Abstract
Purpose
Few studies examining the clinical features and gene mutations in lung cancer patients 30 years of age or younger have been published. A trend towards increasing morbidity has been noted in young patients; thus, an urgent need exists to explore this subgroup of patients.
Methods
Patients aged ≤30 years with pathologically diagnosed lung cancer were retrospectively evaluated. We reviewed the clinical features, gene mutations and prognosis of each patient.
Results
Forty-one patients were included in this study. The mean age was 26.4±3.5 years. Cough, tightness/dyspnea and chest pain were common symptoms, and 58.5% of patients presented with advanced stages of lung cancer. Adenocarcinoma was the predominant histologic type noted in these young patients. Masses and nodules were the dominant imaging features observed upon lung computed tomography (CT). Thoracic lymphadenopathy occurred very frequently in these patients. Five of 6 patients with echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) gene fusions presented solid masses with no ground-glass opacity (GGO) and thoracic multifocal lymphadenopathy. Six of 22 (27.2%) cases contained EML4-ALK gene fusions. In addition, 5 of 22 (22.7%) patients harbored epidermal growth factor receptor (EGFR) mutations, and 2 of 17 patients exhibited KRAS and ROS1 gene mutations. The median survival times were 44.2 months for patients with early stage disease and 8 months for patients with advanced NSCLC disease. The one-year and 5-year survival rates were 56.6% and 38.6%, respectively.
Conclusions
Increased gene mutation frequencies are noted in these very young lung cancer patients. This finding indicates that the detection of gene mutations in these patients is important and will help to determine the appropriate targeted therapy.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with a 5-year survival rate of only 15% [1]. The annual lung cancer mortality rate in China is estimated to reach 1 million individuals by 2025 [2]. The majority of lung cancer cases occur in patients over 50 years old, most often between 60 and 80 years of age [3]. The incidence of lung cancer in young adults is reportedly relatively low. The incidence is approximately 1.2 to 6.2% in patients under 40 years of age [4,5], 5.3% in those under 45 [6,7], and 13.4% in those under 50 years [8]. However, some recent reports have indicated that the incidence of lung cancer in young patients is increasing worldwide [7,9–13]. Various studies have discussed the characteristics and prognosis of lung cancer in young patients <45 or <40 years old. Furthermore, lung cancer patients younger than 30 years old with poor prognoses have been reported [14–16]. Indeed, in recent years, we have also observed increasing numbers of very young lung cancer patients. Unfortunately, little research has been conducted regarding the clinical features, gene mutations and prognosis of this subgroup of patients due to the limited documented cases.
In recent years, targeted therapy has served as a major breakthrough in the management of non-small cell lung cancer (NSCLC) [17–19]. Patients with epidermal growth factor receptor (EGFR) mutations or echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) gene fusions exhibit increased progression-free survival (PFS) and overall survival (OS) after EGFR-tyrosine kinase inhibitor (TKI) (erlotinib or gefitinib) or crizotinib treatment [20–26]. However, data describing gene mutations in young patients are rare, including patients younger than 30 years of age. In this study, we retrospectively analyzed the clinical features, gene mutations and prognosis of 41 lung cancer patients aged 30 years or younger. We hope that this study will aid clinicians in improving awareness as well as diagnosis and treatment strategies in this patient population.
Methods
Patients and Data Collection
We retrospectively collected data from 41 patients 30 years of age or younger with pathologically diagnosed lung cancer between January 2008 and July 2014 at The First Affiliated Hospital, College of Medicine, Zhejiang University. The medical records of all patients included in this study were analyzed for the following data: (1) demographic data, including age and sex; (2) symptoms; (3) smoking status and family history; (4) tumor histology and disease stage; (5) radiological imaging; (6) gene abnormalities; and (7) overall survival. Disease was staged according to the seventh edition of the TNM (tumor, node, and metastasis) classification system. Smoking status was divided into two categories: non-smokers and smokers (including current and previous smokers). Family history was considered positive if any member of a patient’s family had a history of lung cancer. The Ethics Committee of The First Affiliated Hospital, College of Medicine, Zhejiang University approved this study. All patients provided written informed consent for the use of their clinical data and tumor tissues for research.
Imaging Techniques and Analysis
Computed tomography (CT) was performed on 64-slice systems (Brilliance iCT and 64-channel systems), and intra-venous contrast was used in all patients. All CT scans were assessed for the presence of a mass (>30 mm in maximum dimension), nodule (≤30 mm in maximum dimension), ground-glass opacity (GGO) and lymphadenopathy. GGO was defined as a hazy increase in attenuation that did not obscure normal lung markings. Lymphadenopathy was defined as hilar or mediastinal lymph nodes >15 mm in the short-axis dimension.
Gene Mutation Assessment
We examined EGFR mutations in exons 18 to 21 and KRAS mutations in codons 12 and 13 using a PCR-based pyrosequencing assay. Sequence analysis was performed using the PyroMark ID system (Qiagen, Hilden, Germany). Each case was identified as positive or negative by comparison with the wild-type sequence. EML4-ALK rearrangements were examined by fluorescence in situ hybridization (FISH) with a break-apart ALK probe (Vysis LSI Dual Color, Break Apart Rearrangement Probe; Abbott Molecular, Abbott Park, IL, USA). EML4-ALK rearrangements were classified as positive if greater than 15% of the tumor cells displayed split signals or isolated signals containing a kinase domain. ROS1 expression was assessed by immunohistochemistry.
Statistical Analysis
The patients were followed until September 31, 2014 or the date of their death. OS was defined as the time from the date of diagnosis to the date of death or last visit. Survival curves were calculated according to the Kaplan-Meier method and compared using log-rank tests. Statistical analysis was performed using SPSS 18.0 software (SPSS, Chicago, IL).
Results
Patient Characteristics
A summary of the characteristics of 41 lung cancer patients is provided in Table 1. The age of the lung cancer patients assessed ranged from 17 to 30 years with a mean age of 26.4±3.5 years. In the entire cohort, 23 (56.1%) cases were males, and 18 (43.9%) cases were females. Only five patients (12.2%) reported a history of smoking, and only 1 patient had a family history of lung cancer. Cough was the most common initial presenting symptom, which occurred in 25 patients (61.0%), followed by chest tightness/dyspnea (24.4%), chest pain (21.9%) and bone/muscle pain (17.1%). Specifically, 12 patients (29.3%) were asymptomatic with abnormal chest radiological findings. Regarding disease stage, 22 (56.3%) NSCLC patients had advanced stage tumors (IIIb + IV) at presentation, and two small cell lung cancer (SCLC) patients exhibited extensive disease.
Table 1. The clinical characteristics of 41 patients.
| Clinical characteristics | No. | (%) |
|---|---|---|
| Mean age, years (range) | 26.4±3.5 | |
| Sex | ||
| Male | 23 | 56.1 |
| Female | 18 | 43.9 |
| Smoking | ||
| Non-smoker | 36 | 87.8 |
| Current or ex-smoker | 5 | 12.2 |
| Symptoms present | ||
| Cough | 25 | 61.0 |
| Chest tightness/dyspnea | 10 | 24.4 |
| Chest pain | 9 | 22.0 |
| Hemoptysis | 5 | 12.2 |
| Bone/muscle pain | 7 | 17.1 |
| Fever | 6 | 14.6 |
| No symptom | 12 | 29.3 |
| Family history | 1 | 2.4 |
| NSCLC TNM stage | ||
| I | 6 | 15.3 |
| II | 6 | 15.3 |
| IIIa | 5 | 12.8 |
| IIIb | 2 | 5.1 |
| IV | 20 | 51.2 |
| SCLC stage | ||
| Extensive | 2 | |
| Limited | 0 |
Furthermore, we analyzed the site of metastasis in 22 patients with advanced stage NSCLC, including four patients with ALK rearrangements, two with EGFR mutations, one with ROS1 mutations and 15 with unknown driver oncogenes. The majority of patients, 16 of 22 (72.7%), exhibited pulmonary nodules. Similar to a previous report [27], a large proportion of patients, 15 of 22 (68.2%), displayed metastasis to intrathoracic lymph nodes, whereas only 9 of 22 (40.9%) showed involvement of extrathoracic lymph nodes. Other common metastatic sites included bone (8/22, 36.4%), pleura (4/22, 18.2%) and brain (3/22, 13.6%). Additionally, we found an average of 2.33 metastatic sites among the 15 patients with unknown driver oncogenes and an average of 4 sites among the ALK rearrangement-positive patients; this difference was not statistically significant.
Radiographic Findings
All 41 patients underwent contrast CT scans. The pulmonary abnormalities observed on the initial CT scans are summarized in Table 2. Masses were observed in 19 patients, with five patients exhibiting multiple nodules. Nodules were observed in 19 patients, with seven patients exhibiting solitary nodules and 12 patients exhibiting multi-scattered nodules; in addition, GGOs were observed in two patients. Most of the masses/nodules were ill-defined with irregular margins, and no cavitations were observed. Lymphadenopathy was observed in 27 patients. Other associated findings included segmental/lobar atelectasis (n = 4), pleural effusion (n = 6) and pericardial fluid build-up (n = 2).
Table 2. Radiological characteristics of 41 patients.
| Major Radiographic Findings | No. of patients | % |
|---|---|---|
| Mass | 19 | 46.3 |
| Nodule | 19 | 46.3 |
| Multiple peripheral shadows | 13 | 31.7 |
| Lymphadenopathy | 27 | 65.9 |
| Segmental/lobar atelectasis | 4 | 9.8 |
| Pleural effusion | 6 | 14.6 |
| Pericardial fluid | 2 | 4.9 |
Five of 6 patients with EML4-ALK gene fusions presented solid masses with no GGO, and 1 of these patients exhibited multiple nodules. Five of 6 patients with EML4-ALK gene fusions also had thoracic multifocal lymphadenopathy (Fig 1).
Fig 1. Thoracic CT findings of representative NSCLC patients with EML4-ALK gene fusions.
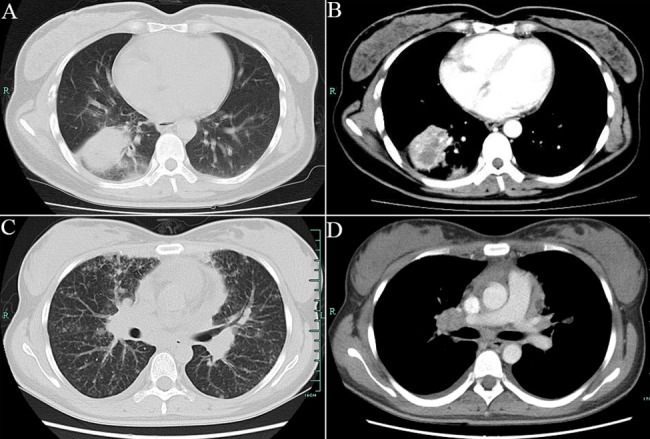
A, B: mass with no GGO; C: multiple nodules; D: multifocal lymphadenopathy.
Three of 5 patients with EGFR mutations showed a solitary nodule or mass, and two of these patients presented a mass or consolidation combined with multiple nodules and intrathoracic lymphadenopathy. All of the nodules were solid, and no GGO was observed in these patients.
Histological Profiles
The predominant histologic type of lung cancer in these young patients was adenocarcinoma, accounting for 78.0% of cases (n = 32), followed by neuroendocrine carcinoma (n = 3, 7.3%), SCLC (n = 2, 4.9%) and undifferentiated NSCLC (n = 2, 4.9%). Specifically, only one patient exhibited squamous cell histology (Table 3).
Table 3. Pathological type of 41 patients.
| Male | Female | |||
|---|---|---|---|---|
| Pathological type | N | % | N | % |
| Adenocarcinoma | 18 | 43.9 | 14 | 34.1 |
| Neuroendocrine carcinoma | 1 | 2.4 | 2 | 4.8 |
| SCLC | 2 | 4.9 | 0 | 0.0 |
| Undifferentiated NSCLC | 1 | 2.4 | 1 | 2.4 |
| Squamous cell carcinoma | 0 | 0.0 | 1 | 2.4 |
| Mucoepidermoid carcinoma | 1 | 2.4 | 0 | 0.0 |
The histologic characteristics of the lung adenocarcinoma cases could be evaluated in 26 patients. Acinar predominant adenocarcinoma was the most common subtype (14/26, 53.8%), followed by solid predominant adenocarcinoma (8/26, 30.8%), lepidic predominant adenocarcinoma (1/26, 3.8%) and other subtypes (3/26, 11.5%). No papillary or micropapillary predominant adenocarcinomas were found in our study. Most patients with EGFR mutations presented as acinar predominant adenocarcinoma, while patients with EML4-ALK gene fusions tended to present as solid predominant adenocarcinoma. A lepidic growth pattern was found in two patients, both of whom harbored EGFR mutations. A signet ring cell feature was observed in three patients, 2 of whom harbored EML4-ALK gene fusions (S1 Table).
Lung Adenocarcinoma Gene Mutations
Of the 32 adenocarcinoma patients studied, 22 had specimens available for gene mutation assessment (Table 4). A total of 22 cases were evaluated for EGFR mutations and EML4-ALK gene fusions. Five patients (3 males and 2 females) (5/22, 22.7%) harbored EGFR mutations, including two with L858R mutations and three with exon 19 deletions. Six of 22 cases (2 males and 4 females) (6/22, 27.2%) possessed EML4-ALK gene fusions. KRAS and ROS1 mutations were assessed in 17 patients. Two KRAS mutations and 2 ROS1 mutations were identified; both KRAS mutations were Gly12Asp.
Table 4. Gene alterations of 22 lung adenocarcinoma patients.
| No. | Age | Sex | T | N | M | Stage | EGFR | ALK | KRAS | ROS1 | Initial treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | M | 4 | 3 | 1a | IV | L858R | - | - | - | untreated |
| 2 | 27 | F | 2 | 2 | 0 | IIIa | L858R | - | - | - | radical surgery |
| 3 | 30 | M | 4 | 3 | 1 | IV | exon 19-del | - | - | - | gefitnib |
| 4 | 27 | M | 2a | 0 | 0 | Ib | exon 19-del | - | - | - | radical surgery |
| 5 | 27 | F | 1a | 2 | 0 | IIIa | exon 19-del | - | - | - | radical surgery |
| 6 | 23 | F | 4 | 3 | 1b | IV | - | + | - | - | chemotherapy |
| 7 | 29 | F | 4 | 3 | 1b | IV | - | + | - | - | chemotherapy |
| 8 | 30 | M | 2a | 2 | 0 | IIIa | - | + | - | - | radical surgery |
| 9 | 30 | F | 1b | 2 | 0 | IIIa | - | + | - | - | radical surgery |
| 10 | 30 | M | 3 | 0 | 1a | IV | - | + | - | - | palliative surgery |
| 11 | 23 | F | 4 | 3 | 1 | IV | - | + | - | - | chemotherapy |
| 12 | 30 | M | 2b | 0 | 0 | IIa | - | - | Gly12Asp | - | radical surgery |
| 13 | 27 | F | 3 | 0 | 0 | IIb | - | - | Gly12Asp | - | radical surgery |
| 14 | 30 | M | 1a | 0 | 0 | Ia | - | - | - | + | radical surgery |
| 15 | 27 | M | 1 | 3 | 1b | IV | - | - | - | + | crizotinib |
| 16 | 30 | F | 4 | 2 | 1a | IV | - | - | - | - | chemotherapy |
| 17 | 30 | F | 1a | 0 | 0 | Ia | - | - | - | - | radical surgery |
| 18 | 26 | M | 2a | 0 | 0 | Ib | - | - | n.d. | n.d. | radical surgery |
| 19 | 18 | M | 1a | 1 | 0 | IIa | - | - | n.d. | n.d. | radical surgery |
| 20 | 25 | M | 4 | 3 | 1b | IV | - | - | n.d. | n.d. | untreated |
| 21 | 28 | F | 3 | 0 | 0 | IIb | - | - | n.d. | n.d. | radical surgery |
| 22 | 30 | M | 3 | 3 | 1b | IV | - | - | n.d. | n.d. | chemotherapy |
n.d.: not detected
Treatment and Overall Survival Analysis
For the initial treatment of the NSCLC patients, 18 patients received surgical therapy, 14 patients received chemotherapy, 2 patients received targeted agents (gefitinib and crizotinib for each) and 5 patients quit therapy (Table 5). For second line therapy, 3 patients with ALK rearrangements received crizotinib. Both SCLC patients initially received chemotherapy.
Table 5. The treatment of 39 NSCLC patients.
| Treatment | No. |
|---|---|
| Surgery | 18 |
| no adjuvant therapies | 5 |
| surgery+adjuvant therapies | 5 |
| unclear adjuvant therapies | 7 |
| Chemotherapy | 14 |
| Targeted therapy (2nd line) | 3 |
| Targeted therapy (1st line) | 2 |
| Untreated | 5 |
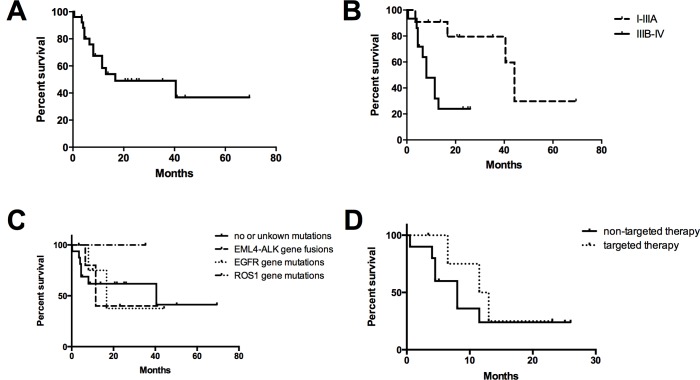
Long-term follow-up data were available for 25 NSCLC patients (Fig 2). The follow-up time ranged from 1 to 70 months (median time of 14 months). Twelve deaths occurred during the follow-up period. The median survival time was 44.2 months for patients with early stage disease and 8 months for those with advanced disease. The one-year and 5-year survival rates were 56.6% and 38.6%, respectively. Fifteen NSCLC patients with advanced disease were available for survival analysis, in which 5 patients received targeted agents (1 with gefitinib and 4 with crizotinib). The median survival time was 12.25 months and 8 months for the targeted therapy cohort and non-targeted therapy cohort, respectively. However, no statistical significance was observed.
Fig 2. Survival curves for lung cancer patients are presented.
A: Overall survival curves for 25 NSCLC patients; B: Survival by stage; C: Survival by oncogenic drivers; D: Survival by targeted therapy.
Discussion
Here, we report the clinical features and gene mutations of lung cancer patients 30 years of age or younger. We found that no specific clinical symptoms were ascribed to these patients. Cough was the most common initial clinical presentation, followed by chest tightness/dyspnea and chest pain, which is similar to observations reported in previous studies [28,29]. The manifestations of lung cancer on chest CT in younger patients were similar to those observed in older patients. According to our study, masses or nodules were the most common CT manifestations. Multiple peripheral shadows, which may be easily confused with inflammatory lung disease, were also frequently noted. In particular, thoracic lymphadenopathy frequently occurred in these patients, and this finding may be related to thoracic lymph node metastasis. Recently, Hsu and Togashi [30,31] reported that lung adenocarcinomas in patients with EGFR mutations tend to have an invasive solid pattern at early stages and diffuse and random pulmonary metastases at advanced stages. This trend was also observed in our patients. Furthermore, few studies have described the imaging findings related to EML4-ALK gene fusions in lung cancer. In the present study, 5 of 6 patients with EML4-ALK mutations exhibited solid masses with no GGO lesions, and 1 of 6 patients harbored multiple nodules. Five of 6 ALK-positive patients had thoracic lymphadenopathy. These features indicate that most ALK-positive tumors exhibit a solid growth pattern without GGO and have a tendency to infiltrate into localized lymph nodes. Furthermore, compared to patients with EGFR mutations, lymphadenopathy was more common and remarkable in patients with EML4-ALK gene fusions in our study. Therefore, a solid mass and thoracic lymphadenopathy may be the most common imaging features of ALK-positive patients, as indicated by Fukui and Park et al. [32,33]. Given the nonspecific clinical presentations, these very young patients are typically misdiagnosed with pneumonia or pulmonary tuberculosis. Moreover, disease in a younger patient is less likely to be considered cancer when symptoms occur because physicians typically consider cancer at the end of a differential diagnosis. As a result, the correct diagnosis of lung cancer in patients younger than 30 years of age is often delayed. The median interval time from symptom onset to diagnosis was greater than 30 days in our series.
In our study, adenocarcinoma was the leading histologic type, accounting for greater than 70% of all cases. In addition, squamous cell carcinoma was rarely observed. Previous studies [14–16,28] have also demonstrated that younger patients exhibit a greater incidence of adenocarcinoma (33–82.6%) and a low proportion of squamous cell carcinoma lesions (6.3–22%). Although the reason for the high percentage of adenocarcinoma in younger patients is unclear, we believe that smoking is one factor involved in this phenomenon. Given that the development of lung cancer may occur over decades after beginning to smoke, an increased number of adenocarcinoma lesions and fewer smoking-related squamous cell carcinoma lesions may be expected in young patients. Subramanian et al. [5] and McDuffie et al. [34] reported that lung cancer patients with no history of smoking appear to develop lung cancer at earlier ages compared with lung cancer patients with a history of smoking. Genetic and environmental factors have been suggested to play important roles in young patients with lung cancer. Furthermore, in studies by Y. Pan and Kim [35,36], the most common histologic patterns observed were acinar and solid predominant adenocarcinoma. In addition, ALK-rearranged tumors more frequently showed a solid predominant pattern and signet ring cells, which is similar to observations reported in previous studies [36,37].
Interestingly, our study demonstrated that EGFR and EML4-ALK mutations were observed in 50% (11/22) of the lung adenocarcinoma patients. EGFR mutation frequencies in lung adenocarcinoma reported in various studies have exhibited significant variations due to ethnicity, sex and smoking status. In unselected lung adenocarcinoma cases, EGFR mutations are present in ~15% of Caucasian cases and 30 to 50% of East Asian cases [17,18,38]. Moreover, ALK rearrangement is observed in 3 to 5% of unselected NSCLC cases [19,39]. VandenBussche et al. [40] reported that the frequency of EGFR mutations and ALK translocations is increased among Caucasian patients aged <50 years. Interestingly, studies have also demonstrated that EML4-ALK and ROS1 gene rearrangement mutations are significantly more common in young Asian patients [41,42]. Our study revealed that EML4-ALK rearrangement was the most common mutation (27.2% of cases), followed by EGFR mutations (25% of patients). Compared with overall gene alterations in Asian lung adenocarcinoma patients, EML4-ALK alterations in our study were relatively increased, and EGFR mutations were relatively reduced. In addition, EML4-ALK alterations predominantly occurred in female patients [41,42]. Ye T and his colleagues [43] reported the molecular characteristics of 36 resected lung adenocarcinomas from young patients under 40 years old. The mean age was 34.53±4.63, and only one patient had advanced stage disease. Their research showed that EGFR mutations occurred in greater than 50% of patients, and ALK rearrangements occurred in only 5.6% of patients. Several reasons may exist for the discrepancies between their study and our study: (1) This results may be influenced by the age of the population studied. The mean age in our study was 26.4±3.5, compared to 34.53±4.63 in the study by Ye T et al. Meanwhile, Nagashima [41] and Sholl [42] demonstrated that EML4-ALK and ROS1 gene rearrangement mutations are significantly more common in young patients. (2) A higher percentage of advanced stage disease was observed in our study (greater than 50% of all patients). (3) Of note, the study size may have been an important influential factor in both studies. ROS1 rearrangement is observed in 1 to 2% of unselected NSCLC cases [37,44,45]. KRAS mutations are present in 25–40% of adenocarcinoma patients, and these mutations are rarely observed in never-smokers [46]. In this study, 2 of 17 patients harbored KRAS and ROS1 mutations. ROS1 gene mutations occurred more frequently in our study compared with the overall lung cancer patient population. The high frequency of gene mutations in our study indicates that the detection of gene mutations in these very young patients is important and will help to identify the appropriate targeted drug therapy, such as EGFR-TKI and crizotinib.
In this study, long-term follow-up data were available for 25 patients. The median survival time was 8 and 44.2 months in advanced disease stages and early disease stages, respectively. According to a previous study that included 20 patients 30 years of age or younger [16] with either stage III or IV disease, the median survival was only 5.5 months, and no 5-year survivors were noted. The poor prognosis was partially related to the lack of targeted therapies at that time. The 1-year and 5-year survival rates in our study were 56.6% and 38.6%, respectively. Zhang et al. [7] have reported 1-year and 5-year survival rates of 49.87% and 23.12%, respectively, in patients younger than 45 years. The results in our study should be interpreted carefully given the small number of patients studied.
Conclusions
Higher gene mutation frequencies are found in these very young lung cancer patients. EML4-ALK gene fusion was the most common mutation. This study indicates that it is very important to detect the gene mutations in young patients, and it will help to determine the appropriate therapy. As far as we know, no study has described the gene mutation characteristics of lung cancer among patients 30 years of age or younger, and our study has filled this gap. However, this study has several limitations. First, the study utilized a retrospective design. Second, the sample size was small. Thirdly, only a small number of patients received the targeted therapy. Further prospective studies are needed to identify gene alterations and therapeutic strategies in this subgroup of patients.
Supporting Information
(XLSX)
Data Availability
Data are available from the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, for researchers who meet the criteria for access to confidential data. Readers can contact the corresponding author (zjyhz@zju.edu.cn) to request the data.
Funding Statement
This study was partly supported by the Fund of Science Technology Department of Zhejiang Province (No. 2012C33064) and the Fund of Education Department of Zhejiang Province (No. Y201120841).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65: 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. She J, Yang P, Hong Q, Bai C (2013) Lung cancer in China: challenges and interventions. Chest 143: 1117–1126. 10.1378/chest.11-2948 [DOI] [PubMed] [Google Scholar]
- 3. Nugent WC, Edney MT, Hammerness PG, Dain BJ, Maurer LH, et al. (1997) Non-small cell lung cancer at the extremes of age: impact on diagnosis and treatment. Ann Thorac Surg 63: 193–197. [DOI] [PubMed] [Google Scholar]
- 4. Liam CK, Lim KH, Wong CM (2000) Lung cancer in patients younger than 40 years in a multiracial Asian country. Respirology 5: 355–361. [PubMed] [Google Scholar]
- 5. Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, et al. (2010) Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol 5: 23–28. 10.1097/JTO.0b013e3181c41e8d [DOI] [PubMed] [Google Scholar]
- 6. Kuo CW, Chen YM, Chao JY, Tsai CM, Perng RP (2000) Non-small cell lung cancer in very young and very old patients. Chest 117: 354–357. [DOI] [PubMed] [Google Scholar]
- 7. Zhang J, Chen SF, Zhen Y, Xiang J, Wu C, et al. (2010) Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer 116: 3656–3662. 10.1002/cncr.25100 [DOI] [PubMed] [Google Scholar]
- 8. Ak G, Metintas M, Metintas S, Yildirim H, Erginel S, et al. (2007) Lung cancer in individuals less than 50 years of age. Lung 185: 279–286. [DOI] [PubMed] [Google Scholar]
- 9. Hsu CL, Chen KY, Shih JY, Ho CC, Yang CH, et al. (2012) Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer 12: 241 10.1186/1471-2407-12-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozielski J, Kaczmarczyk G, Porebska I, Szmygin-Milanowska K, Golecki M (2012) Lung cancer in patients under the age of 40 years. Contemp Oncol (Pozn) 16: 413–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marugame T, Yoshimi I, Kamo K, Imamura Y, Kaneko S, et al. (2005) Trends in lung cancer mortality among young adults in Japan. Jpn J Clin Oncol 35: 177–180. [DOI] [PubMed] [Google Scholar]
- 12. Strand TE, Malayeri C, Eskonsipo PK, Grimsrud TK, Norstein J, et al. (2004) Adolescent smoking and trends in lung cancer incidence among young adults in Norway 1954–1998. Cancer Causes Control 15: 27–33. [DOI] [PubMed] [Google Scholar]
- 13. Tian DL, Liu HX, Zhang L, Yin HN, Hu YX, et al. (2003) Surgery for young patients with lung cancer. Lung Cancer 42: 215–220. [DOI] [PubMed] [Google Scholar]
- 14. Duan L, You Q, Chen X, Wang H, Zhang H, et al. (2013) Outcome and prognosis for patients younger than thirty with primary lung cancer. Minerva Chir 68: 175–182. [PubMed] [Google Scholar]
- 15. Mizushima Y, Yokoyama A, Ito M, Manabe H, Hirai T, et al. (1999) Lung carcinoma in patients age younger than 30 years. Cancer 85: 1730–1733. [PubMed] [Google Scholar]
- 16. Whooley BP, Urschel JD, Antkowiak JG, Takita H (2000) Bronchogenic carcinoma in patients age 30 and younger. Ann Thorac Cardiovasc Surg 6: 86–88. [PubMed] [Google Scholar]
- 17. Pao W, Girard N (2011) New driver mutations in non-small-cell lung cancer. Lancet Oncol 12: 175–180. 10.1016/S1470-2045(10)70087-5 [DOI] [PubMed] [Google Scholar]
- 18. Sharma SV, Bell DW, Settleman J, Haber DA (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7: 169–181. [DOI] [PubMed] [Google Scholar]
- 19. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, et al. (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566. [DOI] [PubMed] [Google Scholar]
- 20. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, et al. (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361: 947–957. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 21. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 22. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 23. Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, et al. (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13: 1011–1019. 10.1016/S1470-2045(12)70344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, et al. (2013) Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368: 2385–2394. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 25. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, et al. (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16: 141–151. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 26. Lara MS, Brunson A, Wun T, Tomlinson B, Qi L, et al. (2014) Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): a California Cancer Registry analysis. Lung Cancer 85: 264–269. 10.1016/j.lungcan.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 27. Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, et al. (2012) Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 118: 4502–4511. 10.1002/cncr.27409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang W, Kang Y, Shi GY, Zhang HY, Cai L, et al. (2012) Comparisons of multiple characteristics between young and old lung cancer patients. Chin Med J (Engl) 125: 72–80. [PubMed] [Google Scholar]
- 29. Mauri D, Pentheroudakis G, Bafaloukos D, Pectasides D, Samantas E, et al. (2006) Non-small cell lung cancer in the young: a retrospective analysis of diagnosis, management and outcome data. Anticancer Res 26: 3175–3181. [PubMed] [Google Scholar]
- 30. Hsu KH, Chen KC, Yang TY, Yeh YC, Chou TY, et al. (2011) Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 6: 1066–1072. 10.1097/JTO.0b013e31821667b0 [DOI] [PubMed] [Google Scholar]
- 31. Togashi Y, Masago K, Kubo T, Sakamori Y, Kim YH, et al. (2011) Association of diffuse, random pulmonary metastases, including miliary metastases, with epidermal growth factor receptor mutations in lung adenocarcinoma. Cancer 117: 819–825. 10.1002/cncr.25618 [DOI] [PubMed] [Google Scholar]
- 32. Fukui T, Yatabe Y, Kobayashi Y, Tomizawa K, Ito S, et al. (2012) Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 77: 319–325. 10.1016/j.lungcan.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 33. Park J, Yamaura H, Yatabe Y, Hosoda W, Kondo C, et al. (2014) Anaplastic lymphoma kinase gene rearrangements in patients with advanced-stage non-small-cell lung cancer: CT characteristics and response to chemotherapy. Cancer Med 3: 118–123. 10.1002/cam4.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDuffie HH, Klaassen DJ, Dosman JA (1987) Female-male differences in patients with primary lung cancer. Cancer 59: 1825–1830. [DOI] [PubMed] [Google Scholar]
- 35. Kim L, Kim KH, Yoon YH, Ryu JS, Choi SJ, et al. (2012) Clinicopathologic and molecular characteristics of lung adenocarcinoma arising in young patients. J Korean Med Sci 27: 1027–1036. 10.3346/jkms.2012.27.9.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan Y, Zhang Y, Li Y, Hu H, Wang L, et al. (2014) ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer 84: 121–126. 10.1016/j.lungcan.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 37. Cha YJ, Lee JS, Kim HR, Lim SM, Cho BC, et al. (2014) Screening of ROS1 rearrangements in lung adenocarcinoma by immunohistochemistry and comparison with ALK rearrangements. PLoS One 9: e103333 10.1371/journal.pone.0103333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, et al. (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97: 339–346. [DOI] [PubMed] [Google Scholar]
- 39. Zhou J, Zhao J, Sun K, Wang B, Wang L, et al. (2014) Accurate and economical detection of ALK positive lung adenocarcinoma with semiquantitative immunohistochemical screening. PLoS One 9: e92828 10.1371/journal.pone.0092828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. VandenBussche CJ, Illei PB, Lin MT, Ettinger DS, Maleki Z (2014) Molecular alterations in non-small cell lung carcinomas of the young. Hum Pathol 45: 2379–2387. 10.1016/j.humpath.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 41. Nagashima O, Ohashi R, Yoshioka Y, Inagaki A, Tajima M, et al. (2013) High prevalence of gene abnormalities in young patients with lung cancer. J Thorac Dis 5: 27–30. 10.3978/j.issn.2072-1439.2012.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sholl LM (2015) Biomarkers in lung adenocarcinoma: a decade of progress. Arch Pathol Lab Med 139: 469–480. 10.5858/arpa.2014-0128-RA [DOI] [PubMed] [Google Scholar]
- 43. Ye T, Pan Y, Wang R, Hu H, Zhang Y, et al. (2014) Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old. J Thorac Dis 6: 1396–1402. 10.3978/j.issn.2072-1439.2014.08.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, et al. (2012) RET, ROS1 and ALK fusions in lung cancer. Nat Med 18: 378–381. 10.1038/nm.2658 [DOI] [PubMed] [Google Scholar]
- 45. Zhao C, Li X, Li J, Zhang Y, Ren S, et al. (2014) Detecting ALK, ROS1 and RET Fusion Genes in Cell Block Samples. Transl Oncol 7: 363–367. 10.1016/j.tranon.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Korpanty GJ, Graham DM, Vincent MD, Leighl NB (2014) Biomarkers That Currently Affect Clinical Practice in Lung Cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front Oncol 4: 204 10.3389/fonc.2014.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data are available from the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, for researchers who meet the criteria for access to confidential data. Readers can contact the corresponding author (zjyhz@zju.edu.cn) to request the data.