Abstract
Adenosine is generated from adenosine triphosphate, which is released by stressed and damaged cells. Adenosine levels are significantly increased in patients with bronchial asthma (BA) and mediate mast cell degranulation and bronchoconstriction. Over the last decade, increasing evidence has shown that adenosine can modulate the innate immune response during monocytes differentiation towards mature myeloid cells. These adenosine-differentiated myeloid cells, characterized by co-expression of monocytes/macrophages and dendritic cell markers such as CD14 and CD209, produce high levels of pro-inflammatory cytokines, thus contributing to the pathogenesis of BA and chronic obstructive pulmonary disease. We found that expression of ADORA2A and ADORA2B are increased in monocytes obtained from patients with BA, and are associated with the generation of CD14posCD209pos pro-inflammatory cells. A positive correlation between expression of ADORA2B and IL-6 was identified in human monocytes and may explain the increased expression of IL-6 mRNA in asthmatics. Taken together, our results suggest that monocyte-specific expression of A2 adenosine receptors plays an important role in pro-inflammatory activation of human monocytes, thus contributing to the progression of asthma.
Introduction
Monocytes rapidly infiltrate lung tissue after injury [1] and contribute to initiation and amplification of inflammation via mechanisms, including acceleration of neutrophil migration to the lungs and generation of reactive oxygen/nitrogen species [2,3]. It has been also shown that monocytes promote inflammation through differentiation into inflammatory macrophages and dendritic cells (DCs) [4,5,6]. DCs play a crucial role in primary and secondary immune responses in lung inflammation and development of BA [7]. Under inflammatory conditions, monocytes contribute to the DCs pool and promote Th2-mediated immune response in asthma [8,9,10,11]. Using animal models of lung inflammation, it has been established that the local microenvironment plays an essential role in the regulation of monocytes differentiation towards mature myeloid cells (macrophages and DC) with different properties.
Adenosine is an endogenous purine nucleoside with a broad spectrum of immunomodulatory activities. Adenosine production is significantly increased during lung inflammation, resulting in enhanced extracellular levels of adenosine in patients with BA [12,13]. Adenosine mediates its action via signaling through adenosine receptors (ADORA), namely A1, A2A, A2B and A3. We have shown previously that adenosine, acting via A2B receptors, directs differentiation of monocytes into inflammatory cells characterized by co-expression of monocytes/macrophages and DC markers [14]. We demonstrated a pro-inflammatory role of A2B during chronic pulmonary inflammation using a mouse model of allergen-induced chronic pulmonary inflammation [15]. The role of A2B signaling in pro-inflammatory activation of monocytes has been further demonstrated in an allergic-airway inflammation with myeloid cell specific deletion of Adora2b in mouse models [16]. However, little is known about the expression of ADORA in monocytes and their functional properties in patients with BA. In the current study, we performed analysis of ADORA expression in peripheral blood monocytes obtained from patients with BA and healthy subjects. We used an established model of adenosine-driven monocyte differentiation in vitro [14] to demonstrate the functional significance of ADORA expression for differentiation towards cells promoting inflammation. We hypothesized that the expression levels of adenosine receptors in circulating monocytes may predict their differentiation towards pro-inflammatory mature myeloid cells in asthmatics. We found that the expression of ADORA2 mRNA is increased in BA patients and adenosine induces their differentiation towards pro-inflammatory CD14posCD209pos cells. We documented a positive correlation between expression of ADORA2B and IL-6 in human monocytes. These findings may have implications for the rational employment of ADORA antagonists to target adenosine-induced inflammation in patients with BA. Our results support the hypothesis that the expression of A2B receptors in circulating monocytes can play a significant role in the pathogenesis of chronic airway inflammation in asthmatics.
Methods
Subjects
We recruited mild-moderate allergic asthmatic and control subjects (Table 1). Individuals were defined as mild-moderate asthmatics according to “The Global Initiative for Asthma (GINA, 2010)” as previously described [17]. The protocol was approved by The Ethical Committee of the Siberian State Medical University, and written informed consent was obtained from all patients included in the study. All subjects met the following inclusion criteria: males and females aged 18 years or older and 65 years or younger. Asthmatic subjects had no history of other cardiopulmonary diseases. Healthy subjects were negative for allergies and respiratory diseases. The relatively small number of included patients was attributed to the invasive procedure used in the study.
Table 1. Patient demographics.
| Criteria | Bronchial asthma patients |
Control subjects |
|
|---|---|---|---|
| Mild (n=14) | Moderate (n=5) | (n=20) | |
| Age (years) | 37.3 (30.7-43.8) |
37.6 (25.6-49.6) |
32.2 (28.7-35.7) |
| Gender (M/F) | (5/9) | (2/3) | (6/14) |
|
Duration of
disease (years) |
10.4 (6.1-14.6) |
16.8 (0.6-31.1) |
N/A |
|
FEV1, % of
predicted |
87.8 (83.4-92.1) |
68.4 (51.8-85.0) |
ND |
Values reported as mean with mean with 95% confidence intervals.
Definition of abbreviations: N/A – not applicable; ND – not determined; F = females, FEV1 = forced expiratory volume in 1 second, M = males.
Purification of peripheral blood monocytes
Human peripheral blood monocytes purification was performed by two-step gradient centrifugation procedure as described [18] with modifications. Briefly, peripheral blood was diluted with Hank’s balanced salt solution (HBSS) (1:1), loaded on Ficoll–Hypaque gradient (Sigma, Moscow, Russia) and centrifuged for 30 min at 600 × g at room temperature. Peripheral blood mononuclear cells (PBMC) were collected, washed twice in HBSS (pH 7.4), resuspended in serum-free RPMI and mixed with 1.5× volume of isotonic Percoll solution (IPS) (percoll:PBS, 9:1 v/v, p=1,123 g/ml). Then cells was carefully overlaid with Percoll-RPMI solution 1 (IPS:RPMI, p= 1,064g/ml) and Percoll-RPMI solution 2 (IPS:RPMI, p=1,032g/ml). Monocytes were collected from RPMI/percoll interface after centrifugation at 2000 ×g for 50 min at 20°C. The purity of monocytes was 75–85% as determined after analysis of CD14pos cells using a FACSCalibur flow cytometer.
In vitro culture and stimulation of human peripheral blood monocytes
Isolated monocytes were resuspended in 10% FBS RPMI medium containing 20 mM HEPES, 50 μM β-mercaptoethanol, 1× antibiotic antimycotic mix (Sigma, Moscow, Russia) and supplemented with 10 ng/ml of human GM-CSF and human IL-4 (both from ProSpec-Tany technoGene, Ness Ziona, Israel). Monocytes were seeded in 24 well plates at concentration of 2 × 105 cell/well and stimulated in the absence (DMSO) or presence of 30 μM stable adenosine analog NECA for 3 days at 37°C in CO2-incubator.
Real-time reverse transcription-polymerase chain reaction
Total RNA was isolated from purified monocytes using RNeasy Mini kit (Qiagen, Valencia, CA). Real-time RT-PCR was performed on a DT-96 Sequence Detection System (Dna-technology, Moscow, Russia). For human ADORA1 forward primer was: 5′-CTACTTCCACACCTGCCTC-3′, and the reverse primer was – 5′-GTCACCACCATCTTGTAC-3′; human ADORA2A: 5′-GAGCTCCATCTTCAGTCTCC-3′ (forward), 5′-GCATGGGAGTCAGGCCGATG-3 (reverse); human ADORA2B: 5′-GTCGACAGATACCTGGCCATC-3′ (forward), 5′-CAGTTGTTGGTGGCACTGTC-3′ (reverse); human ADORA3 primers: 5′-GTTGTCCGCAAGGCTGACC-3′(forward), 5′-CAAATGACTGATTACAGAG-3′(reverse). The human VEGFA forward primer was 5′-GGGCAGAATCATCACGAAGTG-3′, and the reverse primer was 5′-ATTGGATGGCAGTAGCTGCG-3′; for human IL-8, the forward primer was 5′-TGCCAAGGAGTGCTAAAG-3′ and the reverse primer was 5′-TCCACAACCCTCTGCAC-3′, human IL-6 forward: 5′-CACAGACAGCCACTCACCTC-3′, reverse – 5′-TTTTCTGCCAGTGCCTCTTT-3′. For human ACTB the forward primer was 5′-CGCCCCAGGCACCAGGGC-3′, and the reverse primer was 5′-GGCTGGGGTGTTGAAGGT-3′.
The relative mRNA quantity for a given gene measured from a single reverse transcription reaction was divided by the value obtained for β-actin to correct for fluctuations in input RNA levels and varying efficiencies of reverse transcription reactions.
Flow cytometry
After treatment with FcR Blocking Reagent (Miltenyi Biotec Inc., Auburn, CA), monocytes (106 cells/ml) were labeled using relevant antibodies for 20 minutes on ice. All antibodies were obtained from BD Bioscience Pharmingen (San Jose, CA). Data acquisition was performed on a FACScalibur flow cytometer, and the data were analyzed with WinList 5.0 software. Non-viable cells were excluded by using 7-amino actinomycin D. Antigen negativity was defined as having the same fluorescent intensity as the isotype control.
Statistical Analysis
Normally distributed variables are expressed as mean ± SEM. Comparisons between two groups were performed using two-tailed unpaired t tests. Data are expressed as median (Me) values with interquartile range (IQR) when distributions are skewed. For variables with skewed distributions, pairwise comparisons of median values were examined using Mann-Whitney test. Comparisons made between three or more groups were carried out using the Kruskal-Wallis test, followed by Dunn’s multiple-comparison posttest. A P value < .05 was considered significant.
Results
Expression of adenosine A2 receptors is increased in monocytes obtained from patients with BA
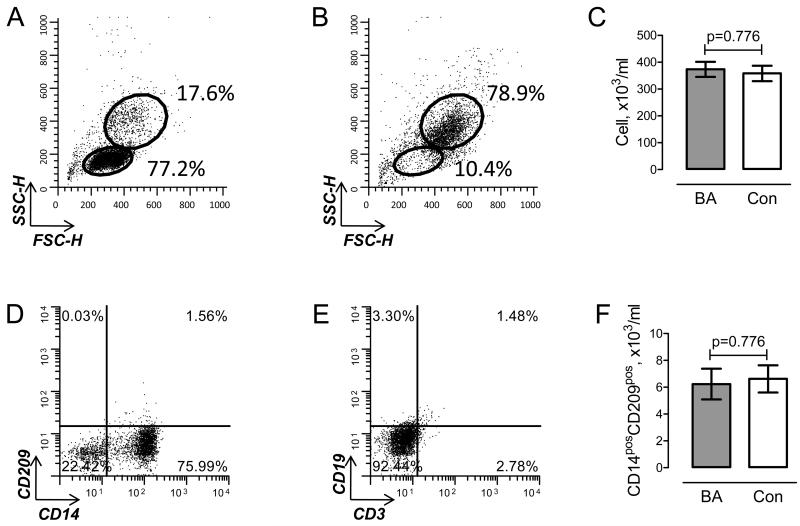
We have previously shown that all four subtypes of adenosine receptor are expressed in human peripheral blood monocytes [14]. Several methods are currently used to isolate human monocytes from peripheral blood. Isolation of CD14-positive cells using FACS sorting or immunoprecipitation provides both high yield and purity of monocytes. However, CD14 is a co-receptor of toll-like receptors and its activation has been found to modify the expression of ADORA [19,20]. To avoid risks associated with antibody-mediated activation, we used a two-step gradient centrifugation procedure [18] to obtain “untouched” monocyte. Figure 1 illustrates our strategy to monitor isolation and characterize the purity of monocytes. An aliquot of mononuclear cells obtained after enrichment using Ficoll–Hypaque gradient was used to determine the percentage of monocytes based on their side and forward scatter characteristics (Fig. 1A). No difference was found in the number of monocytes between two groups (Figure 1C). The purity of monocytes was examined after collecting cells from the interphase of percoll gradient (Fig. 1B). In addition, we characterized the surface expression of CD14, CD209, CD3 and CD19 markers (Fig 1D and E). No difference was seen in the percentage of CD14pos cells between BA and control groups (76.7±1.4 and 80.6±1.8 %, respectively; p=0.102, unpaired t test). Also, no significant presence of CD3pos T cells or CD19pos B lymphocytes was detected after two-step enrichment of monocytes.
Figure 1. Isolation of human peripheral blood monocytes.
Representative flow cutometric dot plots demonstrating SSC-H/FSC-H scatters following Fycoll-Hypaque (A) and Percoll (B) gradient. The upper gate corresponds to “monocyte” and lower gate to “lymphocyte” cell populations. C. Number of monocytes, calculated from percentage of cells in “monocyte” gate and the total number of cells, in patients with bronchial asthma (BA, grey bar) and controls (open bar). D, E. Representative dot plots showing cell surface expression of CD14 and CD209 (D), and CD3 and CD19 (E) in subpopulation of isolated monocytes. F. Number of CD14posCD209pos monocytes in BA and control. P values are indicated; unpaired t test.
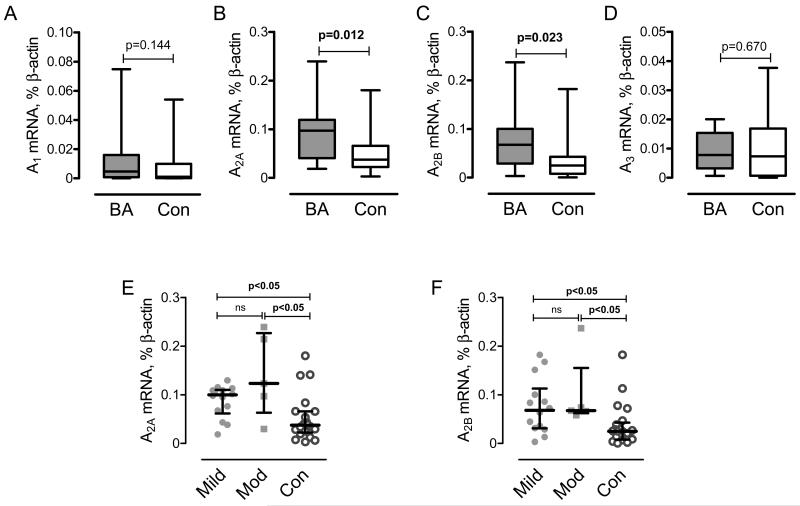
Examination of ADORA expression in monocytes from the study groups revealed that mRNA levels of both ADORA2A and ADORA2B were significantly increased in the asthmatics compared to control subjects (Fig 2B and 2C). However, we found no statistically significant difference in the expression of A2 receptors between patients with mild and moderate asthma (Fig. 2E and F). Therefore, our data indicate that changes in the expression of A2 receptors on monocytes may be an early event in BA development. In contrast, ADORA1 and ADORA3 were characterized by lower levels of mRNA expression compared to A2 receptors and no statistical difference between all groups was found (Fig. 2A and D).
Figure 2. Characterization of adenosine receptors expression in human monocytes obtained from patients with bronchial asthma or healthy donors.
Human monocytes were purified from peripheral blood; real-time RT-PCR analysis of mRNA encoding A1 (A), A2A (B), A2B (C) and A3 (D) adenosine receptor subtypes was performed as described under the Materials and Methods section. Data are presented in standard percentile format (minimum, 25th percentile, 50th percentile, 75th percentile, and maximum). Differences between patients with bronchial asthma (BA, n=19) and healthy donors (Con, n=20) were analyzed using Mann-Whitney test. E, F. Expression of A2A (E) and A2B (F) receptor mRNA in patients with mild (Mild) and moderate (Mod) BA and control subjects (Con). P values indicate significance level calculated by Dunn’s multiple comparison post-test after Kruskal-Wallis test (p=0.008 for A2A; p=0.009 for A2B). ns, non-significant.
Stimulation of adenosine receptors results in higher yield of CD14posCD209pos cells in patients with BA
We have previously shown that adenosine-dependent activation of A2B receptors results in differentiation of human monocytes towards distinct cell population characterized by co-expression of monocytes/macrophages and dendritic cell markers, CD14 and CD209. These adenosine-differentiated DCs produce high levels of pro-inflammatory cytokines and growth factors [14]. To determine if the increased expression of adenosine receptors in BA patients leads to accumulation of CD14posCD209pos cells in peripheral blood in vivo, we determined their number in purified subpopulation of monocytes obtained from BA patient and control subjects (Fig. 1D). No difference in the number of CD14posCD209pos cells was found in peripheral blood between these groups (Fig 1F). Considering that concentrations of adenosine in peripheral blood are low (<100 nM) [21], and that differentiation of infiltrating monocytes into pulmonary dendritic cell occurs in the lung tissue where adenosine levels in asthmatics can reportedly reach concentrations in the high micromolar range [12,13], we next explored if the increased expression of A2 receptors on monocytes of BA patients could potentially lead to elevated generation of CD14posCD209pos cells.
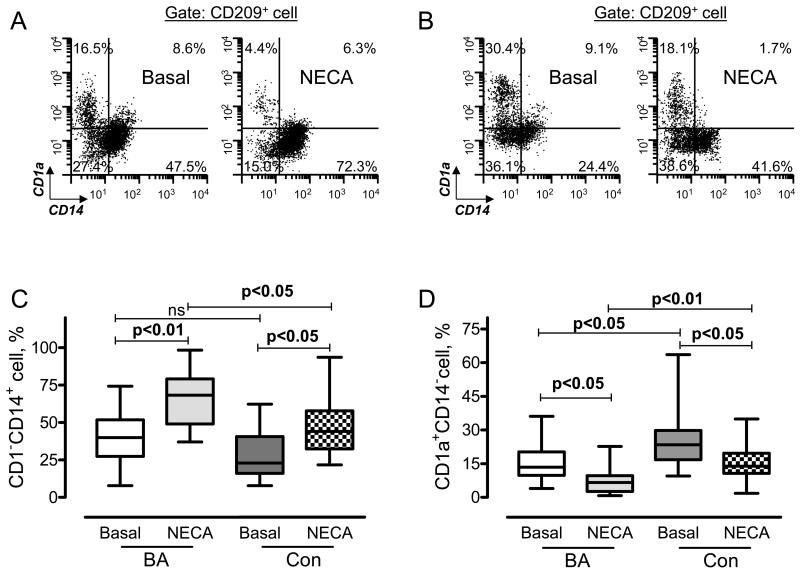
To mimic differentiation of human monocytes into DC in the lung tissue, we used a well-characterized culture system [22]. When cultured in vitro in the presence of GM-CSF and IL-4, human monocytes differentiate into DC. They acquire specific morphological features and markers including CD1a and CD209, but lose their CD14 marker. In our previous study, we demonstrated that stable cell-impermeable adenosine analog NECA affects differentiation of monocytes and dramatically decreases production of CD1a-positive cells while preserving the surface expression of the monocyte marker CD14 [14].
In the present study, we incubated monocytes obtained from asthmatics and control subjects in conditions that favor their differentiation into DCs in the absence or presence of NECA for three days. This time point was chosen based on previously performed characterization of time-course changes with a maximal effect of NECA seen by 72 h [14]. Cells expressing CD14 or CD1a antigens were determined within CD209pos cell population (Fig. 3A and B). No difference was found in the percentage of CD209-expressing cells between these two groups in the absence of NECA (70.5±2.1 and 68.8±2.6 for BA and control, respectively (d3); p=0.612, unpaired t test). As expected, stimulation of cells with NECA preserved the surface expression of CD14 in both asthmatics and controls, compared to unstimulated cells. However, NECA was more effective in the group of BA patients, as indicated by a 1.4-fold increase in the percentage of CD14pos cells, compared to the control subjects (Fig. 3C). Elevated capability of NECA to preserve the expression of CD14 in asthmatics was accompanied by a 2.2-fold decrease in the number of CD1apos cells (Fig. 3D), demonstrating the functional significance of the upregulation of A2 adenosine receptors on monocytes in patients with BA for promotion of their potential differentiation towards pro-inflammatory CD14posCD209pos cells.
Figure 3. Effect of stimulation of adenosine receptors on CD1a and CD14 cell markers expression.
The expression of CD1a, CD14 and CD209 cell surface markers was assessed by flow cytometry after incubation of PB monocytes with 20 ng/ml of GM-CSF and 20 ng/ml of IL-4 in the absence (Basal) or presence of 30 μM NECA for 72 hours. Representative cytofluorographic dot plots showing the percentage of CD1aposCD14neg (upper left quadrant) and CD1anegCD14pos (lower right quadrant) cells within the CD209 positive cell population in patients with bronchial asthma (A) and healthy donors (B). (C) Graphic representation of data from flow cytometry analysis of CD1anegCD14pos cells obtained from nineteen patients with bronchial asthma (BA) or twenty control subjects (Con). (D) The percentage of CD1a+CD14− cells in patients with bronchial asthma (BA, n=19) or healthy donors (Con, n=20). Data are presented as minimum, 25th percentile, 50th percentile, 75th percentile, and maximum. The Kruskal-Wallis test was significant (p <0.0001) for between group differences. Dunn’s multiple comparison post-test P values between groups are shown.
IL-6 expression is elevated in patients with BA
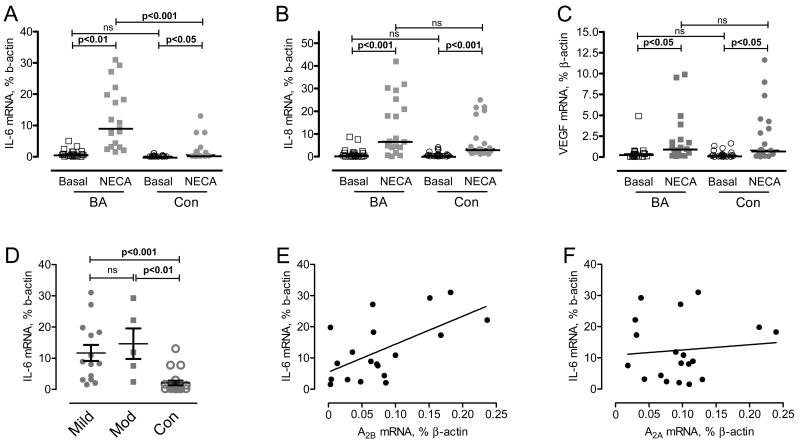
We also examined a potential link of the increased expression of A2 adenosine receptors in asthmatics with the expression of IL-6, IL-8 and VEGF, factors contributing to the pathogenesis of BA [23,24,25]. In agreement with previously documented adenosine actions [14,26,27,28], stimulation of adenosine receptors resulted in the induction of IL-6, IL-8 and VEGF mRNA expressions in both asthmatics and control subjects (Fig. 4). Our analysis, however, revealed higher levels of IL-6 mRNA in cells stimulated with NECA in the group of BA patients compared to healthy controls (Fig. 4A). While no difference was found between patients with mild and moderate asthma, the expression of IL-6 was increased in each asthma severity group compared to control subjects (Fig. 4D). A positive correlation was found between levels of NECA-induced IL-6 and ADORA2B mRNA expression (Fig. 4E). It is likely, therefore, that the increased expression of IL-6 mRNA in asthmatics is due to a higher level of A2B receptors. In contrast, no correlation was found between the levels of IL-6 cytokine and A2A receptors (Fig. 4F), confirming our previously reported data about the specific role of A2B receptors in regulation of IL-6 production in monocytes [26]. No significant differences were found in the expression of both IL-8 and VEGF in cells stimulated with NECA between these two study groups.
Figure 4. Expression of IL-6, IL-8 and VEGF in monocytes.
Real-time RT-PCR analysis of mRNA transcripts of IL-6 (A), IL-8 (B) and VEGF (C) was performed as described under the Materials and Methods section. Graphs are presented as scatter dot plots and the horizontal line indicate the median values for each group. P values indicate significance level calculated by Dunn’s multiple comparison post-test after Kruskal-Wallis test (p<0.001 for IL-6 and VEGF; p<0.007 for IL-8). D. Expression of IL-6 in patients with mild (Mild), moderate (Mod) asthma and controls (Con). E, F. Correlation between NECA-induced IL-6 and A2B receptor mRNA (rs=0.467, p=0.041) (E) or A2a (rs=0.040, p=0.869)(F) in BA patients.
Discussion
In the current study, we investigated the expression of adenosine receptors in monocytes obtained from patients with BA and control subjects. We have shown previously that adenosine, acting via A2B receptors, was involved in the augmentation of pro-inflammatory activation of monocytes [14,26,29].
The important role of adenosine and adenosine receptors in activation of monocytes and monocyte-derived mature myeloid cells (macrophages and inflammatory DCs) has been demonstrated in many studies using animal models of lung inflammation [15,16,30,31]. Growing evidence indicates that levels of adenosine receptors expression are changed in the lungs of patients with BA and chronic obstructive pulmonary disease [32,33]. It has also been shown that stimulation of A1 and A2B receptors leads to pro-inflammatory activation of monocytes whereas signaling through A2A or A3 is associated with anti-inflammatory effects of adenosine [34]. In humans, however, different genomic backgrounds considerably contribute to the pro-inflammatory activation and differentiation of monocytes. Understanding the heterogeneity of inflammatory mechanisms underlying the lung inflammation will help to develop a personalized treatment targeting inflammation in patients with BA. Our current study indicates that the expression of ADORA2A and ADORA2B but not ADORA1 or ADORA3 is increased in monocytes from asthmatics. We have shown previously that stimulation of A2B receptors promotes differentiation of human monocytes into pro-inflammatory adenosine-differentiated DCs [14], suggesting that enhanced A2B signaling can further promote inflammation. Indeed, our current study demonstrated that an increase in ADORA2 expression in monocytes from patients with BA is associated with their elevated capability to differentiate into CD14posCD209pos pro-inflammatory cells. It would be interesting in the future to determine specific contributions of A2A and A2B receptor subtypes in the promotion of pro-inflammatory phenotype of DCs in the lung tissue of patients with BA.
To the best of our knowledge, this is the first work that demonstrates positive correlation between the expression of ADORA2B and IL-6 in human monocytes. It has been shown that the level of IL-6 protein is increased in asthmatics [23]. IL-6 contributes to impaired lung function in allergic asthma and therefore plays a more significant role than a pro-inflammatory marker in the lung, contributing (at least in part) to the progression of asthma. The pathophysiology of asthma is complex and involves different genomic background. The expression of adenosine receptors are characterized by large inter-individual variability. It is very likely that patients with the increased expression of ADORA2 receptors will be at higher risk for adenosine-induced inflammation. Our results suggest that IL-6 could be used as a marker for rational employment of ADORA antagonist therapy and monitoring of adenosine-induced inflammation. Adenosine receptor antagonists are currently under development by the pharmaceutical industry [35,36,37].
Supplementary Material
We performed analysis of ADORA expression on monocytes from patients with bronchial asthma and healthy subjects.
Expression of ADORA2A and ADORA2B is increased in asthmatics.
Adenosine induces higher accumulation of pro-inflammatory CD14posCD209pos cells in asthmatics.
Positive correlation between ADORA2B and IL-6 expression is exist in human monocytes.
Acknowledgements
A special thanks to Rutwik Rath, MS for assistance in preparation of the manuscript. This research was supported in part via 2010-1.5-504-004-012 Russian Federal Special Program Grant, Vanderbilt Institute for Clinical and Translational Research CTSA grant VR2497, NIH/NIGMS P30 GM106391, COBRE in Stem & Progenitor Cell Biology and Regenerative Medicine (Pilot Project funding and flow cytometry analyses via the Progenitor Cell Analysis Core Facility) and COBRE P30GM30992 Pilot Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Feoktistov is a co-author on a patent: I. Biaggioni, I. Feoktistov, J.N. Wells. Selective Antagonists of A2B Adenosine Receptors. US 6,806,270 B2 issued on October 19 and US 6,815,446 B1 issued on November 9, 2004 with royalties paid by Gilead Palo Alto, Inc.
References
- [1].Henderson RB, Hobbs JA, Mathies M, et al. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- [2].Maus UA, Waelsch K, Kuziel WA, et al. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol. 2003;170:3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- [3].Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- [4].Lambrecht BN, Hammad H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat Rev Immunol. 2003;3:994–1003. doi: 10.1038/nri1249. [DOI] [PubMed] [Google Scholar]
- [5].Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- [6].Chen L, Zhang Z, Barletta KE, et al. Heterogeneity of lung mononuclear phagocytes during pneumonia: contribution of chemokine receptors. Am J Physiol Lung Cell Mol Physiol. 2013;305:L702–711. doi: 10.1152/ajplung.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Rijt LS, Jung S, Kleinjan A, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Osterholzer JJ, Chen GH, Olszewski MA, et al. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–8053. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- [10].Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- [11].Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38:322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- [12].Driver AG, Kukoly CA, Ali S, et al. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- [13].Huszar E, Vass G, Vizi E, et al. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- [14].Novitskiy SV, Ryzhov S, Zaynagetdinov R, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zaynagetdinov R, Ryzhov S, Goldstein AE, et al. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol. 2010;42:564–571. doi: 10.1165/rcmb.2008-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Belikoff BG, Vaickus LJ, Sitkovsky M, et al. A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic-airway inflammation. J Immunol. 2012;189:3707–3713. doi: 10.4049/jimmunol.1201207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Selivanova PA, Kulikov ES, Kozina OV, et al. Differential expression of the beta2-adrenoreceptor and M3-cholinoreceptor genes in bronchial mucosa of patients with asthma and chronic obstructive pulmonary disease. Ann Allergy Asthma Immunol. 2012;108:39–43. doi: 10.1016/j.anai.2011.10.002. [DOI] [PubMed] [Google Scholar]
- [18].de Almeida MC, Silva AC, Barral A, et al. A simple method for human peripheral blood monocyte isolation. Mem Inst Oswaldo Cruz. 2000;95:221–223. doi: 10.1590/s0074-02762000000200014. [DOI] [PubMed] [Google Scholar]
- [19].Murphree LJ, Sullivan GW, Marshall MA, et al. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Panther E, Idzko M, Herouy Y, et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- [21].Vizi E, Huszar E, Csoma Z, et al. Plasma adenosine concentration increases during exercise: a possible contributing factor in exercise-induced bronchoconstriction in asthma. J Allergy Clin Immunol. 2002;109:446–448. doi: 10.1067/mai.2002.121955. [DOI] [PubMed] [Google Scholar]
- [22].Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8:1281–1290. doi: 10.7150/ijbs.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yalcin AD, Bisgin A, Gorczynski RM. IL-8, IL-10, TGF-beta, and GCSF levels were increased in severe persistent allergic asthma patients with the anti-IgE treatment. Mediators Inflamm. 2012;2012:720976. doi: 10.1155/2012/720976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kanazawa H, Hirata K, Yoshikawa J. Involvement of vascular endothelial growth factor in exercise induced bronchoconstriction in asthmatic patients. Thorax. 2002;57:885–888. doi: 10.1136/thorax.57.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ryzhov S, Zaynagetdinov R, Goldstein AE, et al. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- [27].Ryzhov S, Biktasova A, Goldstein AE, et al. Role of JunB in adenosine A2B receptor-mediated vascular endothelial growth factor production. Mol Pharmacol. 2014;85:62–73. doi: 10.1124/mol.113.088567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ryzhov S, Goldstein AE, Biaggioni I, et al. Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol. 2006;70:727–735. doi: 10.1124/mol.106.022780. [DOI] [PubMed] [Google Scholar]
- [29].Ryzhov SV, Pickup MW, Chytil A, et al. Role of TGF-beta Signaling in Generation of CD39+CD73+ Myeloid Cells in Tumors. J Immunol. 2014;193:3155–3164. doi: 10.4049/jimmunol.1400578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mohsenin A, Mi T, Xia Y, et al. Genetic removal of the A2A adenosine receptor enhances pulmonary inflammation, mucin production, and angiogenesis in adenosine deaminase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L753–761. doi: 10.1152/ajplung.00187.2007. [DOI] [PubMed] [Google Scholar]
- [31].Young HW, Sun CX, Evans CM, et al. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006;35:549–558. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Y, Murthy JN, Zeng D, et al. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One. 2010;5:e9224. doi: 10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Selman M, Carrillo G, Estrada A, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wilson CN. Adenosine receptors and asthma in humans. Br J Pharmacol. 2008;155:475–486. doi: 10.1038/bjp.2008.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Polosa R, Holgate ST. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets. 2006;7:699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- [36].Hasko G, Linden J, Cronstein B, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pastorin G, Federico S, Paoletta S, et al. Synthesis and pharmacological characterization of a new series of 5,7-disubstituted-[1,2,4]triazolo[1,5-a][1,3,5]triazine derivatives as adenosine receptor antagonists: A preliminary inspection of ligand-receptor recognition process. Bioorg Med Chem. 2010;18:2524–2536. doi: 10.1016/j.bmc.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.