Abstract
Binge eating disorders are characterized by episodes of intense consumption of high-calorie food. In recently developed animal models of binge eating, rats given intermittent access to such food escalate their consumption over time. Consumption of calorie-dense food is associated with neurochemical changes in the nucleus accumbens, including dopamine release and alterations in dopamine and opioid receptor expression. Therefore, we hypothesized that binge-like consumption on intermittent access schedules is dependent on opioid and/or dopamine neurotransmission in the accumbens. To test this hypothesis, we asked whether injection of dopamine and opioid receptor antagonists into the core and shell of the accumbens reduced consumption of a sweet high-fat liquid in rats with and without a history of intermittent binge access to the liquid. Although injection of a μ opioid agonist increased consumption, none of the antagonists (including μ opioid, δ opioid, κ opioid, D1 dopamine and D2 dopamine receptor antagonists, as well as the broad-spectrum opioid receptor antagonist naltrexone) reduced consumption, and this was the case whether or not the animals had a prior history of intermittent access. These results suggest that consumption of sweet, fatty food does not require opioid or dopamine receptor activation in the accumbens even under intermittent access conditions that resemble human binge episodes.
Keywords: binge eating disorder, nucleus accumbens, opioids, dopamine
1. Introduction
Binge eating disorders are common in the American population. These disorders, which are associated with other medical problems such as depression and obesity, are characterized by intermittent binge episodes during which large amounts of highly palatable food (typically sweet, fat and calorie-dense) are consumed [1]. Because currently available pharmacotherapies are only minimally effective in preventing binges from occurring, there is a pressing need for new treatment options based on the neurobiological mechanisms of the disorder. Recent work in this area has focused on opioid antagonists, which reduce consumption of sweet and fatty foods in animals [2]. Indeed, the “gain of function” A118G allele of the μ opioid receptor gene is overrepresented in people with binge eating disorders vs obese controls [3], and several studies have tested the efficacy of opioid antagonists to reduce calorie-dense food consumption or binge eating in humans, with mixed success [4-6]. A more detailed understanding of the contributions of opioid receptors to caloric intake regulation, and particularly to binge consumption, may help to identify more specific and efficacious targets for therapeutic development.
Recently, several animal models of binge consumption have been developed [7]. In one subset of these models, rats are allowed intermittent access to a highly palatable food, such as sucrose solution [8-10], fat or high-fat food [11-14], or mixtures of sugar and fat [15-18]. Over 1 to 7 weeks, binge eating develops: animals increase consumption of the palatable food when it is available, such that their total consumption matches or exceeds that of animals given continuous access to the palatable food. A critical difference between animal and human bingeing is that humans decide themselves when to initiate a binge, whereas the interval between animals’ binges is set by the experimenter. However, in both humans and animals, binges occur even in the absence of a biological need for nutrients (hunger), the food consumed during binges is almost always sweet and/or high-fat food, and a reduction in consumption of less palatable food occurs between binges. These parallels suggest that similar neural mechanisms in humans and animals regulate consumption both during and between binges [1, 19-23].
Pharmacological studies of animal models point towards a role for endogenous opioids in promoting binge consumption. Somatic and behavioral signs of withdrawal can be precipitated in sucrose-bingeing rats by either withholding sucrose or giving an opioid receptor antagonist [24]. Moreover, opioid antagonist treatment reduces consumption of highly palatable food in rats previously exposed to an intermittent access or stress regimen that leads to binge eating [13, 18, 25-28]. These effects are more pronounced for high-fat than sweet food [27], and in some cases normal chow consumption is also not as strongly affected [13, 18, 25-27]. Because these studies used systemic injections of broad-spectrum opioid antagonists, which are likely to have blocked opioid receptors of all subtypes in many brain regions, an important next step is to determine which opioid receptors and brain areas are involved in promoting binge consumption.
The nucleus accumbens (NAc) may be an important locus where opioid receptors of the μ subtype may contribute to binge eating. Binding studies demonstrate greater numbers of μ opioid receptors in the NAc shell of rats given prolonged intermittent access to glucose [29], and similar limited access schedules for sucrose or a sweet/fat liquid (Ensure®) causes reduced expression of the opioid peptide enkephalin in the NAc [30, 31]. Injection of opioid agonists (especially μ-specific) into the core or shell of the NAc is potently orexigenic [32-37], and increases consumption of high-calorie food more than less palatable food [38, 39]. However, opioid receptor antagonist effects are less clear. Injection of the broad-spectrum antagonist naltrexone into the NAc typically results in reduction of sweet or fatty food intake only at very high doses [38, 40-42], and the δ receptor antagonist naltrindole actually increases sucrose consumption [42]. Moreover, although some studies show that injection of μ receptor-specific antagonists reduces consumption [41-44], another found no effect [45].
Notably, in rodent binge eating models, consumption escalates over weeks of intermittent access, and this escalation only occurs with extremely calorie-dense food [16]. One attractive hypothesis, then, is that this escalation is due to plasticity in the NAc opioid system, such that binge consumption comes to depend on activation of NAc opioid receptors by endogenous opioids. This hypothesis predicts that opioid antagonist injection in the NAc should block binge-like consumption whereas it should be less effective in reducing non-binge consumption. To test this hypothesis, we subjected rats to 5 weeks of intermittent access to an emulsion consisting of cream, corn oil and sugar (COS), a procedure that produces a binge-like escalation of consumption [16], and asked whether these animals’ binge consumption of COS was more affected by injection of opioid receptor antagonists in the NAc than that of control animals that were given intermittent access to water alone (but not COS).
One additional prediction of our hypothesis is that if μ opioid receptor numbers are increased in bingeing animals [29], then activation of NAc μ receptors should cause a greater increase in consumption in animals with a history of binge access than in those with a history of intermittent access to water alone. We tested this prediction by injecting the μ agonist DAMGO into animals previously given intermittent access to COS or water. We also injected DAMGO into a group of animals given 5 weeks of continuous access to COS. These animals become obese [16]; thus, comparing the effects of DAMGO in intermittent and continuous access groups allows us to assess whether the NAc opioid consumption-promoting effects are similarly escalated in models of bingeing and obesity, which would suggest a similar neural mechanism.
In addition to a potential role for NAc opioid receptors in binge consumption, several lines of evidence suggest that NAc dopamine receptors may be involved as well. For instance, bingeing rats exhibit increased dopamine D1 receptor binding [29], reduced D2 and increased D3 receptor expression [31], and increased expression of the dopamine transporter [46] in the NAc. In addition, microdialysis studies show that sucrose binges are invariably accompanied by an increase in dopamine levels in the NAc, whereas animals given continuous access to sucrose, or insufficient sucrose access to develop binging, show no or much smaller increases [8, 10, 47]. Although the dopamine response to palatable food habituates with repeated episodes of consumption [48], binge-associated dopamine release does not [8, 10, 47]. Moreover, locomotor sensitization to psychostimulants – a NAc dopamine-dependent process – is enhanced in animals binging on sucrose [49-51], and amphetamine-sensitized animals show more locomotion in response to a brief taste of sucrose, and consume more freely-available sucrose, than controls [52]. Finally, abnormalities in dopamine turnover, dopamine transporters and dopamine receptors have been observed in humans with binge eating disorders [53].
The foregoing studies suggest that NAc dopamine may contribute to binge consumption. Food consumption is typically not strongly affected by disruption of NAc dopamine [54-60], but the effects of NAc dopamine manipulations on binge consumption have not been tested. Therefore, in this study we assessed the effects of NAc injection not only of opioid receptor ligands, but of dopamine receptor antagonists on binge intake of COS.
2. Materials and methods
2.1 Animals
Male Long–Evans rats (n=233, Harlan) weighing 275–300 g were housed in a room with a 12 h light cycle. Experiments were conducted during the light phase. Animals were handled daily for at least one week before experiments; chow intake and body weight were measured daily. Prior to the start of the experiment, three groups of rats were matched by average amount of chow consumed and average body weight. All animal procedures were consistent with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
2.2 Behavior
2.2.1. Operant chambers
Behavioral experiments were run in standard Med Associates operant chambers (30 × 25 cm). The chambers were illuminated with one 28 V white house light, and white noise (65 dB) was played through a dedicated speaker. Operant chambers were equipped with a lickometer filled with either water or a cream-oil-sucrose emulsion (COS). Photobeams across the lickometers were used to detect the precise times of licks.
2.2.2 Ingestants
The COS emulsion was prepared daily and consisted of 50% (by volume) each of heavy cream and corn oil, 8% (weight per volume) sucrose, and 1 g per L of sodium stearoyl lactylate (Niacet Corporation), an emulsifier. The emulsion, prepared using a wire whisk, was stable for > 24 hr. The calorie content of COS was 5.99 kCal/mL (chow was 3.02 kCal/g).
2.2.3. Acquisition phase
As described previously [16], rats were divided in three group, the intermittent access (binge) group (IA; n=93) and two control groups: the water access group (WA; n=83) and the continuous access group (CA; n=38). For 5 weeks, each group had access to the lickometer in the operant chambers three times per week (Monday, Wednesday and Friday; M-W-F) for 90 min. During these sessions, the IA and CA groups had access to the COS solution while the WA group had access to water. The CA group also had ad libitum access to COS at all times in their home cages. Licks and COS or water intake were recorded during the sessions.
Throughout all phases, a given rat was always placed in the access chambers at roughly the same time of day (either morning or afternoon).
2.3 Cannula implantation surgery
After the bingeing procedure, rats were implanted with bilateral guide cannulae for microinjection. These consisted of stainless steel guide cannulae (27 ga) with plastic hubs (Plastics One), cut such that the end of the guide was 2 mm above the target. Animals were anesthetized with isoflurane and placed in a stereotactic frame. The scalp was retracted and holes drilled above the targets. Target coordinates for the tips of the injectors were as follows (in mm below bregma): core: AP 1.2, ML 2.0, DV 7.8; shell: AP 1.2, ML 0.75, DV 7.3. Cannulae were fixed to the skull with bone screws and dental cement. All animals were treated with enrofloxacin, ketoprofen, and Neopredef topical antibiotic powder. Stainless steel wires were inserted into the guides and remained there at all times except during injections. Animals were allowed 4–7 d to recover from the surgery prior to beginning experiments.
2.4 Microinjection experiments
After surgery and recovery, baseline performance was reestablished over one week of M-W-F 90 min COS access sessions. All three groups had access to COS during these sessions; this procedure was followed in order to eliminate neophobia in the WA group as a potential confound during the experimental phase. In the experimental phase, animals in all groups (including WA) continued to receive M-W-F 90 min COS access sessions, and microinjections were performed prior to every session. Rats were gently restrained, and a 33 ga injector cannula extending 2 mm below the guide was inserted; 0.5 μl of drug solution was infused bilaterally over 3 min; and after a 2 min diffusion period, the injectors were withdrawn and rats were immediately placed into the operant chamber and the session started. Drugs were purchased from Tocris. SCH23390, raclopride, [D-Ala2,N-MePhe4,Gly-ol]-enkephalin (DAMGO), naltrexone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) were dissolved in sterile 0.9% saline; naltrindole and norbinaltorphimine (nor-BNI) were dissolved in saline with 15% and 10% dimethyl sulfoxide, respectively. Each animal received injections of vehicle as well as two drugs. Each pair of drugs was injected in the same order for each rat that received the pair, as described in Table 1. Three doses of each of these drugs were injected, and their order was randomized. The doses were (per hemisphere): 0.1, 1.0, and 2.0 μg of SCH23390, 0.2, 2.0, and 4.0 μg of raclopride, 0.025, 0.25, and 2.5 μg of DAMGO; vehicle, 6.5, 20, and 30 μg of naltrexone; vehicle, 2, 4, and 8 μg of CTAP; vehicle, 1, 10, and 20 μg of naltrindole; vehicle, 0.1, 1 and 10 μg of nor-BNI [32, 41, 42, 44, 45, 61, 62]. Each animal received a single vehicle injection; the order of these was randomized with the constraint that at least one vehicle injection occurred every day on which injections occurred, except the last one. Each rat was consistently run at roughly the same time in either the morning or the afternoon, but groups of rats (as defined in Table 1) were split across morning and afternoon sessions (i.e., no drug was injected only in the morning or only in the afternoon).
Table 1.
Subject groups, drugs injected and injection order.
| Group | Cannula location | 5 week access history | First drug | Second drug | N |
|---|---|---|---|---|---|
| 1 | core | IA | DAMGO | raclopride | 9 |
| 2 | core | WA | DAMGO | raclopride | 7 |
| 3 | core | CA | DAMGO | raclopride | 7 |
| 4 | shell | IA | DAMGO | raclopride | 7 |
| 5 | shell | WA | DAMGO | raclopride | 7 |
| 6 | shell | CA | DAMGO | raclopride | 6 |
| 7 | core | IA | naltrexone | SCH23390 | 11 |
| 8 | core | WA | naltrexone | SCH23390 | 8 |
| 9 | core | CA | naltrexone | SCH23390 | 9 |
| 10 | shell | IA | naltrexone | SCH23390 | 7 |
| 11 | shell | WA | naltrexone | SCH23390 | 6 |
| 12 | shell | CA | naltrexone | SCH23390 | 8 |
| 13 | core | IA | CTAP | 11 | |
| 14 | core | WA | CTAP | 11 | |
| 15 | shell | IA | CTAP | 10 | |
| 16 | shell | WA | CTAP | 8 | |
| 17 | core | IA | naltrindole | nor-BNI | 12 |
| 18 | core | WA | naltrindole | nor-BNI | 11 |
| 19 | shell | IA | naltrindole | nor-BNI | 11 |
| 20 | shell | WA | naltrindole | nor-BNI | 8 |
| 21 | core | none | (CTAP vs DAMGO) | - | 19 |
| TOTAL | 193 |
Three doses of the first drug were injected in random order, followed by three doses of the second drug in random order. See section 2.4 for doses. Each rat received a single vehicle (saline) injection on a randomly-determined day.
To test whether CTAP antagonized the effects of DAMGO, 19 additional rats were habituated to drink COS during 90 min access sessions in the experimental boxes during one week. After cannulae implantation in the NAc core, they had access to the COS in the experimental boxes for 3 sessions before the injection experiment. Each rats received two consecutives injections of either saline or CTAP 8 μg and either saline or DAMGO (0.25 μg or DAMGO 2.5 μg) in randomized order.
2.5 Data analysis
2.5.1. Statistics
Repeated-measures ANOVA with one factor (dose) or two factors (dose and group) were used to compare the effects of bingeing and control procedures. ANOVAs were followed by Holm–Sidak post hoc tests; an adjusted p < 0.05 was considered a significant difference, and, provided the overall ANOVA result was considered significant (p < 0.05), a Holm–Sidak post hoc unadjusted p < 0.1 was considered a trend toward significance. Most consumption occurred in the first 22.5 minutes (i.e., first quarter) of the session, with a stereotypical rapid decline in responding across this period. In the remaining 67.5 minutes of the session, animals typically consumed at relatively constant, low rates (Figs. 3A,B, 4A,B). To capture these distinct epochs, we divided the session into first quarter and last three quarters and analyzed them separately. All analyses were performed using the R software environment.
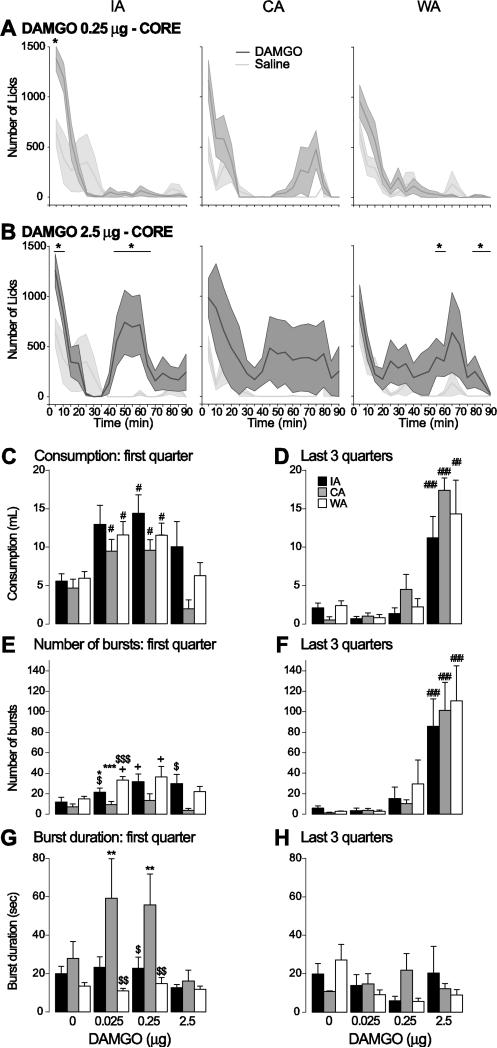
Figure 3. Effect of DAMGO injection in the core on COS consumption.
A,B: Average time course of licking (5 min bins) after injection of the middle dose (A) and the high dose (B) of DAMGO (dark grey) and saline (light grey) into the NAc core in the IA (left), CA (middle) and WA (right) groups. Stars indicate bins in which the number of licks was significantly different between DAMGO and saline injections.
C-H: The amount of COS consumed (C,D), number of lick bursts (E,F) and lick burst duration (G,H) are shown for the first quarter of the session (when most of the licking takes place; left) and the last 3 quarter of the session (right) for the IA (black), WA (white) and CA (grey) groups. Symbols in C-H show the results of post-hoc tests:
*: significant difference from the WA group (p < 0.05).
$, $$, $$$: significant difference from the CA group (p < 0.05, 0.01, 0.001).
#, #, ###: significant difference from control injection (p < 0.05, 0.01, 0.001); + trend to significance.
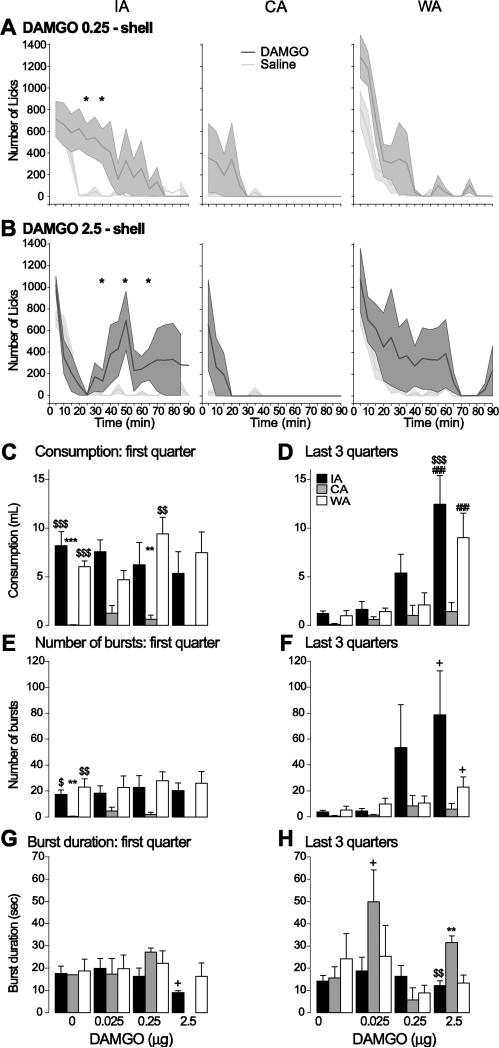
Figure 4. Effect of DAMGO injection in the shell on COS consumption.
A,B: Average time course of licking (5 min bins) after injection of the middle dose (A) and the high dose (B) of DAMGO (dark grey) and saline (light grey) into the NAc shell in the IA (left), CA (middle) and WA (right) groups. Stars indicate bins in which the number of licks was significantly different between DAMGO and saline injections.
C-H: The amount of COS consumed (C,D), number of lick bursts (E,F) and lick burst duration (G,H) are shown for the first quarter of the session (when most of the licking takes place; left) and the last 3 quarter of the session (right) for the IA (black), WA (white) and CA (grey) groups.
**, ***: significant difference from the WA group (p < 0.01, 0.001).
$$, $$$: significant difference from the CA group (p < 0.01, 0.001).
###: significant difference from control injection (p < 0.001); + trend to significance.
2.5.2. Consumption
Overall consumption was measured by counting the number of licks. At the end of the session we also measured the amount of liquid consumed during the session (initial lickometer reservoir volume minus ending volume). Consumption in the first quarter and last 3 quarters was determined by multiplying the number of licks in the given time period by the calculated volume obtained per lick.
2.5.3. Licking micros tructure
Lick rate was defined as the number of licks per second. The initial lick rate was defined as the lick rate during the first minute of the first meal [63-65]. Bursts were defined by ILI <1 s. Termination of a burst was defined by the onset of an ILI >1 s. Burst duration refers to the time spanned by a burst. Only bursts of three or more licks were considered.
2.6 Histology
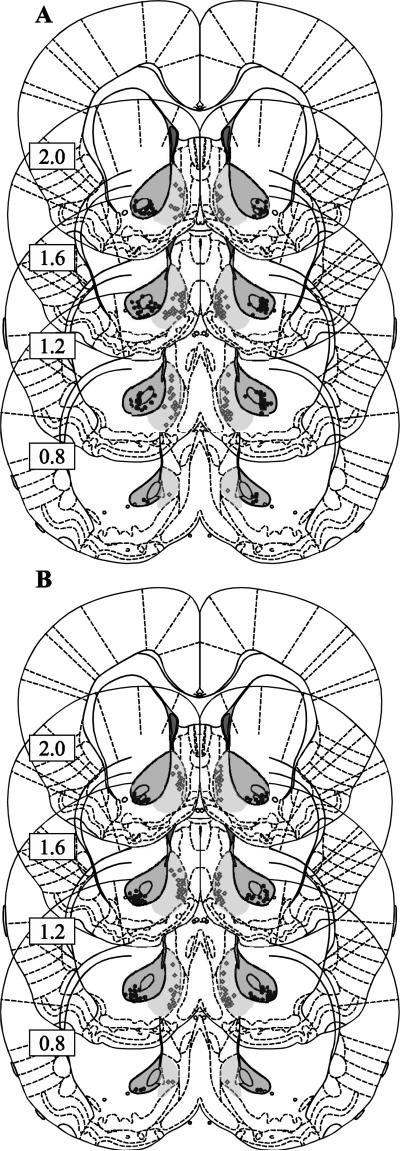
Animals were deeply anesthetized with pentobarbital and decapitated. Brains were removed and fixed in formaldehyde. They were then dehydrated in 30% sucrose, sectioned (50 μm), and stained for Nissl substance to locate injection sites. Injector tip locations are shown in Fig. 1.
Figure 1. Histology.
The placement of the tip of the cannula is represented by a black circle for the core and a grey diamond for the shell for the animals in the first experiment (DAMGO, naltrexone, SCH23390 and raclopride experiments) in A and the second experiment (CTAP, naltrindole, nor-BNI) in B. Dark shading indicates the region that was acceptable for core cannula locations, and light shading indicates the same for the shell. Rats with one or both placements outside these regions were rejected from the analysis.
3. Results
3.1 Subjects
Of the 233 rats used in this study, 214 were given intermittent access acquisition sessions (see Section 3.2) and 19 were not given acquisition sessions but instead used to test whether CTAP blocks the ability of DAMGO to increase consumption (see section 3.6 and Fig. 7). A total of 40 animals were removed from the analysis of pharmacological effects: 20 rats died or lost their caps before the end of the experiment; the cannulae were misplaced in 17 rats (3 directed towards the core); and 3 IA rats did not meet the criteria for binge-like consumption because they drank very little COS. Table 1 shows the pharmacological treatments given to each group and the final N (after removing animals) for each group.
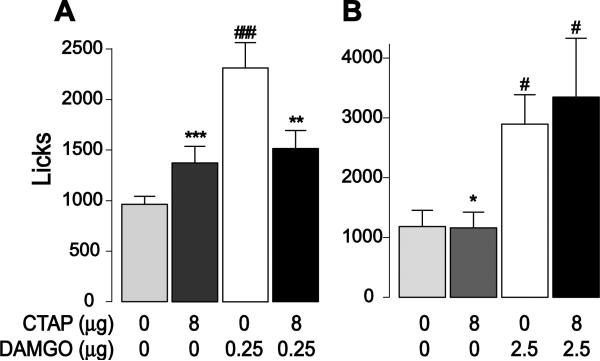
Figure 7. Effect of CTAP on DAMGO-induced COS consumption.
CTAP and DAMGO were injected in the NAc core alone or in combination. The graphs show the effects of CTAP injection (8 μg) on the increase in licking caused by 0.25 μg DAMGO (A) or 2.5 μg DAMGO (B).
#, ###: significant difference from saline injection (p < 0.05, 0.001).
*, **, ***: significant statistical difference from DAMGO injection group (p < 0.05, 0.01, 0.001).
3.2 Acquisition phase
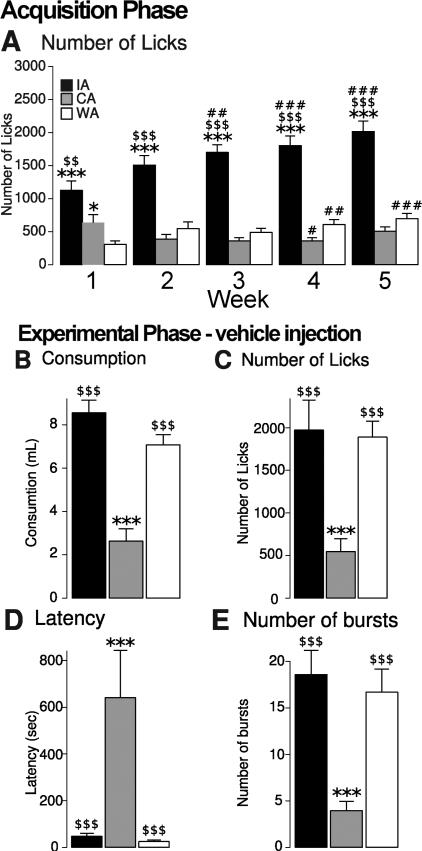
Our previous study, which used a subgroup (n=144) of the rats used in the present study (n=214), demonstrated that rats given MWF access to COS gradually escalate their COS consumption over 5 weeks of access [16]. The same effects as reported previously were observed in the superset used here: the IA group escalated its consumption whereas the CA group (which had access to COS not only in the operant chambers, but in their home cages as well) showed no escalation of COS consumption and the WA group (which received no COS, but instead 5 weeks of access to the operant chambers but with only water available) showed no change in water consumption (Fig. 2A). In addition, the CA group gained significantly more weight over the 5 weeks than the other groups, and the IA and WA groups’ weight gain was statistically indistinguishable from each other (IA: 137.9g ± 3.94, WA: 132.8g ± 5.2, CA: 196.8g ± 7.8; group effect between IA and WA: F(1,3989) = 0.82, p > 0.05).
Figure 2. training and control injections.
A: Number of licks per session (averaged across each week) during the training phase of the experiments. The IA (black) and CA (grey) groups licked for COS, whereas the WA (white) group licked for water.
B-E: COS consumption parameters during vehicle injection sessions: B, amount of COS consumed; C, number of licks during the session; D, latency to first lick; and E, number of bursts.
Symbols indicate results of post-hoc tests:
*, ***: significant difference from the WA group (p < 0.05 0.001).
$$, $$$: significant difference from the CA group (p < 0.01, 0.001).
#, ##, ###: significant difference from week 1 (p < 0.05, 0.01, 0.001).
3.3 Differences in COS licking among groups
To assess differences in COS licking across groups, we analyzed licking behavior for all rats after the vehicle injection, combining data across NAc core- and shell-injected rats. The amount of COS consumed and the number of licks in the 90 min session were greater in the IA and WA groups than in the CA group (group effect: licks: F(2,79) = 8.65, p < 0.001; consumption: F(2,79) = 37.9; p < 0.001), and these values did not significantly differ between IA and WA groups (Fig. 2B,C). The observation that IA consumption was not greater than WA consumption may seem at odds with the escalation of consumption observed in the IA group during the acquisition phase (Fig. 2A). A potential explanation is that because vehicle injections occurred at random time points during the experimental phase, some of them occurred after two or more weeks of COS access sessions – by which point the WA group's consumption could have escalated. However, this explanation is unlikely to be correct because when we compared COS consumption across WA rats in vehicle injection sessions, there was no effect of the position of the vehicle injection in the injection order (1 through 7) (order effect: F(7,60) = 0.503, p > 0.05). Thus, the majority of the escalation of consumption in the WA group is likely to have occurred in the first week of COS access (three M-W-F sessions of COS access given to all rats after surgery but before injections were performed); the escalation could have occurred more rapidly in the WA group than in the IA group because the WA group was already familiar with the experimental procedure when COS access sessions began, whereas the IA group was not.
The CA group exhibited a greater latency to begin licking than the other two groups (group effect: F(2,73) = 13.98, p < 0.001; Fig. 2D) and fewer lick bursts throughout the session (group effect: F(2,79) = 12.34, p < 0.001; Fig. 2E). Burst duration, in contrast, was statistically indistinguishable across the three groups [not shown, but see 16]. Because latency and burst number are reflective of motivation, these results suggest that motivation to consume COS was high in the IA and WA groups but low in the CA group, whereas palatability (as reflected by burst duration) was equivalent.
3.4 μ-opioid agonist: DAMGO
DAMGO injection into the core increased COS consumption and licks in all groups (Fig. 3). Because COS intake is initially high but rapidly declines to reach a low, steady rate (Fig. 3A,B), we quantified these effects separately for the first quarter and last three quarters. At low and intermediate doses (0.025 and 0.25 μg), the increase occurred at the beginning of the session (drug effect: F(3,80) = 9.0, p < 0.001) but not the end, as seen in licking time courses (Fig. 3A) and total COS consumption in the first quarter (Fig.3C) and last three quarters (Fig. 3D) of the session. In contrast, the high dose (2.5 μg) had no effect in the first quarter but dramatically increased consumption by causing a large second meal in the last three quarters (drug effect: F(3,80) = 39.72; p < 0.001; Fig. 3B-D). These effects of DAMGO on amount of COS consumed were largely similar across the three groups (Fig. 3C,D). Analysis of lick microstructure in the first quarter showed that low and intermediate DAMGO doses caused trends towards increased burst number in the IA and WA groups, whereas there were no effects in the CA group (drug effect: F(3,80) = 3.84, p < 0.05; group effect: F(2,80) = 10.49, p < 0.001; Fig. 3E). The high DAMGO dose dramatically increased burst number in all three groups (Fig. 3F). Burst duration was generally not affected by DAMGO in the first quarter; although some CA animals showed a large increase in burst duration after the low and intermediate dose, these effects were inconsistent and did not reach our threshold for a trend towards significance (drug effect: F(3,75) = 12.12, p < 0.001; group effect: F(2,75) = 2.28, p > 0.05; Fig. 3G). Burst duration in the last three quarters was not affected (drug effect: F(3,52) = 0.79, p > 0.05; group effect: F(2,52) = 0.08, p > 0.05; Fig. 3H). These results indicate that core DAMGO injection increases COS consumption primarily by increasing the number of lick bursts, not their duration. Moreover, the general similarity of DAMGO effects across groups means that COS access history does not alter sensitivity to core DAMGO's effects.
DAMGO injection in the shell increased COS consumption in the IA and WA groups, but this effect was consistent only at the highest dose and only in the last three quarters of the session (drug effect: F(3,67) = 15.47, p < 0.001; group effect: F(2,67) = 8.68, p < 0.001; interaction effect: F(6,67) = 2.53, p < 0.05; Fig. 4A-D). Lower doses increased licking at some time points (Fig. 4A). The increase in consumption in the last three quarters was accompanied by a trend towards an increase in number of bursts with no change in burst duration (burst number: drug effect: F(3,67) = 3.58, p < 0.05; group effect: F(2,67) = 4.53, p < 0.05; burst duration: drug effect: F(3,44) = 2.38, p > 0.05; group effect: F(2,44) = 2.28, p > 0.05; Fig. 4E-H). In contrast to the IA and WA groups, shell DAMGO injection in the CA group did not significantly increase the amount of COS consumed or lick rate at any of the time points examined (Fig. 4C,D); however, COS consumption in the CA group was unusually low in the control (vehicle injection) condition. These results indicate that shell DAMGO injections are generally less effective in increasing COS consumption than core injections.
3.5 General opioid antagonist: naltrexone
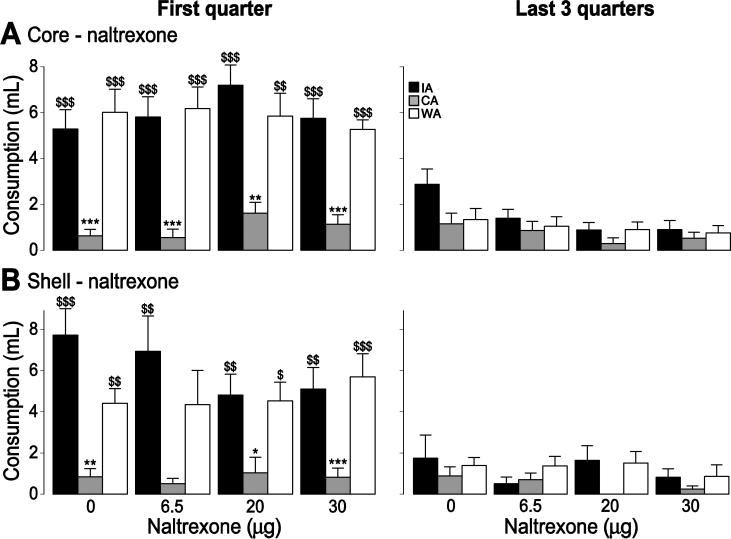
Despite the potency of intra-NAc μ agonist injection to promote food intake observed here and previously [32-39, 45], blockade of opioid receptors in the NAc using broad-spectrum antagonists (naltrexone or naloxone) typically reduces consumption of highly palatable food in ad libitum-fed animals only at very high doses [40-42]. To test the hypothesis that consumption becomes dependent on NAc opioids after a history of binge-like intake or obesity, we injected naltrexone into the NAc core or shell prior to COS access sessions. In no group did any dose of naltrexone affect COS consumption (core: drug effect: F(3,96) = 1.11, p > 0.05; drug x group effect: F(6,96) = 0.35, p > 0.05; shell: drug effect: F(3,72) = 0.93, P > 0.05; drug x group effect: F(6,72) = 0.81, p > 0.05; Fig. 5). Moreover, the number of lick bursts and burst duration were unaffected (not shown).
Figure 5. Effect of naltrexone o n COS consumption.
The effect of naltrexone injection in the core (A) and the shell (B) on COS consumption during the first quarter of the session (left) and the last 3 quarters of the session (right) for the IA (black), WA (white) and CA (grey) groups.
*, **, ***: significant difference from the WA group (p < 0.05, 0.01, 0.001).
$, $$, $$$: significant difference from the CA group (p < 0.05, 0.01, 0.001).
3.6 μ-opioid antagonist: CTAP
Although the ability of core injections of DAMGO to increase consumption suggests that μ receptor activation is sufficient to promote COS feeding behavior, the observation that naltrexone did not reduce consumption suggests that μ receptors are not necessary for this behavior. However, naltrexone blocks not only μ receptors, but other opioid receptors as well. Blockade of δ receptors can increase consumption [42], which could have masked an inhibitory effect of naltrexone. Therefore, we injected antagonists specific for μ, δ and κ opioid receptors to assess whether they differentially altered COS consumption. We performed these experiments only in IA and WA groups because we expected that at least one of these drugs would decrease consumption, an effect that would be difficult to observe in the CA group because of its low baseline consumption.
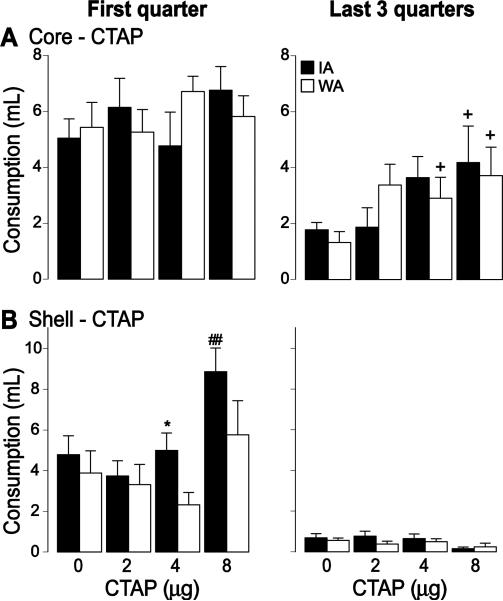
CTAP injection into the core had only minimal effect on COS consumption. Although there was a significant effect of drug (F(3,80) = 3.95, p < 0.05), in both groups, post-hoc tests showed only trends towards an increase, which was observed only in the last three quarters of the session (Fig. 6A). Injection of the highest dose of CTAP (8 μg) into the shell caused an increase in consumption in the first quarter in the IA group only (drug effect: F(3,64) = 6.06, p < 0.01; group effect: F(1,64) = 5.7, p < 0.05); there were no effects at lower doses, in the last three quarters, or in the WA group (Fig. 6B). The consumption increase in IA rats caused by the highest dose was accompanied by an increase in the number of lick bursts (drug effect: F(3,64) = 8.27, p < 0.001; group effect: F(1,64) = 6.72, p < 0.05), but not their duration (not shown). Thus, counter to our expectations, CTAP injections clearly did not inhibit COS consumption, but rather mildly stimulated it.
Figure 6. Effect of CTAP on COS consumption.
The effect of CTAP injection in the core (A) and the shell (B) on COS consumption during the first quarter of the session (left) and the last 3 quarters of the session (right) for the IA (black) WA (white) groups.
*: significant difference from the WA group (p < 0.05).
##: significant difference from control injection (p < 0.01); + trend to significance.
A potential explanation for CTAP's failure to reduce consumption is that it did not block μ receptors, although the doses we used are standard [44, 45, 66, 67]. As a positive control to test its efficacy, we determined whether CTAP (8 μg) antagonized the effects of the middle (0.25 μg) and high (2.5 μg, n=8) dose of DAMGO. Both drugs were injected in the NAc core. As expected, CTAP alone either had no effect or mildly increased the number of licks in the entire session, whereas both DAMGO doses potently increased licking (Fig. 7). CTAP blocked the increased licking caused by the 0.25 μg (drug effect: F(3,40) = 9.97, p < 0.001), but not the 2.5 μg dose of DAMGO (Fig. 7), indicating that, in our hands, CTAP effectively blocks μ receptors.
3.7 δ-opioid antagonist: naltrindole
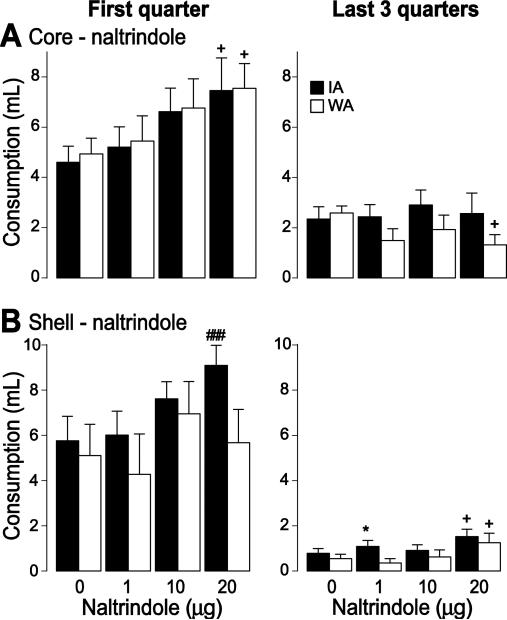
After core injection of the highest naltrindole dose (20 μg), COS intake tended to increase in the first quarter of the session in both groups, but this effect did not reach statistical significance, nor was it observed at lower doses (drug effect: F(3,84) = 3.39 p < 0.05). In the last three quarters after core injection, the high dose caused a trend towards a decrease in consumption in the WA group (drug effect: F(3,84) = 0.5, p > 0.05; Fig. 8A). After shell injection, the high dose caused a trend towards increased COS consumption in the IA group in the first quarter (drug effect: F(3,68) = 2.4, p = 0.08), and a trend towards an increase in both groups in the last three quarters (drug effect: F(3,68) = 2.88, p < 0.05; Fig. 8B). The small effects of naltrindole, the trend towards increased (not decreased) consumption, and the fact that the effects were limited to the highest dose together suggest that COS consumption is not dependent on NAc δ receptor activation.
Figure 8. Effect of naltrindole on COS consumption.
The effects of naltrindole injection in the core (A) and the shell (B) on COS consumption during the first quarter of the session (left) and the last 3 quarters of the session (right) for the IA (black) WA (white) groups.
*: significant statistical difference from the WA group (p < 0.05).
###: significant statistical difference from control injection (p < 0.001); + trend to significance.
3.8 κ-opioid antagonist: nor-BNI
Although there was a significant effect of nor-BNI dose injected in the NAc core on COS consumption in the first quarter (drug effect: F(3,83) = 2.98, p < 0.05), there were no significant post-hoc effects. Both IA and WA groups showed a trend towards increased consumption only after the highest dose (10 μg); no trends or effects were observed at lower doses or in the last three quarters of the session (drug effect: F(3,83) = 0.82, p > 0.05) (not shown). Shell injections of nor-BNI were without effect at any dose or time point (first quarter: drug effect: F(3,67) = 1.05, p > 0.05; last 3 quarter: drug effect: F(3,67) = 0.21, p > 0.05; not shown).
3.9 Dopamine receptor antagonists
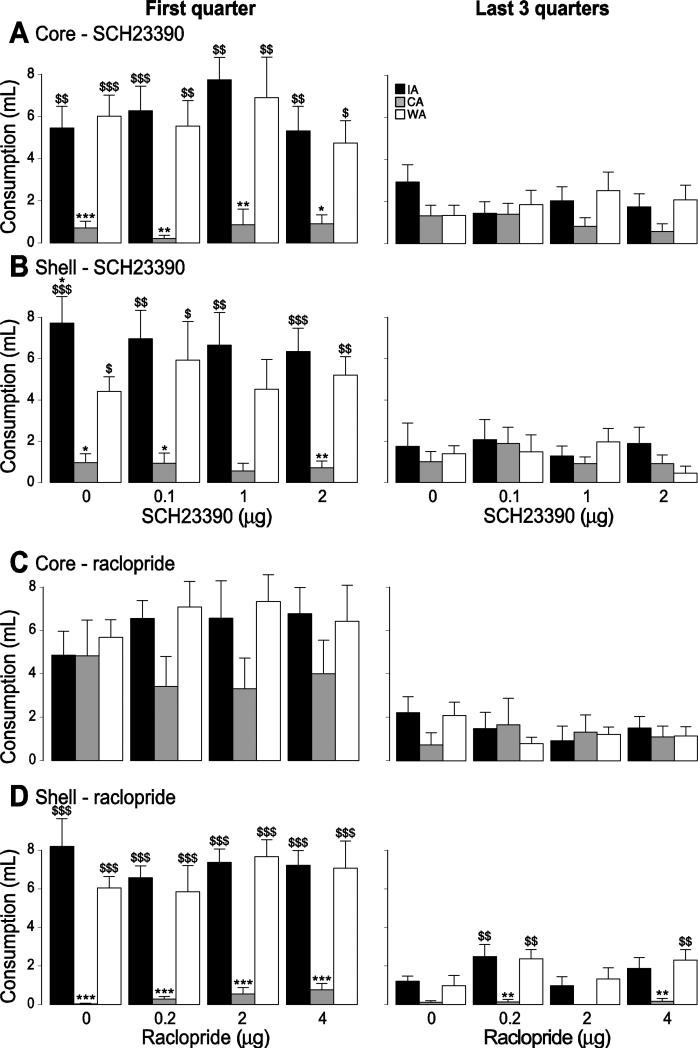
Free intake of solid or liquid food is generally not impaired by disruption of NAc dopamine function. However, both binge access to highly palatable food and access to a high-fat diet that results in obesity cause changes in dopamine neurotransmission in the striatum and NAc, suggesting that free intake may become NAc dopamine-dependent after a history of binge-like consumption or diet-induced obesity. To test this hypothesis, we injected the D1 receptor antagonist SCH23390 and the D2 receptor antagonist raclopride into the NAc core and shell prior to COS access sessions. None of these injections significantly impacted consumption in any of the three groups despite the use of doses high enough to severely impair reward-seeking behavior in several other tasks [61, 62] (core D1 antagonist: drug effect: F(3,85) = 1.2, drug x group effect: F(6,85) = 0.37; core D2 antagonist: drug effect: F(3,68) = 0.62, drug x group effect: F(6,68) = 0.14; shell D1 antagonist: drug effect: F(3,67) = 0.74, drug x group effect: F(6,67) = 0.21; shell D2 antagonist: drug effect: F(3,68) = 0.5, drug x group effect: F(6,68) = 0.54; all p values > 0.05; Fig. 9). Aside from a small increase in burst duration in the WA group caused by SCH23390 in the core (drug x group effect: F(6,68) = 2.30, p < 0.05 ) in the last three quarters of the session, no dose of either antagonist affected the number of lick bursts or burst duration (not shown).
Figure 9. Effect of dopamine antagonists on COS consumption.
The effect of the D1 antagonist SCH23390 (A,B) and the D2 antagonist raclopride (C,D) injection in the core (A,C) and the shell (B,D) on COS consumption during the first quarter of the session (left) and the last 3 quarters of the session (right) for the IA (black), WA (white) and CA (grey) groups.
*, **, ***: significant difference from the WA group (p < 0.05, 0.01, 0.001).
$, $$, $$$: significant difference from the CA group (p < 0.05, 0.01, 0.001).
4. DISCUSSION
Using pharmacological treatment of the NAc, we show here that neither opioid nor dopamine receptors in the core or shell are necessary for rats to express high levels of caloric intake during intermittent access to a sweet high-fat emulsion (COS). We interpret these results to mean that consumption of calorie-dense food is most likely not under the control of NAc opioid or dopamine receptors even after prolonged intermittent access has escalated consumption in a binge-like manner. Thus, although systemic injections of both opioid and dopamine receptor antagonists alter binge intake in similar behavioral paradigms [13, 18, 25-28], our results suggest that the effects of systemic injections are not due to actions on opioidergic and dopaminergic synaptic transmission within the NAc, but rather elsewhere in the brain. Furthermore, our findings impact the interpretation of earlier observations that binge access to calorie-dense food causes neurochemical and molecular changes related to NAc opioid and dopamine neurotransmission [7]. As described in more detail below, we suggest that these changes could influence behaviors other than free consumption.
Our dopamine antagonist results are consistent with many earlier studies showing that reduction of dopamine function within the NAc only minimally reduces consumption of freely available food, if at all: neither blockade of dopamine receptors in the NAc nor depletion of dopamine from the NAc using the toxin 6-hydroxydopamine causes food consumption to be reduced [54-60]. Our results add to this literature by showing that neither a D1 nor a D2 receptor antagonist injected in the NAc core or shell reduces consumption of COS: whereas most previous studies used standard chow, we find that NAc dopamine disruption is also ineffective when subjects consume an extremely palatable, sweet, very high fat liquid emulsion, indicating that consumption of such a food is not dependent on mesolimbic dopamine despite the supposedly greater “hedonic” experience of COS consumption over chow consumption. In contrast, when we inject the same dopamine antagonists into the NAc, particularly the core, at the same or similar doses as those used here, they cause profound impairment in a variety of reward-seeking tasks, including cued lever approach and high-effort fixed ratio tasks [61, 62, 68-71]. We conclude that food intake in the free-access consumption test is simply not dependent on mesolimbic dopamine whether or not the food is highly palatable, and whether or not subjects have had a history of intermittent binge-like access to it.
Similarly, our results suggest that even though activation of μ opioid receptors in the NAc is sufficient to promote consumption of COS, consumption is not dependent on these receptors even after a history of binge-like access. Injection of the μ agonist DAMGO increased COS consumption, particularly in the NAc core (Fig. 3) – an effect similar to the previously reported potent orexigenic effects of NAc μ agonist injection [32-37] which are especially pronounced when the food is calorie-dense, highly palatable, and/or high in fat [38, 39, 45]. Because the increases in COS consumption caused by DAMGO were similar across IA, WA and CA groups, it appears that neither a 5 week history of intermittent binge-like access to COS (IA group) nor 5 weeks of continuous access to COS (CA group, which became obese) increases the sensitivity of NAc μ opioid receptors such that activating them produces more COS consumption. This lack of difference among groups may have been due to a ceiling effect; however, consumption decreased dramatically in the first quarter of each access period even after DAMGO injection (Fig. 3A,B), indicating that total consumption could have been much higher had DAMGO been more effective in attenuating this decline.
Given DAMGO's potent orexigenic effects, it is somewhat surprising that neither the broad-spectrum opioid receptor antagonist naltrexone (Fig. 5) nor the μ-specific antagonist CTAP (Fig. 6) reduced COS consumption in either IA or WA groups. These results are not, however, without precedent. For instance, at low doses (5 – 10 μg/side), injection of naltrexone in the NAc reduced chow consumption in food-restricted rats [41], but to reduce sucrose consumption in ad libitumfed animals, much higher doses (20 – 50 μg/side naltrexone; 30 μg naloxone) were required [40-42]. When animals had a choice of high-carbohydrate and high-fat food, consumption of both was reduced by 20 μg naltrexone, but a dose-response function was not described [38]. Because these doses are far higher than the typical intra-NAc naltrexone doses (<10 μg) required to observe effects in other behavioral paradigms [41, 72-76], the high-dose effects may not be specific to opioid receptors. In the present study, doses up to 30 μg/side in either core or shell were ineffective in reducing COS consumption in any group, all of which were given chow ad libitum in the home cage. Injection of naltrexone doses up to 30 μg into the NAc did reduce performance in a different behavioral task conducted in our lab (K. Caref and SMN, in preparation). Thus, the reasons for the lack of effect of our highest dose on COS intake are unclear, but could be due to procedural differences from the previous consumption studies (e.g., specific food, intermittency of access, duration of access). The key observation, however, is that even when very high, likely non-specific naltrexone doses were tested, consumption in the IA group was not affected, suggesting that intermittent access binge-like consumption does not depend on NAc opioid receptors.
The μ-specific antagonist CTAP either had no effect on COS consumption or it produced a small, inconsistent increase, especially when injected in the NAc core (Fig. 6A). CTAP was likely to have blocked μ receptors in these experiments because co-injection of 8 μg CTAP with DAMGO effectively blocked DAMGO's potentiation of consumption (Fig. 7). Previous studies examining the effects of intra-NAc CTAP injection on consumption have produced conflicting results: although one study found (in rabbits) that CTAP reduced sucrose consumption [44], another (in rats) observed no effects of CTAP on fat consumption at doses that blocked DAMGO's potentiating effects [45]. Similarly, intra-NAc naloxonazine, a μ1 receptor-specific antagonist, potently increased deprivation-induced chow consumption in one study [41] but reduced it in another while also having no effect on sucrose intake in free-fed rats [42]. NAc injection of the long-lasting μ antagonist β-FNA appears to be more consistently effective in reducing palatable food intake [40-44] as well as deprivation-induced chow consumption [41, 42]. Notably, intra-NAc β-FNA spares intake of less palatable chow [43] and of sucrose at less preferred concentrations [40], suggesting that μ receptors may be required only for very high rates of consumption. However, this hypothesis is clearly not supported by our CTAP results in that consumption of COS – a highly palatable liquid that engenders very high rates of caloric intake – was not reduced by CTAP even in animals with a history of intermittent binge access to COS.
A reasonable interpretation of the existing literature and the present findings is that treatment of the rat NAc with β-FNA reduces palatable food consumption whereas CTAP is less effective, if at all. One possible explanation for the differential effectivity of these drugs is that each one blocks a different cohort of opioid (or perhaps other) receptors. Indeed, even at the lower end of the dose ranges used in microinjection studies, the concentrations of injected drug (typically high μM to low mM) far exceed the Ki for the target receptor (typically nM). Resolving the conflicting antagonist results will require more specific approaches, such as knockdown of receptors via viral expression of inhibitory RNAs. In the meantime, our results suggest that the hypothesis that NAc μ receptors are required for palatable or high-rate food intake is not correct in its simplest form: we propose that blockade of more than one type of receptor is likely required to reduce intake.
Endogenous opioids are notoriously promiscuous in that multiple peptide splice variants derived from preprodynorphin and preproenkephalin mRNA are present in the striatum [77], and many of these opioid peptides bind with high affinity to multiple opioid receptors [78]. Therefore, we tested whether NAc injection of the δ receptor- and κ receptor-specific antagonists naltrindole and nor-BNI, respectively, reduced COS consumption. Consistent with prior observations [41, 42], nor-BNI had no discernible effect, and naltrindole caused a trend towards increased COS consumption that was similar in IA and WA groups (Fig. 8). These results argue that alterations in neither δ nor κ receptor expression or sensitivity underlie consumption in animals with a history of intermittent binge-like access to COS.
In sum, our results indicate that a 5 week history of intermittent binge access to COS does not cause consumption to become dependent on either dopamine or opioid receptors in the NAc core or shell. This interpretation could be criticized based on the observation that in the control (vehicle injection) condition, the IA group did not consume more COS than the WA group, which had only 1 week of COS access sessions prior to the injection sessions. This lack of difference calls into question whether the IA group was, in fact, bingeing on COS by the end of their 5 weeks of intermittent access. However, the IA group did escalate its consumption substantially across this period (Fig. 2A); as discussed in detail previously, this escalated consumption meets an operational definition of binge intake [16]. In fact, the absence of a difference between the IA and WA groups’ control sessions is explained not by an absence of escalation in the IA group, but by unusually high consumption in the WA group from their first week of COS access. Several explanations for their high intake are possible: for instance, in contrast to the IA group, the WA group's first access to COS occurred shortly after surgery, and these rats were already very familiar with the access environment as a safe place. Thus, although we cannot rule out the possibility that the absence of antagonist effects in the WA group is a consequence of their unusually high rates of consumption, our results generally parallel earlier observations in non-bingeing rats. Most importantly, the high consumption of the WA group does not detract from our main conclusion: that the escalated COS consumption resulting from a history of intermittent access in the IA group is not dependent on NAc opioid or dopamine receptors.
Systemic treatment with opioid or dopamine receptor antagonists reduces consumption in animals with a prior history of intermittent binge access [13, 18, 25-28] and opioid antagonists reduce food intake in non-bingeing animals as well [reviewed in 2]. Our results suggest that the antagonists produce their anorectic effects not by actions within the NAc, but elsewhere.
Candidates include the central nucleus of the amygdala and hypothalamic paraventricular nucleus, where relatively low dose (3 – 10 μg) naltrexone infusions reduce palatable food intake [79, 80]; and the dorsal striatum, where dopamine depletion reduces food intake [81, 82] unlike in the NAc [57]. We propose that the NAc could play one or more roles in binge consumption that are not captured by examining the effects of dopamine or opioid receptor-active drugs on consumption of freely available food. One possibility derives from observations that pharmacological inhibition of the NAc potently increases consumption independent of palatability [83-91] whereas electrical stimulation of the NAc induces a pause in consumption [92, 93]. These results suggest that the activity of some NAc projection neurons, perhaps those that are inhibited just prior to and during consumption [93-96], exerts a tonic inhibitory influence on consumption. This consumption-inhibitory activity could, in theory, be attenuated during binge episodes via a mechanism independent of opioids or dopamine; however, there is no direct evidence to support this hypothesis.
A second possibility is that NAc opioid and/or dopamine receptors contribute to binge intake, but the behavioral paradigm we used to measure intake was incapable of capturing their contributions. Intriguingly, in an operant limited daily access binge model, low-dose naltrexone treatment of the NAc shell reduced the breakpoint of animals on a progressive ratio schedule of palatable food reinforcement, whereas higher doses were required to reduce lower effort fixed ratio 1 operant performance [72]. These results parallel findings that NAc dopamine depletion or dopamine receptor blockade are far more effective in reducing high-effort operant performance than both FR1 responding and free feeding [61, 97-102]. Indeed, the specific behavior during high-effort task performance that is NAc dopamine-dependent is approach to the operandum from variable starting locations [61, 71, 103], a behavior that, in sated animals, may also be dependent on μ opioid receptor activation in the NAc [104]. Thus, NAc dopamine and opioid receptors contribute to a specific form of food-seeking behavior that is not engaged by the free access paradigm, perhaps explaining the absence of effects of antagonists of these receptors in our study.
We propose that the degree to which NAc dopamine and opioid systems contribute to binge eating in both humans and animal models is a function of the dependence of disordered eating on the specific food-seeking behaviors that these systems control. Animal studies have revealed changes in expression of genes related to dopamine and opioid neurotransmission in the NAc of binge-eating subjects [29-31, 46] as well as neurochemical evidence for enhanced dopamine release during binges [8, 10, 47]; PET imaging studies in humans further support the hypothesis that dopaminergic synaptic transmission is abnormal in patients with eating disorders [105, 106]. These changes may contribute to some aspect of binge eating – perhaps enhanced palatable food-seeking behavior and hence greater frequency of binges – but our results suggest that this contribution cannot be easily detected with simple free-access consumption tests. More elaborate food-seeking tasks, including but not necessarily limited to those used previously [61, 72, 107] will be required to determine how NAc dopamine and opioids contribute to binge-like behavior.
5. Acknowledgements
This work was supported by NIH grants (MH092757, DA019473 and DA038412), NARSAD Young Investigator awards and Klarman Family Foundation awards to SMN; and by a Davis Foundation postdoctoral fellowship to SL.
Abbreviations
- NAc
nucleus accumbens
- COS
cream, oil, and sugar emulsion
- B-FNA
β-funaltrexamine
- nor-BNI
nor-binaltorphimine
- DAMGO
[D-Ala2,N-MePhe4,Gly-ol]-enkephalin
- CTAP
D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2
- M-W-F
Monday-Wednesday-Friday
- IA
intermittent access group
- CA
continuous access group
- WA
water access group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52:545–53. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taha SA. Preference or fat? Revisiting opioid effects on food intake. Physiol Behav. 2010;100:429–37. doi: 10.1016/j.physbeh.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, et al. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–5. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- 4.Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–12. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- 5.McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, et al. A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int J Eat Disord. 2013;46:239–45. doi: 10.1002/eat.22114. [DOI] [PubMed] [Google Scholar]
- 6.Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR, et al. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry. 2013;73:887–94. doi: 10.1016/j.biopsych.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–20. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–74. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Buda-Levin A, Wojnicki FH, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86:176–84. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 13.Rao RE, Wojnicki FH, Coupland J, Ghosh S, Corwin RL. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol Biochem Behav. 2008;89:581–90. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojnicki FH, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiol Behav. 2008;94:627–9. doi: 10.1016/j.physbeh.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with limited access to a sweet-fat diet. Obesity (Silver Spring) 2008;16:1998–2002. doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- 16.Lardeux S, Kim JJ, Nicola SM. Intermittent access to sweet high-fat liquid induces increased palatability and motivation to consume in a rat model of binge consumption. Physiol Behav. 2013;114-115:21–31. doi: 10.1016/j.physbeh.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berner LA, Bocarsly ME, Hoebel BG, Avena NM. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav Pharmacol. 2009 doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong KJ, Wojnicki FH, Corwin RL. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92:528–36. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–8. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–30. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Nathan PJ, Bullmore ET. From taste hedonics to motivational drive: central mu-opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol. 2009:1–14. doi: 10.1017/S146114570900039X. [DOI] [PubMed] [Google Scholar]
- 24.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- 25.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–14. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano C, Robbins TW, Nathan PJ, Bullmore ET, Everitt BJ. Inhibition of opioid transmission at the mu-opioid receptor prevents both food seeking and binge-like eating. Neuropsychopharmacology. 2012;37:2643–52. doi: 10.1038/npp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–48. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- 28.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–35. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 29.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 30.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–8. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 31.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–60. [PubMed] [Google Scholar]
- 33.Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993;111:207–14. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 35.Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–24. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- 36.Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 37.Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–14. [PubMed] [Google Scholar]
- 39.Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–60. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- 40.Katsuura Y, Taha SA. Mu opioid receptor antagonism in the nucleus accumbens shell blocks consumption of a preferred sucrose solution in an anticipatory contrast paradigm. Neuroscience. 2014;261:144–52. doi: 10.1016/j.neuroscience.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–12. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- 42.Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–507. [PubMed] [Google Scholar]
- 43.Lenard NR, Zheng H, Berthoud HR. Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rats. Int J Obes (Lond) 2010;34:1001–10. doi: 10.1038/ijo.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–13. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- 45.Katsuura Y, Heckmann JA, Taha SA. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am J Physiol. 2011;301:R244–54. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1260–8. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- 47.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156:865–71. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–97. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 49.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 50.Foley KA, Fudge MA, Kavaliers M, Ossenkopp KP. Quinpirole-induced behavioral sensitization is enhanced by prior scheduled exposure to sucrose: A multi-variable examination of locomotor activity. Behav Brain Res. 2006;167:49–56. doi: 10.1016/j.bbr.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74:635–9. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 53.Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010;97:25–33. doi: 10.1016/j.pbb.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakshi VP, Kelley AE. Dopaminergic regulation of feeding behavior. I. Differential effects of haloperidol infusion into three striatal subregions. Psychobiology. 1991;19:223–32. [Google Scholar]
- 55.Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, et al. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin's effect on food reward but not food intake. Neuropharmacology. 2013;73:274–83. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–77. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 57.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–27. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 58.Salamone JD, Kurth PA, McCullough LD, Sokolowski JD, Cousins MS. The role of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on the local rate of instrumental responding. Brain Res. 1993;628:218–26. doi: 10.1016/0006-8993(93)90958-p. [DOI] [PubMed] [Google Scholar]
- 59.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 60.Yun IA, Nicola SM, Fields HL. Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci. 2004;20:249–63. doi: 10.1111/j.1460-9568.2004.03476.x. [DOI] [PubMed] [Google Scholar]
- 61.Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–33. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- 64.Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–94. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- 65.Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009;161:718–33. doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of mu-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–10. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perry CJ, McNally GP. mu-Opioid receptors in the nucleus accumbens shell mediate context-induced reinstatement (renewal) but not primed reinstatement of extinguished alcohol seeking. Behav Neurosci. 2013;127:535–43. doi: 10.1037/a0032981. [DOI] [PubMed] [Google Scholar]
- 68.Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res. 2004;154:19–30. doi: 10.1016/j.bbr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Nicola SM, Taha SA, Kim SW, Fields HL. Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience. 2005;135:1025–33. doi: 10.1016/j.neuroscience.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 70.Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–61. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.du Hoffmann J, Nicola SM. Dopamine invigorates reward seeking by promoting cue-evoked excitation in the nucleus accumbens. J Neurosci. 2014;34:14349–64. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blasio A, Steardo L, Sabino V, Cottone P. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol. 2013 doi: 10.1111/adb.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides. 2005;26:621–9. doi: 10.1016/j.peptides.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 74.MacDonald CJ, Cheng RK, Williams CL, Meck WH. Combined organizational and activational effects of short and long photoperiods on spatial and temporal memory in rats. Behav Processes. 2007;74:226–33. doi: 10.1016/j.beproc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Vaccarino FJ, Corrigall WA. Effects of opiate antagonist treatment into either the periaqueductal grey or nucleus accumbens on heroin-induced locomotor activation. Brain Res Bull. 1987;19:545–9. doi: 10.1016/0361-9230(87)90071-2. [DOI] [PubMed] [Google Scholar]
- 76.Varaschin RK, Wazlawik E, Morato GS. Systemic and intra-accumbens microinjections of naltrexone interfere with tolerance to ethanol in rats. Psychopharmacology (Berl) 2005;182:366–74. doi: 10.1007/s00213-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 77.Decaillot FM, Che FY, Fricker LD, Devi LA. Peptidomics of Cpefat/fat mouse hypothalamus and striatum: effect of chronic morphine administration. J Mol Neurosci. 2006;28:277–84. doi: 10.1385/JMN:28:3:277. [DOI] [PubMed] [Google Scholar]
- 78.Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- 79.Glass MJ, Billington CJ, Levine AS. Naltrexone administered to central nucleus of amygdala or PVN: neural dissociation of diet and energy. Am J Physiol Regul Integr Comp Physiol. 2000;279:R86–92. doi: 10.1152/ajpregu.2000.279.1.R86. [DOI] [PubMed] [Google Scholar]
- 80.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- 81.Marshall JF, Richardson JS, Teitelbaum P. Nigrostriatal bundle damage and the lateral hypothalamic syndrome. J Comp Physiol Psychol. 1974;87:808–30. doi: 10.1037/h0037223. [DOI] [PubMed] [Google Scholar]
- 82.Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc Natl Acad Sci U S A. 2006;103:8858–63. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–36. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 84.Kelley AE, Swanson CJ. Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: a microinfusion mapping study. Behav Brain Res. 1997;89:107–13. doi: 10.1016/s0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 85.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newman S, Pascal L, Sadeghian K, Baldo BA. Sweetened-fat intake sensitizes gamma-aminobutyric acid-mediated feeding responses elicited from the nucleus accumbens shell. Biol Psychiatry. 2013;73:843–50. doi: 10.1016/j.biopsych.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–70. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soderpalm AH, Berridge KC. Food intake after diazepam, morphine or muscimol: microinjections In the nucleus accumbens shell. Pharmacol Biochem Behav. 2000;66:429–34. doi: 10.1016/s0091-3057(00)00220-3. [DOI] [PubMed] [Google Scholar]
- 89.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res. 1998;93:43–50. doi: 10.1016/s0166-4328(97)00140-x. [DOI] [PubMed] [Google Scholar]
- 91.Ward BO, Somerville EM, Clifton PG. Intraaccumbens baclofen selectively enhances feeding behavior in the rat. Physiol Behav. 2000;68:463–8. doi: 10.1016/s0031-9384(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 92.Halpern CH, Tekriwal A, Santollo J, Keating JG, Wolf JA, Daniels D, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33:7122–9. doi: 10.1523/JNEUROSCI.3237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci. 2010;30:4746–56. doi: 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol. 2004;91:1866–82. doi: 10.1152/jn.00658.2003. [DOI] [PubMed] [Google Scholar]
- 95.Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–97. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 96.Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25:1193–202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–52. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 98.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–8. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 99.Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav. 1999;64:21–7. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 100.Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–82. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 101.Salamone J, Aberman J, Sokolowski JD, Cousins MS. Nucleus accumbens dopamine and rate of responding: neurochemical and behavioral studies. Psychobiology. 1999;27:236–47. [Google Scholar]
- 102.Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–70. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 103.McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward-seeking by cue and proximity encoding in the nucleus accumbens. Neuron. 2013;78:910–22. doi: 10.1016/j.neuron.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caref K, Nicola SM. Nucleus accumbens opioids drive conditioned approach to high calorie reward only in the absence of homeostatic drive.. Annual Meeting of the Society for Neuroscience; Washington, DC. 2014. [Google Scholar]
- 105.Broft A, Shingleton R, Kaufman J, Liu F, Kumar D, Slifstein M, et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int J Eat Disord. 2012;45:648–56. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–8. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smith KL, Rao RR, Velazquez-Sanchez C, Valenza M, Giuliano C, Everitt BJ, et al. The Uncompetitive N-methyl-D-Aspartate Antagonist Memantine Reduces Binge-Like Eating, Food-Seeking Behavior, and Compulsive Eating: Role of the Nucleus Accumbens Shell. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]