Abstract
The amniotic membrane is the innermost layer of fetal membrane and is attached to the chorion in the placenta. This membrane has been used for nearly a century in varied fields such as ophthalmology, reconstructive surgery, and burn treatment. In this case report, we used a human amniotic membrane to repair an iatrogenic oroantral communication that occurred during the extraction of the patient's right upper second molar. A splint was given after the perforation was covered with human amniotic membrane and healing was clinically evaluated at various intervals. The outcome of the study revealed that the human amniotic membrane was an efficient graft material for repairing the defect caused by an iatrogenic oroantral communication following tooth extraction.
Keywords: Oroantral communication, Amnion
I. Introduction
An oroantral communication is a relatively common yet serious complication in exodontia. Its incidence ranges from 0% to 68% as reported by various authors1. Multiple treatment modalities exist for the repair of oroantral communications, including the use of local and free flaps, tissue expansion, allogenic tissues, and biomaterials2.
Fresh human amniotic membrane (HAM) has been used in reconstructive surgery for nearly a century2. Due to its low immunogenicity and minimal inflammation, scarring, and enhancement of epithelialization, its use has been advocated in various ophthalmological studies2. Oral and maxillofacial surgeons have used it in the healing of gingival wounds3, in vestibuloplasty, and in the prefabrication of flaps 4,5,6. Kruse et al.7 advocated the use of a multilayered application of HAM for deep corneal defects. We evaluated the use of multilayered HAM as a grafting material in a patient for the repair of an oroantral communication.
II. Case Report
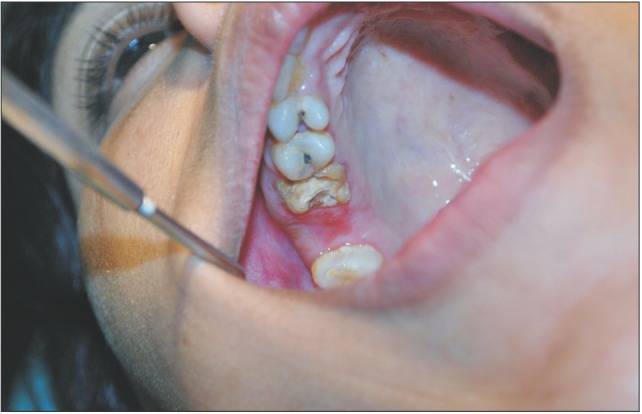
A 29-year-old female patient had undergone removal of a grossly decayed upper right second molar tooth. Preoperative intraoral radiographs ruled out any periapical pathology. The patient reported back to the department after 3 days with a complaint of nasal regurgitation of food and liquids. On clinical examination, oroantral perforation was observed in relation to the extraction socket. The size of the oroantral communication was measured at about 4 to 6 mm in diameter.(Fig. 1) A confirmatory mirror test was performed over the extraction site, which revealed a communication between the oral cavity and the right antrum.(Fig. 2) Typically, a persistent oroantral communication of this size would have been repaired with a buccal advancement flap in our department. For this case, oroantral communication repair with amniotic membrane (AM) was planned for the patient.
Fig. 1. Picture shows persistent 4 to 6 mm wide oroantral communication.
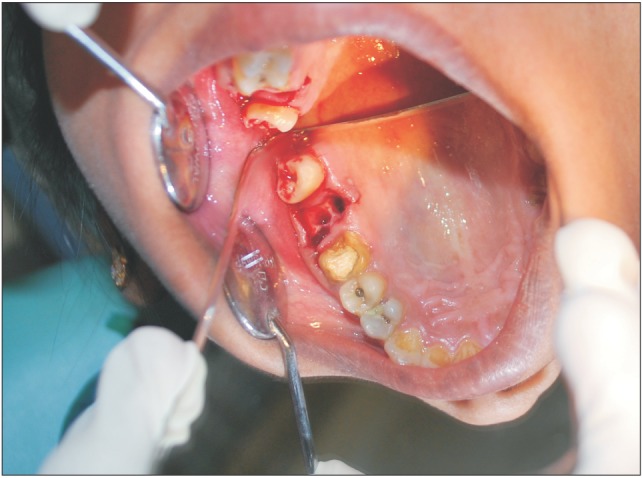
Fig. 2. Picture shows mirror fog test of oralantral communication.
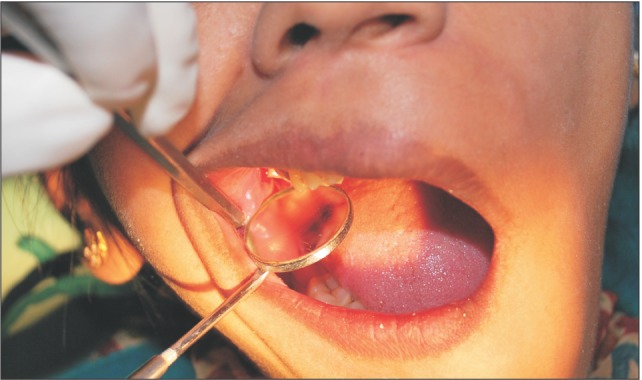
A donor mother was identified who was to undergo cesarean section in the Department of Obstetrics and Gynaecology in Chigatere Hospital (Davangere, India). The donor was screened for infections such as HIV and hepatitis B surface antigens, and other routine investigations were carried out. Prior to the surgery, clearance was obtained from the institutional ethical committee. The intended use of the AM was explained to the patient, and consents were obtained for the procedure. The repair of the oroantral communication in our patient was planned a day after the donor's cesarean section. In the operating room during the cesarean, the placenta was collected in a tray by the obstetrician and handed over to the oral and maxillofacial surgeon. In the same operating room, the oral and maxillofacial surgeon then carefully separated the AM from the chorion of the placenta under sterile aseptic conditions in the presence of the obstetrician. The AM was cleared of all blood clots and gross tissue attachment with copious amounts of tap water.(Fig. 3) The membrane was stored overnight in a large bottle containing 85% of glycerol and 5 mL of gentamicin 80 mg/mL and stored in refrigerator at a temperature of 4℃. On the next day, just prior to its application over the extraction socket of the recipient patient, the membrane was soaked in normal saline for a period of 10 minutes.(Fig. 4) This method of preparing and preserving the AM has been described by Kesting et al.2 for the application of closing oroantral communications in minipigs. For our procedure, the AM was then folded in layers and applied over the extraction socket and secured with sutures.(Fig. 5, 6)
Fig. 3. Amniotic membrane being prepared before repair of oroantral communication.
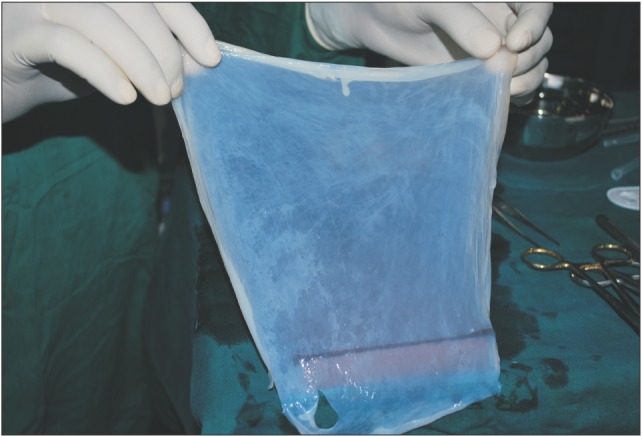
Fig. 4. Picture showing amniotic membrane stored in gentamycin solution.

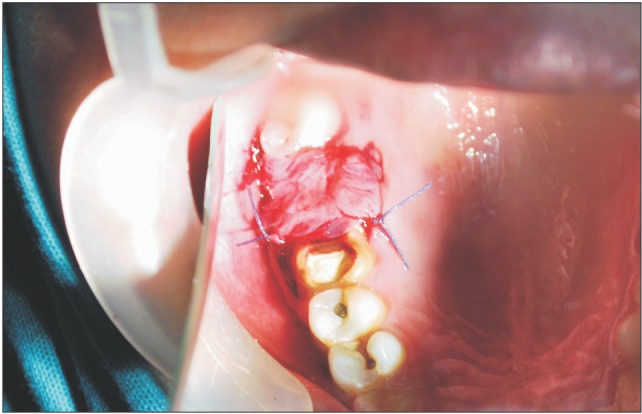
Fig. 5. Amniotic membrane being used in layers and sutured to close the oroantral communication.
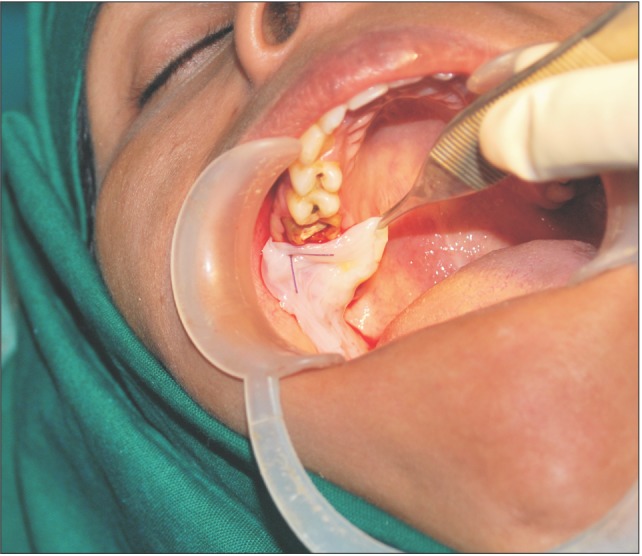
Fig. 6. Closure of oroantral communication achieved with multi-layered amniotic membrane.
The patient was followed up after 3 days, 5 days, and 7 days, during which we observed a surrounding area of granulation tissue. After 14 days, the defect was completely covered by normal healing epithelium.(Fig. 7)
Fig. 7. Complete healing of the oroantral communication after 14 days of closure.
III. Discussion
The treatment of oroantral fistulas has evolved over a long period of time with various modalities, including bone grafts, tissue transfer, flaps, and even stem cells. Considering all methods, the closure of oroantral communications can be divided into four groups: local flaps, pedicled flaps (e.g., a flap from the tongue), free flaps, and biomaterials2. The use of flaps is accompanied with donor site morbidity. Biomaterials seem to offer an ideal treatment modality but no known biomaterial has been able to fulfill the complexity of biocompatibility and integration. In addition, incomplete coverage and the formation of scars are complications associated with oroantral communications, which further complicate their treatment.
The HAM is a thin semitransparent tissue forming the innermost layer of the fetal membrane and was first introduced as a dressing in 1910 by Davis8. The AM consists of an epithelial monolayer, a thick basement membrane, and an avascular stroma. The AM contains no blood vessels or nerves; instead, the nutrients it requires are supplied directly by diffusion out of the amniotic fluid and/or from the underlining decidua. It is the innermost layer, nearest to the fetus, and consists of a single layer of cells uniformly arranged on the basement membrane. The basement membrane is one of the thickest membranes found in all human tissues. The support provided to the fetus throughout gestation by the basement membrane provides testimony to the structural integrity of this remarkable membrane. The compact layer of stromal matrix adjacent to the basement membrane forms the main fibrous skeleton of the AM. The collagens of the compact layer are secreted by mesenchymal cells situated in the fibroblast layer. Interstitial collagens (types I and III) predominate and form parallel bundles that maintain the mechanical integrity of AM. Collagens type V and VI form filamentous connections between interstitial collagens and the epithelial basement membrane.
The HAM and its extracellular matrix contain components such as growth factors and antimicrobial peptides which make it an ideal scaffold material, and unlike other biomaterials, it is cheap to obtain. These peptides also induce the migration of epithelial cells, the reinforcement of basal cell adhesion, and the induction of epithelial differentiation. The AM also has unique antibacterial and pain reduction properties. Moreover, harvesting AM is a simple procedure and does not require any special arrangements. HAM is also known to have low immunogenic characteristics making it a suitable tissue for transplantation, and clinical signs of acute rejection have only rarely been observed when HAM has been used as graft material.
Kesting et al.2 evaluated the use of multilayer HAM as a grafting material for the repair of oroantral fistulas in minipigs. The result of this experiment provided evidence for the use of HAM as a simple and effective method for the closure of such communications. Sharma et al.9 suggested that AM can be favorable graft materials for vestibuloplasty, promoting healing and preventing relapse. Sham and Sultana10 described the role of AM in biological wound healing in a buccal mucosal defect in an operative case of leukoplakia.
In another study by Ahn et al.11, the quantification of epidermal growth factor (EGF) in AM and the effect of the AM-collagen complex on full-thickness skin defects was examined in rats. The concentration of EGF in fresh, deep frozen, and freeze-dried AM was evaluated by ELISA. EGF receptor (EGF-R) immune-staining was performed in freeze-dried AM. Three full-thickness skin defects (28 mm in diameter) were made on the dorsal surface of Sprague-Dawley rats. The control group was covered by Vaselin gauze while the AM-collagen complex and Terudermis was grafted onto the two other defects. The areas of healing were evaluated by Cinamon's score before biopsy. The grafted sites were retrieved at 3 days, 1 week, 2 weeks, and 4 weeks after the operation. H&E and factor VIII immunohistochemical staining was performed to evaluate the microscopic adhesions, structural integrity, and micro-vessel formation. It was concluded that the EGF concentration of the fresh, deep frozen, and freeze-dried AM showed similar levels and that EGF-R staining was observed in the epithelial layer of the freeze-dried AM. EGF and EGF-R were well-preserved in the freeze-dried AM. The AM attached to collagen acted as an excellent biologic dressing with a similar effect as Terudermis. The AM showed anti-inflammatory activity and healing was completed at 4 weeks after the full-thickness skin defect11.
In our case, the HAM proved to be an excellent scaffold layer for covering the defect of an iatrogenic oroantral communication. The membrane enhanced wound healing and complete functional and esthetic rehabilitation was achieved with no noticeable complications.
Biological substitutes such as HAMs can be efficient scaffolds for promoting the repair of oroantral communications. Its ease of availability, cost-effectiveness, simple preparation, and simple storage requirements facilitates its application in most conventional dental and maxillofacial units. We have found this method to be a good alternative to the conventional methods in the treatment of oroantral communications.
Footnotes
Conflict of Interest: No potential confl ict of interest relevant to this article was reported.
References
- 1.Kirschner RE, Cabiling DS, Slemp AE, Siddiqi F, LaRossa DD, Losee JE. Repair of oronasal fistulae with acellular dermal matrices. Plast Reconstr Surg. 2006;118:1431–1440. doi: 10.1097/01.prs.0000239612.35581.c3. [DOI] [PubMed] [Google Scholar]
- 2.Kesting MR, Loeffelbein DJ, Classen M, Slotta-Huspenina J, Hasler RJ, Jacobsen F, et al. Repair of oronasal fistulas with human amniotic membrane in minipigs. Br J Oral Maxillofac Surg. 2010;48:131–135. doi: 10.1016/j.bjoms.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Rinastiti M, Harijadi, Santoso AL, Sosroseno W. Histological evaluation of rabbit gingival wound healing transplanted with human amniotic membrane. Int J Oral Maxillofac Surg. 2006;35:247–251. doi: 10.1016/j.ijom.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty: a preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:574–578. doi: 10.1016/S107921040400006X. [DOI] [PubMed] [Google Scholar]
- 5.Güler R, Ercan MT, Ulutunçel N, Devrim H, Uran N. Measurement of blood flow by the 133Xe clearance technique to grafts of amnion used in vestibuloplasty. Br J Oral Maxillofac Surg. 1997;35:280–283. doi: 10.1016/s0266-4356(97)90048-6. [DOI] [PubMed] [Google Scholar]
- 6.Kethley JL, Jr, Gamble JW. The lipswitch: a modification of Kazanjian's labial vestibuloplasty. J Oral Surg. 1978;36:701–705. [PubMed] [Google Scholar]
- 7.Kruse FE, Rohrschneider K, Völcker HE. Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology. 1999;106:1504–1510. discussion 1511. doi: 10.1016/S0161-6420(99)90444-X. [DOI] [PubMed] [Google Scholar]
- 8.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307. [Google Scholar]
- 9.Sharma Y, Maria A, Kaur P. Effectiveness of human amnion as a graft material in lower anterior ridge vestibuloplasty: a clinical study. J Maxillofac Oral Surg. 2011;10:283–287. doi: 10.1007/s12663-011-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sham E, Sultana NS. Biological wound dressing: role of amniotic membrane. Int J Dent Clinics. 2011;3:71–72. [Google Scholar]
- 11.Ahn KM, Lee JH, Lee UL, Lee JH, Lee JW, Kim SP, et al. Development of biocompatible dressing material made of collagen and amniotic membrane and wound healing experiment in rat. J Korean Assoc Oral Maxillofac Surg. 2006;32:189–199. [Google Scholar]


