Abstract
In this work, we compared the use of repeated cycles of centrifugation at conventional speeds for enrichment of exosomes from human serum compared to the use of ultracentrifugation. After removal of cells and cell debris, a speed of 110,000×g or 40,000×g was used for the ultracentrifugation or centrifugation enrichment process, respectively. The enriched exosomes were analyzed using the BCA assay, 1-D gel separation, transmission electron microscopy, Western blotting, and high resolution LC-MS/MS analysis. It was found that a five cycle repetition of ultracentrifugation or centrifugation is necessary for successful removal of non-exosomal proteins in the enrichment of exosomes from human serum. More significantly, 5×centrifugation enrichment was found to provide similar or better performance than 5×ultracentrifugation enrichment in terms of enriched exosome protein amount, Western blot band intensity for detection of CD-63 and numbers of identified exosome-related proteins and CD proteins. A total of 478 proteins were identified in the LC-MS/MS analyses of exosome proteins obtained from 5×ultracentrifugations and 5×centrifugations including many important CD membrane proteins. The presence of previously reported exosome-related proteins including key exosome protein markers demonstrates the utility of this method for analysis of proteins in human serum.
Keywords: Exosomes, mass spectrometry, human serum, ultracentrifugation, centrifugation
1. Introduction
Exosomes are small endosomal-derived membrane microvesicles (~30–100 nm in diameter) secreted by most cell types. Exosomes are found in many biological fluids, such as blood [1, 2], urine [3–7], saliva [8, 9], and breast milk [10]. Exosomes have received much attention recently since exosomes are believed to have important roles in intercellular communications [11]. There are several recent review papers in the literature providing an overview of the current status of exosome research [12–16], among which Simpson and his coworkers [12] provided proteomic insights and diagnostic potentials of exosomes and reported twenty four proteins commonly identified in most exosome studies.
The most common exosome enrichment method involves using ultracentrifugation at high speeds such as 110,000×g. Prior to the ultracentrifugation, whole cells and large cell debris are removed by low speed centrifugations or by filtration using a 0.22 µm filter. Ultracentrifugation is performed one [17, 18], two [19–22], or three times [23], where the supernatant is removed followed by addition of a buffer solution after each ultracentrifugation. After the initial ultracentrifugation, density-gradient ultracentrifugation using sucrose [22] or iodixanol [2, 23] is often applied to improve the purity of exosomes. Ultracentrifugation on a sucrose cushion has also been performed to isolate exosomes [24, 25].
There are several commercially available kits used to enrich exosomes such as the Total Exosome Isolation kit (Life Technologies) [26], ExoQuick (System Bioscience) [10, 21, 27], Exo-spin (Cell Guidance System) [28], and PureEXO (101Bio). An immuno-affinity pull-down method can also be performed where an exosome-specific antibody is used to selectively enrich exosomes [7]. Recently, two types of antibodies on photosensitizer-beads were utilized to perform a rapid and sensitive detection of extracellular vesicles including exosomes [29]. A filtration device has also been used to enrich exosomes where ultrafiltration devices with 10,000 Da MW cut-off membranes were used [30]. Field-free Fractionation [31] or size-exclusion chromatography where particles are separated based on their size has also been applied to enrich exosomes.
A combination of two or more enrichment methods has often been used to isolate exosomes with varying degrees of success. These may include ultracentrifugation with ExoQuick precipitation [10], size exclusion chromatography with immuno-affinity [32], filtration using a 100,000 MW cut-off filter with ultracentrifugation [33, 34], filtration using a 100,000 MW cut-off filter with the application of a commercial enrichment kit [35], filtration using 100,000 MW cut-off filter with immuno-affinity and ultracentrifugation [18], filtration using 100,000 MW cut-off filter with sucrose density-gradient ultracentrifugation, or ultracentrifugation followed by a commercial kit [36]. However, exosome enrichment from human serum using ultracentrifugation or a commercial kit often suffers from impurities [24, 25], due to the presence of high abundant proteins such as albumin and immunoglobulin G in human serum. Although density gradient ultracentrifugation is often used to improve the purity, it is a relatively long (~18 h) process [2, 10].
Recently, three different isolation methods (density gradient (DG), ultracentrifugation (UC), EpCAM-based immunoaffinity pull-down (EI)) were compared for isolating exosomes from normal human plasma, where a total of 213 exosome proteins were identified [2]. The authors mentioned that DG was superior in isolating pure exosomes since it successfully removed highly abundant plasma proteins compared to the other two techniques. The LC-MS/MS analyses revealed 148 (69.5%), 78 (36.6%), and 39 (18.3%) exosome proteins from DG, UC, and EI, respectively.
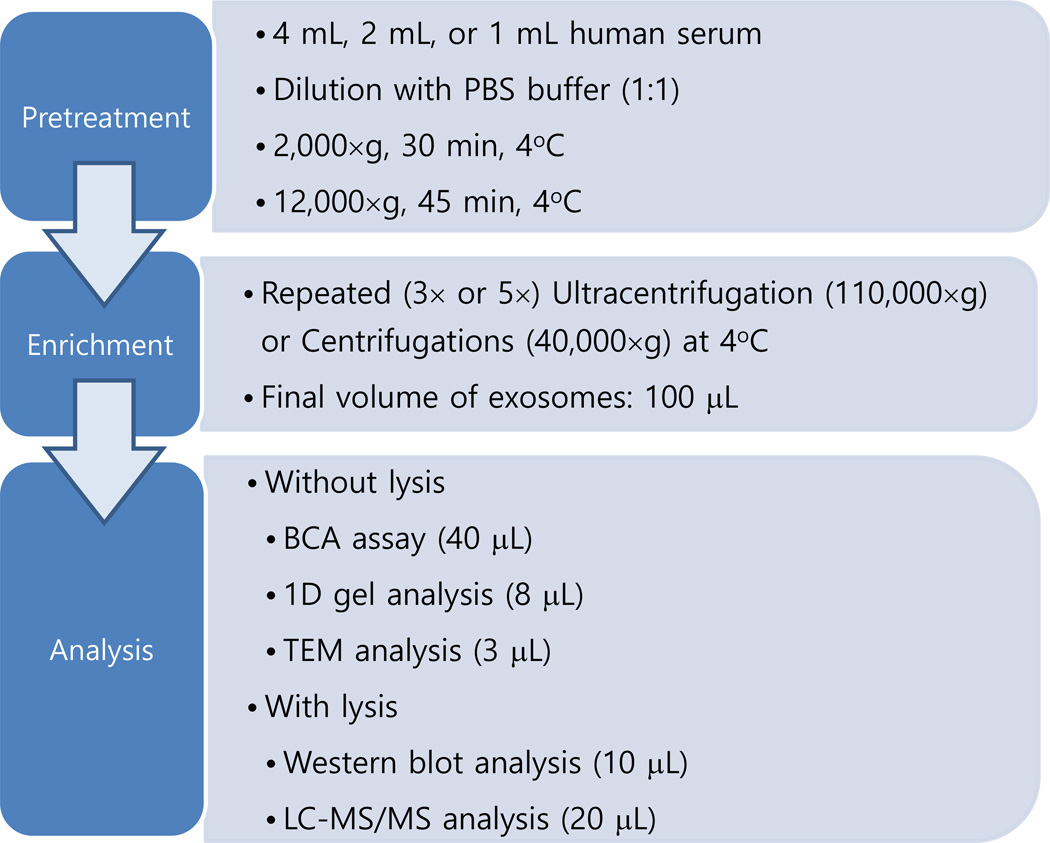
In the current study, we have explored the application of centrifugation at a speed of 40,000×g which is much more affordable and accessible to most scientists than that of ultracentrifugation and whether it can enrich exosomes from a human serum sample comparable to that obtained by ultracentrifugation. Centrifugation at 40,000×g was applied three and five times to study the effect of multiple cycles of centrifugation. The results from centrifugation were compared with those from conventional ultracentrifugation based on several methods including 1-D gel analysis, TEM, Western blotting and LC-MS/MS analysis on a high resolution Orbitrap mass spectrometer (see Scheme 1). It was found that centrifugation at 40,000×g could provide comparable or improved results relative to ultracentrifugation by using multiple cycles of centrifugation. The current results show that the exosome enrichment can be successfully achieved in a rather inexpensive centrifuge instrument.
Scheme 1.
Summary of the current investigation
2. Materials and Methods
2.1. Materials
Pooled normal human serum samples were obtained from Innovative Research (Novi, MI, USA). Anti-CD63 antibody (ab59479, Mouse monoclonal to CD63), Goat Anti-Mouse IgG H&L (HRP) preadsorbed (ab9704) were from Abcam (Cambridge, MA). Phosphate buffered Saline (PBS) (P-5368) was obtained from Sigma-Aldrich (St Louis, MO).
2.2. Exosome enrichment - Pretreatment of serum
The serum sample was first diluted with an equal volume of PBS buffer solution to decrease viscosity. The diluted serum sample was then centrifuged at 2000×g for 30 min at 4°C. The supernatant was transferred into 1-mL tubes and centrifuged at 12,000×g for 45 min at 4°C. The supernatant was filtered through a 0.22 µm filter to remove any remaining cell particles or cell debris.
2.3. Exosome enrichment - Ultracentrifugation
Ultracentrifugation was performed using a Beckman Optima XL-70 Ultracentrifuge with a speed of 110,000×g at 4°C for 120 min (the first ultracentrifugation step) or 70 min (the subsequent ultracentrifugation steps). Ultra-Clear™ tubes (catalog number: 344057, from Beckman Coulter) were used with a SW 55 Ti rotor in the process of ultracentrifugation. The total volume of each tube was limited to 4.0 mL to avoid any overflowing or contamination during sample preparation steps.
Five consecutive ultracentrifugation steps were performed to improve the purity of exosomes obtained. For the exosomes obtained starting from 4.0 mL serum (corresponding to 8.0 mL of 2×diluted serum), the two pellets were combined after the first ultracentrifugation. For the exosomes obtained starting from 1.0 mL serum (2.0 mL of 2×diluted serum), 2.0 mL PBS buffer was added to the tube containing 2.0 mL of 2×diluted serum prior to the first ultracentrifugation step. After each ultracentrifugation step, supernatant was removed, followed by addition of 4 mL PBS buffer. After the fifth ultracentrifugation step, the pellet was resuspended in 100 µL PBS buffer after the supernatant was removed.
2.4. Exosome enrichment - Centrifugation
Centrifugation to enrich exosomes was performed using a Sorvall Stratos Centrifuge from Thermo at a speed of 40,000×g (20,762 rpm) at 4°C for 120 min (first run) or 70 min (for subsequent runs) with a microcentrifuge tube (Axygen MCT-175-L-C) from Axygen.
For exosomes prepared from 1.0 mL serum (2.0 mL of 2×diluted serum), two tubes were used where each tube contains 1.0 mL of 2×diluted serum. For exosomes prepared from 2.0 mL serum, three tubes were used where each tube contains 1.33 mL of 2×diluted serum. For exosomes prepared from 4.0 mL serum, six tubes were used where each tube contains 1.33 mL of 2×diluted serum. After the first ultracentrifugation, the pellets were combined. After each centrifugation, 1.2 mL PBS buffer was added to the pellet after removing the supernatant. After the fifth centrifugation step, the pellet was resuspended in 100 µL PBS buffer.
2.5. Quantitation and gel analysis
The quantity of proteins in the supernatants and final pellet was determined using the BCA Assay (Pierce Biotechnology, Pittsburgh, PA), where two of 20 µL for each sample was used. For the 1-D gel analysis, sample (8 µL) was mixed with a lane marker non-reducing sample buffer (5×) from Thermo Scientific Pierce (catalog number: PI-39001), followed by incubation at 70°C for 10 min. Electrophoresis was then performed on a mini-protean TGX precast gel (Bio-Rad) at 90 V for 10 min, followed by 200 V for 25 min. The gel was stained with the Sigma silver staining kit following the manufacturer’s instruction.
2.6. Transmission Electron Microscopy (TEM) analysis
Carbon film (CFTH200-Cu) was obtained from Electron Microscopy Sciences (Hatfield, PA, USA). Glow discharge on the carbon film was performed to make the surface of the carbon film hydrophilic. The sample (3 µL) was then loaded on the carbon film and incubated for 2 min. After removing the supernatant liquid by absorbing it using filter paper, 5 µL of 2.5% (w/v) glutaldehyde in PBS was loaded for the fixation of the exosomes. After 5 min incubation, the supernatant liquid was removed and the carbon film was washed with water 3 times. After removing the last water, the film was stained with 5 µL of 1% uranyl acetate for 1 min. The TEM image was obtained using a CM-100 TEM instrument from Philips.
2.7. Lysis and Western blotting
The lysis of the enriched exosomes involved incubation of the exosomes at 4°C for 30 min in a 1:1 ratio with a 2×RIPA buffer. The 2×RIPA buffer solution was composed of 100 mM TrisHCl, 300 mM NaCl, 2.0% NP-40 (USBiological), 1.0% sodium deoxychlorate, 0.2% SDS, 1 mM EDTA, and protease inhibitors (cOmplete, EDTA-free Protease Inhibitor Cocktail Tablets, Roche).
For Western blot analysis, the lysed exosome proteins (20 µL each) were separated on a gel as described above and transferred onto a PVDF membrane (catalog number: 162-0177, Bio-Rad). Blots were then first incubated in PBS blocking buffer containing 5% milk for 1 hr at room temperature and then with primary mouse anti-CD63 (catalog number: ab59479, Abcam) diluted in a 1:500 ratio in PBST (0.1% Tween 20 in PBS buffer solution) overnight at 4°C. The blots were then washed three times with PBST and incubated with secondary goat anti-mouse IgG H&L (HRP) preadsorbed (ab97040, Abcam) in PBST (1:1000 dilution) and visualized by incubating sections with 3,3-diaminobenzidine tetrahydrochloride (ImmPACT DAB peroxidase substrate; Vector Laboratories, Burlingame, CA, USA)
2.8. Tryptic digestion
Following the lysis of the exosome samples, the FASP method was used to perform tryptic digestion. The lysed sample was reduced with 100 mM dithiothreitol for 10 min at 70°C. The solution was allowed to cool down and then was mixed with 200 µL of 8 M urea in 100 mM TrisHCl (pH 8.5), transferred to a centrifugal spin filter with a MW cutoff of 30 kDa (YM-30, Millipore), and centrifuged for 15 min at 14,000×g. The same centrifugation conditions were used for the following steps: The sample was washed again with 200 µL of the urea buffer. Alkylation was performed by adding 100 µL of 50 mM iodoacetamide (IAA) in the urea buffer, followed by vortexing for 1 min and incubation for 20 min in the dark at room temperature. To remove the remaining IAA, the protein mixture was centrifuged and washed twice with the urea buffer. The sample was washed three times with 100 µL of 50 mM ammonium bicarbonate. Then, tryptic digestion was performed overnight at 37 °C by adding trypsin (Sequencing grade modified, Promega) in a 1:20 ratio (w/w). Digested peptides were collected by centrifugation with 40 µL of 50 mM ammonium bicarbonate three times. After tryptic digestion, the samples were desalted using Thermo Scientific Pierce C18 Spin Columns before LC-MS/MS analysis.
2.9. LC-MS/MS analysis
The samples were analyzed in duplicate. For each LC-MS/MS analysis, ~0.5 µg exosome proteins were used. Peptide mixtures dissolved in 0.5% formic acid (FA) were loaded onto a Proxeon Easy-nLC II system (Thermo) with a flow rate of 400 nL/min. The samples were first desalted on a reversed-phase trap column (100 µm × 20 mm, C18AQ particles, 5µm, 200Å, Michrom Bioresources, Auburn, CA) and separated on a C18 analytical column (75µm × 250 mm, C18AQ particles, 5 µm, 200 Å) coupled to an Orbitrap Elite mass spectrometer (ThermoFisher Scientific, San Jose, CA). Peptides were separated with 0.1% FA in water (solvent A) and 0.1% FA in acetonitrile (solvent B) using a 70 min linear gradient from 5 to 35% solvent B at a flow rate of 400 nL/min.
The mass spectrometer was operated in positive ion mode with an electrospray voltage of +2.5 kV and a capillary temperature of 300°C. Full scan mass spectra were acquired from m/z 400.0–1800.0 in the Orbitrap analyzer with a resolution R = 120000, followed by HCD MS/MS scans with resolution R = 15000 on the top 15 most intense ions. The isolation width was set to 1.5 and the normalized collision energy was 35.0%. Dynamic exclusion was enabled with a ±10 ppm exclusion window with a repeat count of 1 using an exclusion duration of 30 s.
All MS/MS spectra were searched against the human Uniprot database (downloaded June, 2014) containing 26,152 entries using SEQUEST (Proteome Discoverer 1.4, Thermo Fisher Scientific). The search parameters were as follows: (1) static carbamidomethylation of cysteine residues (+57.021 on Cys); (2) dynamic oxidation of methionine residues (+15.995 on Met); (3) allowing two missed cleavages; (4) peptide ion mass tolerance 10 ppm (Isotopic MW); (5) fragment ion mass tolerance 0.6 Da (Isotopic MW). Identified peptides were filtered using a 1% FDR.
2.10. Ingenuity Pathway Analysis (IPA)
IPA (Ingenuity Systems) was performed to obtain the detailed molecular information. The identified protein lists were uploaded into the IPA tool and analyzed. The result files contained gene symbols, descriptions, locations, and types of the proteins. The location has four different categories, such as extracellular, cytoplasm, plasma membrane, nucleus, and other.
2.11. CD antigen list and comparison
The common CD antigen list was obtained from the cdlist on Uniprot (http://www.uniprot.org/docs/cdlist) released on 09-Jul-2014. The CD antigen list from CD1 through CD-363 was used for comparison. A total of 445 entries from the common CD antigen list were used for comparison where some CD antigens have more than one entry; e.g. CD-235a and CD-235b. The Swiss-Prot Entry Names from the common CD antigen list and from the currently identified protein list were compared to obtain the CD antigen name for each identified protein in the currently identified protein list.
3. Results
3.1. Enrichment of exosomes
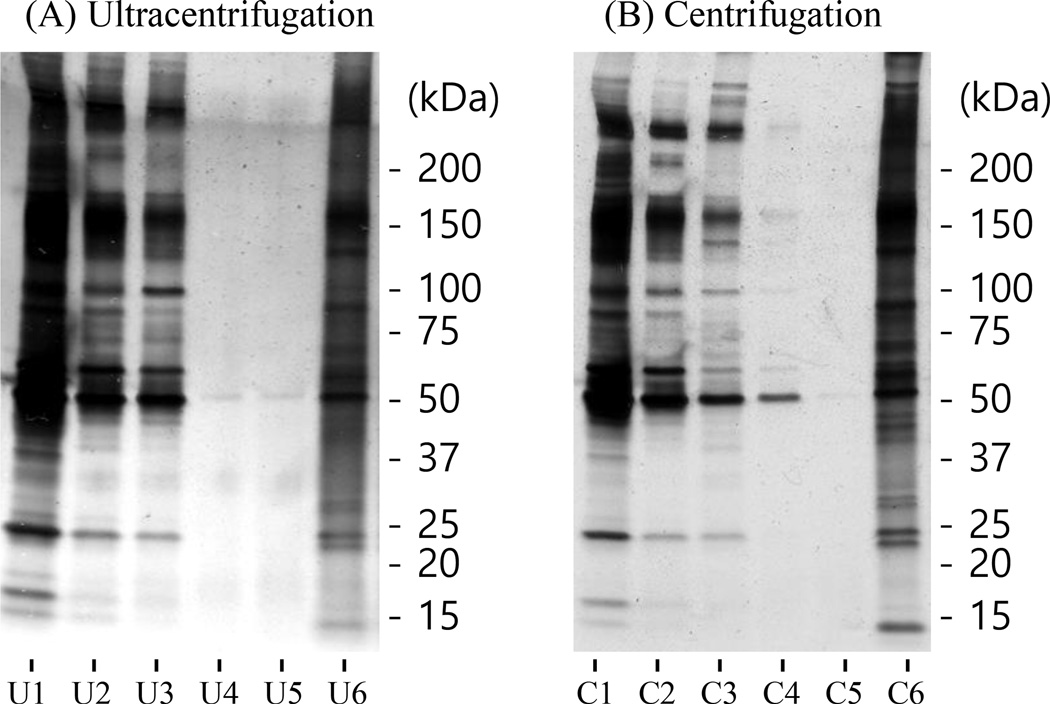
Currently, the most common enrichment method of exosomes is using ultracentrifugation with a speed of 110,000×g. In this study, we have explored whether a reduced speed (e.g. 40,000×g) would provide similar performance for the enrichment of exosomes. Figure 1 shows the 1-D gel images of the samples obtained from the ultracentrifugation and centrifugation procedures for the enrichment of exosomes from 2.0 mL human serum. Samples 1 through 5 are from the supernatants from the first through the fifth enrichment steps while sample 6 is from the enriched exosome pellet. The enriched exosome proteins were visualized using silver-staining. As shown in Figure 1, the protein separation patterns for the corresponding samples of supernatants and enriched exosomes between ultracentrifugation and centrifugation were very similar, showing that these two enrichment methods provided similar efficiencies for exosome enrichment.
Figure 1.
1-D gel images for the samples from (A) ultracentrifugation and (B) centrifugation processes of 2.0 mL human serum. The samples from 1 through 5 (U1–U5 and C1–C5) are the supernatants from the corresponding enrichment processes. The samples of U6 and C6 are from the enriched exosomes. The samples of 1 and 2 were diluted 500-fold and 20-fold with a PBS buffer solution prior to loading to reduce their concentrations and provide weaker bands.
The concentrations of the first, second, and third supernatants for both ultracentrifugation and centrifugation were ~50, ~1, and ~0.01 mg/mL, respectively, based on the BCA assay. In the fourth and fifth supernatant samples, no protein was detected using BCA assay. Most proteins are believed to be eliminated after four ultracentrifugation steps or centrifugation steps. Few bands were still visualized on gel using sliver-staining (Fig. 1), which illustrates 3×ultracentrifugation enrichment or 3×centrifugation enrichment is not sufficient to remove non-exosomal proteins.
3.2. Exosome protein yield
The amount of exosome proteins obtained from 1 mL, 2 mL, or 4 mL human serum was around 2.2 µg, 14.3 µg, or 28.5 µg, respectively, from the 3×ultracentrifugation enrichment and 2.1 µg, 8.3 µg, or 20.7 µg from the 5×ultracentrifugation enrichment while 3.8 µg, 8.6 µg, or 21.1 µg from the 3×centrifugation enrichment, and 2.9 µg, 8.5 µg, or 16.3 µg from the 5×centrifugation enrichment was obtained using the BCA assay. Around 20% less exosome proteins were observed from the five-cycle enrichment process compared to the corresponding three-cycle enrichment process. Based on the amount of exosome proteins obtained from 2 mL serum where both enrichment methods provided a similar yield of ~ 8.5 µg proteins (Table 1), the current yield is around 0.005%, assuming the protein concentration in human plasma is 60 ~ 80 mg/mL. The yields from all the enrichment conditions in this study were within the reasonable range between 0.001% and 0.01% [1] [37].
Table 1.
Quantities of exosomes obtained from 2 mL serum using 5×ultracentrifugations and 5×centrifugations.
| Sample number | Ultracentrifugation (µg) | Centrifugation (µg) |
|---|---|---|
| 1 | 3.4 | 4.3 |
| 2 | 4.0 | 5.6 |
| 3 | 7.5 | 7.8 |
| 4 | 10.9 | 9.7 |
| 5 | 11.3 | 11.5 |
| 6 | 12.7 | 14.7 |
| Average ± standard deviation | 8.3 ± 4.0 | 8.9 ± 3.9 |
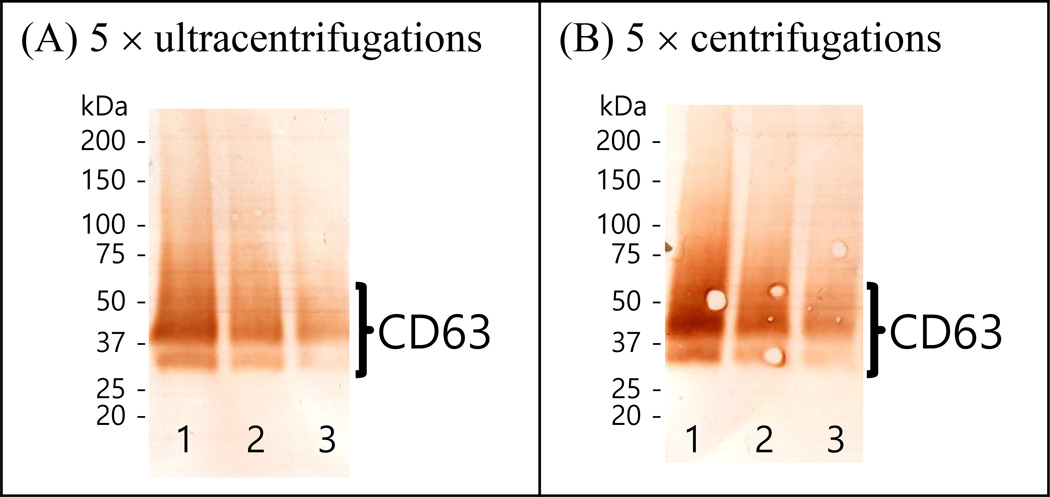
3.3. Western blot analysis
Figure 2 shows the Western blot analyses detecting CD-63 in the exosome proteins isolated from human serum using ultracentrifugation or centrifugation, where the intensities of the bands become weaker as the starting amount decreases. Similar intensities in Western blot analysis were observed from the ultracentrifugation and the centrifugation enrichment procedures for the same starting amounts of exosome proteins, confirming the similar performances between ultracentrifugation and centrifugation. The broad band ~ 50 kDa is characteristic of CD-63 [19, 26]. In the current investigation, CD-63 was only detected when the exosome proteins were not reduced. In additional Western blot analyses, CD-9 and CD-81 were also detected from reduced exosomes proteins and non-reduced proteins, respectively (Data not shown).
Figure 2.
Western blot analyses detecting CD-63 in exosomes purified from human serum using (A) the ultracentrifugation enrichment for 5 times and (B) the centrifugation enrichment for 5 times. The columns of “1”, “2”, and “3” for each image are from the exosome proteins obtained from 4 mL, 2 mL, and 1 mL human serum, respectively.
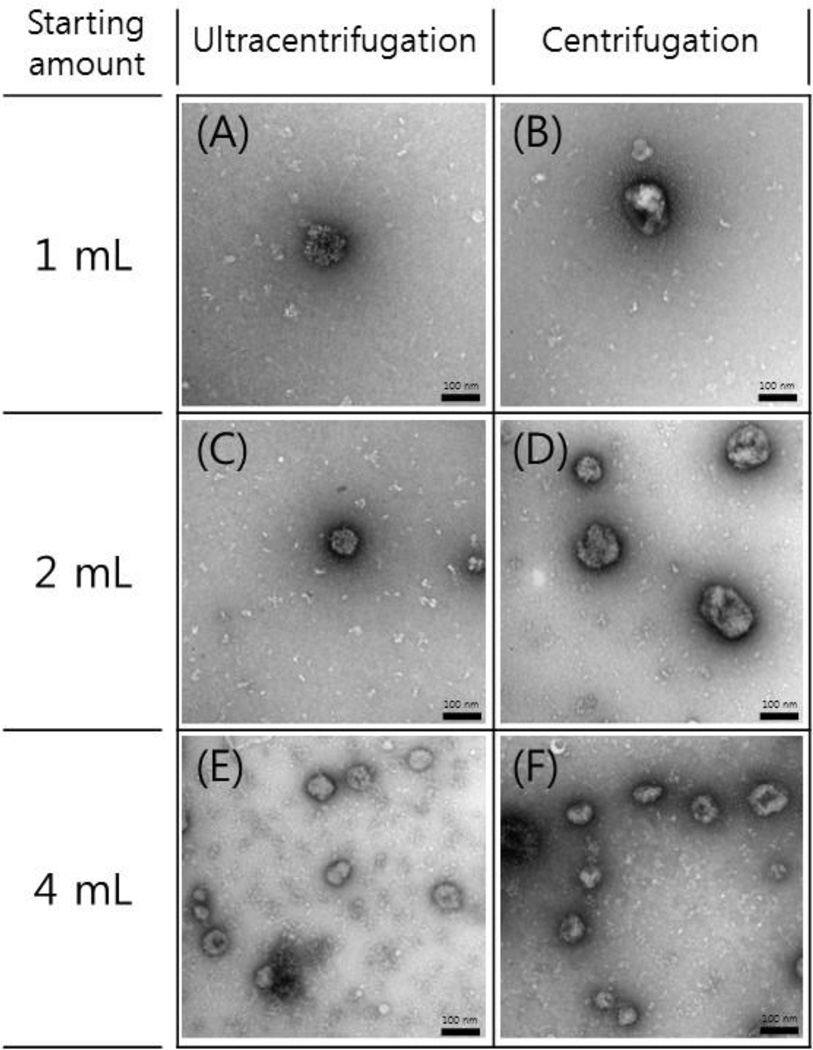
3.4. Size distribution of exosomes
Figure 3 shows the TEM images of the enriched exosome samples from different starting amounts using either 5×ultracentrifugations or 5×centrifugations. Exosomes were observed in a size range of around 100 nm. The number of exosomes increased with all experimental conditions as the amount of starting serum increased.
Figure 3.
TEM images of exosome samples enriched from human serum using 5×ultracentrifugations and 5×centrifugations. The first, the second and the third rows show the images for the exosomes enriched from starting amounts of 1 mL, 2 mL, and 4 mL, respectively. Scale bars, 100 nm.
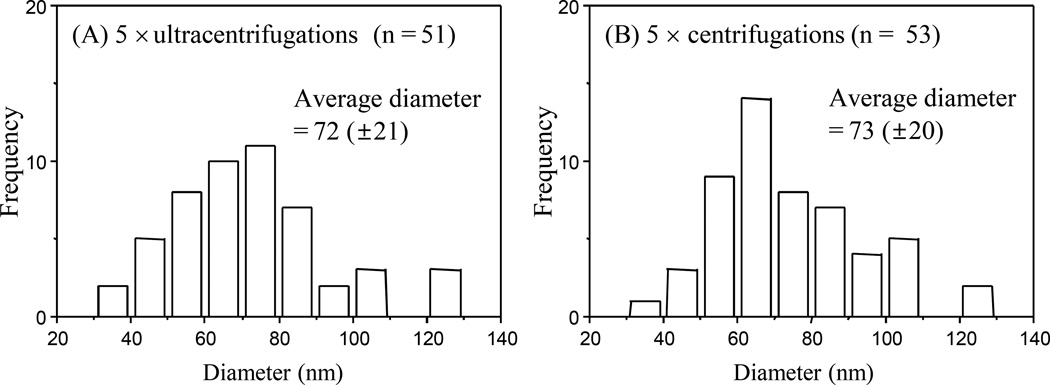
Figure 4 shows the histograms of size distribution of exosomes enriched from human serum using 5×ultracentrifugations and 5×centrifugations. The size distributions from the 5×ultracentrifugations and the 5×centrifugations were found to be very similar where the average diameters of the purified exosomes were 72 (±21) nm and 73 (±20) nm, respectively.
Figure 4.
Histograms showing the diameter distribution of exosomes enriched from 4 mL human serum using (A) 5×ultracentrifugation enrichment and (B) 5×centrifugation enrichment. The total number of exosome particles used for each histogram is shown as “n”.
3.5. Application of FASP
The filter-aided sample preparation (FASP) method has recently been published for the successful digestion of the sample containing sodium dodecyl sulfate (SDS), where SDS is exchanged to urea on a standard filtration device [38, 39]. In the digestion of exosome proteins, in-gel digestion is frequently used since in-gel digestion can circumvent problems associated with SDS [30, 40].
In the current investigation, protease inhibitors were added during the lysis step to avoid any protease activity during lysis. The protease inhibitors in the sample also inhibit trypsin activity. With the application of the FASP method, we successfully digested the exosome proteins, while without the use of the FASP method, the digestion was not successful. The successful digestion using the FASP method appears to be due to the protease inhibitors along with detergents such as SDS and NP-40 being removed during the filtration processes.
3.6. Proteins identified from LC-MS/MS analysis
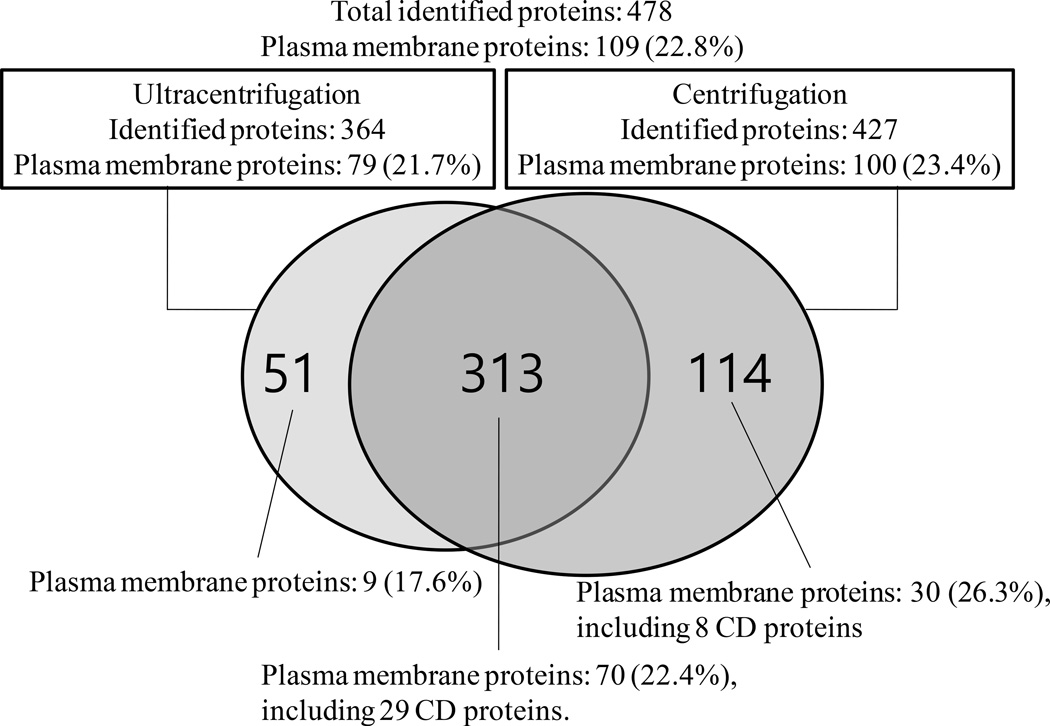
A total of 478 proteins were identified from the LC-MS/MS analyses of exosome proteins enriched from 4 mL serum using 5×ultracentrifugations and 5×centrifugations. Figure 5 shows the Venn diagram showing the overlap of exosome proteins enriched using 5×ultracentrifugations or 5×centrifugations, where 313 proteins (65.5%) were commonly observed in both enrichment processes. The complete list of the 478 proteins can be found in Supplemental Table S1. The total number of plasma membrane proteins is 109, where 9 plasma membrane proteins were only observed from the exosomes enriched using 5×ultracentrifugations and 30 plasma membrane proteins were only observed from the exosomes enriched using 5×centrifugations.
Figure 5.
Venn diagram showing the overlap of exosome proteins enriched from 5×ultracentrifugations and 5×centrifugations. A total of 37 CD proteins were identified.
Among the 478 proteins, 196 proteins (41.0%) were identified with a single unique peptide of each protein. The high mass accuracy of an Orbitrap Elite mass spectrometer (peptide ion mass tolerance<10 ppm) and high peptide confidence level (FDR<1%) are believed to be sufficient to provide a confident peptide list and corresponding protein list even with a single unique peptide for the identification of proteins. The proteins identified with single unique peptides are assumed to be low-abundance proteins [41].
Cluster of differentiation (CD) antigens are cell surface molecules recognized by specific monoclonal antibodies [42]. CD antigens are defined when surface molecules on human cells interact with at least one new monoclonal antibody [43]. CD antigens perform a variety of roles in immune reactions of organisms [44]. A total of 37 CD proteins were identified from 5×centrifugations, while 29 CD proteins were identified from 5×centrifugations (Table 2). Additional identification of CD proteins from the centrifugation purification process showed that the centrifugation is more efficient in isolating exosomes than the ultracentrifugation. The detailed information of the 37 CD proteins and their related peptides are shown in Table 2 and Supplemental Table S2, respectively. The MS/MS spectra of the identified peptides from the 37 CD proteins are included in Supplemental Figure S1.
Table 2.
List of 37 CD proteins identified in the current investigation.
| Number | CD number |
Swiss Prot Name | Accession Number |
Gene Name | Description | na) | Ub) | Cc) |
|---|---|---|---|---|---|---|---|---|
| 1 | CD9 | CD9_HUMAN | P21926 | CD9 | CD9 antigen | 5 | V | V |
| 2 | CD10 | NEP_HUMAN | P08473 | MME | Neprilysin | 1 | -d) | V |
| 3 | CD11b | ITAM_HUMAN | P11215 | ITGAM | Integrin alpha-M | 1 | -d) | V |
| 4 | CD13 | AMPN_HUMAN | P15144 | ANPEP | Aminopeptidase N | 7 | V | V |
| 5 | CD18 | ITB2_HUMAN | P05107 | ITGB2 | Integrin beta-2 | 4 | -d) | V |
| 6 | CD29 | ITB1_HUMAN | P05556 | ITGB1 | Integrin beta-1 | 12 | V | V |
| 7 | CD31 | PECA1_HUMAN | P16284 | PECAM1 | Platelet endothelial cell adhesion molecule | 4 | V | V |
| 8 | CD36 | CD36_HUMAN | P16671 | CD36 | Platelet glycoprotein 4 | 6 | V | V |
| 9 | CD41 | ITA2B_HUMAN | P08514 | ITGA2B | Integrin alpha-IIb | 26 | V | V |
| 10 | CD42a | GPIX_HUMAN | P14770 | GP9 | Platelet glycoprotein IX | 4 | V | V |
| 11 | CD42b | GP1BA_HUMAN | P07359 | GP1BA | Platelet glycoprotein Ib alpha chain | 2 | V | V |
| 12 | CD42c | GP1BB_HUMAN | P13224 | GP1BB | Platelet glycoprotein Ib beta chain | 4 | V | V |
| 13 | CD43 | LEUK_HUMAN | P16150 | SPN | Leukosialin | 1 | V | V |
| 14 | CD45 | PTPRC_HUMAN | P08575 | PTPRC | Receptor-type tyrosine-protein phosphatase C | 3 | V | V |
| 15 | CD47 | CD47_HUMAN | Q08722 | CD47 | Leukocyte surface antigen CD47 | 3 | V | V |
| 16 | CD49b | ITA2_HUMAN | P17301 | ITGA2 | Integrin alpha-2 | 4 | -d) | V |
| 17 | CD49f | ITA6_HUMAN | P23229 | ITGA6 | Integrin alpha-6 | 19 | V | V |
| 18 | CD53 | CD53_HUMAN | P19397 | CD53 | Leukocyte surface antigen CD53 | 1 | V | V |
| 19 | CD59 | CD59_HUMAN | P13987 | CD59 | CD59 glycoprotein | 3 | V | V |
| 20 | CD61 | ITB3_HUMAN | P05106 | ITGB3 | Integrin beta-3 | 27 | V | V |
| 21 | CD63 | CD63_HUMAN | P08962 | CD63 | CD63 antigen | 2 | V | V |
| 22 | CD66b | CEAM8_HUMAN | P31997 | CEACAM8 | Carcinoembryonic antigen-related cell adhesion molecule 8 | 1 | -d) | V |
| 23 | CD71 | TFR1_HUMAN | P02786 | TFRC | Transferrin receptor protein 1 | 23 | V | V |
| 24 | CD82 | CD82_HUMAN | P27701 | CD82 | CD82 antigen | 2 | -d) | V |
| 25 | CD91 | LRP1_HUMAN | Q07954 | LRP1 | Prolow-density lipoprotein receptor-related protein 1 | 11 | V | V |
| 26 | CD92 | CTL1_HUMAN | Q8WWI5 | SLC44A1 | Choline transporter-like protein 1 | 5 | V | V |
| 27 | CD98 | 4F2_HUMAN | P08195 | SLC3A2 | 4F2 cell-surface antigen heavy chain | 1 | V | V |
| 28 | CD107a | LAMP1_HUMAN | P11279 | LAMP1 | Lysosome-associated membrane glycoprotein 1 | 2 | -d) | V |
| 29 | CD107b | LAMP2_HUMAN | P13473 | LAMP2 | Lysosome-associated membrane glycoprotein 2 | 1 | V | V |
| 30 | CD148 | PTPRJ_HUMAN | Q12913 | PTPRJ | Receptor-type tyrosine-protein phosphatase eta | 7 | V | V |
| 31 | CD151 | CD151_HUMAN | P48509 | CD151 | CD151 antigen | 2 | V | V |
| 32 | CD156c | ADA10_HUMAN | O14672 | ADAM10 | Disintegrin and metalloproteinase domain-containing protein 10 | 13 | V | V |
| 33 | CD225 | IFM1_HUMAN | P13164 | IFITM1 | Interferon-induced transmembrane protein 1 | 1 | V | V |
| 34 | CD233 | B3AT_HUMAN | P02730 | SLC4A1 | Band 3 anion transport protein | 17 | V | V |
| 35 | CD240CE | RHCE_HUMAN | P18577 | RHCE | Blood group Rh(CE) polypeptide | 1 | -d) | V |
| 36 | CD241 | RHAG_HUMAN | Q02094 | RHAG | Ammonium transporter Rh type A | 1 | V | V |
| 37 | CD321 | JAM1_HUMAN | Q9Y624 | F11R | Junctional adhesion molecule A | 3 | V | V |
Number of identified unique peptides
Detection in the samples from 5×ultracentrifugations
Detection in the samples from 5×centrifugations
Not detected
3.7. Comparison with other identified proteins
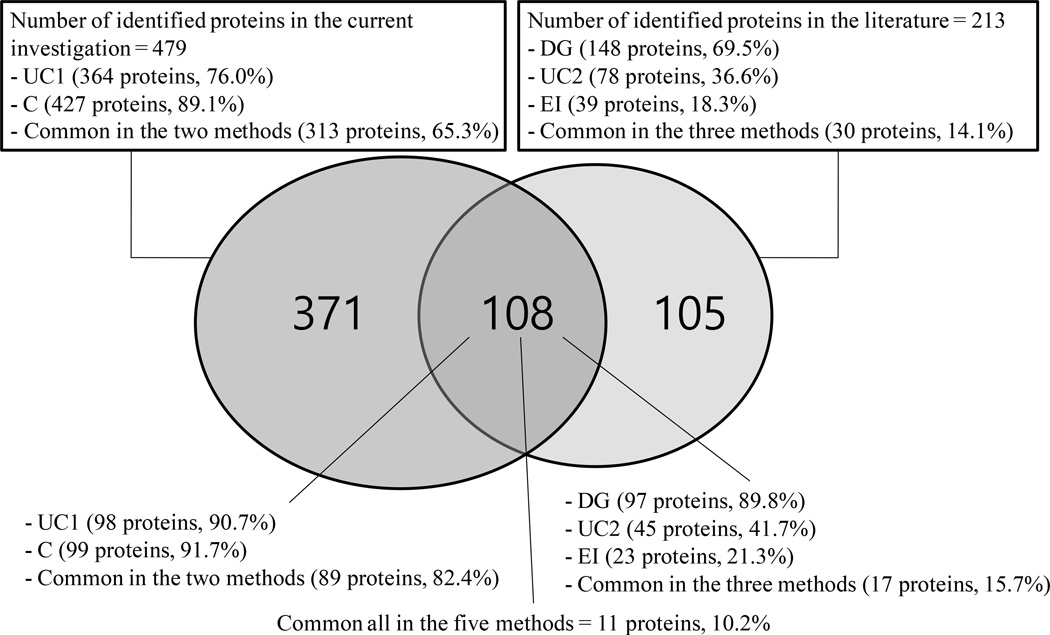
Comparison of the 213 human plasma exosome proteins identified from three different isolation methods (DG, UC, and EI) [2] with the currently identified 479 exosome proteins revealed 108 common exosome proteins as shown in the Supplementary Table S3. Among the 108 common exosome proteins, seven proteins were found to be CD proteins (CD31, CD41, CD42c, CD61, CD71, CD233, and CD321). Eleven proteins were found in all of the five enrichment methods as shown in Table 3. Among the eleven commonly observed proteins, four proteins (alpha-2-macroglobulin, Albumin, Fibrinogen alpha chain, and Haptoglobin) are well-known abundant plasma proteins [45], which might have been enriched as impurities. Five proteins (immunoglobulin J polypeptide, keratin 6A, keratin 14, keratin 16, and keratin 17) were already identified in the exosome fraction of human parotid saliva [2]. Ten proteins (All proteins except immunoglobulin lambda-like polypeptide 5 in Table 3) were also previously identified in the exosome fraction of normal human urine [7]. In Figure 6 is shown the Venn diagram comparing the overlap of exosome proteins from the three different isolation methods and the current investigation, where 97 (89.8%), 45 (41.7%), and 23 (21.3%) exosome proteins were from proteins from DG, UC, and EI, respectively.
Table 3.
List of 11 proteins identified from all of the five different exosome enrichment methods (two methods in the current investigation and three methods in the previous investigation from human plasma [2])
| Number | Swiss-Prot Name | Gene Name | Description |
|---|---|---|---|
| 1 | A2MG_HUMAN | A2M | alpha-2-macroglobulin |
| 2 | ALBU_HUMAN | ALB | Albumin |
| 3 | FIBA_HUMAN | FGA | fibrinogen alpha chain |
| 4 | HPT_HUMAN | HP | Haptoglobin |
| 5 | IGJ_HUMAN | IGJ | immunoglobulin J polypeptide |
| 6 | K2C6A_HUMAN | KRT6A | keratin 6A |
| 7 | K1C14_HUMAN | KRT14 | keratin 14 |
| 8 | K1C16_HUMAN | KRT16 | keratin 16 |
| 9 | K1C17_HUMAN | KRT17 | keratin 17 |
| 10 | DCD_HUMAN | DCD | Dermcidin |
| 11 | IGLL5_HUMAN | IGLL5 | immunoglobulin lambda-like polypeptide 5 |
Figure 6.
Venn diagram showing the overlap of exosome proteins enriched from the current investigation and from the three different enrichment methods [2]. UC, ultracentrifugation; C, centrifugation; DG, density gradient; EI, EpCAM-based immunoaffinity pull-down.
Among the twenty four common exosomal proteins reported by Simpson and his coworkers [12], eighteen proteins were identified in the current investigation, where fourteen proteins were identified in both the 5×ultracentrifugation and 5×centrifugation procedures (Table 4). Two proteins (HSP90AB1 and YWHAG) were only identified from 5×ultracentrifugations and the other two proteins (HSP90AA1 and PGK1) were only identified from 5×centrifugations. The other six unidentified proteins were identified in similar forms as shown in Table 4.
Table 4.
Comparison between the twenty four commonly identified exosomal proteins [12] and proteins identified in the current analysis.
| Gene Name | Description | Current analysis |
|---|---|---|
| ACTB | Actin, cytoplasmic 1 | U, C |
| ACTC1 | Actin, alpha cardiac muscle 1 | U, C |
| ACTG1 | Actin, cytoplasmic 2 | ACTN1 (U, C), ACTR2 (C), ACTR3 (C) |
| ANXA11 | Annexin A11 | U, C |
| ANXA6 | Annexin A6 | U, C |
| ARF1 | ADP-ribosylation factor GTPase-activating protein 1 | ARF3 (U, C) |
| CFL1 | Cofilin-1 | U, C |
| ENO1 | Alpha-enolase | U, C |
| GNAI3 | Guanine nucleotide-binding protein G(k) subunit alpha | GNAI2 (U, C), GNAQ (C), GNAZ (C) |
| GNB1 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | U, C |
| HSP90AA1 | Heat shock protein HSP 90-alpha | C |
| HSP90AB1 | Heat shock protein HSP 90-beta | U |
| HSPA8 | Heat shock cognate 71 kDa protein | U, C |
| PDCD6IP | Programmed cell death 6-interacting protein | U, C |
| PGK1 | Phosphoglycerate kinase 1 | C |
| PKM2 | Pyruvate kinase PKM | PKMb) (U, C) |
| RAB5A | Ras-related protein Rab-5A | RAB5C (U, C) |
| RAB5B | Ras-related protein Rab-5B | RAB5C (U, C) |
| RAB5C | Ras-related protein Rab-5C | U, C |
| RAP1B | Ras-related protein Rap-1b | U, C |
| YWHAB | 14-3-3 protein beta/alpha | U, C |
| YWHAE | 14-3-3 protein epsilon | U, C |
| YWHAG | 14-3-3 protein gamma | U |
| YWHAZ | 14-3-3 protein zeta/delta | U, C |
U and C mean detection from 5×ultracentrifugation and 5×centrifugation purifications, respectively. For the proteins that were not identified in the current analysis, the gene names of the identified similar proteins were provided.
The alternative name is PKM2.
4. Discussion
A recent study showed that a single cycle by ultracentrifugation or the ExoQuick kit to purify exosomes from human serum was not sufficient to remove high amounts of albumin and immunoglobulin G, where it was suggested that two or more cycles were required to increase exosome purity [24]. Another study showed that two cycles of ultracentrifugation is not sufficient to increase the purity of exosomes from non-exosomal protein contamination in the enrichment of exosomes from human serum [25]. The current methodology using ultracentrifugation twice for enriching exosomes may be effective for enriching exosomes from cells, while for serum or plasma samples which contain several high abundant proteins, multiple cycles (more than 4) of ultracentrifugation or centrifugation are necessary.
The TEM image showed that the exosome enrichment using 5×centrifugations is similar to 5×ultracentrifugations in removing proteins and protein aggregates, providing a similar average diameter of exosomes.
There are several advantages to the use of centrifugation in that a centrifuge instrument is relatively inexpensive and widely disseminated compared to an ultracentrifugation instrument. In addition, it is easy to handle the samples without contamination since most tubes for centrifugation have lids, while extra care is required for sample handling using ultracentrifugation since most tubes for ultracentrifugation do not have lids.
5. Conclusion
In the current investigation we have shown that five cycle repetition use of ultracentrifugation or centrifugation is necessary for a successful enrichment of exosomes from human serum based on 1-D gel analysis and the comparison of protein yield between 3 cycles and 5 cycles. In addition, we have shown that 5×centrifugations provided comparable results to those obtained using 5×ultracentrifugations. Both enrichment procedures provided similar performances in terms of exosome protein amounts and Western blot analyses detecting CD-63 antigen, while significantly higher numbers of identified exosome proteins and CD proteins were obtained from 5×centrifugations. A comparison between the exosome protein list from the current investigation with the previously reported exosome protein list shows that the current method is successful in isolating exosomes from human serum. Additionally, a total of 37 CD proteins were identified, which will be important in future exosome research for providing a means for rapid detection of exosomes using targeted antibodies or mass spec assays. This will be especially important in biomarker studies of disease states and therapeutic response based on monitoring of proteins from exosomes in serum.
Supplementary Material
Acknowledgements
We acknowledge support of this work by the National Institutes of Health under grant R01GM49500 and grant R21 CA189775 and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010–0010776).
Abbreviations
- DG
Density gradient
- UC
ultracentrifugation
- EI
EpCAM-based immunoaffinity pull-down
- HRP
horseradish peroxidase
- PBS
Phosphate buffered Saline
- BCA
Bicinchoninic acid
- TEM
Transmission Electron Microscopy
- PBST
Phosphate Buffered Saline with Tween-20
- IAA
Iodoacetamide
- FA
Formic acid
- MW
Molecular weight
- FDR
False Discovery Rate
- IPA
Ingenuity Pathway Analysis
- FASP
Filter-aided sample preparation
- SDS
Sodium dodecyl sulfate
- CD
Cluster of differentiation
Footnotes
The authors have declared no conflict of interest.
References
- 1.Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Int. Immuno. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 2.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 3.Moon PG, You S, Lee JE, Hwang D, Baek MC. Mass Spectrom. Rev. 2011;30:1185–1202. doi: 10.1002/mas.20319. [DOI] [PubMed] [Google Scholar]
- 4.Moon P-G, Lee J-E, You S, Kim T-K, Cho J-H, Kim I-S, Kwon T-H, Kim C-D, Park S-H, Hwang D, Kim Y-L, Baek M-C. Proteomics. 2011;11:2459–2475. doi: 10.1002/pmic.201000443. [DOI] [PubMed] [Google Scholar]
- 5.Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, Semmes OJ, Troyer DA, Lance RS, Kislinger T, Drake RR. Proteomics. 2013;13:1667–1671. doi: 10.1002/pmic.201200561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dear JW, Street JM, Bailey MA. Proteomics. 2013;13:1572–1580. doi: 10.1002/pmic.201200285. [DOI] [PubMed] [Google Scholar]
- 7.Prunotto M, Farina A, Lane L, Pernin A, Schifferli J, Hochstrasser DF, Lescuyer P, Moll S. J. Proteomics. 2013;82:193–229. doi: 10.1016/j.jprot.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. J. Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau CS, Wong DT. PLoS ONE. 2012;7:e33037. doi: 10.1371/journal.pone.0033037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada T, Inoshima Y, Matsuda T, Ishiguro N. J. Vet. Med. Sci. 2012;74:1523–1525. doi: 10.1292/jvms.12-0032. [DOI] [PubMed] [Google Scholar]
- 11.Choi DS, Kim DK, Kim YK, Gho YS. Mass Spectrom. Rev. 2014 doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 12.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Expert Rev. Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 13.Drake RR, Kislinger T. Expert Rev. Proteomics. 2014;11:167–177. doi: 10.1586/14789450.2014.890894. [DOI] [PubMed] [Google Scholar]
- 14.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Biochim. Biophys. Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Bobrie A, Colombo M, Raposo G, Thery C. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai RC, Yeo RW, Tan KH, Lim SK. Biotechnol. Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Pisitkun T, Shen RF, Knepper MA. Proc. Natl. Acad. Sci. USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rappa G, Mercapide J, Anzanello F, Pope RM, Lorico A. Mol. Cancer. 2013;12:62. doi: 10.1186/1476-4598-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiba M, Kimura M, Asari S. Oncol. Rep. 2012;28:1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laulagnier K, Vincent-Schneider H, Hamdi S, Subra C, Lankar D, Record M. Blood Cells Mol. Dis. 2005;35:116–121. doi: 10.1016/j.bcmd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette RJ, Anchordoquy TJ, Bemis LT, Graner MW. PLoS ONE. 2012;7:e42064. doi: 10.1371/journal.pone.0042064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Sci. Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 23.Mathivanan S, Lim JW, Tauro BJ, Ji H, Moritz RL, Simpson RJ. Mol. Cell. Proteomics. 2010;9:197–208. doi: 10.1074/mcp.M900152-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caradec J, Kharmate G, Hosseini-Beheshti E, Adomat H, Gleave M, Guns E. Clin. Biochem. 2014;47:1286–1292. doi: 10.1016/j.clinbiochem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Webber J, Clayton A. J. Extracell. Vesicles. 2013;2:19861. doi: 10.3402/jev.v2i0.19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oksvold MP, Kullmann A, Forfang L, Kierulf B, Li M, Brech A, Vlassov AV, Smeland EB, Neurauter A, Pedersen KW. Clin. Ther. 2014;36:847–862. e841. doi: 10.1016/j.clinthera.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 27.de Hoog VC, Timmers L, Schoneveld AH, Wang JW, van de Weg SM, Sze SK, van Keulen JK, Hoes AW, den Ruijter HM, de Kleijn DP, Mosterd A. Eur. Heart J. Acute Cardiovasc. Care. 2013;2:53–60. doi: 10.1177/2048872612471212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T. Nat. Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merchant ML, Powell DW, Wilkey DW, Cummins TD, Deegens JK, Rood IM, McAfee KJ, Fleischer C, Klein E, Klein JB. Proteomics Clin. Appl. 2010;4:84–96. doi: 10.1002/prca.200800093. [DOI] [PubMed] [Google Scholar]
- 31.Kang D, Oh S, Ahn SM, Lee BH, Moon MH. J. Proteome Res. 2008;7:3475–3480. doi: 10.1021/pr800225z. [DOI] [PubMed] [Google Scholar]
- 32.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Clin. Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 33.Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Furst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH, Kabelitz D. Int. J. Cancer. 2013;133:1557–1566. doi: 10.1002/ijc.28174. [DOI] [PubMed] [Google Scholar]
- 34.Adamczyk KA, Klein-Scory S, Tehrani MM, Warnken U, Schmiegel W, Schnolzer M, Schwarte-Waldhoff I. Life Sci. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Cui Y, Luan J, Zhang G, Zhang X, Han J. Biosci. Trends. 2013;7:144–151. [PubMed] [Google Scholar]
- 36.Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu Y, Feng J. PLoS ONE. 2014;9:e89534. doi: 10.1371/journal.pone.0089534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren Y, Yang J, Xie R, Gao L, Yang Y, Fan H, Qian K. Transfusion. 2011;51:1002–1011. doi: 10.1111/j.1537-2995.2010.02909.x. [DOI] [PubMed] [Google Scholar]
- 38.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Nat. Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Nie S, Wu J, Lubman DM. J. Proteome Res. 2013;12:2791–2804. doi: 10.1021/pr400139c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Looze C, Yui D, Leung L, Ingham M, Kaler M, Yao X, Wu WW, Shen RF, Daniels MP, Levine SJ. Biochem. Biophys. Res. Commun. 2009;378:433–438. doi: 10.1016/j.bbrc.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallick P, Kuster B. Nat. Biotechnol. 2010;28:695–709. doi: 10.1038/nbt.1658. [DOI] [PubMed] [Google Scholar]
- 42.Knapp W, Rieber P, Dörken B, Schmidt RE, Stein H, Borne AEGKvd. Immunol. Today. 1989;10:253–258. doi: 10.1016/0167-5699(89)90135-7. [DOI] [PubMed] [Google Scholar]
- 43.Barber N, Gez S, Belov L, Mulligan SP, Woolfson A, Christopherson RI. FEBS Lett. 2009;583:1785–1791. doi: 10.1016/j.febslet.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Woolfson A, Ellmark P, Chrisp JSMAS, Christopherson RI. Pharmacogenomics. 2006;7:759–771. doi: 10.2217/14622416.7.5.759. [DOI] [PubMed] [Google Scholar]
- 45.Anderson NL, Anderson NG. Mol. Cell. Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.