Abstract
Assessment with 18F-fluorodeoxy glucose (FDG)—positron emission tomography (PET) before hematopoietic cell transplantation (HCT) for lymphoma may be prognostic for outcomes. Patients with chemotherapy-sensitive non—Hodgkin lymphoma (NHL) undergoing allogeneic HCT reported to the Center of International Blood and Marrow Transplantation Registry between 2007 and 2012 were included. Pre-HCT PET status (positive versus negative) was determined by the reporting transplantation centers. We analyzed 336 patients; median age was 55 years and 60% were males. Follicular lymphoma (n = 104) was more common than large cell (n = 85), mantle cell (n = 69), and mature natural killer or T cell lymphoma (n = 78); two thirds of the cohort received reduced-intensity conditioning; one half had unrelated donor grafts. Patients underwent PET scanning a median of 1 month (range, .07 to 2.83 months) before HCT; 159 were PET positive and 177 were PET negative. At 3 years, relapse/progression, progression-free survival (PFS), and overall survival (OS) in PET-positive versus PET-negative groups were 40% versus 26%; P = .007; 43% versus 47%; P = .47; and 58% versus 60%; P = .73, respectively. On multivariate analysis, a positive pretransplantation PET was associated with an increased risk of relapse/progression (risk ratio [RR], 1.86; P = .001) but was not associated with worse OS (RR, 1.29, 95% confidence interval [CI], .96 to 1.7; P = .08), PFS (RR, 1.32; 95% CI, .95 to 1.84; P = .10), or nonrelapse mortality (RR, .75; 95% CI, .48 to 1.18; P = .22). PET status conferred no influence on graft-versus-host disease. A positive PET scan before HCT is associated with increased relapse risk but should not be interpreted as a barrier to a successful allograft. PET status does not appear to predict survival after allogeneic HCT for NHL.
Keywords: Non-Hodgkin lymphoma, Allogeneic transplantation, Positron emission tomography
Introduction
Allogeneic hematopoietic cell transplantation (HCT) can provide long-term survival for patients with various subtypes of lymphoma; however, relapse remains the predominant cause of treatment failure [1-4]. The use of 18F-fluorodeoxy glucose (FDG)-positron emission tomography (PET) after front-line or salvage chemotherapy is a valuable prognostic tool to assess the depth of remission before autologous HCT [5-9]. FDG-PET scan metabolic positivity is associated with a higher post-autograft relapse risk and worse survival in patients with diffuse large B cell lymphoma (DLBCL) and Hodgkin lymphoma (HL) [5,9]. However, it is unclear whether FDG-PET before allogeneic HCT can be reliably used to predict post-transplantation outcomes among non-Hodgkin lymphoma (NHL) patients. Several single-institution studies have found conflicting data on relapse and long-term survival among allogeneic HCT recipients according to pretransplantation PET status; however, these studies were based on smaller cohorts of patients (58 to 88 patients) and often included patients with both HL and NHL [10-13]. We conducted a retrospective, multicenter, registry-based analysis of a large cohort of NHL patients to determine whether FDG-PET performed before allogeneic HCT can be used to predict post-transplantation outcomes.
Patients and Methods
Data Sources
The Center of International Blood and Marrow Transplantation Registry (CIBMTR) is a working group of more than 450 transplantation centers worldwide that contribute detailed data on HCTs longitudinally with yearly follow-up to a statistical center at the Medical College of Wisconsin. Centers report HCTs consecutively, with compliance monitored by on-site audits. The study was performed in compliance with federal regulations and the institutional review board of the Medical College of Wisconsin.
Patients
We included adults undergoing first allogeneic HCT for a histologically proven diagnosis of follicular lymphoma (FL), DLBCL, mantle cell lymphoma (MCL), or mature T cell or natural killer (NK) cell neoplasm between 2007 and 2012. Eligible histological subtypes were restricted to either routinely FDG-avid lymphomas or subtypes where expected FDG avidity rates ranged from 80% to 100% [14,15]. Patients not responding (ie, not achieving a complete or partial remission [CR or PR]) to the last line of therapy (n = 104), with an untreated relapse (n = 50) before allogeneic HCT, or undergoing ex vivo graft manipulation (n = 4) or post-transplantation cyclophosphamide (n = 1) were excluded. We identified 998 potential cases and contacted transplantation centers for additional information about availability, date, and status of the last FDG-PET scan performed before allogeneic HCT (Supplemental Appendix Figure). Among the 815 (81.2%) responses received, 367 patients met the eligibility criteria of the protocol, including the final designation of FDG-PET status as assessed by the local radiology team in individual centers. Cases where the interval between the FDG-PET scan and day 0 of allogeneic HCT was > 3 months were excluded (n = 31).
Definitions
The CIBMTR form defines CR after the last line of therapy before HCT as complete resolution of all known disease on radiographic (computerized tomography [CT] scan) assessments. PR required ≥ 50% reduction in the greatest diameter of all sites of known disease and no new sites of disease. Pre-HCT PET scan status determination (positive scan versus negative scan) was performed by the reporting transplantation center according to routinely used criteria at individual centers.
Conditioning regimens were categorized by intensity using established consensus criteria [16]. Previously established criteria for categorizing the degree of HLA matching were used for unrelated donor transplantations [17]. Well-matched patients had either no identified HLA mismatching and informative data at 4 loci or allele matching at HLA-A, -B, and -DRB1 (6/6). Partially matched pairs had a defined, single-locus mismatch, and/or missing HLA data. Mismatched cases had ≥ 2 allele or antigen mismatches.
Study Endpoints
Primary outcomes were relapse/progression and progression-free survival (PFS); secondary outcomes were nonrelapse mortality (NRM) and overall survival (OS). NRM was defined as death without evidence of lymphoma relapse; relapse/progression was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For PFS, treatment failure occurred at the time of relapse or death from any cause. Patients alive without evidence of disease relapse were censored at last follow-up. OS was defined as the interval from the date of transplantation to the date of death or last follow-up. Acute graft-versus-host disease (GVHD) was defined and graded based on the pattern and severity of organ involvement using established criteria [18]. Chronic GVHD was defined as the development of any evidence of chronic GVHD based on clinical criteria [19].
Statistical Analysis
Probabilities of PFS and OS were calculated using the Kaplan-Meier estimator. Probabilities of NRM and lymphoma relapse were calculated using cumulative incidence curves to account for competing risks. Patient-, disease-, and transplantation-related factors were compared between PET-positive and PET-negative groups using the chi-square test for categorical variables and the Wilcoxon sample test for continuous variables. Associations among patient-, disease-, and transplantation-related variables and outcomes of interest were evaluated using multivariate Cox proportional hazards regression. A stepwise selection multivariate model was built to identify covariates that influenced outcomes. Covariates with a P < .05 were considered significant. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were stratified in the Cox regression model. Results are expressed as relative risk (RR) or the relative rate of occurrence of the event.
Variables considered in multivariate analysis included positive or negative PET status (the main effect) and clinical factors listed in Tables 1 and 2 (denoted by an asterisk sign). Potential interactions among the main effect and all significant covariates were tested. CR versus PR and bulky disease (≥5 cm) at HCT were not included in multivariate analysis owing to the strong correlation of CR with PET-negative status and presence of bulky disease with PET-positive status.
Table 1. Patient and Disease Characteristics.
| Variable | FDG-PET− | FDG-PET+ | P Value |
|---|---|---|---|
| No. of patients | 177 | 159 | |
| Age at transplantation, yr* | .950 | ||
| Median (range) | 54 (19-71) | 55 (18-70) | |
| Karnofsky score before HCT* | .054 | ||
| <90% | 45 (25) | 48 (30) | |
| ≥90% | 128 (72) | 100 (63) | |
| Missing | 4 (2) | 11 (7) | |
| Sex* | .571 | ||
| Male | 110 (62) | 94 (59) | |
| Female | 67 (38) | 65 (41) | |
| Histology* | .009 | ||
| FL | 44 (25) | 60 (38) | |
| DLBCL† | 41 (23) | 44 (28) | |
| MCL | 41 (23) | 28 (18) | |
| Mature T cell and NK cell neoplasm‡ | 51 (29) | 27 (17) | |
| No. of prior chemotherapy lines* | .025 | ||
| 1-2 | 68 (43) | 44 (30) | |
| ≥3 | 92 (58) | 102 (70) | |
| Missing | 17 | 13 | |
| Disease status before transplantation§ | <.001 | ||
| CR‖ | 147 (83) | 6 (4)‖ | |
| PR | 30 (17) | 153 (96) | |
| Extranodal involvement before transplantation* | <.001 | ||
| No | 154 (87) | 99 (62) | |
| Yes | 20 (11) | 58 (36) | |
| Unknown | 3 (2) | 2 (1) | |
| Bulky disease before transplantation (nodal) | <.001 | ||
| <5 cm | 8 (5) | 68 (43) | |
| ≥5 cm | 1 (1) | 16 (10) | |
| No nodal involvement before transplantation | 153 (86) | 33 (21) | |
| Missing | 15 (8) | 42 (26) | |
| BM involvement before transplantation | <.001 | ||
| No | 12 (7) | 35 (22) | |
| Yes | 8 (5) | 22 (14) | |
| Unknown | 157 (89) | 102 (64) | |
| Symptoms at diagnosis | .328 | ||
| A | 84 (47) | 72 (45) | |
| B | 59 (33) | 46 (29) | |
| Missing | 34 (19) | 41 (26) | |
| Elevated LDH before transplantation* | 43 (26) | 47 (33) | .179 |
| Missing | 11 | 16 | |
| Interval from diagnosis to transplantation (range), mo* | 26 (3-208) | 28 (4-352) | .320 |
| Interval from FDG-PET to transplantation (range), mo | 1 (.20-2.80) | 1 (.07-2.83) | .595 |
| Prior autologous transplantation* 41 (23) | 28 (18) | .208 | |
| Time from autoHCT to alloHCT, (range) mo | 24 (7-133) | 21 (7-66) | .447 |
| ≤12 | 8 (5) | 5 (3) | |
| >12 | 33 (19) | 23 (14) |
BM indicates bone marrow; LDH, lactate dehydrogenase; auto, autologous; allo, allogeneic.
Variables considered in multivariate analysis.
Thirty-two transformed patients were included (32 out of 85 DLBCL).
FDG-PET−: mycosis fungoides (n = 1), anaplastic large T cell (n = 12),peripheral T cell (n = 9), angioimmunoblastic T cell (n = 9), adult T cellleukemia/lymphoma (n = 3), extranodal NK/T cell (n = 4), other NK (n = 9),hepatosplenic gamma delta T-cell (n = 2), subcutaneous panniculitis T-cell(n = 2); FDG-PET+: peripheral T cell (n = 10), angioimmunoblastic T cell(n = 7), extranodal NK/T cell (n = 2), other NK cell (n = 4), subcutaneouspanniculitis T cell (n = 2), anaplastic large T cell (n = 2).
Disease status: FDG-PET−: CR (CR1 = 40 and CR2+ = 107), PR (PIF sensitive = 14 and REL sensitive = 16); FDG-PET+: CR (CR1 = 1 and CR2+ = 5), PR (PIF sensitive = 54 and REL sensitive = 99).
FDG-PET/CT reports of 6 patients who were in CR by CT criteria but with PET+ scans were reviewed. In all patients, CR by CT criteria was confirmed. All cases had metabolic activity in nonenlarged lymph nodes.
Table 2. Transplantation and Treatment Characteristics.
| Variable | FDG-PET− | FDG-PET+ | P Value |
|---|---|---|---|
| No. of patients | 177 | 159 | |
| Donor type* | .402 | ||
| Cord blood | 27 (15) | 26 (16) | |
| HLA-identical siblings | 65 (37) | 57 (36) | |
| Unrelated well matched | 59 (33) | 57 (36) | |
| Unrelated partially matched | 17 (10) | 17 (11) | |
| Unrelated matching missing | 9 (5) | 2 (1) | |
| Graft type* | .331 | ||
| Bone marrow | 7 (4) | 12 (8) | |
| Peripheral blood | 143 (81) | 121 (76) | |
| Cord blood | 27 (15) | 26 (16) | |
| Conditioning intensity* | .814 | ||
| Myeloablative | 50 (28) | 40 (25) | |
| Cyclophosphamide + TBI | 30 (17) | 19 (12) | |
| Busulfan + fludarabine | 10 (6) | 7 (4) | |
| Other/busulfan + cyclophosphamide | 5/4 (4/2) | 6/8 (4/5) | |
| Reduced intensity | 70 (40) | 66 (42) | |
| TBI + other | 14 (9) | 5 (4) | |
| Fludarabine + melphalan | 18 (10) | 21 (13) | |
| Melphalan ± others† | 16 (9) | 9 (6) | |
| Busulfan + others | 20 (12) | 27 (17) | |
| Nonmyeloablative | 57 (32) | 53 (33) | |
| Fludarabine + cyclophosphamide + TBI | 17 (10) | 20 (13) | |
| Fludarabine + cyclophosphamide or TBI | 37 (21) | 33 (20) | |
| Radiation ± ATG | 6 (3) | 2 (1) | |
| Radiation before HCT* | .094 | ||
| No | 134 (76) | 127 (80) | |
| Yes | 43 (24) | 32 (20) | |
| Rituximab at conditioning* | .189 | ||
| Yes | 34 (19) | 40 (25) | |
| No | 143 (81) | 119 (75) | |
| Donor-recipient CMV status* | .336 | ||
| Positive donor | 25 (14) | 17 (11) | |
| Positive recipient | 88 (49) | 81 (51) | |
| Donor-recipient negative | 42 (24) | 40 (25) | |
| Missing | 22 (12) | 21 (13) | |
| Year of transplantation | .125 | ||
| 2007-2008 | 84 (47) | 66 (42) | |
| 2009-2010 | 57 (32) | 68 (43) | |
| 2011-2012 | 36 (20) | 25 (16) | |
| ATG/alemtuzumab* | .434 | ||
| ATG alone | 36 (20) | 30 (19) | |
| Alemtuzumab alone | 12 (7) | 11 (7) | |
| GVHD prophylaxis* | .325 | ||
| Tacrolimus + MMF ± others | 48 (27) | 29 (18) | |
| Tacrolimus + MTX ± others (except MMF) | 58 (33) | 66 (42) | |
| Tacrolimus + others (except MTX, MMF) | 12 (7) | 17 (10) | |
| CSA + MMF ± others (except tacro) | 31 (18) | 24 (15) | |
| CSA + MTX ± others (except tacro, MMF) | 9 (5) | 10 (6) | |
| CSA + others (except tacro, MTX, MMF) | 5 (3) | 3 (2) | |
| Other GVHD prophylaxis‡ | 14 (8) | 10 (6) | |
| Planned post-transplantation radiation | 1 (1) | 3 (2) | |
| Median follow-up of survivors, mo | 49 (3-75) | 48 (12-82) |
TBI indicates total body irradiation; ATG, antithymocyte globulin; CMV, cytomegalovirus; MMF, mycophenolate mofetil; CsA, cyclosporine; MTX, methotrexate.
Data presented are n (%), unless otherwise indicated.
Variables considered in multivariate analysis.
Negative PET: Ara-C + VP16 + melphalan+nitro (n = 9), melphalan alone (n = 2); Positive PET: Ara-C + VP16 + melphalan + nitro (n = 5), Ara-C + VP16 + melphalan + nitro + Velcade (n = 2), melphalan alone (n = 1), melphalan + clorabine (n = 1).
MTX + MMF = 1, KGF + MTX = 1, MAB + MMF + Campath = 2, MTX + Siro = 1, not specified = 19.
Results
Patients Characteristics
We examined data on 336 eligible patients from 81 reporting centers (Tables 1 and 2). Median age was 55 years (range, 18 to 71); 60% were males. FL (n = 104) was more common than DLBCL (n = 85), MCL (n = 69), and mature NK or T cell (n = 78) lymphomas. Patients underwent FDG-PET scanning a median of 1 month (range, .07 to 2.83 months) before allografting; 159 were FDG-PET positive (FDG-PET+) and 177 FDG-PET negative (FDG-PET−). As expected, there were differences in disease characteristics between FDG-PET+ and FDG-PET− groups (Table 1). FDG-PET+ patients more often had FL (38% versus 25%), ≥3 lines of prior therapy (70% versus 58%), extranodal disease before HCT (36% versus 11%), marrow involvement before HCT (14% versus 5%), and bulky disease before HCT (10% versus 1%) compared with the FDG-PET− cohort. Pretransplantation radiation was administered for 20% of FDG-PET+ patients and 24% of FDG-PET – patients before PET imaging. The interval from diagnosis to transplantation was similar (median 28 versus 26 months). In addition, similar proportions of patients in both groups received rituximab-containing conditioning (25% versus 19%) and peritransplantation antithymocyte globulin/alemtuzumab (26% versus 27%), and only a few had radiation (2% versus 1%) after transplantation (Table 2). For the entire cohort, most patients received reduced-intensity (RIC) or nonmyeloablative conditioning. Less than 25% had a prior autologous HCT, with DLBCL being the most common histology (undergoing an autologous transplantation previously) in both PET groups (FDG-PET+ 46% and FDG-PET – 37%; P = .44) and similar distribution of other histologies. There was no significant difference between the FDG-PET+ and FDG-PET− cohorts in graft and donor type (Table 2). Median follow-up of survivors was 48 months (12 to 82 months; PET+ group) and 49 months (range, 3 to 75 months; PET– group).
NRM and GVHD
The cumulative incidence of NRM at 1 year was 14% (95% confidence interval [CI], 9% to 20%] in FDG-PET+ and 19% (95% CI, 13% to 25%; P = .23) in FDG-PET− groups (Table 3, Figure 1A). The respective figures at 3 years were 17% versus 27% (P = .03). On multivariate analysis, FDG-PET status was not predictive of NRM risk (Table 4). Unrelated donor (RR, 3.59; 95% CI, 1.96 to 6.58; P < .0001) and cord blood (RR, 2.69; 95% CI, 1.2 to 6.03; P = .01) HCT were associated with increased NRM risk (Table 4). The cumulative incidences of grade II to IV acute GVHD at day 100 (26% and 27%) and chronic GVHD at 1 year (43% versus 43%) were not significantly different between the 2 cohorts (Table 3).
Table 3. Univariate Analysis*.
| Outcomes | FDG-PET− | FDG-PET+ | P Value |
|---|---|---|---|
|
|
|
||
| Cumulative Incidence (CI) | |||
| Acute GVHD (grade II-IV) | |||
| 100 Days | 26 (20-33) | 27 (21-34) | .874 |
| Chronic GVHD | |||
| 1 Year | 43 (36-51) | 43 (36-51) | .997 |
| 3 Years | 52 (44-59) | 54 (46-62) | .717 |
| NRM | |||
| 1 Year | 19 (13-25) | 14 (9-20) | .236 |
| 3 Years | 27 (20-34) | 17 (11-23) | .031 |
| Relapse/progression | |||
| 1 Year | 17 (12-23) | 32 (25-40) | .002 |
| 3 Years | 26 (19-33) | 40 (32-48) | .007 |
| PFS | |||
| 1 Year | 64 (57-71) | 54 (46-62) | .064 |
| 3 Years | 47 (40-55) | 43 (36-51) | .472 |
| OS | |||
| 1 Year | 75 (68-81) | 72 (65-79) | .581 |
| 3 Years | 60 (52-67) | 58 (50-65) | .731 |
Probabilities of acute GVHD, chronic GVHD, treatment-related mortality, and relapse were calculated using the cumulative incidence estimate. PFS and OS were calculated using the Kaplan-Meier product limit estimate. P values reflect point-wise comparison at defined times.
Figure 1.
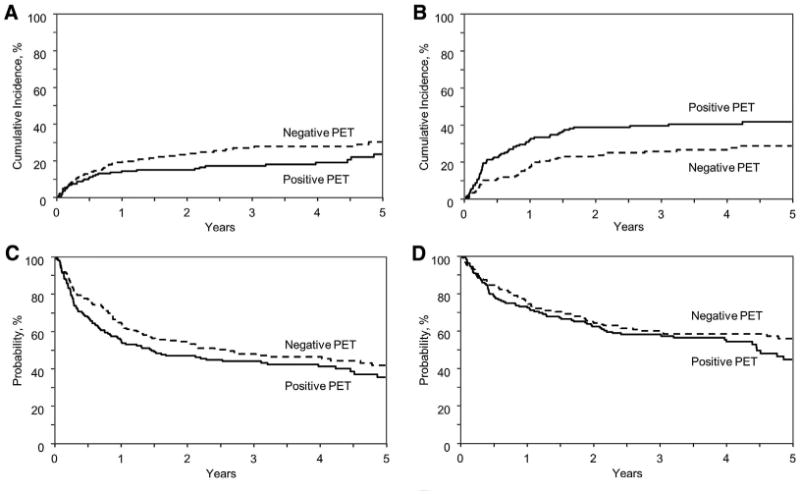
Cumulative incidence of NRM (A) and relapse (B) and Kaplan-Meyer estimates of PFS (C) and OS (D).
Table 4. Multivariate Analysis.
| Factor | N | RR (95% CI) | P Value |
|---|---|---|---|
| NRM | |||
| Main effect | |||
| FDG-PET− | 177 | 1 | |
| FDG-PET+ | 156 | .754 (.479-1.185) | .2202 |
| Donor type | |||
| HLA-identical sibling | 120 | 1 | |
| Cord blood | 53 | 2.691 (1.2-6.032) | .0162 |
| Unrelated | 160 | 3.595 (1.964-6.583) | <.0001 |
| Stem cell source | |||
| Bone marrow | 18 | 1 | |
| Peripheral blood | 262 | .33 (.16-.67) | .0025 |
| Relapse/progression | |||
| Main effect | |||
| FDG-PET− | 177 | 1 | |
| FDG-PET+ | 156 | 1.862 (1.263-2.745) | .0017 |
| Histology | |||
| FL | 104 | 1 | |
| DLBCL | 82 | 2.365 (1.378-4.059) | .0018 |
| MCL | 69 | 2.122 (1.209-3.726) | .0088 |
| T and NK neoplasm | 78 | 1.882 (1.051-3.369) | .0333 |
| Prior auto transplantation | |||
| Yes | 66 | 1 | |
| No | 267 | .578 (.38-.881) | .0109 |
| Therapy failure (PFS) | |||
| Main effect | |||
| FDG-PET− | 177 | 1 | |
| FDG-PET+ | 156 | 1.297 (.966-1.741) | .0833 |
| Donor type | |||
| HLA-identical sibling | 120 | 1 | |
| Cord blood | 53 | 1.896 (1.228-2.929) | .0039 |
| Unrelated | 160 | 1.521 (1.083-2.136) | .0155 |
| Stem cell source | |||
| Bone marrow | 18 | 1 | |
| Peripheral blood | 262 | .54 (.3-.5) | .03 |
| Histology | |||
| FL | 104 | 1 | |
| DLBCL | 82 | 2.094 (1.401-3.131) | .0003 |
| MCL | 69 | 1.873 (1.23-2.853) | .0035 |
| T and NK neoplasm | 78 | 1.548 (.995-2.408) | .0527 |
| Mortality (OS) | |||
| Main effect | |||
| FDG-PET− | 177 | 1 | |
| FDG-PET+ | 159 | 1.321 (.946-1.844) | .1028 |
| Donor type | |||
| HLA-identical sibling | 122 | 1 | |
| Cord blood | 53 | 2.098 (1.266-3.476) | .004 |
| Unrelated | 161 | 2.064 (1.379-3.09) | .0004 |
| Stem cell source | |||
| Bone marrow | 18 | 1 | |
| Peripheral blood | 262 | .38 (.21-.67) | .0008 |
| Histology | |||
| FL | 104 | 1 | |
| DLBCL | 85 | 2.393 (1.489-3.846) | .0003 |
| MCL | 69 | 1.844 (1.118-3.041) | .0166 |
| T and NK neoplasm | 78 | 1.706 (1.01-2.883) | .0458 |
| Conditioning regimen | |||
| Myeloablative | 90 | 1 | |
| Non-myeloablative/RIC | 246 | .642 (0.447-0.921) | .0161 |
Relapse/Progression
The cumulative incidence of relapse at 1 year of PET+ patients was higher than that of the PET– group (32% versus 17%; P = .002) (Table 3) and the relapse difference persisted at 3 years (40% versus 26%; P = .007) (Figure 1B). On multivariate analysis, a positive pretransplantation PET scan was associated with increased the risk of relapse by almost 2-fold (RR, 1.86; 95% CI, 1.26 to 2.74; P = .002) (Table 4). Whereas higher relapse risk with PET+ status was seen in all histological subtypes (3-year cumulative incidence rates: DLBCL, 51% versus 34%; P = .10; MCL, 54% versus 27%, P = .025; NK/T lymphoma, 44% versus 24%; P = .07); the trend was negligible in patients with FL (22% versus 17%, P = .50). Other clinical factors independently prognostic of relapse risk were lymphoma histology other than FL (RR, 1.88 to 2.36 for different subsets) and prior autologous transplantation (RR, 1.73; P = .01) and use of bone marrow grafts (RR, 3.0) (Table 4). The median time to relapse in PET– and PET+ groups were 10 months (range, 1 to 50) and 4 months (range, .1 to 51), respectively.
PFS and OS
At a median follow-up of 4 years (range, .25 to 6.8), FDG-PET status before allograft did not affect survival. Three-year PFS and OS for PET+ and PET– groups were similar at 43% (95% CI, 36% to 51%) versus 47% (95% CI, 40% to 55%); P = .47 and 58% (50% to 65%) versus 60%; (95% CI, 52% to 67%); P = .73, respectively (Figure 1C,D). On multivariate analysis, FDG-PET+ status was not associated with increased risk of therapy failure (ie, inferior PFS; RR, 1.29; 95% CI, .96 to 1.74; P = .08) or mortality (ie, inferior OS; RR, 1.32; 95% CI, .94 to 1.84; P = .10). Factors significantly associated with therapy failure (donor type, stem cell source, and lymphoma subgroup) and mortality (donor type, stem cell source, lymphoma subgroup, and conditioning intensity) are summarized in Table 4.
Causes of Death
In the FDG-PET+ group, 75 patients died. The most common causes of death were primary disease (55%) followed by GVHD (19%), organ failure (8%), and infection (8%). FDG-PET− patients (n = 73) died most often of primary disease (36%), GVHD (25%), organ failure (18%), and infections (8%) (Table 5).
Table 5. Causes of Death.
| Cause of Death | FDG-PET− | FDG-PET+ |
|---|---|---|
| Total no. of deaths | 73 | 75 |
| Primary disease | 26 (36) | 41 (55) |
| Infection | 6 (8) | 6 (8) |
| Idiopathic pneumonia syndrome | 0 | 3 (4) |
| GVHD | 17 (23) | 14 (19) |
| Organ failure | 13 (18) | 6 (8) |
| Second malignancy | 3 (4) | 3 (4) |
| Hemorrhage | 0 | 1 (1) |
| Severe platelet transfusion reaction | 1 (1) | 0 |
| Not specified | 7 (10) | 1 (1) |
Data presented are n (%).
Discussion
In our multicenter retrospective analysis of 336 patients, the largest cohort studied for the association between FDG-PET status and allogeneic HCT outcomes to our knowledge, we found that patients with residual lymphoma, as detected by FDG uptake on PET imaging, had a modestly increased risk of disease relapse after transplantation. Long-term survival, however, was similar for all lymphoma patients receiving allogeneic HCT in our cohort regardless of PET status.
Our results showed a link between a positive FDG-PET scan and clinical factors before transplantation, including extranodal involvement, presence of bulky disease, marrow involvement, and more prior lines of therapy, suggesting biologic differences in the compared PET status groups. Whereas 3-year NRM appeared to be higher in PET– patients compared with PET+ patients on univariate analysis, the difference, which is partially attributable to competing risk of early progressive disease in PET+ patients, was not confirmed after adjusting for potential confounding factors in multivariate analysis. It is important to highlight that all patients included in the current study were chemosensitive by CT criteria and, regardless of metabolic depth of remission immediately before transplantation, allogeneic HCT yielded 3-year survival close to 60%. Our results suggest that in NHL patients demonstrating chemosensitive disease by conventional radiographic criteria, a FDG-PET (at least as clinically applied in individual centers across the world) is not predictive of post-allogeneic HCT survival outcomes. Disease control long-term benefits from graft-versus-lymphoma (GVL) responses and the availability of effective salvage therapies in the case of post-allogeneic HCT relapse of NHL. The GVL effect in our series is further implied by improved survival using peripheral blood compared with marrow graft source.
We recognize that variations in PET techniques and interpretation among centers in different countries exist and evolve over time. In general, PET/CT interpretation guidelines from the International Harmonization Project in Lymphoma recommend using visual assessment of residual mass (positive versus negative) with mediastinal blood pool activity or background activity as the reference [20]. We collected additional supplemental data from centers and utilized the pre-HCT PET status as determined by the reporting transplantation center using their institutional practice and criteria. This strategy allowed us to examine the utility of pre-allograft PET scan, as practiced and utilized in the “real-world.” Whether our observations would be applicable to PET images interpreted centrally or by using standardized 5-point scale criteria is not known and likely beyond the scope of a registry analysis [20,21]. This limitation highlights the future need to use standardized 3- or 5-point scale PET imaging in forthcoming studies [14]. It is also important to highpoint that the Deauville criteria were published in late 2009 and nearly one half of the subjects included in our analysis underwent transplantation before the availability of these guidelines. Until future prospective studies are conducted in NHL histological subsets using standardized PET imaging methods, our analysis provides clinically relevant insights on the predictive value of PET after allograft for patients with NHL.
Current published data contain limited and contrasting findings on the predictive value of FDG-PET imaging. Dodero et al. reviewed 80 patients (34 with high-grade NHL and 46 with HL) before RIC allogeneic HCT [10]. PET positivity predicted survival, but over one half of the patients had HL, a disease where PET-CT provides the most reliable assessment of chemosensitivity and possibly weaker GVL effects [22,23]. Our cohort appears more homogenous, as HL patients were not included. A study from the University College London that reported outcomes of 88 patients with predominantly indolent NHL treated with alemtuzumab-containing RIC conditioning and risk-adapted post-HCT donor lymphocyte infusion showed a lack of difference in relapse and similar PFS between PET+ patients versus PET– groups; however, persistence of PET activity after transplantation most often predicted imminent relapse and reduced PFS [11-13]. More recent single-institution studies from the University of Minnesota and Memorial Sloan Kettering used Deauville score PET interpretation in their cohorts (78 and 58 NHL allograft recipients, respectively) who were chemosensitive by CT criteria and found no difference in event-free survival or OS between FDG-PET positive (Deauville 4, 5) and FDG-PET negative (Deauville 1 to 3) patients [12,13]. It is important to highlight that most aforementioned series studying PET in allogeneic HCT, including ours, comprised predominantly indolent NHL histologies, whereas publication on autologous HCT [5,8,9] included predominantly aggressive histologies. Biologic differences inherent to histologic subtypes clearly impact on predictive utility of PET; however, more data will be needed to assess implications of pretransplantation functional imaging in specific histologic subsets.
Our results provide potentially useful clinical information for interpreting the prognostic meaning of FDG-PET imaging results in the setting of allogeneic HCT. For example, whereas PET negativity leads to a lower risk of relapse, PET positivity may guide decisions about post-transplantation interventions to reduce relapse. Importantly, a positive PET scan should not be interpreted as a barrier to a successful allograft. It is a potentially modifiable variable affecting early relapse and, unlike histology or prior autograft, can be targeted by pre- or peritransplantation strategies [24,25]. Our study also highlights the need to standardize interpretation of PET scans and examine the utility of the Deauville scoring system in NHL and within the context of allogeneic HCT [14,26].
Supplementary Material
Highlights.
Patients with non-Hodgkin lymphoma and positive positron emission tomography scan before allogeneic hematopoietic cell transplantation are at higher risk of relapse.
In chemosensitive non—Hodgkin lymphoma patients by computed tomography criteria, positron emission tomography scan does impact allogeneic hematopoietic cell transplantation outcomes.
Acknowledgments
The authors thank the following authors for their contributions to the manuscript: Mahmoud D. Aljurf, Christopher Dandoy, John Gibson, Mark S. Hertzberg, Richard F. Olsson, Bipin N. Savani, Harry C. Schouten, Leo F. Verdonck and Ravi Vij. The authors also thank Maggie Simaytis for administrative support.
The CIBMTR is supported by Public Health Service grant/cooperative agreement [U24-CA076518] from the National Cancer Institute, the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement [5U10HL069294] from National Heart, Lung, and Blood Institute and National Cancer Institute; a contract [HHSH250201200016C] with Health Resources and Services Administration; 2 grants [N00014-12-1-0142] and[N00014-13-1-0039] from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen; anonymous donation to the Medical College of Wisconsin; Ariad; Be The Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc.; Roswell Park Cancer Institute; Histo-Genetics; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co., Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Bio-vitrum; Tarix Pharmaceuticals; *Terumo BCT; Teva Neuroscience, Inc.; Therakos; University of Minnesota; University of Utah; and *WellPoint. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration or any other agency of the US Government.
*Corporate Members
Footnotes
Financial disclosure statement: There are no relevant conflicts of interest to disclose.
Conflict of interest statement: There are no conflicts of interest to report.
Supplementary Data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2015.05.007.
References
- 1.Maloney DG. Graft-versus-lymphoma effect in various histologies of non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S99–S105. doi: 10.1080/10428190310001623694. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus HM, Zhang MJ, Carreras J, et al. A comparison of HLA-identical sibling allogeneic versus autologous transplantation for diffuse large B cell lymphoma: a report from the CIBMTR. Biol Blood Marrow Transplant. 2010;16:35–45. doi: 10.1016/j.bbmt.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Besien K, Carreras J, Bierman PJ, et al. Unrelated donor hematopoietic cell transplantation for non-Hodgkin lymphoma: long-term outcomes. Biol Blood Marrow Transplant. 2009;15:554–563. doi: 10.1016/j.bbmt.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachanova V, Burns LJ, Wang T, et al. Alternative donors extend transplantation for patients with lymphoma who lack an HLA matched donor. Bone Marrow Transplant. 2015;50:197–203. doi: 10.1038/bmt.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson M, Hoyt R, Roberts AW, et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br J Haematol. 2010;150:39–45. doi: 10.1111/j.1365-2141.2010.08162.x. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz CH, Yahalom J, Zelenetz AD, et al. High-dose chemoradiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148:890–897. doi: 10.1111/j.1365-2141.2009.08037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulou LS, Thanos L, Ziakas PD. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and metaanalysis of published trials. Eur J Nucl Med Mol Imaging. 2010;37:156–162. doi: 10.1007/s00259-009-1258-y. [DOI] [PubMed] [Google Scholar]
- 9.Derenzini E, Musuraca G, Fanti S, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer. 2008;113:2496–2503. doi: 10.1002/cncr.23861. [DOI] [PubMed] [Google Scholar]
- 10.Dodero A, Crocchiolo R, Patriarca F, et al. Pretransplantation [18-F] fluorodeoxyglucose positron emission tomography scan predicts outcome in patients with recurrent Hodgkin lymphoma or aggressive non-Hodgkin lymphoma undergoing reduced-intensity conditioning followed by allogeneic stem cell transplantation. Cancer. 2010;116:5001–5011. doi: 10.1002/cncr.25357. [DOI] [PubMed] [Google Scholar]
- 11.Lambert JR, Bomanji JB, Peggs KS, et al. Prognostic role of PET scanning before and after reduced-intensity allogeneic stem cell transplantation for lymphoma. Blood. 2010;115:2763–2768. doi: 10.1182/blood-2009-11-255182. [DOI] [PubMed] [Google Scholar]
- 12.Bachanova V, Cao Q, Ustun C, et al. Pretransplantation fluorine-18-deoxyglucose-positron emission tomography scan has no influence on relapse and survival in non-Hodgkin lymphoma patients undergoing allo-SCT. Bone Marrow Transplant. 2015;50:142–144. doi: 10.1038/bmt.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauter CS, Lechner L, Scordo M, et al. Pretransplantation fluorine-18-deoxyglucose—positron emission tomography scan lacks prognostic value in chemosensitive B cell non-Hodgkin lymphoma patients undergoing nonmyeloablative allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:881–884. doi: 10.1016/j.bbmt.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–3058. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 19.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 20.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 21.El-Jurdi N, Reljic T, Kumar A, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy. 2013;5:457–466. doi: 10.2217/imt.13.31. [DOI] [PubMed] [Google Scholar]
- 22.Ram R, Gooley TA, Maloney DG, et al. Histology and time to progression predict survival for lymphoma recurring after reduced-intensity conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1537–1545. doi: 10.1016/j.bbmt.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warlick ED, Tomblyn M, Cao Q, et al. Reduced-intensity conditioning followed by related allografts in hematologic malignancies: long-term outcomes most successful in indolent and aggressive non-Hodgkin lymphomas. Biol Blood Marrow Transplant. 2011;17:1025–1032. doi: 10.1016/j.bbmt.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA. Decade in review-cancer immunotherapy: Entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11:630–632. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


