Abstract
Aim
To evaluate the efficacy and safety of bevacizumab in the adjuvant cancer therapy setting within different subset of patients.
Methods & Design/ Results
PubMed, EMBASE, Cochrane and Clinical trials.gov databases were searched for English language studies of randomized controlled trials comparing bevacizumab and adjuvant therapy with adjuvant therapy alone published from January 1966 to 7th of May 2014. Progression free survival, overall survival, overall response rate, safety and quality of life were analyzed using random- or fixed-effects models according to the PRISMA guidelines. We obtained data from 44 randomized controlled trials (30,828 patients). Combining bevacizumab with different adjuvant therapies resulted in significant improvement of progression free survival (log hazard ratio, 0.87; 95% confidence interval (CI), 0.84–0.89), overall survival (log hazard ratio, 0.96; 95% CI, 0.94–0.98) and overall response rate (relative risk, 1.46; 95% CI: 1.33–1.59) compared to adjuvant therapy alone in all studied tumor types. In subgroup analyses, there were no interactions of bevacizumab with baseline characteristics on progression free survival and overall survival, while overall response rate was influenced by tumor type and bevacizumab dose (p-value: 0.02). Although bevacizumab use resulted in additional expected adverse drug reactions except anemia and fatigue, it was not associated with a significant decline in quality of life. There was a trend towards a higher risk of several side effects in patients treated by high-dose bevacizumab compared to the low-dose e.g. all grade proteinuria (9.24; 95% CI: 6.60–12.94 vs. 2.64; 95% CI: 1.29–5.40).
Conclusions
Combining bevacizumab with different adjuvant therapies provides a survival benefit across all major subsets of patients, including by tumor type, type of adjuvant therapy, and duration and dose of bevacizumab therapy. Though bevacizumab was associated with increased risks of some adverse drug reactions such as hypertension and bleeding, anemia and fatigue were improved by the addition of bevacizumab.
Introduction
Bevacizumab (BV), a humanized recombinant monoclonal antibody against vascular endothelial growth factor (VEGF), was approved by the US food and drug administration (FDA) on the market based on its effectiveness in metastatic cancers. Bevacizumab specifically binds to the VEGF-A protein, thereby inhibiting the process of angiogenesis.
Many randomized controlled trials (RCTs) and several meta-analyses on the efficacy and safety of BV in different tumor types have been published. From these studies, while BV added to chemotherapy improved progression free survival (PFS) and overall survival (OS), there was no significant influence on quality of life (QOL) but there were increased risks of serious adverse drug reactions (ADRs). There was controversy on the dose-effect relations of BV and ADRs: while some studies found increased risks of the occurrence of some ADRs e.g. all grade hypertension (RR: 7.5, 95% CI: 4.2–13.4 vs. RR: 3.0, 95%CI: 2.2–4.2), and high-grade bleeding (RR: 3.02, 95% CI:1.85–4.95 vs. RR: 1.27,95%CI: 0.95–1.7) [1,2] for the high-dose BV compared to low-dose, whereas a recent safety meta-analysis of 13 heterogeneous trials did not [3]. However, defining which of any benefited more or less from BV has not been extensively studied. Therefore, and because there have been new RCTs published after the latest published meta-analysis [4], we conducted a large meta-analysis to examine predictive factors for BV efficacy and safety by performing a series of subgroup, meta-regression and sensitivity analyses. In addition, we systematically assessed heterogeneity and publication bias.
Methods
Data source
All published RCTs on the efficacy and safety of BV in different tumor types were collected by conducting a literature search using PubMed, EMBASE, Cochrane and Clinical trials.gov database with the keywords shown in S1 Table (See Appendix). Furthermore, we searched abstracts and virtual meeting presentations from websites of the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), Federation of European Cancer Societies (FECS) and San Antonio Breast Cancer Symposium (SABCS) to identify relevant RCTs. Further information was retrieved through a manual search of references from recent meta-analyses and relevant published trials.
Inclusion and exclusion criteria
All phase 2 or 3 RCTs were included in our study if there was a direct comparison between BV in combination with adjuvant therapy and adjuvant therapy alone available (experimental arm: BV plus adjuvant therapy agent (s); control arm: adjuvant therapy with or without placebo) in patients with metastatic cancers. Only publications in English language and from January 1966 to 7th of May 2014 were considered. Trials in pediatric populations and trials where BV was used for the treatment of macular retinopathy and brain tumors were excluded from this meta-analysis because of different outcome measurements. Two investigators (FA and AdB) independently applied the inclusion and exclusion criteria to select the relevant trials.
Data extraction and clinical end points
Data extraction was conducted in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance (S1 PRISMA Checklist) [5]. The clinical end points used for this study were PFS, defined as the time from random assignment to first reported progression or all-cause mortality in the absence of previously documented tumor progression, OS, defined as the time from random assignment to death which can be from any cause, censoring patients who had alive at the date last visit, overall response rate (ORR), defined as the sum of partial and complete response rates (according to the Response Evaluation Criteria in Solid Tumors) [6], ADRs were graded according to the Common Toxicity Criteria version 3 (http://ctep.cancer.gov) and QOL assessed at baseline and during the follow-up time until disease progression.
Hazard Ratios (HRs) for PFS and OS, median PFS (mPFS) and median OS (mOS), the number of patients with ORR and the number of adverse drug reactions (ADRs) were extracted from the papers. Furthermore, the first author, year of publication, trial design characteristics (study phase, outcome measures, tumor type, therapy regime for each arm, dose of treatment, median time of follow-up, median duration of BV therapy and time points of response assessment), patient characteristics (median age and number of patients evaluated for efficacy and safety in each arm) were extracted. In case of missing data for HR as a point estimate in trials, authors were contacted via email to provide the necessary information.
Risk of bias assessment
Quality assessment of the publications included was performed independently by three investigators using the Cochrane Collaboration’s tool (http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm). This means that the trials were rated for domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of primary outcomes (PFS, OS, ORR) assessment, blinding of secondary outcomes (safety and QOL) assessment, incomplete PFS, OS and ORR data, incomplete safety data, selective reporting and other biases. In the case of any disagreement between investigators to rate the quality of each trial consensus was reached. When there was insufficient information to permit the evaluation of the quality it was rated as unclear (uncertain risk of bias).
Data analysis
Overall pooled estimates, together with 95% confidence intervals (CI) of the PFS, OS, ORR and safety outcomes were obtained using either a fixed-effects model or in the event of heterogeneity, a random-effects model. Relative risks (RRs) and 95% CIs were calculated to assess the ORR and safety of BV compared with control group. Subgroup analyses to identify the overall impact of patient and trial characteristics on BV efficacy were performed for the following characteristics: different tumor types, BV dose (high-dose (5 mg/kg weekly) and low-dose (2.5 mg/kg weekly)), types of adjuvant therapies (platinum (cisplatin, carboplatin, or oxaliplatin) and taxanes (paclitaxel or docetaxel) versus non-platinum (non-platinum and nontaxane-based)), age of participants (50–55, 56–60, 61–66, >66 years old), median duration of follow-up (6–12, 13–24, 25–36, >37 months), median duration of BV therapy (<12, 12–24, 25–36, >37 weeks) and timing of response assessment (6, 8–12, 24 weeks). These subgroup analyses were performed for all trials combined, colorectal cancer, non-small cell lung cancer (NSCLC) and breast cancer patients separately. The time interval between mOS and mPFS was measured as survival post progression (SPP) in different tumor types including colorectal cancer, NSCLC, breast cancer and ovarian cancer. The validation of PFS as a surrogate endpoint for OS in all combined trials was tested using the Spearman’s rank correlation. Linearity between the log HRs of PFS and OS was also assessed in a linear regression model. The correlation between PFS and OS was further studied in subgroup analyses in different tumor types including colorectal cancer, NSCLC and breast cancer. Publication bias was evaluated by using funnel plots and the Egger test was applied to measure any asymmetry. Heterogeneity of the studies was tested by the I2 measure of inconsistency with 25% corresponding to low heterogeneity, 50% to moderate and 75% to high. If heterogeneities existed, one of the following techniques was used to explain them: random-effects models for meta-analysis, subgroup analyses, or meta-regression analyses. Meta-regression analyses were also performed for the BV dose, median age of participants, median duration of follow-up and median duration of BV therapy. To evaluate the relation between BV doses and the risk of ADRs a subgroup analysis was performed. In addition, sensitivity analysis was applied by omitting one study in each turn and investigated the influence of a single study on the overall meta-analysis estimate [7] when necessary. All statistical analyses were conducted using STATA 10/SE (StataCorp. 2007. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP).
Results
Search results
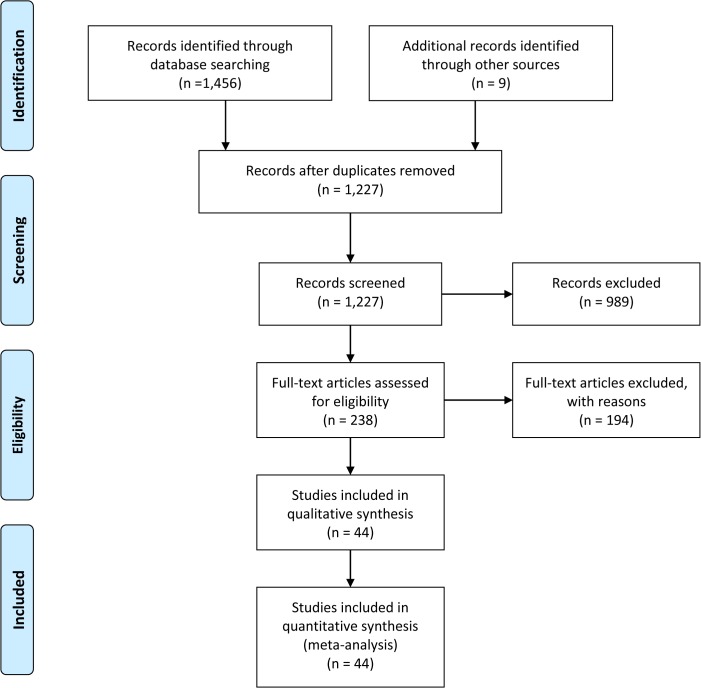
Fig 1 shows a flow chart for the selection procedure of the trials. Our literature search yielded 1,465 published articles on BV safety and efficacy and after applying the inclusion and exclusion criteria a total of 44 RCTs [8–11,11–19,19–28,29–51] were selected for the meta-analyses (Fig 1).
Fig 1. A Flow Diagram showing the RCTs selection.
Study characteristics
The characteristics of the included trials are summarized in Table 1. A total of 30,828 patients (BV, n = 16,266; control, n = 14,562) from 44 RCTs were included in the meta-analysis. Underlying malignancies were included colorectal cancer (13 studies), breast cancer (10 studies), NSCLC (7 studies), ovarian cancer (4 studies), renal cell cancer (2 studies), pancreatic cancer (2 studies), gastric cancer (1 study), melanoma cancer (1 study), prostate cancer (1 study), mesothelioma cancer (1 study), cervical cancer (1 study) and Follicular lymphoma (1 study). Five trials evaluated BV in different arms, either different doses of BV [14,26,27,35] or its combination with different adjuvant therapy agents [34] with a control group. Sample sizes ranged from 23 to 2,867 patients, with 24 trials including more than 500 patients each. In all trials, patients were randomly assigned to either the control or the BV group. Eleven (25%) trials were phase II and 33 (75%) were phase III studies. Trial treatment regimens varied by tumor types and the BV dose ranged from 2.5 to 5 mg/kg weekly. The median age of patients in all trials combined was almost 59 years in both groups. All studies recruited both male and female participants (except trials of metastatic breast cancer, ovarian cancer, prostate cancer and cervical cancer) however outcomes were not reported stratified by gender.
Table 1. Characteristics of trials included in the meta-analysis.
| RCTs | Tumor type | Allocation sequences | Trial phase | Enrolled patients | Adjuvant therapy | BV dose, mg/kg/week | Median duration of follow–up (months) | Time points of response assessment (weeks) |
|---|---|---|---|---|---|---|---|---|
| Bennouna, J et al, 2013 | MCRC | C | III | 820 | Oxaliplatin plus Irrinotecan | 2.5 | 11.1 | NA |
| Cunnigham, D et al, 2013 | MCRC | C | III | 280 | Capecitabine | 2.5 | 24.8 | 9 |
| De Gramont, A et al, 2012 | MCRC | C | III | 2867 | Oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) | 2.5 | 48 | 24 |
| Dotan, E et al, 2012 | MCRC | C | II | 23 | Capecitabine plus Oxaliplatin plus Cetuximab | 2.5 | 25.9 | 6 |
| Kemeny, NE et al, 2011 | MCRC | C | II | 73 | Oxaliplatine plus Fluorouracil plus Leucovorin | 2.5 | 30 | NA |
| Guan, ZZ et al, 2011 | MCRC | C | III | 214 | Irinotecan plus leucovorin, and 5-fluorouracil | 2.5 | NA | 6 |
| Allegra,C J et al, 2011 | MCRC | C | III | 2710 | FOLFOX6 | 2.5 | 35.6 | NA |
| Tebbutt, N et al, 2009 | MCRC | C | III | 471 | Capecitabine | 2.5 | 31 | 6 |
| Stathopulos, GP et al, 2010 | MCRC | C | III | 222 | leucovorin plus 5-fluorouracil plus irinotecan | 2.5 | 36 | 8 |
| Saltz, LB et al, 2008 | MCRC | B | III | 1401 | FOLFOX-4 / XELOX | 2.5 | 27.6 | 6 |
| Giantanio, BJ et al, 2007 | MCRC | C | III | 829 | FOLFOX4 | 5 | 28 | 12 |
| Hurwitz, HI et al, 2004 | MCRC | B | III | 813 | Irinotecan plus fluorouracil, and leucovorin | 2.5 | 18 | 6 |
| Kabbinavar, F et al, 2003 | MCRC | C | II | 104 | Fluorouracil l/ leucovorin | 2.5, 5 | 17.6 | 8 |
| Cameron, D et al, 2013 | MBC | C | III | 2591 | Antracycline plus Taxane | 5 | 31.5 | NA |
| Luca, G et al, 2013 | MBC | C | III | 424 | Docetaxel plus Trastuzumab | 5 | 26 | 9 |
| Von Minckwitz, G et al, 2012 | MBC | C | III | 1948 | Epirubicin plus cyclophosphamide plus docetaxel | 5 | NA | 6 |
| Bear, H et al, 2012 | MBC | C | III | 1206 | Docetaxel plus Capecitabine | 5 | NA | NA |
| Brufsky, AM et al, 2011 | MBC | A | III | 684 | Capecitabine plusa Taxane | 5 | 15 | 6 |
| Robert, NJ et al, 2011 | MBC | A | III | 618 | Capecitabine plus Taxane–based | 5 | 15.6, 19.2 | 9 |
| Martin, M et al, 2011 | MBC | A | II | 191 | Paclitaxel | 2.5 | NA | 8 |
| Miles, DW et al, 2010 | MBC | A | III | 736 | Docetaxel | 2.5, 5 | 25 | 9 |
| Miller, K et al, 2007 | MBC | C | III | 722 | Paclitaxel | 5 | 41.6 | 12 |
| Miller, KD et al, 2005 | MBC | C | III | 462 | Capecitabine | 5 | 14.8 | 6 |
| Niho, S et al, 2012 | NSCLC | C | II | 180 | Carboplatin plus Paclitaxel | 5 | NA | 6 |
| Herbst, RS et al, 2011 | NSCLC | C | III | 636 | Erlotinib | 5 | 19 | 6 |
| Spigel, DR et al, 2011 | NSCLC | A | II | 102 | Cisplatin or Carboplatin plus Etoposide | 5 | 7.8 | 6 |
| Reck, M et al, 2009 | NSCLC | B | III | 1043 | Cisplatin plus Gemcitabine | 2.5, 5 | 7 | 9 |
| Herbst,R et al, 2007 | NSCLC | C | II | 120 | Docetaxel or Pemetrexed | 5 | 15.8 | 6 |
| Sandler, A et al, 2006 | NSCLC | C | III | 878 | Paclitaxel plus Carboplatin | 5 | 19 | 6 |
| Johnson, DH et al, 2004 | NSCLC | C | II | 99 | Carboplatin plus Paclitaxel | 2.5, 5 | 14.7 | 6 |
| Eric, PL et al, 2014 | OC | C | III | 361 | Doxorubicin plus Paclitaxel plus Topotecan | 5 | 13.9 | 8 |
| Aghajanian, C et al, 2012 | OC | A | III | 484 | Carboplatin plus Gemcitabine | 5 | 24 | 9 |
| Perren,T et al, 2011 | OC | C | III | 1528 | Carboplatin plus Paclitaxel | 2.5 | 28 | 6 |
| Burger, RA et al, 2011 | OC | A | III | 1873 | Paclitaxel plus Carboplatin | 5 | 17.4 | NA |
| Kindler, HL et al, 2010 | MPC | A | III | 602 | Gemcitabine | 2.5 | 11.3 | 6 |
| Cutsem, E et al, 2009 | MPC | A | III | 607 | Gemcitabine plus Erlotinib | 2.5 | 6.7 | 8 |
| Rini, BI et al, 2010 | RCC | C | III | 732 | Interferon alpha | 5 | 46.2 | 12 |
| Escudier, B et al, 2007 | RCC | A | III | 649 | Interferon alfa-2a | 5 | 13.3 | 8 |
| Krishnansu, ST et al, 2014 | CC | C | III | 452 | Cisplatin plus Paclitaxel | 5 | 20.8 | NA |
| John, DH et al, 2014 | RFL | C | II | 60 | Rituximab | 5 | 34 | NA |
| Kim, KB et al, 2012 | MC | A | II | 214 | Carboplatin plus paclitaxel | 5 | 13 | 6 |
| Kelly, WK et al, 2012 | PC | A | III | 1050 | Docetaxel plus prednisone | 5 | 24 | 12 |
| Kindler, HL et al, 2012 | AM | A | II | 115 | Gemcitabine plus Cisplatin | 5 | NA | 6 |
| Ohtsu, A et al, 2011 | GC | A | III | 774 | Cisplatin plus Capecitabine | 2.5 | 11.4 | 6 |
Abbreviations: RCTs: randomized control trials; BV: bevacizumab; MCRC: metastatic colorectal cancer; GC: gastric cancer; MPC: metastatic pancreatic cancer; RCC: renal carcinoma cancer; NSCLC: non-small-cell lung carcinoma; MBC: metastatic breast cancer; AM: advanced mesothelioma; PC: prostate cancer; MC: melanoma carcinoma; OC: ovarian cancer; CC: cervical cancer; RFL: Relapsed Follicular Lymphoma, A: Double-blinded- placebo and active treatment control, B: Placebo and active treatment control, C: Active treatment control.
Risk of bias assessment in all trials combined
Randomized treatment allocation sequences were generated in 44 RCTs. Fourteen trials were double blinded with placebo and active treatment controls, 3 trials had placebo and active treatment controls and the rest of trials had active treatment controls. S2 Table presents the risk of bias judgments for the 44 RCTs. According to our methodological assessment, the results showed a low risk of bias in most domains except for blinding across all outcomes; therefore, the overall quality of all trials combined was acceptable.
Efficacy analyses in all trials combined
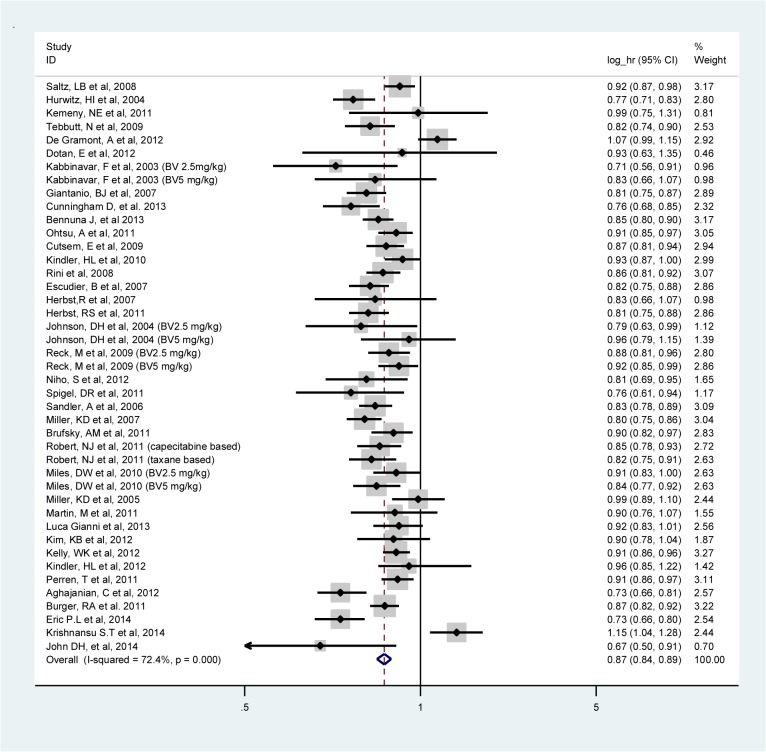
The meta-analysis of PFS was based on 38 RCTs (Table 2). Combining BV with different adjuvant therapies resulted in a 13% risk reduction of PFS events (log HR, 0.87; 95% CI, 0.84–0.89; I2:72.4%, random-effects model) (Fig 2) with high heterogeneity attributed to the colorectal cancer (I2: 82.3%) and ovarian cancer (I2: 92.8%) trials. PFS statistically significantly improved in patients for all types of tumors except for patients with melanoma, mesothelioma and cervical cancers. No statistically significant differences between logs HRs of PFS were observed between the different tumor types.
Table 2. BV efficacy assessment in trials included in the meta-analysis.
| PFS | OS | ORR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | No. of patients in BV group/sample size | No. of patients in control group/sample size | Log HR (95% CI) | I2 | No. of studies | No. of patients in BV group/sample size | No. of patients in control group/sample size | Log HR (95% CI) | I2 | No. of studies | No. of patients in BV group/sample size | No. of patients in control group/sample size | RR (95% CI) | I2 | |
| All trial combined | 38 | 12221/24696 | 10756/24696 | 0.87 (0.84–0.89) | 72.4 | 34 | 11611/23821 | 10491/23821 | 0.96 (0.94–0.98) | 22.2 | 24 | 7898/14596 | 6385/14596 | 1.46 (1.33–1.59) | 71.3 |
| Colorectal cancer | 10 | 3364/7681 | 3346/7681 | 0.85 (0.79–0.92) | 82.3 | 9 | 3224/7401 | 3206/7401 | 0.93 (0.87–0.99) | 61.1 | 6 | 1606/3384 | 1514/3384 | 1.40 (1.01–1.95) | 84.2 |
| NSCLC | 7 | 1712/3058 | 1279/3058 | 0.85 (0.82–0.89) | 13.0 | 6 | 1016/2015 | 932/2015 | 0.94 (0.90–0.98) | 0 | 4 | 1188/1961 | 773/1961 | 1.60 (1.37–1.87) | 9.4 |
| Breast cancer | 7 | 2670/4456 | 1737/4456 | 0.87 (0.84–0.91) | 50.5 | 6 | 3658/6432 | 2725/6432 | 0.98 (0.94–1.01) | 0 | 6 | 2454/4032 | 1529/4032 | 1.36 (1.25–1.47) | 10.4 |
| Pancreatic cancer | 2 | 608/1209 | 601/1209 | 0.90 (0.85–0.96) | 36.5 | 2 | 608/1209 | 601/1209 | 1.01 (0.94–1.08) | 0 | 2 | 608/1209 | 601/1209 | 1.41 (1.02–1.95) | 0 |
| Ovarian cancer | 4 | 2035/4698 | 2038/4698 | 0.86 (0.76–0.99) | 92.8 | 3 | 1044/2718 | 1049/2718 | 0.97 (0.91–1.03) | 0 | 3 | 1185/2373 | 1188/2373 | 1.47 (1.25–1.73) | 68.7 |
| Renal Cancer | 2 | 696/1381 | 685/1381 | 0.84 (0.80–0.89) | 0 | 2 | 696/1381 | 685/1381 | 0.95 (0.90–0.99) | 0 | 1 | 1016/2015 | 932/2015 | 2.55 (1.81–3.61) | - |
| Gastric Cancer | 1 | 387/774 | 387/774 | 0.91 (0.85–0.97) | - | 1 | 387/774 | 387/774 | 0.94 (0.87–1.01) | - | 1 | 387/774 | 387/774 | 1.29 (1.05–1.58) | - |
| Prostate Cancer | 1 | 524/1050 | 526/1050 | 0.91 (0.86–0.96) | - | 1 | 524/1050 | 526/1050 | 0.96 (0.90–1.02) | - | - | - | - | - | - |
| Melanoma Cancer | 1 | 143/214 | 71/214 | 0.90 (0.78–1.04) | - | 1 | 143/214 | 71/214 | 0.84 (0.71–0.99) | - | 1 | 143/214 | 71/214 | 1.49 (0.83–2.68) | - |
| Mesothelioma Cancer | 1 | 53/115 | 55/115 | 0.96 (0.79–1.15) | - | 1 | 53/115 | 55/115 | 1.02 (0.85–1.22) | - | - | - | - | - | - |
| Cervical cancer | 1 | 227/452 | 225/452 | 1.15 (1.04–1.28) | - | 1 | 227/452 | 225/452 | 1.08 (0.92–1.28) | - | - | - | - | - | - |
| Lymphoma | 1 | 29/60 | 31/60 | 0.67 (0.50–0.91) | - | 1 | 29/60 | 31/60 | 0.67 (0.44–1.02) | - | - | - | - | - | - |
Abbreviations: PFS: progression free survival; OS: overall survival; ORR: overall response rate; HR: hazard ratio; RR: relative risk; BV: bevacizumab; NSCLC: non-small cell lung cancer.
Fig 2. Meta-analysis of the log hazard ratios of progression free survival comparing bevacizumab and standard therapy in all trials combined.
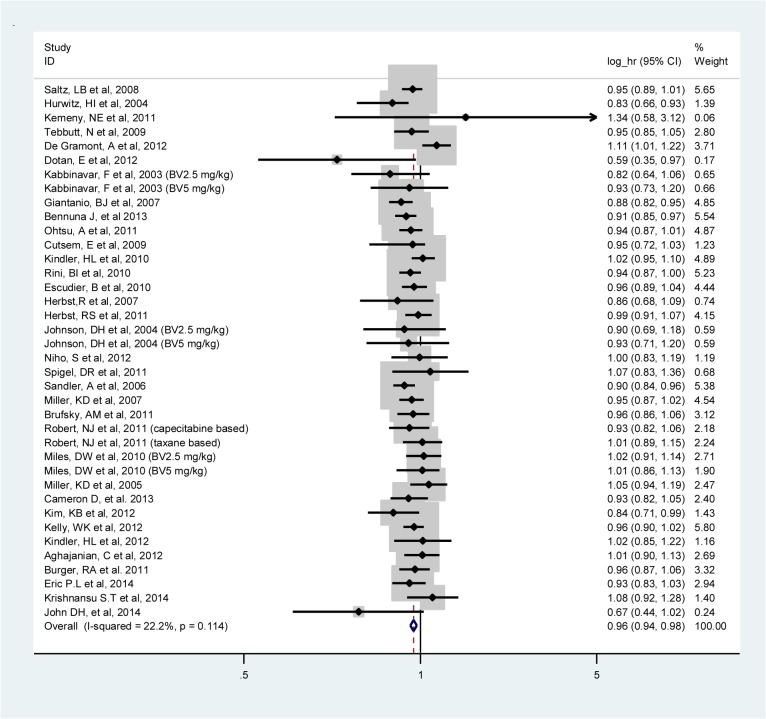
The meta-analysis of OS was based on 34 RCTs. Adding BV caused a 4% risk reduction of OS events as compared with regimens without BV (log HR, 0.96; 95% CI, 0.94–0.98; I2:22.2%, fixed-effects model) (Fig 3). When investigating by type of tumor, OS was significantly improved only in colorectal cancer, NSCLC, renal cancer and melanoma cancer. Again the results showed no significant difference between logs HRs of OS in different tumor types.
Fig 3. Meta-analysis of the logs hazard ratios of overall survival comparing bevacizumab and standard therapy in all trials combined.
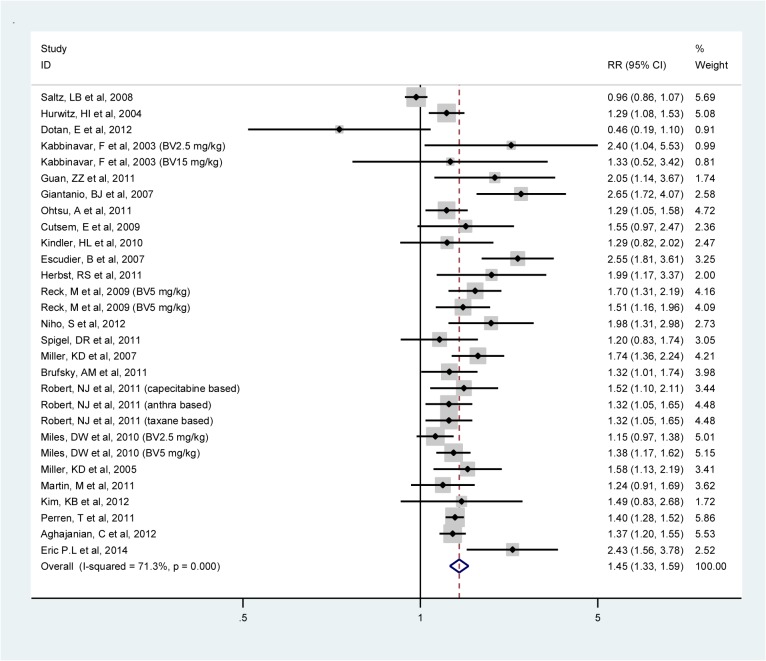
The meta-analyzed RR of the ORR associated with the addition of BV to adjuvant therapy in 24 studies was 1.46 (95% CI: 1.33–1.59; I2: 71.3%, random-effects model) (Fig 4). High heterogeneity was observed in the colorectal cancer (I2: 84.2%) trials. The ORR was statistically significantly improved in all tumor types except in patients with melanoma cancer. Furthermore, the highest improvement of ORR was observed among renal cancer patients (RR, 2.55; 95% CI, 1.81–3.61). This was statistically significant different from the other tumor types.
Fig 4. Meta-analysis of the risk ratios of overall response rate comparing bevacizumab and standard therapy in all trials combined.
Efficacy subgroup analyses
As shown in Tables 3 and 4, patient and trial characteristics did not modify the effects of BV on PFS and OS. For specific tumor types including colorectal cancer, NSCLC and breast cancer, the overall pooled estimates of logs HRs of PFS (Figures A, B and C in S1 File) and OS (Figures A, B and C in S2 File) and RRs of ORR (Figures A, B and C in S3 File) comparing BV and standard chemotherapy are shown in forest plots.
Table 3. Efficacy subgroup analyses in all trials combined.
| PFS | OS | ORR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Log HR (95% CI) | I2 | No. of studies | Log HR (95% CI) | I2 | No. of studies | RR (95% CI) | I2 | |
| BV dose | |||||||||
| 2.5 mg/kg | 17 | 0.88 (0.84–0.92) | 72.3 | 13 | 0.96 (0.92–1.01) | 50.4 | 11 | 1.26 (1.09–1.45) | 74.6 |
| 5 mg/kg | 25 | 0.86 (0.83–0.89) | 71.6 | 24 | 0.95 (0.93–0.97) | 0 | 15 | 1.58 (1.43–1.74) | - |
| Chemotherapy regimen | |||||||||
| Platinum | 30 | 0.88 (0.85–0.91) | 74.5 | 25 | 0.96 (0.93–0.98) | 27.5 | 17 | 1.40 (1.26–1.56) | 77.0 |
| Non-platinum | 13 | 0.84 (0.80–0.88) | 62.6 | 11 | 0.96 (0.93–1.00) | 13.7 | 10 | 1.58 (1.36–1.84) | 43.6 |
| Age (years) | |||||||||
| 50–55 | 8 | 0.88 (0.83–0.93) | 55.6 | 6 | 0.98 (0.93–1.03) | 0 | 7 | 1.32 (1.20–1.44) | 0 |
| 56–60 | 14 | 0.87 (0.83–0.92) | 80.5 | 11 | 0.96 (0.92–1.01) | 52.4 | 12 | 1.34 (1.18–1.52) | 77.9 |
| 61–65 | 10 | 0.84 (0.80–0.88) | 58.2 | 10 | 0.95 (0.92–0.98) | 15.9 | 7 | 2.04 (1.67–2.49) | 33.1 |
| >66 | 3 | 0.84 (0.75–0.95) | 68.9 | 3 | 0.94 (0.87–1.02) | 25.6 | - | ||
| Median Follow-up (months) | |||||||||
| 6–12 | 6 | 0.88 (0.85–0.92) | 26.1 | 5 | 0.96 (0.91–1.01) | 26.5 | 5 | 1.40 (1.22–1.60) | 0 |
| 13–24 | 18 | 0.84 (0.80–0.89) | 79.7 | 17 | 0.95 (0.93–0.98) | 7.6 | 11 | 1.52 (1.35–1.72) | 53.8 |
| 25–36 | 9 | 0.88 (0.84–0.92) | 47.5 | 8 | 0.93 (0.88–0.99) | 37.7 | 6 | 1.32 (1.03–1.69) | 90.4 |
| >37 | 3 | 0.90 (0.77–1.07) | 93.9 | 3 | 0.99 (0.90–1.09) | 78.9 | - | - | - |
| Median duration of therapy (weeks) | |||||||||
| <12 | - | - | - | - | - | - | - | - | - |
| 12–24 | 10 | 0.85 (0.79–0.92) | 83.1 | 9 | 0.97 (0.91–1.03) | 62.2 | 7 | 1.74 (1.41–2.16) | 50.7 |
| 25–36 | 10 | 0.84 (0.80–0.89) | 70.0 | 8 | 0.96 (0.92–0.98) | 0 | 8 | 1.35 (1.14–1.60) | 79.9 |
| >37 | 4 | 0.83 (0.76–0.90) | 61.1 | 3 | 0.91 (0.83–1.00) | 23.3 | 2 | 1.78 (0.90–3.53) | 92.0 |
| Time points of response assessment (weeks) | |||||||||
| 6 | 17 | 0.87 (0.84–0.91) | 55.7 | 16 | 0.95 (0.92–0.98) | 22.9 | 13 | 1.34 (1.16–1.54) | 74.1 |
| 8–12 | 16 | 0.83 (0.80–0.87) | 66.0 | 12 | 0.94 (0.92–0.97) | 0 | 11 | 1.68 (1.41–1.99) | 74.2 |
| 24 | 1 | 1.07 (0.99–1.16) | - | 1 | 1.11 (1.01–1.22) | - | - | - | - |
Abbreviations: PFS: progression free survival; OS: overall survival; ORR: overall response rate; HR: hazard ratio; RR: relative risk; BV: bevacizumab.
Table 4. Efficacy subgroup analyses in different tumor types.
| PFS | OS | ORR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N. of studies | Log HR (95% CI) | I2 | N. of studies | Log HR (95% CI) | I2 | N. of studies | RR (95% CI) | I2 | |
| Metastatic Colorectal Cancer | |||||||||
| BV dose | |||||||||
| 2.5 mg/kg | 9 | 0.86 (0.79–0.94) | 84.8 | 8 | 0.94 (0.87–1.01) | 65.1 | 5 | 1.22 (0.89–1.67) | 80.4 |
| 5 mg/kg | 2 | 0.81 (0.75–0.87) | 0 | 2 | 0.89 (0.82–0.95) | 0 | 2 | 2.15 (1.15–3.99) | 40.7 |
| Chemotherapy regimen | |||||||||
| Platinum | 6 | 0.91 (0.83–1.01) | 84.7 | 6 | 0.95 (0.87–1.04) | 75.1 | 3 | 1.12 (0.49–2.55) | 91.6 |
| Non-platinum | 4 | 0.78 (0.74–0.82) | 0 | 3 | 0.90 (0.83–0.98) | 0 | 4 | 1.51 (1.12–2.04) | 28.0 |
| Age (years) | |||||||||
| 50–55 | 1 | 0.76 (0.68–0.85) | 0 | - | - | - | 1 | 2.05 (1.14–3.67) | - |
| 56–60 | 4 | 0.91 (0.78–1.07) | 91.2 | 4 | 0.94 (0.81–1.08) | 80.5 | 3 | 1.02 (0.80–1.38) | 82.3 |
| 61–65 | 2 | 0.83 (0.79–0.87) | 0 | 2 | 0.90 (0.86–0.94) | 0 | 1 | 2.64 (1.72–4.07) | - |
| >66 | 1 | 0.82 (0.74–0.90) | 0 | 1 | 0.95 (0.85–1.06) | - | - | - | - |
| Median Follow-up (months) | |||||||||
| 6–12 | 1 | 0.85 (0.80–0.90) | 0 | 1 | 0.91 (0.85–0.98) | - | - | - | - |
| 13–24 | 3 | 0.76 (0.71–0.81) | 0 | 2 | 0.83 (0.72–0.96) | 0 | 2 | 1.53 (0.88–2.65) | 51.5 |
| 25–36 | 5 | 0.86 (0.80–0.94) | 58.6 | 5 | 0.92 (0.86–0.98) | 35.1 | 3 | 1.12 (0.49–2.55) | 91.6 |
| >37 | 1 | 1.07 (0.99–1.16) | - | 1 | 1.11 (1.01–1.22) | 0 | - | - | - |
| Median duration of therapy (weeks) | |||||||||
| <12 | - | - | - | — | - | - | - | - | - |
| 12–24 | 2 | 0.93 (0.70–1.23) | 96.2 | 2 | 0.99 (0.79–1.24) | 93.0 | 1 | 2.64 (1.72–4.07) | - |
| 25–36 | 3 | 0.84 (0.74–0.94) | 81.3 | 2 | 0.95 (0.90–1.00) | 0 | 2 | 1.33 (0.63–2.79) | 84.4 |
| >37 | 1 | 0.76 (0.70–0.83) | - | 1 | 0.84 (0.71–0.99) | - | 1 | 1.29 (1.08–1.53) | - |
| Time points of response assessment (weeks) | |||||||||
| 6 | 4 | 0.84 (0.75–0.94) | 78.3 | 4 | 0.91 (0.83–1.00) | 43.5 | 4 | 1.13 (0.82–1.54) | 81.9 |
| 8–12 | 3 | 0.79 (0.74–0.84) | 0 | 2 | 0.88 (0.82–0.94) | 0 | 2 | 2.59 (1.77–3.80) | 0 |
| 24 | 1 | 1.07 (0.99–1.16) | - | 1 | 1.11 (1.01–1.22) | - | - | - | - |
| Metastatic NSCLC | |||||||||
| BV dose | |||||||||
| 2.5 mg/kg | 2 | 0.87 (0.80–0.94) | 0 | 1 | 0.90 (0.69–1.18) | - | - | - | - |
| 5 mg/kg | 7 | 0.85 (0.81–0.89) | 25.3 | 6 | 0.94 (0.90–0.99) | 0 | 5 | 1.60 (1.37–87) | 9.4 |
| Chemotherapy regimen | |||||||||
| Platinum | 8 | 0.86) 0.83–0.90) | 5.3 | 6 | 0.92 (0.87–0.97) | 0 | 3 | 1.57 (1.32–1.86) | 18.6 |
| Non-platinum | 1 | 0.81 (0.75–0.88) | 0 | 1 | 0.99 (0.91–1.07) | - | 1 | 1.99 (1.17–3.37) | - |
| Age (years) | |||||||||
| 50–55 | - | - | - | - | - | - | - | - | - |
| 56–60 | 3 | 0.89 (0.83–0.95) | 20.3 | 1 | 1.07 (0.83–1.36) | - | 3 | 1.51 (1.26–1.80) | 12.8 |
| 61–65 | 3 | 0.81 (0.75–0.87) | 0 | 3 | 0.98 (0.91–1.05) | 0 | 2 | 1.98 (1.43–2.74) | 0 |
| >66 | - | - | - | - | - | - | - | - | - |
| Median Follow-up (months) | |||||||||
| 6–12 | 2 | 0.85 (0.74–0.97) | 39.3 | 1 | 1.07 (0.83–1.36) | - | 2 | 1.47 (1.04–2.06) | 57.2 |
| 13–24 | 4 | 0.82 (0.79–0.86) | 0 | 4 | 0.93 (0.88–0.98) | 2.3 | 1 | 1.99 (1.17–3.37) | - |
| 25–36 | - | - | - | - | - | - | - | - | - |
| >37 | - | - | - | - | - | - | - | - | - |
| Median duration of therapy (weeks) | |||||||||
| <12 | - | - | - | - | - | - | - | - | - |
| 12–24 | 5 | 0.84 (0.80–0.88) | 0 | 4 | 0.92 (0.87–0.98) | 0 | 3 | 1.59 (1.22–2.07) | 44.4 |
| 25–36 | - | - | - | - | - | - | - | - | - |
| >37 | 1 | 0.83 (0.65–1.06) | - | 1 | 0.86 (0.68–1.09) | - | - | - | - |
| Time points of response assessment (weeks) | |||||||||
| 6 | 6 | 0.82 (0.78–0.86) | 0 | 6 | 0.94 (0.90–0.99) | 0 | 3 | 1.64 (1.14–2.35) | 52.6 |
| 8–12 | 1 | 0.88 (0.81–0.96) | - | - | - | - | 1 | 1.69 (1.31–2.19) | - |
| 24 | - | - | - | - | - | - | - | - | - |
| Metastatic Breast Cancer | |||||||||
| BV dose | |||||||||
| 2.5 mg/kg | 2 | 0.91 (0.83–0.98) | 0 | 1 | 1.02 (0.91–1.14) | - | 2 | 1.18 (1.01–1.37) | 0 |
| 5 mg/kg | 6 | 0.87 (0.82–0.92) | 59.8 | 6 | 0.97 (0.93–1.01) | 0 | 7 | 1.42 (1.30–1.55) | 0 |
| Chemotherapy regimen | |||||||||
| Platinum | 6 | 0.86 (0.82–0.90) | 36.6 | 6 | 0.97 (0.93–1.01) | 0 | 5 | 1.34 (1.20–1.49) | 32.0 |
| Non-platinum | 2 | 0.92 (0.79–1.06) | 78.9 | 2 | 0.99 (0.88–1.12) | 47.5 | 2 | 1.43 (1.21–1.68) | 0 |
| Age (years) | |||||||||
| 50–55 | 7 | 0.89 (0.85–0.93) | 31.0 | 5 | 0.98 (0.93–1.03) | 0 | 4 | 1.31 (1.19–1.44) | 0 |
| 56–60 | 2 | 0.82 (0.77–0.87 | 11.8 | 2 | 0.96 (0.90–1.03) | 0 | 3 | 1.51 (1.24–1.85) | 29.5 |
| 61–65 | - | - | - | - | - | - | - | - | - |
| >66 | - | - | - | - | - | - | - | - | - |
| Median Follow-up (months) | |||||||||
| 6–12 | - | - | - | - | - | - | - | - | - |
| 13–24 | 3 | 0.89 (0.82–0.95) | 60.4 | 3 | 1.07 (0.83–1.36) | 0 | 3 | 1.38 (1.22–1.55) | 0 |
| 25–36 | 2 | 0.91 (0.85–0.98) | 0 | 2 | 0.97 (0.93–1.00) | 2.3 | 2 | 1.41 (0.93–2.12) | 86.1 |
| >37 | 1 | 0.80 (0.75–0.86) | - | 1 | 0.93 (0.88–0.98) | - | - | - | - |
| Median duration of therapy (weeks) | |||||||||
| <12 | - | - | - | - | - | - | - | - | - |
| 12–24 | - | - | - | - | - | - | - | - | - |
| 25–36 | 4 | 0.85 (0.80–0.90) | 40.5 | 3 | 0.92 (0.87–0.98) | 0 | 4 | 1.46 (1.25–1.71) | 15.9 |
| >37 | 1 | 0.92 (0.83–1.01) | 0 | - | - | - | - | - | - |
| Time points of response assessment (weeks) | |||||||||
| 6 | 2 | 0.94 (0.85–1.03) | 53.2 | 2 | 0.99 (0.84–1.47) | 28.9 | 2 | 1.42 (1.15–1.75) | 0 |
| 8–12 | 5 | 0.87 (0.82–0.92) | 45.9 | 3 | 0.94 (0.90–0.99) | 0 | 4 | 1.38 (1.12–1.71) | 62.9 |
| 24 | - | - | - | - | - | - | - | - | - |
Abbreviations: PFS: progression free survival; OS: overall survival; ORR: overall response rate; HR: hazard ratio; RR: relative risk; BV: bevacizumab; NSCLC: non-small cell lung cancer.
A statistically significant improvement in ORR was found for 61–65 years old patients compared to the other age groups in all trials combined (RR: 2.04, 95% CI: 1.67–2.49) and in the colorectal cancer trials (RR: 2.64, 95% CI: 1.72–4.07). The highest ORR was observed in studies with a median duration of therapy between 12–24 weeks for colorectal cancer (RR: 2.64, 95% CI: 1.72–4.07). The ORR was higher in colorectal cancer studies if the response was assessed between 8–12 weeks compared to 6 weeks (RR: 2.59, 95% CI: 1.77–3.80 vs. RR: 1.13, 95% CI: 0.82–1.54). For the other subgroup analyses, RR of ORR did not statistically significantly differ.
Survival post progression
SPP calculated in 7 colorectal cancer trials was 10.6 months. In NSCLC (6 studies), breast cancer (4 studies) and ovarian cancer (3 studies) SPP times were 9.2, 15.6 and 21.1 months, respectively.
Evaluating the relationship between PFS and OS
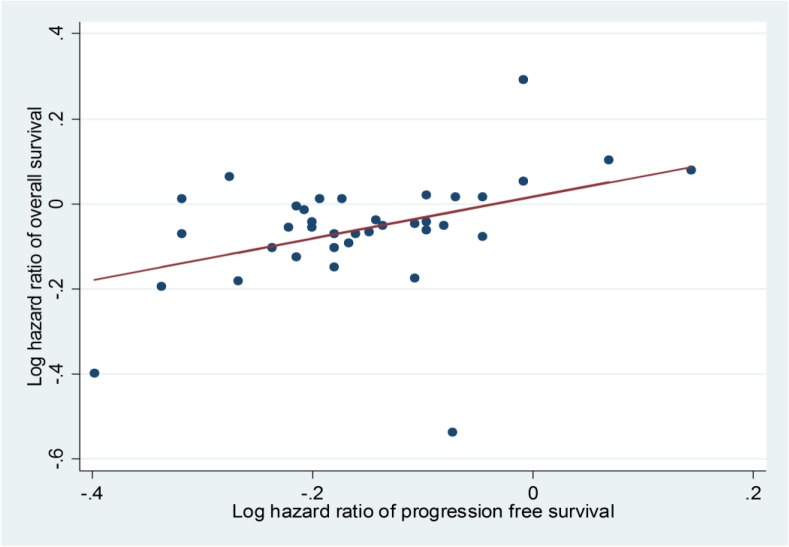
The results showed a significant moderate association between PFS and OS across all combined trials (The Spearman correlation coefficient (r) was 0.41, p-value: 0.01) (Fig 5).
Fig 5. Spearman’s correlation between progression free survival and overall survival.
Results of subgroup analyses showed that the correlation between PFS and OS was stronger in studies of metastatic breast cancers (r: 0.57; p-value: 0.18) compared to metastatic colorectal cancer (r: 0.40; p-value: 0.24) and NSCLC (r:-0.45; p-value: 0.31).
Publication bias
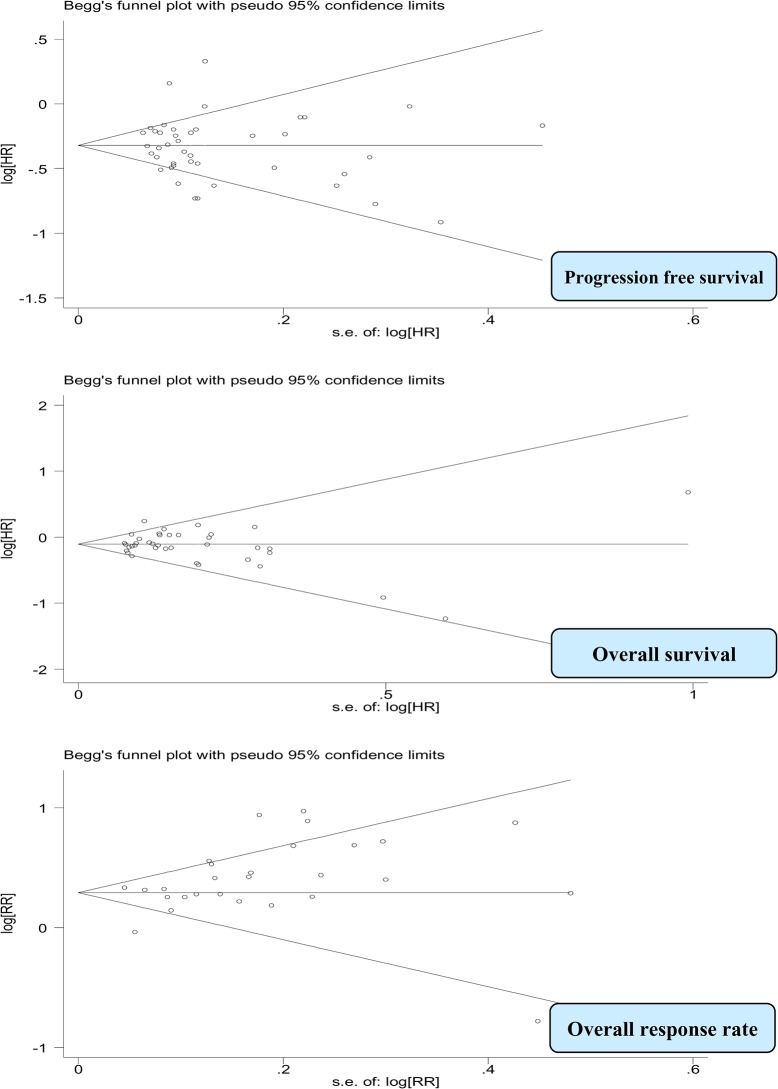
The funnel plots did not show evidence of significant publication bias for PFS and OS (p-values: 0.42, 0.69, respectively). However for ORR, the funnel plot appeared to be asymmetric, and there was evidence of bias using the Egger (weighted regression) method (P for bias was 0.02). It appeared that small trials producing more pronounced effects were missing (Fig 6).
Fig 6. Funnel plots for efficacy assessment in all trials combined.
Heterogeneity
Moderate heterogeneity was observed for PFS (I2: 72.4%) and for ORR (I2: 71.3%) in all combined trials (Table 2). We further explored the causes of the heterogeneity in different tumor types. PFS and ORR were more heterogeneous when analyzed separately in colorectal cancer trials (I2: 82.3% and 84.2%, respectively). Other stratified subgroup analyses were performed and indicated the large differences in the HRs of PFS and RRs of ORR across BV dose and adjuvant therapy agents in colorectal cancer trials (Tables 3&4).
Meta-regression analysis
The potential influence of patient and trial characteristics including BV dose, participant’s age, median duration of follow-up and median duration of BV therapy on study outcomes was explored in meta-regression analyses. The analyses showed that none of these characteristics statistically significantly influenced PFS and OS in all trials combined. However BV dose was found to be a predictor of ORR benefit (p-value: 0.02) (Table 5).
Table 5. Meta-regression analyses.
| Predictors p-value | ||||
|---|---|---|---|---|
| BV dosage | Patient’s age | Median duration of follow-up | Median duration of BV therapy | |
| PFS | 0.48 | 0.05 | 0.53 | 0.87 |
| OS | 0.33 | 0.18 | 0.71 | 0.15 |
| ORR | 0.02 | 0.20 | 0.48 | 1.00 |
Abbreviations: PFS: progression free survival; OS: overall survival; ORR: overall response rate; BV: bevacizumab.
ADRs analyses
ADRs data was available on all grades and on those ADRs with grade 3 (severe) or more (life threatening). ADRs were reported differently among 44 RCTs (Table 6). BV was associated with a higher risk of all grade ADRs e.g. thrombocytopenia, hypertension, bleeding and thromboembolic events. A higher risk of severe grade events such as wound healing complication, epistaxis and stomatitis was also observed in patients treated by BV. The highest risk was found for severe grade hypertension (RR: 5.83, 95% CI: 4.44–7.65) which was reported in 40 trials where 1,149 patients out of 16,437 in the BV treated group and 147 patients out of 15,378 in the control group were diagnosed with this ADR. BV significantly reduced the risk of both all grade (RR: 0.83, 95% CI: 0.71–0.98) and severe grade (RR: 0.78, 95% CI: 0.66–0.93) anemia as well as severe grade fatigue (RR: 0.58, 95% CI: 0.38–0.87) compared with adjuvant therapy alone in cancer patients. No statistically significant differences between patients with and without BV in their regime were found for venous thromboembolic events, fistula abdominal abscess, leukopenia, cardiac events including left ventricular (LV) dysfunction and congestive heart failure and pulmonary events including embolism, dyspnea, pneumonitis and hemorrhages.
Table 6. Safety assessment of ADRs with BV in all trials combined.
| All grade | Severe grade | |||||||
|---|---|---|---|---|---|---|---|---|
| ADRs | No. of studies included | No. of patients in BV group/sample size | No. of patients in control group/sample size | RR (95% CI) | No. of studies included | No. of patients in BV group/sample size | No. of patients in control group/sample size | RR (95% CI) |
| Venous Thromboembolic Events | 6 | 156/10621 | 127/9977 | 1.16 (0.82–1.64) | 19 | 507/10621 | 410/9977 | 1.18 (0.98–1.43) |
| Thrombocytopenia | 8 | 192/6755 | 148/6537 | 1.23 (1.01–1.49) | 21 | 365/6755 | 323/6537 | 1.13 (0.89–1.44) |
| Anemia | 5 | 221/6955 | 257/6744 | 0.83 (0.71–0.98) | 17 | 236/6955 | 295/6744 | 0.78 (0.66–0.93) |
| Hypertension | 13 | 723/16437 | 190/15378 | 3.46 (2.72–4.41) | 40 | 1149/16437 | 147/15378 | 5.83 (4.44–7.65) |
| Bleeding | 7 | 310/14173 | 114/13269 | 2.71 (1.80–4.09) | 30 | 276/14173 | 139/13269 | 1.84 (1.43–2.35) |
| Thromboembolic Events | 4 | 113/4560 | 82/4486 | 1.35 (1.04–1.76) | 11 | 161/4560 | 96/4486 | 1.81 (1.10–2.97) |
| Arterial Thromboembolic Events | 7 | 48/7775 | 24/7162 | 1.49 (0.90–2.45) | 14 | 99/7775 | 51/7162 | 1.68 (1.13–2.50) |
| Gastrointestinal perforation | 7 | 41/11677 | 16/11094 | 1.97 (1.07–3.64) | 23 | 201/11677 | 124/11094 | 2.06 (1.27–3.34) |
| Wound Healing Complication | 3 | 13/7312 | 6/7155 | 1.90 (0.74–4.88) | 12 | 51/7312 | 21/7155 | 1.94 (1.08–3.49) |
| Fistula Abdominal Abscess | 3 | 9/5199 | 1/5092 | 4.51 (0.97–20.91) | 8 | 38/5199 | 21/5092 | 1.51 (0.51–4.42) |
| Neutropenia | 8 | 339/13519 | 259/12492 | 1.20 (1.05–1.37) | 32 | 3913/13519 | 3606/12492 | 1.06 (1.01–1.12) |
| Febrile Neutropenia | 5 | 189/9940 | 104/9208 | 1.55 (1.09–2.19) | 20 | 383/9940 | 247/9208 | 1.42 (1.22–1.66) |
| Leukopenia | 4 | 84/3810 | 65/3692 | 1.26 (0.94–1.68) | 10 | 411/3810 | 183/3692 | 1.52 (0.98–2.36) |
| Proteinuria | 8 | 524/13562 | 142/12621 | 4.98 (2.11–11.71) | 30 | 299/13562 | 36/12621 | 4.90 (3.53–6.80) |
| Diarrhea | 12 | 533/7807 | 476/7600 | 1.09 (0.97–1.22) | 23 | 683/7807 | 533/7600 | 1.21 (1.07–1.37) |
| Epistaxis | 4 | 154/977 | 46/989 | 3.23 (2.38–4.38) | 6 | 14/977 | 3/989 | 3.84 (1.29–11.37) |
| Stomatitis | 4 | 133/2910 | 69/2742 | 1.87 (1.45–2.39) | 6 | 97/2910 | 28/2742 | 3.25 (2.14–4.93) |
| Vomiting | 7 | 28/6787 | 16/6540 | 1.04 (0.87–1.24) | 17 | 3/6787 | 2/6540 | 1.33 (1.00–1.76) |
| Nausea | 8 | 579/6703 | 561/6502 | 1.02 (0.93–1.11) | 18 | 225/6703 | 151/6502 | 1.42 (1.13–1.78) |
| Fatigue | 7 | 766/6518 | 727/6329 | 1.01 (0.93–1.10) | 7 | 566/6518 | 413/6329 | 0.58 (0.38–0.87) |
| Rash | 4 | 196/1815 | 157/1823 | 1.50 (0.93–2.43) | 6 | 87/1815 | 33/1823 | 2.49 (1.69–3.66) |
| Cardiac events * | 6 | 106/9070 | 75/8560 | 1.20 (0.89–1.61) | 16 | 95/9070 | 72/8560 | 1.27 (0.88–1.83) |
| Pulmonary events ** | 3 | 18/2214 | 25/2177 | 0.72 (0.40–1.31) | 6 | 24/2214 | 31/2177 | 0.77 (0.45–1.32) |
Abbreviation: ADRs: adverse drug reactions; RR: relative risk; BV: bevacizumab.
*Cardiac events including: left ventricular (LV) dysfunction and congestive heart failure.
**Pulmonary events including: embolism, dyspnea, pneumonitis and hemorrhages.
ADRs and BV dose
We assessed whether the higher dose of BV is related to the risk for developing ADRs in cancer patients (Table 7). When comparing the risk of ADRs between low and high-doses of BV, the RRs of all grade proteinuria (2.64; 95% CI: 1.29–5.40 vs. 9.24; 95% CI: 6.60–12.94) and severe grade bleeding (1.36; 95% CI: 1.05–1.75 vs. 2.87; 95% CI: 1.97–4.18) was increased significantly when switching from 2.5 mg/kg to 5 mg/kg BV. Although not statistically significant, there was a trend towards a higher risk of several side effects (including all grade and severe grade of hypertension, gastrointestinal perforation, thrombocytopenia, diarrhea, neutropenia and febrile neutropenia, all grade epistaxis and severe grade of ADRs including rash, nausea, vomiting, arterial thromboembolic events and cardiac events) in patients using high-dose compared with low-dose BV.
Table 7. Safety subgroup analyses in all trials combined.
| All grade | Severe grade | |||
|---|---|---|---|---|
| RR (95% CI) by BV dosage (mg/kg) | RR (95% CI) by BV dosage (mg/kg) | |||
| 2.5 (mg/kg) | 5 (mg/kg) | 2.5 (mg/kg) | 5 (mg/kg) | |
| Hypertension | 2.66 (2.15–3.29) | 4.71 (3.10–7.15) | 4.47 (3.05–6.56) | 7.48 (5.04–11.10) |
| Bleeding | 2.35 (1.54–3.60) | 3.53 (2.43–5.13) | 1.36 (1.05–1.75) | 2.87 (1.97–4.18) |
| Gastrointestinal perforation | 2.07 (0.24–18.04) | 2.18 (1.12–4.22) | 1.86 (0.98–3.55) | 2.44 (1.18–5.04) |
| Proteinuria | 2.64 (1.29–5.40) | 9.24 (6.60–12.94) | 4.18 (2.67–6.55) | 6.42 (3.66–11.26) |
| Epistaxis | 2.95 (2.10–4.15) | 4.60 (2.35–8.99) | 4.85 (0.23–10.56) | 3.71 (1.16–11.86) |
| Stomatitis | 1.91 (1.47–2.48) | 1.54 (0.69–3.44) | 3.62 (0.88–14.84) | 3.22 (2.08–4.97) |
| Rash | 1.80 (0.87–3.72) | 1.24 (0.48–3.20) | 1.80 (0.69–4.59) | 2.77 (1.72–4.45) |
| Neutropenia | 1.16 (0.89–1.53) | 1.22 (1.04–1.44) | 1.03 (0.93–1.14) | 1.09 (1.03–1.44) |
| Febrile neutropenia | 1.32 (0.30–5.82) | 1.52 (1.01–2.30) | 1.37 (1.01–1.85) | 1.45 (1.21–1.73) |
| Fatigue | 0.93 (0.78–1.11) | 1.05 (0.95–1.17) | 0.47 (0.27–0.84) | 0.68 (0.33–1.37) |
| Nausea | 1.04 (0.88–1.22) | 0.98 (0.87–1.11) | 1.19 (0.83–1.71) | 1.82 (1.33–2.50) |
| Vomiting | 1.07 (0.88–1.30) | 0.84 (0.47–1.50) | 1.15 (0.83–1.58) | 1.69 (1.04–2.74) |
| Thrombocytopenia | 1.16 (0.88–1.54) | 1.40 (0.96–2.02) | 0.94 (0.63–1.40) | 1.32 (1.00–1.74) |
| Arterial thromboembolic events | 1.91 (0.63–5.79) | 1.42 (0.72–2.79) | 1.50 (0.98–2.29) | 2.78 (1.13–6.85) |
| Diarrhea | 1.04 (0.96–1.14) | 1.46 (0.89–2.40) | 1.21 (1.03–1.42) | 1.23 (0.91–1.67) |
| Cardiac events * | 1.33 (0.19–9.55) | 1.19 (0.89–1.61) | 0.97 (0.48–1.95) | 1.45 (0.93–2.27) |
| Pulmonary events ** | 0.72 (0.38–1.38) | 0.72 (0.16–3.19) | 0.86 (0.36–1.99) | 0.74 (0.27–1.99) |
Abbreviation: RR: relative risk; BV: bevacizumab.
*Cardiac events including: left ventricular (LV) dysfunction and congestive heart failure.
**Pulmonary events including: embolism, dyspnea, pneumonitis and hemorrhages.
Sensitivity analysis
We performed a sensitivity analysis for all grade and severe grade hypertension and proteinuria excluding the 2 trials [22, 23] concerning patients with renal cancer. The magnitude of association was lower after excluding these trials, but remained robustly significant: for all grade and severe grade hypertension (3.06, 95%CI: 2.47–3.79 and 5.72, 95%CI: 4.35–7.51, respectively) and for all grade (3.12, 95%CI: 1.59–6.13) and severe grade proteinuria (4.43, 95%CI: 3.17–6.20).
QOL
QOL was assessed in 7 RCTs [19,31,36,41,47,48,50] at baseline and during follow-up until disease progression. We were not able to conduct a meta-analysis for this outcome because QOL was measured using different instruments in different trials. All trials reported that there were no statistically significant differences in the mean change in QOL between patients treated by BV compared to patients treated with chemotherapeutic agents alone.
Discussion
To the best of our knowledge, this is the largest meta-analysis of BV that evaluated both efficacy and safety in different types of solid tumors in cancer patients. Compared to previous published meta-analyses, our study adds relevant information with respect to the identification of predictors of BV risk and benefit through subgroup and meta-regression analyses. This meta-analysis confirmed that the addition of BV to adjuvant therapy leads to improvement in PFS, OS and ORR in all trials combined. This PFS and OS improvement was observed across various tumor types and BV doses, and across different patient or trial characteristics. In contrast, ORR appeared to be influenced by tumor type and BV dose. Despite increased risk of expected ADRs, the addition of BV to adjuvant therapy does not seem to influence QOL. Though not statistically significant, there was a trend towards a higher risk of several ADRs in patients using high-dose BV compared to patients treated with low-dose.
The quality of each included RCT was assessed by applying the Cochrane Collaboration’s tool, which is a validated assessment instrument. The results showed a high risk of bias in domain of blinding for both primary and secondary outcomes, however because in cancer trials most outcomes (like OS and PFS) are not likely to be influenced by lack of blinding the overall quality of all trials combined was considered to be acceptable [52]. Lack of blinding might have influenced the results of the QOL analyses, and therefore such results should be interpreted with caution.
We demonstrated improvement in PFS in patients with all types of tumors except for patients with melanoma, mesothelioma or cervical cancers. In spite of OS improvement when all trials were combined, no significant OS advantage was observed in certain types of cancer e.g. breast cancer and ovarian cancer although there were trends in the correct direction. Even after adding several new trials to the meta-analyses our findings continued to show this lack of OS benefit in breast cancer [4, 53] and ovarian cancer [54]. A possible explanation according to Broglio et al [55] is that when SPP is long, for example 15.6 months for breast cancer and 21.1 months for ovarian cancer, it is more difficult to show improvement in OS. While in trials with a short SPP such as for colorectal cancer (10.6 months) and NSCLC (9.2 months) there is usually a statistically significant benefit in OS if there is a statistically significant treatment benefit in PFS.
On the other hand, surrogacy of PFS for OS in different cancer types has been evaluated in several studies with different results [56, 57]. In the current study, a significant moderate correlation between PFS and OS was observed in all combined trials which is consistent with the results of previous studies. Therefore, in clinical trials with a PFS benefit, lack of statistical significance in OS does not necessarily mean a lack of improvement in OS.
Our study also suggests that the relationship between PFS and OS varies considerably by cancer type. The correlation between PFS and OS was more pronounced in trials of breast cancer compared with colorectal cancer and in NSCLC there was a surprisingly negative correlation. In breast cancer and colorectal cancer, our results are in line with the results of previous studies [58, 59] while in NSCLC there is evidence for a positive relationship between PFS and OS [60].Most malignant tumors are highly dependent on angiogenesis, therefore it is as expected that BV added to standard chemotherapies substantially improves the ORR in different tumor types.
The dose of BV used in adjuvant therapy was not found to be associated with PFS or OS benefit, consistent with a prior meta-analysis [4]. However, our findings showed that the ORR benefit varied significantly by tumor type. The highest ORR was observed in renal cancer patients, while gastric cancer patients benefited the least of BV treatment. Thus, tumor type likely plays an important role in the response to BV. This variation in response may also be partly due to the combination of BV with different chemotherapeutic agents in different tumor types. However, other reasons might be differences in the number of studies and power of the studies for the different tumor types. Further research is warranted into which tumors benefit most from BV therapy.
Although not significant, there was a trend towards a higher ORR in patients using a high-dose BV compared with patients using a low-dose in all trials combined, in colorectal cancer and in breast cancer trials. The increase in ORR with high-dose BV may have been due to improved BV-induced drug delivery to the tumor site. Our results are in line with the results of previous studies that showed a dose-response relationship in NSCLC and metastatic renal cell carcinoma [61, 62], but not in colorectal cancer [14]. A significantly higher improvement in ORR found in 61–65 years old patients compared with all other age groups in all trials combined and in colorectal cancer trials has no biological or clinical explanation, and is likely a chance finding. An important consideration is the timing of response assessment: the results in the colorectal cancer studies showed a higher ORR in favor of BV if response was assessed between 8–12 weeks compared with 6 weeks. A longer time to response assessment is likely to capture slower tumor responses and be more complete, important for non-cytotoxic agents such as BV.
Our results showed that some ADRs were more common in patients randomized to BV. This is consistent with those of prior safety meta-analyses linking specific ADRs to BV therapy [3,53,63–71]. BV significantly reduced the risk of both all grade and severe grade anemia compared with adjuvant therapy alone in cancer patients with no significant variation among different BV doses which was in line with previous meta-analysis [65]. Several possibilities related to VEGF inhibition may explain the effect of BV on anemia. BV has been shown to promote hepatic erythropoietin (EPO) synthesis and erythrocytosis in preclinical models [72]; also, it may cause tissue hypoxia due to its anti-angiogenesis and vasoconstriction effect, leading to subsequent up-regulation of erythropoietin. Furthermore, we showed that the addition of BV to standard adjuvant therapy was associated with reduced risk of severe grade fatigue in cancer patients compared to those who were treated by adjuvant therapy alone. Increase in several inflammatory markers is associated with an increase in fatigue among cancer patients during and after cancer treatment [73], so BV may reduce these inflammatory markers via an unknown mechanism; alternatively, this may be associated with a reduction in anemia. Since this finding has not been reported before it might be interesting for future research.
Our study adds information to existing literature about the increased risk for the following ADRs: febrile neutropenia, stomatitis, vomiting, nausea and rash as well as decreased risk for severe grade fatigue.
In our study, the most frequent ADRs of BV was hypertension which represents a common finding across 40 trials. Infusion of VEGF has been found to produce hypotension [74] and thus blockade of VEGF may potentially lead to elevation of blood pressure.
We also investigated the associations of BV with ADRs according to different BV dose. Our study indicated a dose dependency although not significantly for the association of most ADRs with BV therapy. Moreover, a significant higher risk of all-grade proteinuria and severe grade bleeding was observed in patients who received high-dose compared with patients treated with low-dose BV (RR: 9.24 vs. 2.64) and (2.87 vs. 1.36), respectively which is in agreement with previous meta-analyses [1,2].
In this study, the following limitations were acknowledged: This study was conducted using published RCTs not individual patient data. Our meta-analysis pooled trials with heterogeneous cancer types and different patient populations, BV doses, antineoplastic agents used, follow-up durations and the timing of response assessment, although by applying a random-effects model we took possible heterogeneity into account. In this meta-analysis we have decided not to include studies on glioblastoma because in this kind of tumor, drug delivery is different from the other types of cancer that were studied. Furthermore, we only compared the efficacy and safety of BV with standard chemotherapy agents not with radiotherapy and other types of anti-neoplasms.
As in all meta-analyses, our results may be biased as a result of potential publication bias however; a funnel plot evaluation for the primary endpoints did not indicate serious publication bias. Finally, most of trials did not report outcomes separately by patient’s gender, so we were not being able to perform a subgroup analysis to evaluate a potential gender treatment interaction.
In summary, this meta-analysis extends the results of previous RCTs and meta-analyses which show a benefit of adding BV to adjuvant therapy compared to patients who received adjuvant therapy alone, but finding no interaction with baseline characteristics or with dose of BV. In contrast, patients treated with high-dose BV had more ADRs but without significant changes in measured QOL. Future studies are needed to evaluate the efficacy and safety of the addition of other angiogenesis inhibitors to standard chemotherapy agents and to compare these results with the effect of BV on PFS and OS.
Supporting Information
(PDF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007; 49 (2):186–193. [DOI] [PubMed] [Google Scholar]
- 2. Xiao Feng Hang, Wen Sheng Xu, Jun Xue Wang, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2011;67:613–623. 10.1007/s00228-010-0988-x [DOI] [PubMed] [Google Scholar]
- 3. Geiger-Gritsch S, Stollenwerk B, Miksad R, Guba B, Wild C, Siebert U. Safety of Bevacizumab in Patients with Advanced Cancer: A Meta-Analysis of Randomized Controlled Trials. The Oncologist 2010;15:1179–1191. 10.1634/theoncologist.2009-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Su Yuan, Yang Wei-Bing., Shi Li, Zhi-Jian Ye, Huan-Zhong Shi, Qiong Zhou. Effect of Angiogenesis Inhibitor Bevacizumab on Survival in Patients with Cancer: A Meta-Analysis of the Published Literature. PLoS ONE. 2012;7(4):e35629 10.1371/journal.pone.0035629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8(5):336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 6. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000. February 2;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 7. Sutton AJ, Abrams KR, Jones DR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. J. Wiley; 2000. [Google Scholar]
- 8. Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008. April 20; 26(12): 2013–2019. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 9. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004. June 3;350(23):2335–2342. [DOI] [PubMed] [Google Scholar]
- 10. Kemeny NE, Jarnagin WR, Capanu M, Fong Y, Gewirtz AN, Dematteo RP, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol 2011. March 1; 29 (7):884–889. 10.1200/JCO.2010.32.5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tebbutt N, Gebski V, Wilson K, Cummins M, Robinson B, Broad A, et al. International randomised phase III study of capecitabine (Cap), bevacizumab (Bev) and mitomycin C (MMC) in first line treatment of metastatic colorectal cancer (mCRC): Final results of the AGITG MAX trial. Eur J Cancer Suppl 2009; 7 (2–3):321. [Google Scholar]
- 12. de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012. December;13(12):1225–1233. 10.1016/S1470-2045(12)70509-0 [DOI] [PubMed] [Google Scholar]
- 13. Dotan E, Meropol NJ, Burtness B, Denlinger CS, Lee J, Mintzer D, et al. A Phase II Study of Capecitabine, Oxaliplatin, and Cetuximab with or Without Bevacizumab as Frontline Therapy for Metastatic Colorectal Cancer. A Fox Chase Extramural Research Study. J Gastrointest Cancer 2012. February 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003. January 1;21(1):60–65. [DOI] [PubMed] [Google Scholar]
- 15. Guan Z-, Xu J-, Luo R-, Feng F-, Wang L-, Shen L, et al. Efficacy and safety of bevacizumab plus chemotherapy in chinese patients with metastatic colorectal cancer:A randomized phase iii artist trial. Chin J Cancer 2011 2011/;30(10):682–689. 10.5732/cjc.011.10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011. January 1;29(1):11–16. 10.1200/JCO.2010.30.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007. April 20;25(12):1539–1544. [DOI] [PubMed] [Google Scholar]
- 18. Stathopoulos GP, Batziou C, Trafalis D, Koutantos J, Batzios S, Stathopoulos J, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010;78(5–6):376–381. 10.1159/000320520 [DOI] [PubMed] [Google Scholar]
- 19. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011. October 20;29(30):3968–3976. 10.1200/JCO.2011.36.2236 [DOI] [PubMed] [Google Scholar]
- 20. Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2009; 27(13):2231–2237. [DOI] [PubMed] [Google Scholar]
- 21. Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010. August 1;28(22):3617–3622. 10.1200/JCO.2010.28.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2010; 28(13):2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007. December 22;370 (9605):2103–2111. [DOI] [PubMed] [Google Scholar]
- 24. Herbst RS, O'Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007. October 20;25(30):4743–4750. [DOI] [PubMed] [Google Scholar]
- 25. Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011. May 28;377(9780):1846–1854. 10.1016/S0140-6736(11)60545-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2004;22(11):2184–2191. [DOI] [PubMed] [Google Scholar]
- 27. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009. March 10;27(8):1227–1234. 10.1200/JCO.2007.14.5466 [DOI] [PubMed] [Google Scholar]
- 28. Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012. June;76(3):362–367. 10.1016/j.lungcan.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 29. Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011. June 1;29(16):2215–2222. 10.1200/JCO.2010.29.3423 [DOI] [PubMed] [Google Scholar]
- 30. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006. December 14;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 31. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007. December 27;357(26):2666–2676. [DOI] [PubMed] [Google Scholar]
- 32. Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2011. November 10;29(32):4286–4293. 10.1200/JCO.2010.34.1255 [DOI] [PubMed] [Google Scholar]
- 33. von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 2012. January 26;366(4):299–309. 10.1056/NEJMoa1111065 [DOI] [PubMed] [Google Scholar]
- 34. Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 2011. April 1;29(10):1252–1260. 10.1200/JCO.2010.28.0982 [DOI] [PubMed] [Google Scholar]
- 35. Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010. July 10;28(20):3239–3247. 10.1200/JCO.2008.21.6457 [DOI] [PubMed] [Google Scholar]
- 36. Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005. February 1;23(4):792–799. [DOI] [PubMed] [Google Scholar]
- 37. Martin M, Roche H, Pinter T, Crown J, Kennedy MJ, Provencher L, et al. Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol 2011. April;12(4):369–376. 10.1016/S1470-2045(11)70037-7 [DOI] [PubMed] [Google Scholar]
- 38. Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, et al. BEAM: A randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2012 2012/January;30(1):34–41. 10.1200/JCO.2011.34.6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol 2012. May 1;30(13):1534–1540. 10.1200/JCO.2011.39.4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kindler HL, Karrison TG, Gandara DR, Lu C, Krug LM, Stevenson JP, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012. July 10;30(20):2509–2515. 10.1200/JCO.2011.41.5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. New Engl J Med 2011 2011/December;365(26):2484–2496. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 42. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012. June 10;30(17):2039–2045. 10.1200/JCO.2012.42.0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013. January;14(1):29–37. 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 44. Bear HD, Tang G, Rastogi P, Geyer CE Jr, Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med 2012. January 26;366(4):310–320. 10.1056/NEJMoa1111097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013. October;14(11):1077–1085. 10.1016/S1470-2045(13)70154-2 [DOI] [PubMed] [Google Scholar]
- 46. Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 2013. September;14(10):933–942. 10.1016/S1470-2045(13)70335-8 [DOI] [PubMed] [Google Scholar]
- 47. Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol 2013. May 10;31(14):1719–1725. 10.1200/JCO.2012.44.7912 [DOI] [PubMed] [Google Scholar]
- 48. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011. December 29;365(26):2473–2483. 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 49. Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014. May 1;32(13):1302–1308. 10.1200/JCO.2013.51.4489 [DOI] [PubMed] [Google Scholar]
- 50. Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014. February 20;370(8):734–743. 10.1056/NEJMoa1309748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hainsworth JD, Greco FA, Raefsky EL, Thompson DS, Lunin S, Reeves J Jr, et al. Rituximab with or without bevacizumab for the treatment of patients with relapsed follicular lymphoma. Clin Lymphoma Myeloma Leuk 2014. August;14(4):277–283. 10.1016/j.clml.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 52. Vale CL, Tierney JF, Burdett S. Can trial quality be reliably assessed from published reports of cancer trials: evaluation of risk of bias assessments in systematic reviews. BMJ 2013. April 22;346:f1798 10.1136/bmj.f1798 [DOI] [PubMed] [Google Scholar]
- 53. Amit Limor, Irit Ben-Aharon, Liat Vidal, Leibovici Leonard, Stemmer Salomon. The Impact of Bevacizumab (Avastin) on Survival in Metastatic Solid Tumors—A Meta-Analysis and Systematic Review. PLOS ONE 2013;8(1):e51780 10.1371/journal.pone.0051780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ye Q, Chen HL. Bevacizumab in the treatment of ovarian cancer: a meta-analysis from four phase III randomized controlled trials. Arch Gynecol Obstet 2013. September; 288(3):655–666. 10.1007/s00404-013-2820-1 [DOI] [PubMed] [Google Scholar]
- 55. Broglio KR BD. detecting an overall survival benefit that is derived from progression free survival. JNCI 2009; 101:1642–1649. 10.1093/jnci/djp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sherrill B, Kaye JA, Sandin R, Cappelleri JC, Chen C. Review of meta-analyses evaluating surrogate endpoints for overall survival in oncology. Onco Targets Ther. 2012;5:287–296. 10.2147/OTT.S36683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davis S, Tappenden P, Cantrell A. A Review of Studies Examining the Relationship between Progression-Free Survival and Overall Survival in Advanced or Metastatic Cancer. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2012. [PubMed] [Google Scholar]
- 58. Beauchemin C.; Cooper D.; Lapierre M.E.; Yelle L.; Lachaine J. Progression-free survival as a potential surrogate for overall survival in metastatic breast cancer. Onco Targets Ther., 2014, 7, 1101–1110. 10.2147/OTT.S63302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tang P.A., Bentzen S.M., Chen E.X., Siu L.L. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. Journal of Clinical Oncology 2007; 25(29):4562–4568. [DOI] [PubMed] [Google Scholar]
- 60. Foster N.R., Qi Y., Shi Q., Krook J.E., Kugler J.W., Jett J.R. et al. Tumor Response and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Extensive Stage Small-Cell Lung Cancer Findings on the Basis of North Central Cancer Treatment Group Trials. Cancer 2011; 117(6):1262–1271. 10.1002/cncr.25526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang K, Wang YJ, Chen XR, Chen HN. Effectiveness and safety of bevacizumab for unresectable non-small-cell lung cancer: a meta-analysis. Clin Drug Investig 2010;30(4):229–241. 10.2165/11532260-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 62. Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 2003. August 15;21(16):3127–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fabio A.B. Schutz a, Denis L.F. Jardim a,c, Youjin Je b, Toni K. Choueiri. Haematologic toxicities associated with the addition of bevacizumab in cancer patients. European Journal Of Cancer 2011; 47:1161–1174. 10.1016/j.ejca.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 64. Schutz FA, Je Y, Azzi GR, Nguyen PL, et al. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol 2010. [DOI] [PubMed] [Google Scholar]
- 65. Sher A, Wu S. Anti-vascular endothelial growth factor antibody bevacizumab reduced the risk of anemia associated with chemotherapy-A meta-analysis. Acta Oncol 2011. October;50(7):997–1005. 10.3109/0284186X.2011.581689 [DOI] [PubMed] [Google Scholar]
- 66. Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2011. June;67(6):613–623. 10.1007/s00228-010-0988-x [DOI] [PubMed] [Google Scholar]
- 67. Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 2010; 21(8):1381–1389. 10.1681/ASN.2010020167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 2009;10(6):559–568. 10.1016/S1470-2045(09)70112-3 [DOI] [PubMed] [Google Scholar]
- 69. Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol 2010. April;49(3):287–297. 10.3109/02841860903524396 [DOI] [PubMed] [Google Scholar]
- 70. Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 2010. May;23(5):460–468. 10.1038/ajh.2010.25 [DOI] [PubMed] [Google Scholar]
- 71. Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007. February;49(2):186–193. [DOI] [PubMed] [Google Scholar]
- 72. Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 2004;7(4):335–345. [DOI] [PubMed] [Google Scholar]
- 73. Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014. October;11(10):597–609. 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol 2008. May;19(5):927–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.