or no impact on CMV-reactivation in our cohort. Analysis of the subset of patients with available samples showed that there was no significant difference in adaptive lymphocytes based on adult donor CMV status alone. Thus CMV seropositive recipients of UCB grafts have significantly higher rates of CMV reactivation compared to recipients of sibling grafts (Figure 6). Protection from CMV reactivation with sibling grafts was not related to the donor CMV status suggesting that sibling grafts contain mature NK or other immune cells which enhance reconstitution of CMV-adaptive NK and T cell subsets. The reconstitution of these adaptive lymphocytes limits CMV reactivation, unlike what was observed in UCB HCT resulting in less protection against CMV reactivation.
Figure 6.
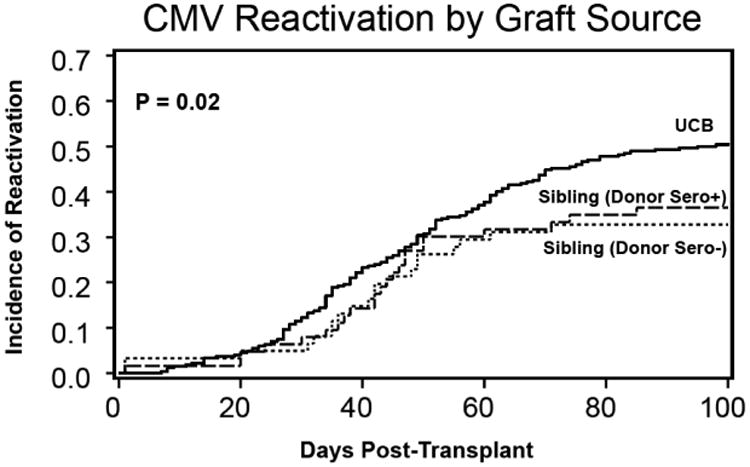
Cumulative incidence of CMV reactivation is lower in sibling vs. UCB grafts, regardless of donor serostatus. Sibling donor Sero+, n=63; 37% CMV-reactivation; donor Sero-, n=61; 33% CMV-reactivation) vs. UCB grafts (n=270; 51% CMV-reactivation).
Discussion
We studied the NK and T cell responses to persistent/non-replicating or reactivated CMV after HCT. We observed that CMV reactivation was associated with marked increases in NK and T cell subsets bearing the CMV adaptive phenotype after allogeneic HCT irrespective of graft source. However, persistent/non-replicating CMV in seropositive recipients (a lower viral antigen exposure) induced adaptive NK cells and T cells when patients were transplanted with sibling but not UCB grafts. This adaptive change was marked by an increased frequency of NKG2C+ cells, with or without CD57 expression, with associated increases in KIR and decreased NKG2A. This suggests that grafts from sibling donors are enriched with mature NK or other accessory cells, which facilitate greater and faster reconstitution of CMV-specific NK and T cells after HCT. We demonstrated that exposure to persistent CMV in seropositive recipients of sibling HCT is sufficient to promote an adaptive NK cell and T cell response although the magnitude of the response is greater in recipients who reactivate CMV. We also observed reduced rates of CMV reactivation in transplants from sibling donors compared to UCB grafts, irrespective of the donor CMV serostatus. Together, these findings suggest that more mature sibling donor immune cells impart some protection against reactivation of CMV in seropositive recipients, even when the sibling donors are CMV naïve.
We also demonstrated that the adaptive response to NK and T cell subsets is CMV specific, as there is no increase in NKG2C+CD57+ cells in CMV seronegative recipients who do not reactivate CMV. However, both persistent/non-replicating and reactivated CMV leads to NK cell maturation evidenced by less frequent NKG2A and increased KIR expression. These findings support a model where CMV primes the immune system based on the response to CMV-modulated HLA-E. NKG2C and NKG2A are most often mutually exclusive, with only rare double positive cells. In recipients who are CMV seropostitive or who reactivate CMV, T lymphocytes undergo programming such that their response to HLA-E transitions from inhibition (with NKG2A+NKG2C- cells) to activation (with NKG2C+NKG2A-cells). This is consistent with previous studies demonstrating an increase of NKG2C+ NK cells with reduced NKG2A and increased KIRs after co-culture with CMV-infected fibroblasts(18). The same phenotype was observed with NK cells from CMV seropositive healthy donors(17). Our previous studies showed that NKG2C+KIR+ NK cells that expanded after CMV reactivation were potent producers of IFN-γ after K562 target cell exposure during the acute phase of infection and long after viral clearance(25). Responses from NKG2C+KIR+ NK cells were also more pronounced when the KIRs expressed were specific for self-HLA(26) though not stratified for donor or recipient HLA. We identified increases in both KIR2DL1+ and KIR2DL2/3+ populations in CMV seropositive patients or after CMV reactivation(25). The self-KIR expressing subset is critical because KIR interaction with self-HLA promotes NK cell education and maintains their function(27).
Exposure to CMV also led to expansions of CD56+CD3+ and CD56-CD3+ T cell subsets expressing CD57 and NKG2C. The interplay between T cells, NK cells and CMV viremia may be important and reflects a polyclonal multi-subset immune response. Expansions of NKG2C+CD57+KIR+ CD56+CD3+ T cells have been reported in individuals who have been infected by CMV(28). This expanded population of KIR+CD3+CD56+ T cells is more cytotoxic than the KIR- population and is enriched for a CMV-pp65 specific TCR(28). However, pp65-tetramer staining was not included in our analysis and will be evaluated in future studies. It is uncertain whether CD57+ or NKG2C+ T cells mediate a direct CMV-specific immune response, or whether they activate the immune response through interaction with NK cells or through secretion of cytokines.
Previous studies have reported an expansion of γδ-TCR+ T cells in individuals who reactivate CMV(29-31). After exposure to CMV-infected fibroblasts, γδT cells show decreased expression of the Vδ2 chain (Vδ2neg) and exhibit increased IFN-γ production(32). γδT cells, as compared to αβT cells, are naturally cytotoxic(33) and are reactive against various leukemia cell lines(32), especially when the γδT cells express KIRs(34). We evaluated the percentages of γδT cells 60 days after HCT in recipients who were CMV seronegative, seropositive, or reactivated and found high frequencies of KIR+γδT cells in all groups, with no differences related to CMV infection (data not shown). Further studies are needed to determine whether these same CMV-adaptive immune subsets mediate other beneficial effects such as protection from malignant relapse.
CMV seropositive recipients of sibling donor transplants yielded enhanced reconstitution of CMV adaptive NK and T cell subsets compared to recipients of UCB transplants who showed no response to persistent/non-replicating recipient CMV. Importantly, UCB-derived NK and T cells developed into adaptive NKG2C+CD57+KIR+ subsets when recipients reactivated CMV, a condition which presumably causes greater density of CMV viral antigens to be presented to the immune system. While potent anti-virals such as gancyclovir have reduced the dangers of CMV reactivation after HCT and limited its mortality(35, 36), we have discovered that non-replicating CMV in seropositive recipients may elicit expansion of NKG2C+CD57+ NK and T cells from sibling donor grafts without the associated morbidity of CMV reactivation. These results may have important clinical implications in light of the reports that CMV reactivation is associated with a reduced risk of post-HCT relapse in patients with acute myeloid leukemia (AML) (37, 38). It is unknown whether these adaptive NK and/or T cells directly mediate an anti-AML response, which needs further study. Alternatively, it is possible that the expanded adaptive NK and T cell populations may have increased cytokine secretion in response to AML, thereby limiting the risk of post-HCT relapse, an area of active investigation in our laboratory.
Supplementary Material
Supplemental Figure 1: General gating strategy for all patient samples. Lymphocytes are gated on using forward scatter vs side scatter. A plot of CD3 vs CD56 is then used to gate on all NK cells (CD3-CD56+), CD56+ T cells (CD3+CD56+) and T cells (CD3+CD56-). Gates from each lymphocyte subset were then analyzed for their expression of NKG2C vs CD57, KIR2DL1, KIR2DL2/3, KIR3DL1 and NKG2A.
Supplemental Table 1. Demographics Among Recipient + CMV Sibling Transplants
Highlights.
CMV sero+ and reactivated HCT recipients have increased levels of adaptive NK cells
Expansion of cells with the adaptive phenotype also seen in CD56+ and CD56- T-cells
Adaptive lymphocytes increase in sero+ recipients of sibling but not UCB HCT
Sero+ sibling HCT recipients have a lower CMV reactivation rate than UCB recipients
Acknowledgments
The authors also acknowledge cell-processing services from the Translational Therapy and FACS Cores of the Masonic Cancer Center. The authors also thank staff in the Cancer Center Clinical Trials Office for sample procurement, in the Oncology Medical Informatics and Service Core for data integration, and in the Biostatistical Core for statistical analysis (P30 CA 77598).
Footnotes
This work was supported by NCI and NIH (P01 CA111412 [JSM, DJW, MRV, SC, TED], CA65493 [JSM, TED, SC, BRB, JW, MRV], R01 CA077544, R01 AI103960, R01 AI63356 [DJD], R01 AI100879 [MRV], R01 CA72669 [BRB]) and T32 HD007062
Authorship Contributions: Z.D. designed and performed experiments, analyzed data and wrote the manuscript; T.E.D. and X.L. performed biostatistical analyses of transplant data; C.B., B.R.B., J.W., M.R.V. and D.J.W supervised clinical research; S.C and J.S.M. supervised research and wrote the manuscript. DJD extensively revised virologic aspects of the manuscript. All authors reviewed, edited and approved the final manuscript.
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature immunology. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell receptors. Annual review of immunology. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 3.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC, Moretta L. Receptors for HLA class-I molecules in human natural killer cells. Annual review of immunology. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 4.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 5.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clinical microbiology reviews. 2009;22:76–98. doi: 10.1128/CMR.00034-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Current topics in microbiology and immunology. 2008;325:297–313. doi: 10.1007/978-3-540-77349-8_17. [DOI] [PubMed] [Google Scholar]
- 7.Konoplev S, Champlin RE, Giralt S, Ueno NT, Khouri I, Raad I, Rolston K, Jacobson K, Tarrand J, Luna M, Nguyen Q, Whimbey E. Cytomegalovirus pneumonia in adult autologous blood and marrow transplant recipients. Bone marrow transplantation. 2001;27:877–881. doi: 10.1038/sj.bmt.1702877. [DOI] [PubMed] [Google Scholar]
- 8.Boeckh M, Stevens-Ayers T, Bowden RA. Cytomegalovirus pp65 antigenemia after autologous marrow and peripheral blood stem cell transplantation. The Journal of infectious diseases. 1996;174:907–912. doi: 10.1093/infdis/174.5.907. [DOI] [PubMed] [Google Scholar]
- 9.Rentenaar RJ, Gamadia LE, van DerHoek N, van Diepen FN, Boom R, Weel JF, Wertheim-van Dillen PM, van Lier RA, ten Berge IJ. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. The Journal of clinical investigation. 2000;105:541–548. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond DJ, York J, Sun JY, Wright CL, Forman SJ. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood. 1997;90:1751–1767. [PubMed] [Google Scholar]
- 11.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of experimental medicine. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouts AE, Chan P, Stephan JP, Vandlen R, Feierbach B. Antibodies against the gH/gL/UL128/UL130/UL131 complex comprise the majority of the anti-cytomegalovirus (anti-CMV) neutralizing antibody response in CMV hyperimmune globulin. Journal of virology. 2012;86:7444–7447. doi: 10.1128/JVI.00467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almehmadi M, Flanagan BF, Khan N, Alomar S, Christmas SE. Increased numbers and functional activity of CD56(+) T cells in healthy cytomegalovirus positive subjects. Immunology. 2014;142:258–268. doi: 10.1111/imm.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 15.Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clinical immunology. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 16.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 18.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. The Journal of experimental medicine. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, Moretta A, Mavilio D. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. Aids. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 21.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. The EMBO journal. 1999;18:4250–4260. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. Journal of immunology. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felices M, Ankarlo DE, Lenvik TR, Nelson HH, Blazar BR, Verneris MR, Miller JS. Notch signaling at later stages of NK cell development enhances KIR expression and functional maturation. Journal of immunology. 2014;193:3344–3354. doi: 10.4049/jimmunol.1400534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Chan WK, Rujkijyanont P, Neale G, Yang J, Bari R, Das Gupta N, Holladay M, Rooney B, Leung W. Multiplex and genome-wide analyses reveal distinctive properties of KIR+ and CD56+ T cells in human blood. Journal of immunology. 2013;191:1625–1636. doi: 10.4049/jimmunol.1300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, Michelson S, Meric C, Hallet MM, Kourilsky P, Potaux L, Bonneville M, Moreau JF. Implication of gammadelta T cells in the human immune response to cytomegalovirus. The Journal of clinical investigation. 1999;103:1437–1449. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couzi L, Lafarge X, Pitard V, Neau-Cransac M, Dromer C, Billes MA, Lacaille F, Moreau JF, Merville P, Dechanet-Merville J. Gamma-delta T cell expansion is closely associated with cytomegalovirus infection in all solid organ transplant recipients. Transplant international : official journal of the European Society for Organ Transplantation. 2011;24:e40–42. doi: 10.1111/j.1432-2277.2010.01181.x. [DOI] [PubMed] [Google Scholar]
- 31.Alejenef A, Pachnio A, Halawi M, Christmas SE, Moss PA, Khan N. Cytomegalovirus drives Vdelta2neg gammadelta T cell inflation in many healthy virus carriers with increasing age. Clinical and experimental immunology. 2014;176:418–428. doi: 10.1111/cei.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, Heijhuurs S, Sebestyen Z, Grunder C, Marcu-Malina V, Marchant A, Donner C, Plachter B, Vermijlen D, van Baarle D, Kuball J. gammadelta T cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328–1338. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira LM. Gammadelta T cells: innately adaptive immune cells? International reviews of immunology. 2013;32:223–248. doi: 10.3109/08830185.2013.783831. [DOI] [PubMed] [Google Scholar]
- 34.Dolstra H, Fredrix H, van der Meer A, de Witte T, Figdor C, van de Wiel-van Kemenade E. TCR gamma delta cytotoxic T lymphocytes expressing the killer cell- inhibitory receptor p58.2 (CD158b) selectively lyse acute myeloid leukemia cells. Bone marrow transplantation. 2001;27:1087–1093. doi: 10.1038/sj.bmt.1703043. [DOI] [PubMed] [Google Scholar]
- 35.Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 36.Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43:869–880. doi: 10.1086/507337. [DOI] [PubMed] [Google Scholar]
- 37.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, Christoph S, Gromke T, Kordelas L, Ottinger HD, Ross RS, Horn PA, Schnittger S, Beelen DW. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 38.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, Riddell SR, Boeckh M. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: General gating strategy for all patient samples. Lymphocytes are gated on using forward scatter vs side scatter. A plot of CD3 vs CD56 is then used to gate on all NK cells (CD3-CD56+), CD56+ T cells (CD3+CD56+) and T cells (CD3+CD56-). Gates from each lymphocyte subset were then analyzed for their expression of NKG2C vs CD57, KIR2DL1, KIR2DL2/3, KIR3DL1 and NKG2A.
Supplemental Table 1. Demographics Among Recipient + CMV Sibling Transplants


