Abstract
Diabetes is associated with dire peripheral sequelae including foot ulcers and amputations. A recent article by Wong et al [2] demystifies this connection by demonstrating that the neutrophil defense mechanism of extruding decondensed chromatin, termed NETosis, mediates delayed wound healing in diabetes and provides a therapeutic strategy for this indication.
A dreaded consequence of diabetes is the prospect of lower leg amputation due to disrupted wound healing in unresolved foot ulcers. Indeed, foot ulcers are the leading cause of hospitalizations for people afflicted with diabetes. Wound healing is an extremely complicated process involving multiple cell types including endothelial cells, fibroblasts, immune cells, platelets and keratinocytes. Remarkably, over 100 known wound healing factors and pathways have been identified as deficient in the diabetic condition [1]. Now a recent report by Wong et al [2] adds significantly to this literature, revealing diabetes actually enhances a neutrophil function that is deleterious to wound healing.
When neutrophils invade a wound, they secrete neutrophil extracellular traps (NETs) to neutralize microorganisms in the form of decondensed chromatin, which are generated by peptidylarginine deiminase 4 (PAD4)-mediated histone citrullination in a process termed “NETosis”. Wong et al show neutrophil PAD4 expression is upregulated by hyperglycemia, while the resultant NETs produced in skin wounds are deleterious to wound healing. Indeed, either loss of neutrophil recruitment in CD18-null mice or PAD4 deficiency enhances wound healing, which under these conditions was not impaired by diabetes. Coupled with recent reports documenting increased circulating NET-related biomarkers in diabetes [3, 4] and a role for neutrophil elastase in mediating insulin resistance [5], the new data by Wong et al highlight a broad impact of neutrophils in the diabetes syndrome.
A particularly intriguing question raised in the study by Wong et al is how do the high glucose concentrations in diabetes prime NETosis? There is abundant evidence linking diabetes pathologies with inflammation, and it is tempting to speculate that perhaps such proinflammatory mediators as reactive oxygen species (ROS) induced by high glucose may play a role in this phenomenon. This hypothesis is especially attractive because phorbol myristate acetate (PMA) and calcium ionophores such as ionomycin, which promote ROS production, are required for NET formation, and Wong et al demonstrate that high glucose exacerbates ionomycin-mediated NETosis. Interestingly, a link between NETosis and pancreatic beta cell destruction has recently been identified in autoimmune type 1 diabetes (T1D) [3]. Taken together, these data suggest a multifaceted role for NETosis in mediating diabetes pathology in response to disrupted cellular pathways of calcium handling and ROS production induced by hyperglycemia.
PAD4 protein levels were enhanced in neutrophils isolated from persons with diabetes, suggesting that increased PAD4 protein may be sufficient to prime neutrophils to undergo NETosis [2]. However, it is unclear whether high glucose upregulates PAD4 protein expression at a transcriptional or post-translational level. A recent study suggests that proinflammatory signaling through Nuclear factor kappa B p50 homodimer binding to the PAD4 promoter actually represses PAD4 transcription, as a negative feedback mechanism [6]. Thus, the transcriptional machinery may mediate the effects of hyperglycemia on PAD4 expression, or perhaps post-translational mechanisms are responsible for this increase. This issue would be important to resolve in future experiments designed to exploit potential therapeutic strategies relevant to this system.
The finding that PAD4 deficiency eliminates the deleterious effect of diabetes on wound healing [2] is particularly remarkable. These data suggest that PAD4 expression and its regulation of NETosis are not only increased in diabetic wounds but also embody the key pathway that delays healing by the diabetes syndrome. But why does the NETosis process delay healing? Perhaps NETs trap neutrophil enzymes such as elastase, which directly damages the extracellular matrix of epithelial and endothelial cells and creates a toxic environment. Another hypothesis put forth by the authors suggests that NETs may prevent keratinocyte migration. Because NETs are comprised of DNA, DNAse treatment is sufficient to degrade them, and systemic DNAse treatment of mice also improved diabetic wound healing [2]. The authors suggest that mutations in DNAse enzymes, which have previously been observed in patients with autoimmune diseases, could render DNAse less active in persons with diabetes. However, some studies indicate that DNAse activity is increased in diabetes, which seems counterintuitive to the authors’ hypothesis [7]. Clearly, future work will be required to determine whether alterations in endogenous DNAse play a role in the altered wound healing in diabetic persons.
Infection and wound healing are intrinsically linked. Loss of PAD4 improved wound healing in both normal laboratory mice and in antibiotic-treated mice [2]. However, the authors suggest that certain bacteria present on skin such as Staphylococcus aureus could degrade NETs and render them ineffective [2]. This could be an important caveat in extrapolating results on NETosis from mice to humans. Because mice housed in a laboratory setting may be relatively germ-free compared with humans, it is unclear what contribution NETs may have to impaired wound resolution if virulent bacterial strains are present. Also relevant is a previous study that found no contribution of PAD4 to systemic infections such as sepsis [8]. Thus, it is unclear what the evolutionary role of PAD4 and NETosis may be to other types of infections and inflammation that are associated with diabetes.
PAD4-mediated histone citrullination is required for NETosis, but that is not the only function of the citrullination process, as histone citrullination can alter cellular gene expression [9]. Therefore, although Wong et al convincingly demonstrated a requirement of NETosis in impaired diabetic wound healing, additional mechanisms may mediate the protection from diabetes-impaired wound resolution in PAD4-null mice. Neither the transcriptome nor the secretome of PAD4-null neutrophils was assessed in this study. Interestingly, anti-citrulline antibodies occur in autoimmune diseases such as rheumatoid arthritis, and promote tissue damage [10]. Such antibodies could also be hypothetically increased in T1D and contribute to the lack of wound resolution.
Consistent with recent findings from other groups [4], Wong et al found that NETosis is increased in persons with type 2 diabetes (T2D), the most prevalent form of diabetes. Two studies suggest a causal role for neutrophils in driving systemic insulin resistance, an underlying syndrome in T2D. Loss of either neutrophil elastase [5], a component of NETs, or of neutrophil ligand P-selectin glycoprotein ligand-1 [11] ameliorates high fat diet-induced insulin resistance in mice. Thus, might increased NETosis be an important trigger for insulin resistance and T2D? Interesting future studies could encompass high fat diet-induced obesity models in PAD4-null mice to determine whether PAD4 contributes to insulin resistance and/or adipose tissue inflammation in T2D. The work of Wong et al may take on even more significance in addressing the fundamental etiology of T2D as well as one of its complications.
In summary, Wong et al provide solid evidence that causally links neutrophil PAD4 expression and NETosis to impaired wound healing in diabetes. Their data provides exciting potential for new therapeutic strategies and ultimate clinical treatment of diabetes-associated wounds such as foot ulcers, a particularly distressful malady.
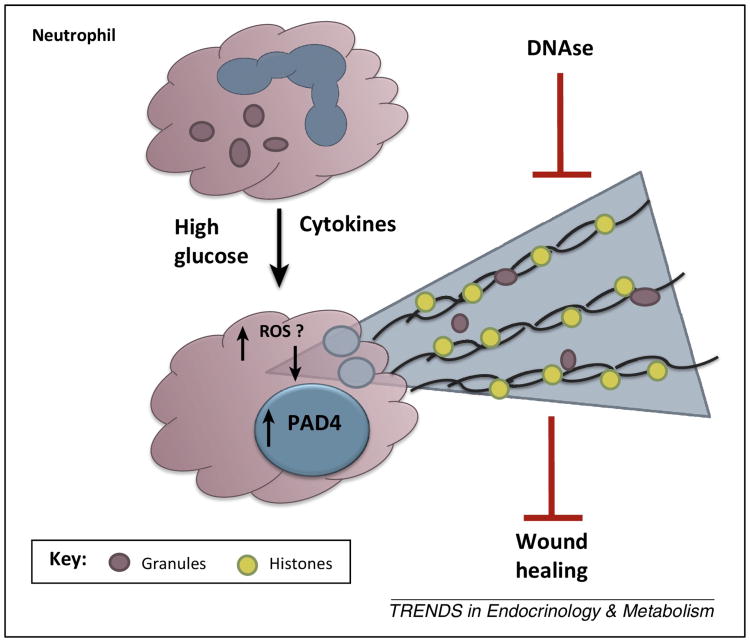
Figure 1. Increased release of neutrophil extracellular traps (NETs) through the process of “NETosis” is triggered by high glucose in diabetes and inhibits wound healing.
High glucose primes neutrophils to undergo NETosis in response to inflammatory stimuli by upregulating PAD4 expression in a manner that potentially is downstream of reactive oxygen species (ROS). PAD4 catalyzes the citrullination of histones that causes decondensation of the chromatin DNA that is released as NETs. The presence of DNAse-sensitive neutrophil NETs in skin wounds impairs wound healing in diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong SL, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with beta-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014;63:4239–4248. doi: 10.2337/db14-0480. [DOI] [PubMed] [Google Scholar]
- 4.Menegazzo L, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta diabetologica. 2015;52:497–503. doi: 10.1007/s00592-014-0676-x. [DOI] [PubMed] [Google Scholar]
- 5.Talukdar S, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas AK, et al. Negative regulation of the peptidylarginine deiminase type IV promoter by NF-kappaB in human myeloid cells. Gene. 2014;533:123–131. doi: 10.1016/j.gene.2013.09.108. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B, et al. Increased DNase I activity in diabetes might be associated with injury of pancreas. Molecular and cellular biochemistry. 2014;393:23–32. doi: 10.1007/s11010-014-2043-1. [DOI] [PubMed] [Google Scholar]
- 8.Martinod K, et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christophorou MA, et al. Citrullination regulates pluripotency and histone H1 binding to chromatin. Nature. 2014;507:104–108. doi: 10.1038/nature12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn KA, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–973. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo HM, et al. P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ Res. 2010;107:388–397. doi: 10.1161/CIRCRESAHA.110.218651. [DOI] [PMC free article] [PubMed] [Google Scholar]