Abstract
Although cisplatin has played a role in “standard-of-care” multimodality therapy for patients with advanced squamous cell carcinoma of the head and neck (HNSCC), the rate of treatment failure remains particularly high for patients receiving cisplatin whose tumors have mutations in the TP53 gene. We found that cisplatin treatment of HNSCC cells with mutant TP53 leads to arrest of cells in the G2 phase of the cell cycle, leading us to hypothesize that the wee-1 kinase inhibitor MK-1775 would abrogate the cisplatin-induced G2 block and thereby sensitize isogenic HNSCC cells with mutant TP53 or lacking p53 expression to cisplatin. We tested this hypothesis using clonogenic survival assays, flow cytometry, and in vivo tumor growth delay experiments with an orthotopic nude mouse model of oral tongue cancer. We also used a novel TP53 mutation classification scheme to identify which TP53 mutations are associated with limited tumor responses to cisplatin treatment. Clonogenic survival analyses indicate that nanomolar concentration of MK-1775 sensitizes HNSCC cells with high-risk mutant p53 to cisplatin. Consistent with its ability to chemosensitize, MK-1775 abrogated the cisplatin-induced G2 block in p53-defective cells leading to mitotic arrest associated with a senescence-like phenotype. Furthermore, MK-1775 enhanced the efficacy of cisplatin in vivo in tumors harboring TP53 mutations. These results indicate that HNSCC cells expressing high-risk p53 mutations are significantly sensitized to cisplatin therapy by the selective wee-1 kinase inhibitor, supporting the clinical evaluation of MK-1775 in combination with cisplatin for the treatment of patients with TP53 mutant HNSCC.
Introduction
Head and neck squamous cell carcinoma (HNSCC) affects over 500,000 patients worldwide annually and half this number of patients will die from the disease each year (1). Multimodality chemotherapy employing cisplatin in the neoadjuvant setting or given concurrently with radiation has become a standard of care for patients with locally advanced HNSCC (2–4). Despite advances in therapy, there is a high rate of treatment failure and the long-term survival in patients with advanced-stage head and neck cancer remains poor (5). Recent genomic data have revealed that TP53 is the most frequently mutated gene in HNSCC, occurring in up to 85% of non–human papillomavirus-positive primary tumors (6–8). Several reports have shown that TP53 mutation is associated with poor therapeutic response and decreased survival in HNSCC (9–12).
Recently, we developed an evolution-based scoring algorithm, called evolutionary action (EA), which stratifies TP53 mutations based upon scores (i.e., high risk vs. low risk) that correlate with HNSCC patient clinical outcomes and response to treatment (unpublished observations). This system (EAp53) has been further validated to predict response to cisplatin-based therapy in patients with HNSCC and in preclinical models of oral tongue cancer using established HNSCC cell lines where we have shown tumors with high-risk TP53 mutations were resistant to cisplatin relative to those with low-risk mutations or wild-type TP53 (unpublished observations). Decreased cisplatin sensitivity associated with these high-risk mutations is driven by their inability to undergo cellular senescence, the primary response for cells with wild-type TP53 (13). Therefore, an important clinical objective is to develop therapeutic strategies for overcoming inherent chemotherapy resistance in tumors from patients with high-risk TP53 mutations. Tumors with loss of p53 function are dependent on activation of the S- and G2-phase checkpoints for mediating the growth arrest needed to repair DNA damage and survive genotoxic stress, making these cells potentially sensitive to G2 checkpoint abrogation (14–17). Conceptually, abrogation of the G2 checkpoint could sensitize cisplatin-resistant mutant TP53 HNSCC cells to DNA-damaging agents and spare normal cells with intact p53 function (18). Thus, developing novel molecularly targeted drugs that abrogate the G2 checkpoint has become an intense area of research.
Wee-1 is a tyrosine kinase involved in DNA damage–induced G2–M arrest, owing to its ability to inactivate the CDC2 also known as cyclin-dependent kinase 1 (CDK1) through phosphorylation of the Tyr15 residue (19). Inhibition of Wee-1 kinase activity can override a G2 cell-cycle arrest, causing an accumulation of cells with extensive DNA damage in the M-phase which can lead to mitotic catastrophe or death (20). Therefore, inhibitors of Wee-1 have been developed as potential anticancer therapeutics (21). Recent work with MK-1775 (currently known as AZD-1775), a specific inhibitor of Wee-1, and siRNA-mediated depletion of this gene (22) suggests that Wee-1 inhibition abrogated the G2 checkpoint and selectively sensitized p53-deficient cells to various DNA-damaging agents, such as gemcitabine, carboplatin, and cisplatin (23, 24), and inhibited tumor growth in in vivo models (24, 25). In light of these preclinical findings, MK-1775 has entered phase I and II clinical trials as a chemosensitizer in combination with gemcitabine, carboplatin, or cisplatin in patients with advanced solid tumors and shows good tolerability and less cytotoxicity (26, 27).
The exact molecular mechanism(s) through which MK-1775 enhances the antitumor efficacy of cisplatin in tumor cells is not completely understood. In addition, the single-agent efficacy of MK-1775 or in combination with cisplatin therapy has not been carefully evaluated in HNSCC. Therefore, we hypothesized that the Wee-1 inhibitor MK-1775 will sensitize HNSCC-bearing high-risk mutant p53 stratified by EAp53 system to cisplatin treatment both in vitro and in vivo in preclinical models of oral cancer. Our data demonstrate that MK-1775 sensitizes high-risk p53 mutant HNSCC cell lines to cisplatin in vitro through abrogation of G2 arrest and accumulation of cells harboring unrepaired DNA lesions in mitosis. Interestingly, the combination therapy leads to aberrant mitosis associated with a senescence-like rather than an apoptotic phenotype. MK-1775 significantly potentiates the efficacy of cisplatin in high-risk mutant p53 in vivo. Furthermore, tumor cells bearing wild-type p53 displayed minimal response to MK-1775 addition, indicating that cisplatin sensitization was linked to p53 loss of function and that MK-1775 may have clinical utility to overcome drug resistance associated with cisplatin-based therapy in patients with HNSCC whose tumors have absent or mutated TP53.
Materials and Methods
Cell culture and reagents
The HNSCC cell line PCI-13 lacking endogenous p53 was obtained from the laboratory of Dr. Jennifer Grandis (University of Pittsburgh, Pittsburgh, PA) in August 2008 and engineered to stably express constructs containing wild-type p53, high-risk EA score mutant p53 (C238F and G245D), which were generated and inserted into a pBabe retroviral vector (pBaBepuro; Addgene) by using standard cloning techniques. The naturally occurring HNSCC cell lines, HN30 (wtp53) and HN31 (mutp53), were obtained in December 2008 from the laboratory of Dr. John Ensley (Wane State University, Detroit, MI). The cell lines and their isogenic derivatives were tested and authenticated against the parental cell lines by our group using short-tandem repeat analysis within 6 months of use for the current study. All cell lines were maintained in DMEM supplemented with 10% FBS, L-glutamine, sodium pyruvate, nonessential amino acids, and vitamins. The Wee-1 inhibitor MK-1775 was provided by Merck Corp. (currently licensed by AstraZeneca and known as AZD-1775), and its chemical structure has been described previously (23). Cells were trapped in mitosis using 0.2 μg/mL of nocodazole (Sigma-Aldrich). For in vitro studies, MK-1775 was prepared as 10 mmol/L stock solution in DMSO and stored at −20°C and diluted in culture medium (0.25 μmol/L) immediately before use. Staurosporine was purchased from Sigma and used at 1:1,000 final concentration
Clonogenic survival assay
For synergy analysis between cisplatin and MK-1775, 500 to 800 cells/well were seeded in 6-well plates and exposed concurrently to different fixed-ratio combinations of cisplatin (dose range, 0.01–2 μmol/L) and MK-1775 (dose range, 0.01–1 μmol/L) for 24 hours. Cells were then washed with 1× PBS to remove the cisplatin followed by addition of fresh MK-1775 for 24 hours and a second washout before culturing 10 to 14 days. Colonies were counted and survival fraction (IC50) was determined as previously described (28).
Analysis of combined drug effects
Drug synergy was determined by the combination index (CI) and isobologram analyses, according to the median-effect method of Chou and Talalay (29) using the CalcuSyn software (Bio-soft). The CI is a quantitative representation of the degree of drug interaction. Although a CI < 1.0 can be considered as synergy, we chose a cutoff point of <0.75 to more rigorously define synergy. Details of the analyses are provided in Supplementary Materials and Methods.
Antibodies and immunoblotting
Cells were treated with cisplatin (1.5 μmol/L), MK-1775 (0.25 μmol/L) either alone or in combination as previously indicated. Cell extracts were prepared and Western blot analysis was conducted as described previously (28). Membranes were blocked for 1 hour at room temperature using 1% powdered milk in 0.1% Tween-20 in TBS, and incubated overnight with the following primary antibodies, including phospho-γH2AX (Ser139; #2577), phospho-CDC2-Tyr15 (#9111), CDC2 (#9112), cyclin B1 (#4138), phospho-CDC25C-Ser216 (#4901), phospho-Histone H3 (p-HH3, #9701), PARP-1, CHK1 (#2345), and CHK1-Ser345 (#2341), all from Cell Signaling Technology; β-actin (#A5316; Sigma-Aldrich). Membranes were then washed with 0.1% Tween-20 in TBS and incubated for 1 hour at room temperature with species-specific horseradish peroxidase–conjugated secondary antibodies, and protein signals were developed using the SuperSignal West chemiluminescent system (Pierce Biotechnology). Membranes were stripped and reprobed with anti–β-actin to verify equal protein loading.
Cell-cycle analysis and apoptosis detection
HNSCC cells (50,000) were seeded in 60-mm dishes, treated the next day with 1.5 μmol/L cisplatin, 0.25 μmol/L MK-1775 either alone or in combination for 48 hours, and then harvested 0, 24, or 48 hours later. Cells were fixed in ice-cold 70% ethanol, permeabilized with 0.25% Triton X-100 in PBS, and incubated with phospho-Histone H3 (Ser10) antibody conjugated to Alexa Flour 488 (#9708; at concentration 1:100, from Cell Signaling Technology) for 2 hours at 4°C. DNA was stained with 20 μg/mL propidium iodide (PI; Sigma-Aldrich) in the presence of 100 μg/mL RNase A (Sigma-Aldrich). For apoptosis assessment, cells were treated as indicated above and DNA strand breaks detected with an APO-BrdU TUNEL assay kit (Life Technologies) according to the manufacturer’s instructions. Samples were analyzed on a Gallios Flow Cytometer (Beckman Coulter, Inc.) combined with Flo-Jo software (FloJo).
Immunofluorescence
Cells were plated on glass coverslips and treated with drugs the following day as described above. Cells were then fixed in 2% paraformaldehyde for 1 hour, washed, permeabilized in 0.2% Triton X-100 in PBS for 20 minutes, washed, and blocked for 1 hour at room temperature in 1× PBS buffer containing 2% normal goat serum, and 0.3% Triton X-100. Next, cells were incubated with primary phospho-H3 (Ser10) antibody overnight at 4°C. After washing with PBS, primary antibody was visualized with secondary Alexa Fluor–conjugated antibody. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired on a Leica confocal microscope. For assessment of mitotic catastrophe, the number of cells with multilobulated nuclei or 2 or more micronuclei per high-power field (hpf) were identified by DAPI and phalloidin (Molecular Probes) staining, counted from four quadrants (in duplicates) and reported as a percentage of total cells per field (800 cells per coverslip).
Senescence-associated-β-gal staining
Briefly, PCI-13 cells plated in 6-well plates were treated with cisplatin and MK-1775 and cultured normally for 72 hours after treatment. Senescence-associated (SA)-beta-gal staining was performed as previously described (28).
Reactive oxygen species measurement
Intracellular reactive oxygen species (ROS) levels were measured according to a published protocol using 5-(and-6)-carboxy-2′,7′-dichlorofluorescein (CM-H2DCFDA) dye (28). Briefly, after treatment with drugs, cells were loaded with CM-H2DCFDA for 60 minutes in culture media and excessive dye removed. Cells were trypsinized, and fluorescence analyzed by flow cytometry was normalized to the control condition and cell number was assayed by total DNA.
Orthotopic mouse model of oral cavity cancer and tumor growth delay
All animal experimentation was approved by the Institutional Animal Care and use Committee of the University of Texas MD Anderson Cancer Center. Our orthotopic nude mouse tongue model has been previously described (30). PCI-13 cells expressing either a high-risk mutant p53, pBabe TP53 null, or wild-type TP53 were injected into the tongues of male athymic nude mice, and 8 days after injection mice were randomized into different groups. Treatment was initiated when tumors were less than 0.5 cm3 in size. Mice were treated with 4 weekly cycles consisting of cisplatin at a dose of 4 mg/kg administered i.v. on day 1, followed by MK-1775 at a dose of 30 mg/kg (in 0.5% methylcellulose) given by oral gavage (p.o.) on days 2 and 4. Tongue tumor size was measured with microcalipers, and tumor volume calculated as previously described (30). For phospho-H3 immunohistochemical analysis, cisplatin treatment was followed by MK-1775 for 3 days (3 doses), with tumors collected 12 hours after the final dose, fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned. Tissue sections were then stained with phospho-H3 (Ser10) rabbit polyclonal antibody (Cat # 06-570; Millipore) at 1:50 concentration using the DAKO Envision + system (Dako Corp.). The percentage of area positively stained in each tumor was calculated for each field.
Statistical analysis
The Student t test was carried out to analyze in vitro data. For mouse studies, the two-tailed t test was used to compare tumor volumes between control and treatment groups. All data were expressed as mean ± SE, and P values <0.05 were considered significant.
Results
The Wee-1 inhibitor MK-1775 synergizes with cisplatin to inhibit in vitro growth of HNSCC cells expressing high-risk TP53 mutations
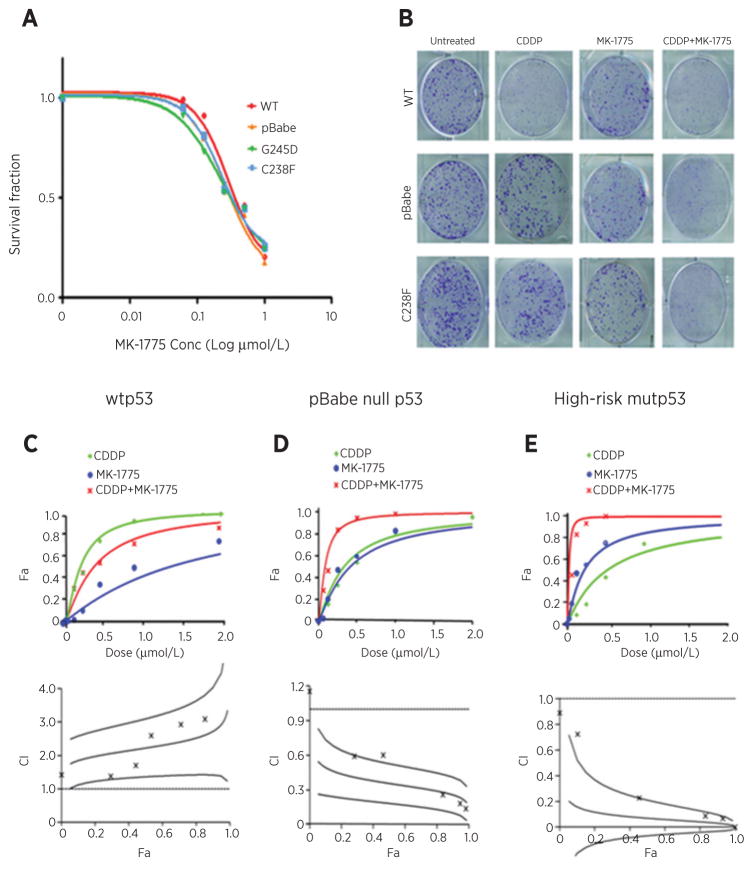
The impact of MK-1775 on HNSCC PCI-13 cells expressing wild-type TP53 or high-risk TP53 mutants treated with cisplatin was assessed using clonogenic survival assays. As shown in Fig. 1A, irrespective of the TP53 mutational status, the PCI-13 isogenic cells displayed similar sensitivity to MK-1775 as a single agent with an average IC50 of 250 nmol/L. We next investigated whether MK-1775 treatment was synergistic with cisplatin treatment in the isogenic PCI-13 cell lines, using the CI method of Chou and Talalay (29). Figure 1B shows representative images of clonogenic survival assays demonstrating the relative resistance of PCI-13 cells expressing the high-risk mutant (C238F) or lacking p53 (pBabe), which could be overcome by addition of MK-1775. In PCI-13 cells expressing wild-type TP53, MK-1775 did not cause shift of the cisplatin response curve and these agents were found to be antagonistic; the CI value [fraction affected (Fa) 0.5, ± SD] was 2.22 ± 0.44 (Fig. 1C, top plot). The results are also displayed as a CI plot (Fig. 1C, bottom plot) and show no synergistic interaction at the more relevant FA values. However, MK-1775 significantly enhanced the cytotoxic effect of cisplatin in p53-deficient HNSCC cells carrying only pBabe control vector (Fig. 1D, top plot) and those expressing the high-risk TP53 mutation (C238F; Fig. 1E, top plot). The combination effect reveals strong synergism manifested by the shift of cisplatin response curves and the IC values (Fa 0.5, ± SD) of 0.35 ± 0.08, and 0.07 ± 0.07, respectively. The CI plots (Fig. 1D and E, bottom plots) in the pBabe p53 null and high-risk p53 mutant cells show a clear synergistic effect at the more relevant FA values (≥50%). The degree of synergy between cisplatin and MK-1775 was also determined in other HNSCC cell lines derived from human tumors with known TP53 status, wild-type HN30 (CI = 1.75 ± 0.32, antagonism), and mutant TP53 HN31 (CI = 0.12 ± 0.15, strong synergism; Supplementary Fig. S1). These data clearly demonstrate that MK-1775 sensitizes the HNSCC tumor cells to cisplatin therapy in a p53-dependent manner.
Figure 1.
The Wee-1 inhibitor MK-1775 synergizes with cisplatin in vitro in HNSCC cells expressing high-risk TP53 mutations. A, clonogenic survival curves for HNSCC PCI-13 isogenic cell lines stably expressing wild-type TP53, pBabe TP53 null control, and high-risk mutant TP53 vectors, treated with a range of MK-1775 concentrations (0–1 μmol/L) for 48 hours to determine the drug IC50. All MK-1775 treatments were performed in triplicate, and each experiment was repeated at least three times. B, representative images of clonogenic survival assays for PCI-13 cells treated with cisplatin (CDDP) followed by MK-1775 according to the treatment protocol described in Materials and Methods. C–E, assessment of the degree of synergy between cisplatin and MK-1775 in PCI-13 cells expressing wild-type TP53, pBabe null TP53 control, and high-risk mutant TP53, respectively, using the Chou and Talalay method. Drug interactions are expressed as fraction affected (Fa) curves and combination index (CI) plots. The CI values were generated over a range of Fa levels from growth inhibition percentages. Synergy was clear at relevant Fa values that are greater than 50%.
Wee-1 inhibition attenuates cisplatin-induced CDC2 phosphorylation and triggers a general DNA damage response
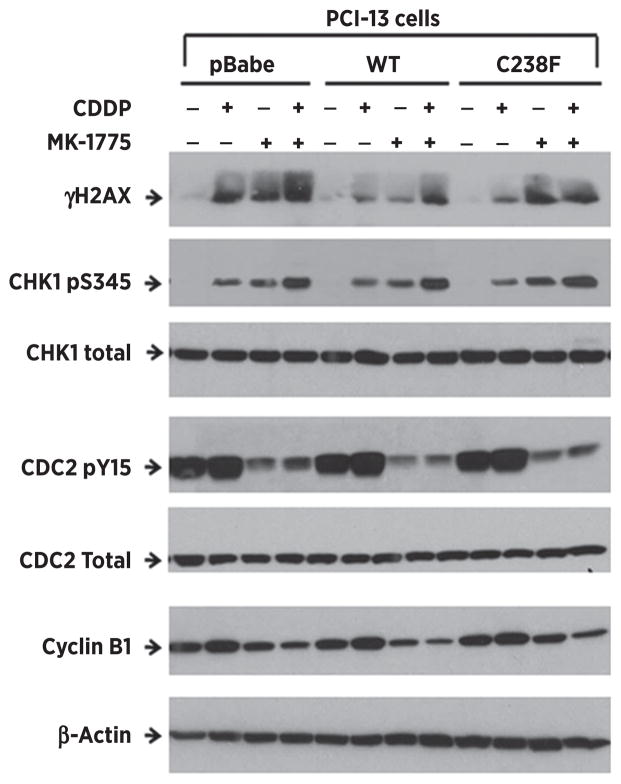
The Wee-1–induced G2 phase arrest results from phosphorylation and inactivation of CDC2 (31). To confirm that MK-1775 affects its downstream target, the isogenic PCI-13 cells were treated as previously described and phosphorylation of CDC2 was examined by Western blot. Following cisplatin, increased phosphorylation of CDC2 was apparent irrespective of p53 status. MK-1775 alone caused substantial suppression of CDC2 phosphorylation accompanied by decreased protein levels of Cyclin B1 (Fig. 2), indicating effective engagement of downstream targets. Combination treatment attenuated CDC2 phosphorylation, indicating that MK-1775 inhibited Wee-1 activity. The combination of cisplatin and MK-1775 significantly increased the levels of phosphorylation of the DNA damage markers, γH2AX and CHK1, at ser139 and ser345, respectively, indicating an increase and persistence of unrepaired DNA damage in all the PCI-13 clones (Fig. 2).
Figure 2.
Wee-1 inhibition attenuates cisplatin-induced CDC2 phosphorylation and triggers a general DNA damage response in high-risk TP53 mutant HNSCC cells. The isogenic HNSCC PCI-13 cells were treated with 1.5 μmol/L cisplatin alone or in combination with 0.25 μmol/L of MK-1775 as described in Materials and Methods. Cells were harvested and lysates analyzed by Western blot with antibodies to the proteins and phosphoproteins indicated. Increased phosphorylation levels of CHK1 and γ-H2AX indicate persistence of unrepaired DNA damage. The levels of phospho-CDC2 and Cyclin B1 expression were significantly suppressed with the MK-1775 treatment, indicating the successful inhibition of Wee-1 kinase activity by this agent.
Wee-1 inhibition induces prolonged mitotic arrest in high-risk TP53 mutant HNSCC cells harboring unrepaired DNA lesions
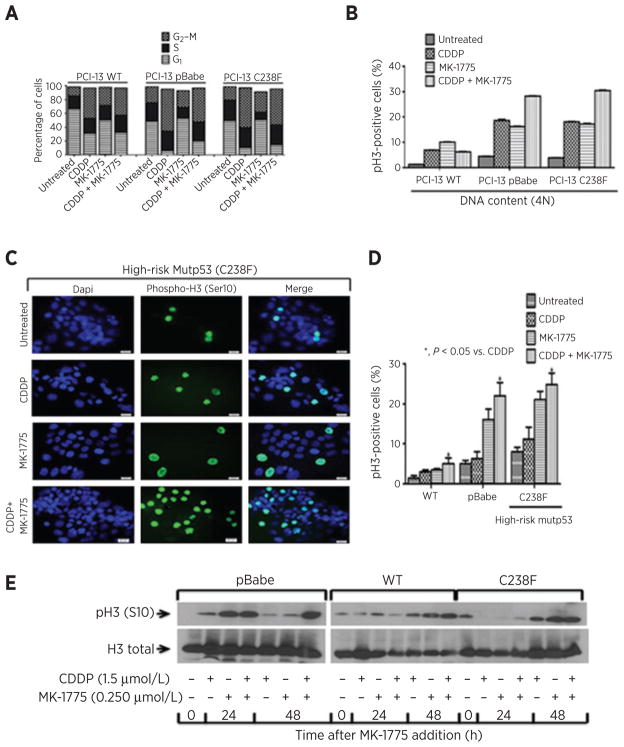
To examine if MK-1775 abrogates a cisplatin-mediated G2 checkpoint in HNSCC PCI-13 cells, unsynchronized cells were treated, and the cell-cycle progression and entry into mitosis were measured as described in Materials and Methods. In response to cisplatin alone, all PCI-13 cells arrested at the G2–M phase (43.6%, 60.9%, and 57.9%, respectively) regardless of their p53 status. However, the total percentage in G1 phase following cisplatin treatment was higher in wtp53 cells (30.9%) compared with cells lacking p53 (pBabe; 5.8%) or mutant p53 cells (10.33%), suggesting that a portion of the wild-type cells may have arrested in G1 phase (Fig. 3A and Supplementary Fig. S2). Addition of MK-1775 to cisplatin did not trigger entry into mitosis in the PCI-13 cells carrying wild-type TP53 (6.91% vs. 6.3% mitotic cells with 4N DNA content; Fig. 3A and Supplementary Fig. S2). However, a substantial proportion of PCI-13 cells carrying (pBabe) and high-risk mutant p53 (C238F) progressed through the G2 checkpoint (18.7% vs. 28.3%, and 18.1% vs. 30.5% mitotic cells with 4N DNA content respectively; Fig. 3A and B and Supplementary Fig. S2) in response to combination treatment. These p53-altered PCI-13 cells did not completely progress to the next cell-cycle phase at 48 or 72 hours following addition of MK-1775. This suggests that abrogation of the G2 block is likely followed by prolonged mitotic arrest and perhaps an exit of some cells with 4N DNA content to the G1 phase through mitotic slippage.
Figure 3.
Wee-1 inhibition induces prolonged mitotic arrest in high-risk TP53 mutant HNSCC cells harboring DNA damage. PCI-13 HNSCC cells were treated with cisplatin (CDDP), MK-1775, or the combination as described in Materials and Methods and subjected to dual phospho-H3 staining for mitotic cells and propidium iodide (PI) flow cytometric analysis (FACS). A 2N DNA content indicates cells in G1 phase and 4N DNA content indicates cells in either G2 or M phase. A, percentage of cell-cycle distribution of phospho-H3/PI FACS analysis shown in Supplementary Fig. S2. Percentage of G2–M phase indicates that pBabe TP53 null and high-risk mutant TP53 (C238F) progressed into mitosis after the G2-block abrogation 48 hours following the combination treatment. B, quantification of mitotic phenotype in phospho-H3–positive HNSCC cells presented in Supplementary Fig. S2 and plotted as mitotic index. TP53 status and treatment type are indicated for each cell line. An increased proportion of cells entering into mitosis with a full 4N complement of DNA was observed. C, representative fluorescence images of PCI-13 HNSCC cells indicating that abrogation of the G2 block results in prolonged mitotic arrest following addition of cisplatin and MK-1775. Cells were plated on cover-glass slips, treated as previously mentioned and incubated in medium containing nocodazole (mitotic trap) for 16 hours before the end of treatment and then subjected to phospho-H3 immunostaining analysis. Scale bars, 20 μm. D, quantification of fluorescence images (phospho-H3–positive staining) shown in C. Error bars, SEM. *, P < 0.05 vs. cisplatin (CDDP). E, Western blot for PCI-13 HNSCC cells with different TP53 mutational status treated at indicated time points and analyzed for pH3 (S10) and total pH3 protein expression.
To determine whether addition of MK-1775 to cisplatin results in premature mitotic entry, we used mitotic trapping followed by phospho-H3 immunostaining analysis to quantitate cells in mitosis. Representative fluorescence images of PCI-13 cell expressing high-risk mutant p53 are shown in Fig. 3C along with quantification in Fig. 3D. The addition of MK-1775 to cisplatin produced significantly higher phospho-H3 (Ser10) staining and expression level (Fig. 3E) in PCI-13 cells null for TP53 (pBabe) and with high-risk mutant TP53 (C238F) compared with cisplatin treatment alone, indicating premature entry of a large proportion of cells into mitosis consistent with the G2 abrogation and prolonged mitotic arrest observed during the cell-cycle analysis (Fig. 3A and B and Supplementary Fig. S2). No significant increase in phospho-H3 staining was seen in PCI-13 cells carrying wild-type TP53 with the drug combination, suggesting that the G2 block in these cells is substantially preserved. No sub-G1 peaks were observed following treatment of any of the cells evaluated, indicating that the cell growth inhibition resulting from the synergistic interaction of cisplatin and MK-1775 is unlikely to be mediated through apoptosis.
Wee-1 inhibition sensitizes high-risk mutant p53 HNSCC cells to cisplatin therapy through induction of mitotic catastrophe associated with a senescence-like phenotype
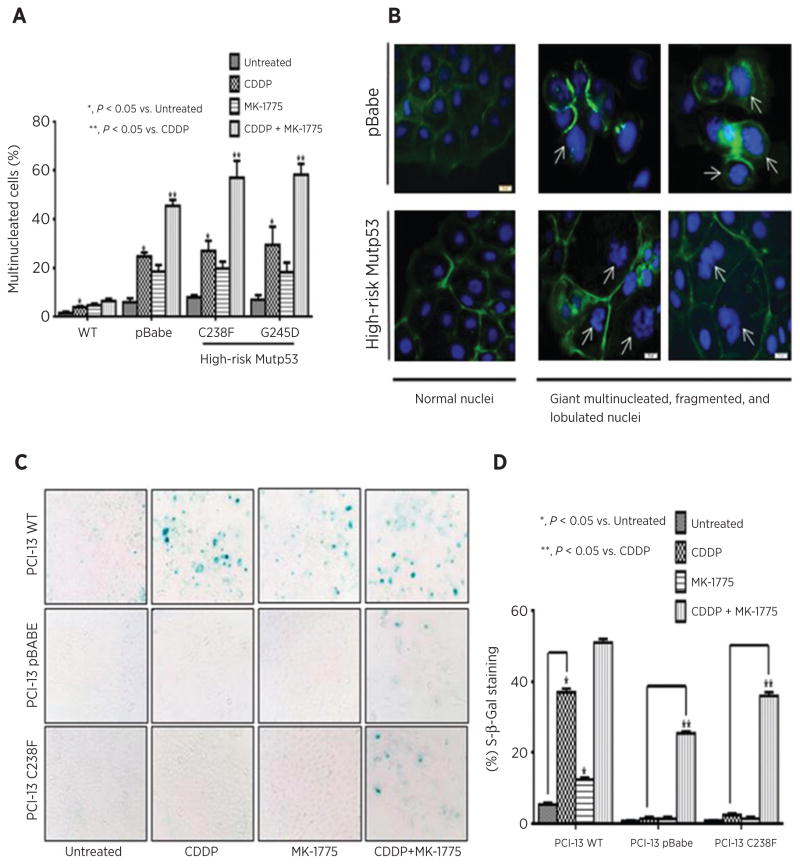
Our data show that MK-1775 addition to cisplatin in p53-mutant or -deficient HNSCC cells results in premature mitotic entry and prolonged mitosis suggestive of mitotic catastrophe, a type of abnormal mitosis. To test this, the isogenic PCI-13 cell lines were grown on glass coverslips and treated as indicated. Cells were stained with phalloidin and DAPI and scored for the presence of multinucleated cells (Fig 4A and B). Treatment of PCI-13 cells deficient for TP53 (pBabe) or expressing high-risk mutant TP53 (C238F or G245D) with the combination of cisplatin and MK-1775 leads to significantly increased number of multinucleated and often giant lobulated multinucleated cells (45.5%, 57%, and 58.3%, respectively) compared with cell treated with cisplatin alone (27.7%, 27.1%, and 29.5%, respectively). These findings are consistent with aberrant mitosis or mitotic catastrophe. However, in PCI-13 cells expressing wild-type TP53, the addition of MK-1775 to cisplatin did not result in more multinuclei formation compared with cisplatin alone (Fig. 4A). Taken together, these results indicate an association between multinuclei formation, TP53 status, and the synergism observed with the combination therapy in the HNSCC PCI-13 cells. Representative immunofluorescence images illustrating the presence of gross multi- and giant lobulated nuclei in isogenic PCI-13 cells following drug treatments are presented in Fig. 4B.
Figure 4.
Cisplatin and MK-1775 synergize in high-risk mutant p53 HNSCC cells and induce mitotic arrest associated with a senescence-like phenotype. A, PCI-13 HNSCC cells expressing different TP53 vectors were treated with cisplatin, MK-1775, or in combination as described in Materials and Methods. The cells were then washed and stained with Phalloidin (green) and DAPI (blue). The proportion of multinucleated and giant lobulated or even fragmented multinucleated cells was determined by immunofluorescent microscopy (pBabe TP53 null control, C238F, and G245D mutant TP53s; CDDP + MK-1775, 45.5%, 57%, and 58.3%, respectively, compared with CDDP alone, 27.7%, 27.1%, and 29.5%, respectively). B, representative immunofluorescent images of normal and giant multinucleated cells illustrating mitotic catastrophe (as described in A) taken after washout of CDDP and MK-1775 combination treatment. Scale bars, 20 μm. C, representative light microscopy showing SA-β-gal staining. Cells were treated with cisplatin and MK-1775 as described in Materials and Methods, and morphologic changes were monitored 4 days later (20× magnification). D, percentage of SA-β-gal–positive cells per total number of cells in an hpf after treatment is graphed. A substantial proportion of cells stained positive for β-Gal was seen in TP53 null (pBabe) and high-risk mutant TP53 cells following combination treatment.
It has been shown that unscheduled mitosis results in micro-nuclei formation and apoptosis in tumor cells treated with gemcitabine and MK-1775 (32, 33). Therefore, we tested whether sensitization of the isogenic PCI-13 cells to cisplatin by addition of MK-1775 led to apoptosis induction. Treated cells were assessed for apoptosis by examining PARP-1 protein cleavage 48 hours after treatment in Western blots. No PARP-1 cleavage was identified in any of the cells, indicating that there was no induction of apoptosis (Supplementary Fig. S3A). The absence of apoptosis was also confirmed with the APO-BrdU tunnel assay, where the percentages of apoptotic cells with positive APO-BrdU staining were very low (Supplementary Fig. S3B). These results further corroborate the absence of the sub-G1 peaks during the cell-cycle analysis in these cells following all treatments. Our results indicate that the underlying synergy between cisplatin and MK-1775 is an abnormal mitosis in the absence of apoptosis.
It has been proposed that cells undergoing mitotic arrest can eventually become senescent (reviewed in ref. 34). To examine for senescence, isogenic PCI-13 cells, treated as indicated, were stained and scored for the SA-β-gal positivity by microscopy. As shown in Fig. 4C and D, cisplatin alone leads to significantly increased levels of SA-β-gal activity only in cells expressing wild-type TP53 but not in cells null for TP53 (pBabe) or with C238F mutation. These results agree with our previously published work (13). Interestingly, a significant increase in the SA-β-gal staining associated with large, flattened cell shape was observed in PCI-13 cells carrying pBabe and in those expressing C238F following combination treatment when compared with cisplatin alone (Fig. 4C and D). A little increase in the SA-β-gal staining was seen in wtp53 cells following combination treatment. These data suggest that inhibition of Wee-1 in the TP53-altered PCI-13 cells exposed to cisplatin results in mitotic arrest associated with senescence-like phenotype and not apoptosis.
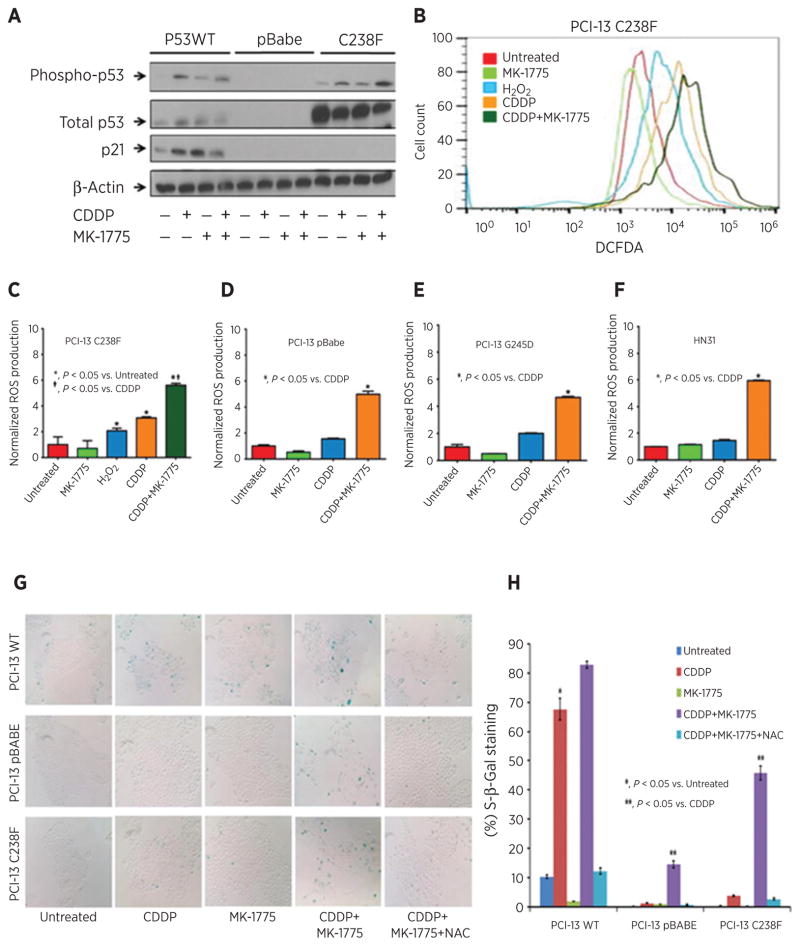
MK-1775–induced senescence is not associated with p21 expression and dependent on sustained ROS production in high-risk p53 mutant HNSCC cells treated with cisplatin
Senescence has also been linked to induction of p21 expression and ROS production, both of which are believed to be necessary for maintenance of the senescent phenotype in HNSCC cells (13, 28). To determine if cisplatin-induced p21 expression or ROS production correlates with the observed senescence phenotype following MK-1775 addition, we assayed p21 protein and ROS levels in cell lines expressing representative TP53 mutations. Compared with cisplatin alone, addition of MK-1775 to cisplatin had no effect on p21 protein expression in cells deficient for TP53 (pBabe) or expressing high-risk mutant TP53 (Fig. 5A). However, production of ROS was significantly increased following the combination treatment in these cells (Fig. 5B–F). Inhibition of ROS using N-acetyl cysteine (NAC) dramatically decreased senescence in cells deficient for TP53 (pBabe) or expressing high-risk mutant TP53 (Fig. 5G and H). These results suggest that sustained ROS production following combination treatment with cisplatin and MK-1775 plays a key role in senescent phenotype.
Figure 5.
MK-1775–induced senescence is not associated with p21 expression and dependent on sustained ROS production in high-risk p53 mutant HNSCC cells treated with cisplatin. A, p21 protein expression in PCI-13 cells expressing the indicated TP53 constructs treated with the indicated doses of cisplatin and MK-1775 unless otherwise stated. B, PCI-13 (C238F) TP53 mutant cells were exposed to cisplatin in the presence or absence of MK-1775, and ROS levels were analyzed using flow cytometry. Hydrogen peroxide (H2O2), a known ROS inducer, was used as a positive control. C, fluorescence from B normalized to control condition and DNA content and presented as ROS levels. D–F, ROS production measured in other cell lines expressing representative TP53 mutations and cell line with known TP53 mutational status. G, representative light microscopy showing SA-β-gal staining in HNSCC cells treated with cisplatin and MK-1775 as previously mentioned in the presence or absence of 10 mmol/L NAC added 2 hours prior to the treatment. H, percentage of cells SA-β-gal staining positive. *, significantly increased over untreated control (P < 0.05); **, significantly different from HNSCC cells in the cisplatin group (P < 0.05).
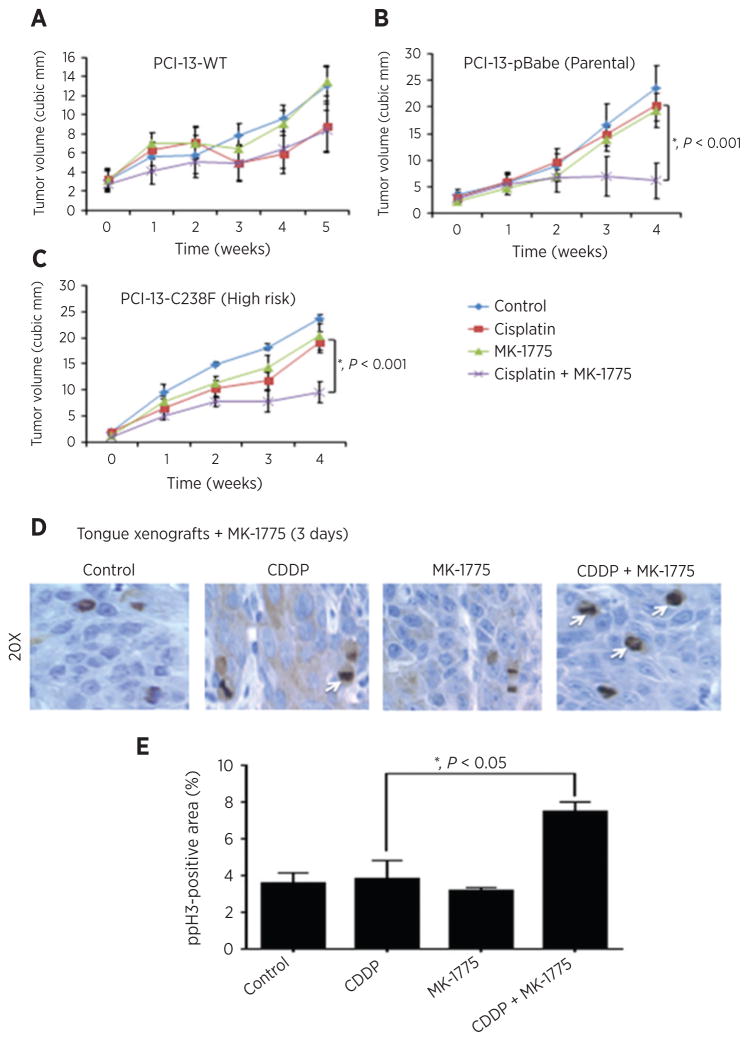
MK-1775 enhances antitumor efficacy of cisplatin in vivo
To determine whether MK-1775 could sensitize HNSCC cells to cisplatin in an orthotopic mouse model of oral cancer, PCI-13 cells expressing the pBabe, wild-type TP53, or high-risk mutant TP53 (C238F) were injected into the tongues of nude mice as previously described (30), and effects of MK-1775 or cisplatin, alone or in combination, on tumor growth over time were examined. MK-1775 did not provide significant improvement over the suppressive effect of cisplatin alone on tumor growth in mice bearing PCI-13 cells with wild-type TP53 (Fig. 6A). However, the combination of cisplatin and MK-1775 displayed significant improvement over cisplatin alone in tumors of mice bearing PCI-13 cells with high-risk mutant TP53 (C238F; Fig. 6C) or absent TP53 (pBabe; Fig. 6B), supporting our previous in vitro findings. To confirm that the enhancement of cisplatin antitumor efficacy by MK-1775 was associated with entry into mitosis, phospho-H3 was evaluated in tongue xenografts bearing tumors with high-risk mutant TP53 (C238F) treated with MK-1775 and/or cisplatin. MK-1775 induced an increase in phospho-H3–positive cells in vivo, suggestive of premature mitotic entry (Fig. 6D and E).
Figure 6.
MK-1775 enhances antitumor efficacy of cisplatin in vivo in an orthotopic mouse model of oral cancer. A–C, xenograft tumors made by orthotopically injecting wild-type TP53, pBabe TP53 null control, or high-risk mutant TP53 PCI-13 cells into the tongues of nude mice. The animals were treated with control, cisplatin, MK1775, or the combination of the two drugs once the tumors reached 5 mm in diameter according to protocol outlined in Materials and Methods. Tumor growth was followed for four weeks and tongue tumor size was measured with microcalipers and illustrated as tumor volume curves. Combination treatment reduced tumor growth to a greater extent than individual treatments (repeated measures Student t test: 30 mg/kg, *, P < 0.001). D, representative images of phospho-H3 immunofluorescence in PCI-13 mutant TP53 (C238F) tumors dosed with MK-1775 (30 mg/kg, p.o.) for 3 days (3 doses) after cisplatin treatment (4 mg/kg, i.v.). Arrows, mitotic phenotype phospho-H3–positive cells and histopathologic drug response. E, proportion of mitotic cells with forced mitotic phenotype in tumors treated as described in D, scored blind in 3 tumors per condition. Control vehicles received only 0.5% methylcellulose solution given by oral gavage. *, P < 0.05. Error bars, SEM tumor diameter for the group on that day and there were 8 to 9 mice in each group.
Discussion
We investigated the chemo-sensitizing abilities of a highly selective inhibitor of the Wee-1 kinase MK-1775 in HNSCC cells treated with cisplatin because platinum-based chemotherapy typically plays a key role in the management of patients with these tumors. We focused our investigations of MK-1775 on isogenic cell lines expressing different high-risk TP53 mutations in human HNSCC tumors. We found that the TP53-deficient (pBabe null) and high-risk mutant TP53 cells are more sensitive to clinically relevant doses of cisplatin when cotreated with nanomolar concentrations of MK-1775. On the other hand, tumor cells expressing wild-type TP53 are already sensitive to cisplatin (13) as a single agent and they were only minimally further sensitized by MK-1775 addition, supporting the concept that the chemo-sensitizing effect of MK-1775 is dependent on TP53 mutational status. We also obtained similar results with HNSCC cells with naturally occurring wild-type or high-risk TP53 mutations, confirming that the chemo-sensitizing effect of MK-1775 depends on the TP53 mutational status. Most tumor cells that harbor p53 alterations are heavily dependent on the G2 checkpoint for DNA repair. We show that cisplatin arrests HNSCC cells deficient for p53 or expressing high-risk mutp53 at the G2 phase and that addition of MK-1775 abrogates the G2 block and pushes the cells prematurely in mitosis. Not surprisingly, these TP53-altered HNSCC cells enter mitosis with extensive DNA lesions and the majority of them suffered prolonged mitotic arrest 48 hours after the addition of MK-1775, accompanied by phosphorylation of the Wee1 downstream target, CDC2. There was no significant increase in the sub-G1 fractions in these cells following combination treatment, indicating that in HNSCC the synergistic interaction of cisplatin and MK-1775 is not mediated through apoptosis, in contrast with what is seen with gemcitabine in colon cancer and sarcoma cell lines (23, 33).
Our recent publication has shown that senescence, rather than apoptosis, is the major mechanism of cisplatin-induced response in wild-type TP53 HNSCC cells and that cisplatin resistance in TP53 null or high-risk mutant TP53 cells is due to a lack of senescence (13). In this study, we found that addition of MK-1775 to cisplatin caused increased growth inhibition only in TP53 null (pBabe) or high-risk mutant TP53 HNSCC cells as a result of premature mitotic entry and prolonged mitosis. No alteration in the mitotic phenotype is seen in wild-type TP53 cells with the combination treatment, implying that wild-type p53 directs HNSCC cells treated with cisplatin alone to undergo senescence. We detected significant induction in phospho-H3 levels with the combination treatment in TP53 null (pBabe) and high-risk mutant TP53 cells, indicating forced entry into mitosis. Furthermore, combination therapy significantly increased the number of giant lobulated multinuclei in TP53 null (pBabe) and high-risk mutant TP53, which is suggestive of mitotic catastrophe.
Aarts and colleagues have shown that unscheduled mitosis results in gross multinuclei formation leading eventually to apoptosis in tumor cells treated with gemcitabine and MK-1775 (32). Recent report has also shown that Wee-1 kinase inhibition with high doses of MK-1775 leads to unscheduled mitotic entry associated with apoptosis in p53 mutant HNSCC cells (35). In our study, neither PARP-1 cleavage nor APO-BrdU tunnel staining was detected in our isogenic cell HNSCC lines with combination therapy, confirming no engagement of apoptosis. Our results strongly suggest that targeted inhibition of Wee-1 kinase with more physiologic dose of MK-1775 preferentially sensitizes TP53-deficient or high-risk mutant TP53 HNSCC cells to cisplatin through induction of mitotic arrest without progression to apoptosis. One possible explanation for such discrepancy is that cisplatin and gemcitabine work through different mechanism(s) when sensitized with MK-1775 addition or perhaps the phenotype is specific for the type of tumor or nature of p53 mutation. In fact, evidence is now accumulating that different p53 mutations possess different functions and responses to therapy in different tissues, potentially reflecting differences in the expression of their cellular targets (36). In addition, the higher doses of MK-1775 used in previous studies may have accounted for extensive DNA damage leading to induction of apoptosis. We have shown that higher concentrations of the drug are extremely toxic to HNSCC cells in vitro. It is also possible that the mutant TP53 HNSCC cells possess major alterations in the BH3 proapoptotic signaling pathways which render them resistance to apoptosis upon chemotherapy (37, 38).
A decrease in apoptosis is often compensated in several tumor cell lines by an increase in cellular senescence after treatment with DNA-damaging agents and radiation (13, 28, 34). In addition, it has been proposed that tumor cells undergoing mitotic catastrophe eventually die through terminal growth arrest known as cellular senescence (39). Treatment with cisplatin alone induced senescence in the HNSCC wild-type TP53 cells, but not in high-risk TP53 mutant or pBabe null cells. Surprisingly, the addition of MK-1775 partially restored cisplatin ability to induce senescence in cells expressing pBabe or high-risk mutant TP53. The induction of ROS appears to be the driving factor for senescence in these cells, whereas the induction of p21 alone does not seem sufficient. ROS induction is most likely caused by excessive DNA damage as it was recently shown that DNA damage induces ROS generation in several tumor cell lines (40).
MK-1775 has shown activity in nude rats bearing WiDr human colon carcinoma xenografts treated with 5-FU, gemcitabine, and cisplatin (23, 24). Moser and colleagues have recently shown that MK-1775 potentiates cisplatin response in mice bearing mutant p53 HNSCC cells established as subcutaneous flanks; however, they have not examined efficacy of MK-1775 in oral tongue xenografts (35). Ectopic subcutaneous xenograft models have proven less useful for studying therapeutic agents than orthotopic models that recapitulate the tumor microenvironment (30). In this study, we examined the antitumor efficacy of MK-1775 in combination with cisplatin in HNSCC xenografts growing in oral tongue of nude mice to mimic the primary tumor site. A significant delay in tumor growth was observed in mice injected with HNSCC cells expressing the pBabe control or high-risk mutant TP53 following combination treatment compared with either cisplatin or MK-1775 alone. No enhanced treatment effect was seen in mice carrying tumors with wild-type TP53. Most likely, tumors harboring wild-type TP53 undergo a G1 or G2 arrest similar to what we demonstrated in vitro, which prevents MK-1775–mediated premature mitotic entry. Thus, our data confirm previous findings that MK-1775 is not effective as a single agent and should be used only in combination with cisplatin or other DNA-damaging agents that induce G2 cell-cycle arrest in HNSCC (23, 24).
Combination of cisplatin and MK-1775 promoted mitotic entry in xenografts obtained from mice bearing HNSCC with high-risk mutant TP53, indicating forced mitotic phenotype. However, the percentage of the phospho-H3 immunostaining in vivo is lower than that observed in vitro, perhaps due to trapping of the cells with nocodazole under the latter conditions (23, 24, 32). On the basis of current understanding of aberrant mitosis (41–43), it is possible that unscheduled entry into mitosis in the presence of unrepaired DNA damage activates the spindle assembly checkpoint and inhibits activation of the anaphase-promoting complex APC/C-Cdc20. This, in turn, could lead to a delay in anaphase and eventually results in either mitotic death or mitotic slippage into G1 phase.
In conclusion, we showed that the Wee-1 kinase inhibitor, MK-1775, selectively sensitized HNSCC cells to cisplatin therapy both in vitro and in vivo based on their TP53 mutational status. The mechanism to explain this sensitization appears to involve a drug-induced, premature acceleration of G2-phase cells into abnormal mitosis. Such cells harbor unrepaired DNA lesions that lead to abnormal cell divisions resulting in a senescence-like process. Our study suggests that forced mitosis with MK-1775 represents a novel therapeutic approach that potentially targets the consequences of oncogenic transformation caused by high-risk mutant TP53 in HNSCC. These pre-clinical data provide compelling evidence that a personalized approach to the treatment of HNSCC based on Wee-1 kinase inhibition in p53-altered cells may be feasible. Further clinical investigations are necessary to determine whether this approach will be useful to improve treatment outcomes for patients with high-risk mutp53 HNSCC.
Supplementary Material
Acknowledgments
The authors thank Mark Blaylock and Cassandra Gonzalez for technical assistance.
Grant Support
This work was supported by the U.T. M.D. Anderson Cancer Center PANTHEON program (philanthropic support to J.N. Myers), the National Institute of Health Specialized Program of Research Excellence Grant P50CA097007 (to J.N. Myers), the National Institute of Health R01 DE14613 (to J.N. Myers), Cancer Prevention and Research Institute of Texas RP120258 (to J.N. Myers), National Research Science Award Institutional Research Training Grant T32CA60374 (to J.N. Myers), the National Institute of Health Program Project Grant C168485 (to J.N. Myers), and the Cancer Center Support Grant CA016672 (to J.N. Myers). This work was also supported by National Institute of Health R01 GM079656 (to O. Lichtarge), R01 GM066099 (to O. Lichtarge), and NSF CCF 0905536 (to O. Lichtarge) and DBI 0851393 (to O. Lichtarge), and Pharmacoinformatics Training Program of the Keck Center of the Gulf Coast Consortia NIH Grant no. 5 R90 DKO71505 (to P. Katsonis).
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ Contributions
Conception and design: A.A. Osman, M.M. Monroe, M.V. Ortega Alves, A.A. Patel, A.L. Fitzgerald, D.M. Neskey, M.J. Frederick, O. Lichtarge, J.N. Myers
Development of methodology: A.A. Osman, M.M. Monroe, M.V. Ortega Alves, A.A. Patel, P. Katsonis, A.L. Fitzgerald, D.M. Neskey, S.H. Woo, O. Lichtarge
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A.A. Osman, M.M. Monroe, M.V. Ortega Alves, A.A. Patel, S.H. Woo
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A.A. Osman, M.M. Monroe, M.V. Ortega Alves, P. Katsonis, D.M. Neskey, M.J. Frederick, S.H. Woo, C. Caulin, T.-K. Hsu, T.O. McDonald, M. Kimmel, J.N. Myers
Writing, review, and/or revision of the manuscript: A.A. Osman, M.M. Monroe, M.V. Ortega Alves, A.A. Patel, P. Katsonis, D.M. Neskey, M.J. Frederick, C. Caulin, T.-K. Hsu, M. Kimmel, R.E. Meyn, J.N. Myers
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A.A. Osman, A.A. Patel, A.L. Fitzgerald, O. Lichtarge, J.N. Myers
Study supervision: A.A. Osman, O. Lichtarge, J.N. Myers
Other (performed most of the experiments): A.A. Osman
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Li Y, Adams GL, Wagner H, Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–8. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes DN, Grandis J, El-Naggar AK. Comprehensive genomic characterization of squamous cell carcinoma of the head and neck in the Cancer Genome Atlas. Cancer Res. 2013;73(suppl):8s. abstr 1117. [Google Scholar]
- 9.Perrone F, Bossi P, Cortelazzi B, Locati L, Quattrone P, Pierotti MA, et al. TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma. J Clin Oncol. 2010;28:761–6. doi: 10.1200/JCO.2009.22.4170. [DOI] [PubMed] [Google Scholar]
- 10.Lindenbergh-van der Plas M, Brakenhoff RH, Kuik DJ, Buijze M, Bloemena E, Snijders PJ, et al. Prognostic significance of truncating TP53 mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2011;17:3733–41. doi: 10.1158/1078-0432.CCR-11-0183. [DOI] [PubMed] [Google Scholar]
- 11.Temam S, Flahault A, Perie S, Monceaux G, Coulet F, Callard P, et al. p53 gene status as a predictor of tumor response to induction chemotherapy of patients with locoregionally advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2000;18:385–94. doi: 10.1200/JCO.2000.18.2.385. [DOI] [PubMed] [Google Scholar]
- 12.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357:2552–61. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadhikar MA, Sciuto MR, Ortega Alves MV, Pickering CR, Osman AA, Neskey DM, et al. Chk1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53. Mol Cancer Ther. 2013;9:1860–73. doi: 10.1158/1535-7163.MCT-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suganuma M, Kawabe T, Hori H, Funabiki T, Okamoto T. Sensitization of cancer cells to DNA damage-induced cell death by specific cell cycle G2 checkpoint abrogation. Cancer Res. 1999;59:5887–91. [PubMed] [Google Scholar]
- 15.Yao SL, Akhtar AJ, McKenna KA, Bedi GC, Sidransky D, Mabry M, et al. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med. 1996;2:1140–3. doi: 10.1038/nm1096-1140. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O’Connor PM. UCN-01: a potent Abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–65. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 17.Powell SN, DeFrank JS, Connell P, Eogan M, Preffer F, Dombkowski D, et al. Differential sensitivity of p53 (−) and p53 (+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 1995;55:1643–8. [PubMed] [Google Scholar]
- 18.Leijen S, Beijnen JH, Schellens JH. Abrogation of the G2 checkpoint by inhibition of Wee-1 kinase results in sensitization of p53-deficient tumor cells to DNA-damaging agents. Curr Clin Pharmacol. 2010;5:186–91. doi: 10.2174/157488410791498824. [DOI] [PubMed] [Google Scholar]
- 19.Squire CJ, Dickson JM, Ivanovic I, Baker EN. Structure and inhibition of the human cell cycle checkpoint kinase, Wee1A kinase: an atypical tyrosine kinase with a key role in CDK1 regulation. Structure. 2005;13:541–50. doi: 10.1016/j.str.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Portugal J, Mansilla S, Bataller M. Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des. 2010;16:69–78. doi: 10.2174/138161210789941801. [DOI] [PubMed] [Google Scholar]
- 21.De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Wurdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–7. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Decker SJ, Sebolt-Leopold J. Knockdown of Chk1, Wee1 and Myt1 by RNA interference abrogates G2 checkpoint and induces apoptosis. Cancer Biol Ther. 2004;3:305–13. doi: 10.4161/cbt.3.3.697. [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 24.Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9:514–22. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 25.Rajeshkumar NV, De Oliveira E, Ottenhof N, Watters J, Brooks D, Demuth T, et al. MK-1775, a potent Wee1 inhibitor, synergizes with gemcitabine to achieve tumor regressions, selectively in p53-deficient pancreatic cancer xenografts. Clin Cancer Res. 2011;17:2799–806. doi: 10.1158/1078-0432.CCR-10-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schellens JH, Leijen S, Shapiro GI, Pavlick AC, Tibes R, O’Day SJ, et al. A phase I pharmacological and pharmacodynamic study of MK-1775, a Wee1 tyrosine kinase inhibitor, in monotherapy and combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumor. J Clin Oncol. 2010;28(suppl):15s. abstr 3067. [Google Scholar]
- 27.Merck Sharp; Dohme Corp. Group. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. A randomized, phase ii study evaluating MK-1775 in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in adult patients with platinum sensitive p53 mutant ovarian cancer. [cited 2014 Aug 11]. Available from: http://clinicaltrials.gov/show/NCT01357161. [Google Scholar]
- 28.Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res. 2012;18:290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 30.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002;8:293–8. [PubMed] [Google Scholar]
- 31.Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. Mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–22. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- 32.Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, et al. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012;6:524–39. doi: 10.1158/2159-8290.CD-11-0320. [DOI] [PubMed] [Google Scholar]
- 33.Kreahling JM, Foroutan P, Reed D, Martinez G, Razabdouski T, Bui MM, et al. Wee1 inhibition by MK-1775 leads to tumor inhibition and enhances efficacy of gemcitabine in human sarcomas. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0057523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–13. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- 35.Moser R, Chang Xu, Kao M, Annis J, Lerma LA, Schaupp CM, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20:4274–88. doi: 10.1158/1078-0432.CCR-13-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–17. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Boehm AL, Miranda MB, Shangary S, Grandis JR, Johnson DE. Targeting antiapoptotic Bcl-2 family members with cell-permeable BH3 peptides induces apoptosis signalling and death in head and neck squamous cell carcinoma cells. Neoplasia. 2007;9:801–11. doi: 10.1593/neo.07394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He L, Torres-Lockhart K, Forster N, Ramakrishnan S, Greninger P, Garnett MJ, et al. Mcl-1 and FBW7 Control a dominant survival pathway underlying HDAC and Bcl-2 inhibitor synergy in squamous cell carcinoma. Cancer Discov. 2013;3:324–37. doi: 10.1158/2159-8290.CD-12-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–62. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 40.Kang MA, So EY, Simons AL, Spitz DR, Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 2012;3:e249. doi: 10.1038/cddis.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitale I, Galluzzi L, Castedo M, Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–92. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 42.On KF, Chen Y, Ma HT, Chow JP, Poon RY. Determinants of mitotic catastrophe on abrogation of the G2 DNA damage checkpoint by UCN-01. Mol Cancer Ther. 2011;10:784–94. doi: 10.1158/1535-7163.MCT-10-0809. [DOI] [PubMed] [Google Scholar]
- 43.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27:3–16. doi: 10.1016/j.molcel.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.