Abstract
Elevated levels of endothelin-1 (ET-1), a potent vasoactive peptide, are implicated as a risk factor for cardiovascular diseases by exerting vasoconstriction. The aim of this study was to address whether ET-1, at sub-vasomotor concentrations, elicits adverse effects on coronary microvascular function. Porcine coronary arterioles (50–100 μm) were isolated, cannulated and pressurized without flow for in vitro study. Diameter changes were recorded using a videomicrometer. Arterioles developed basal tone (60±3 μm) and dilated to the endothelium-dependent nitric oxide (NO)-mediated vasodilators serotonin (1 nmol/L to 0.1 μmol/L) and adenosine (1 nmol/L to 10 μmol/L). Treating the vessels with a clinically relevant sub-vasomotor concentration of ET-1 (10 pmol/L, 60 minutes) significantly attenuated arteriolar dilations to adenosine and serotonin but not to endothelium-independent vasodilator sodium nitroprusside. The arteriolar wall contains ETA receptors and the adverse effect of ET-1 was prevented by ETA receptor antagonist BQ123, the superoxide scavenger Tempol, the NADPH oxidase inhibitors apocynin and VAS2870, the NOX2-based NADPH oxidase inhibitor gp91 ds-tat, or the p38 kinase inhibitor SB203580. However, ETB receptor antagonist BQ788, H2O2 scavenger catalase, scrambled gp91 ds-tat, or inhibitors of xanthine oxidase (allopurinol), PKC (Gö 6983), Rho kinase (Y27632), and c-Jun N-terminal kinase (SP600125) did not protect the vessel. Immunohistochemical staining showed that ET-1 elicited Tempol-, apocynin- and SB203580-sensitive superoxide production in the arteriolar wall. Our results indicate that exposure of coronary arterioles to a pathophysiological, sub-vasomotor concentration of ET-1 leads to vascular dysfunction by impairing endothelium-dependent NO-mediated dilation via p38 kinase-mediated production of superoxide from NADPH oxidase following ETA receptor activation.
Keywords: Arterioles, Endothelin-1, Endothelium, NADPH oxidase, Superoxide
1. Introduction
Endothelin-1 (ET-1) is an endogenous vasoactive peptide that exerts robust and prolonged vasoconstriction of coronary arteries, with reported half maximal effective concentration (EC50) in the nanomolar range from various mammalian species, including mice (~1.0 nmol/L) [1], rats (~12.5 nmol/L) [2, 3], dogs (~12.5 nmol/L) [3], pigs (~6.7 nmol/L) [4, 5], monkeys (~181 nmol/L) [3], and humans (~3.5 nmol/L) [6, 7]. The extent of coronary vasoconstriction to ET-1 appears to be inversely related to vessel size [8, 9]. The normal circulatory level of ET-1 in the peripheral blood is about 1–3 pmol/L (2–8 pg/mL) [10–15]. In patients with hypertension or ischemic heart disease, the plasma level of ET-1 rises 2- to 4-fold [10–15] and the expression of endothelin type A (ETA) and type B (ETB) receptors mRNA in coronary arteries was significantly higher [16]. An increased circulating level of ET-1 is generally associated with poor clinical outcome and survival rate in patients with myocardial infarction [17–20] and is regarded as an independent predictor of myocardial no-flow, reduced left ventricular function, and long-term mortality [17].
The mechanism by which ET-1 exerts its adverse effect on the cardiovascular system has been suggested to involve severe vasoconstriction and/or the disturbance of vascular redox balance. Accordingly, infusion of ET-1 (1.5 nmol/L) potentiates the increase in coronary resistance in the hearts isolated from spontaneous hypertensive rats in a manner sensitive to the administration of nitric oxide (NO) precursor L-arginine. It is likely that the vasoconstriction elicited by ET-1 may compromise the coronary NO-mediated vasodilator function in this disease model [2]. In addition, exposure of the conduit vasculature to high levels of ET-1 (0.1 and 1 nmol/L) leads to increased superoxide anion production in the vessel wall [21] and may consequently contribute to ET-1-mediated vasoconstriction. However, it is unclear whether ET-1 can influence vasodilation elicited by the endothelial NO in the intact coronary microvasculature without the participation of its vasoconstrictor action. In the present study, we addressed whether a pathophysiological, yet sub-vasomotor concentration of ET-1 is capable of exerting oxidative stress and an adverse effect on endothelium-mediated NO function in small coronary arterioles, which control and regulate resistance and flow in the heart [22]. More specifically, we examined the endothelium-dependent NO-mediated dilation of porcine coronary arterioles in the absence and presence of a sub-vasomotor concentration of ET-1 and investigated the relative contribution of superoxide generating enzymes and stress-activated protein kinases to the pathophysiological effect of ET-1 on coronary vasomotor function.
2. Materials and Methods
2.1 Materials
The investigation conforms to the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). All animal procedures were approved by the Scott & White Institutional Animal Care and Use Committee, and have been described in detail previously [23]. In brief, pigs (8 to 12 weeks old of either sex; 8–14 kg) were anesthetized with Telazol® (4 mg/kg, intramuscular injection) and maintained at a surgical plane with 2–3% isoflurane inhalation. The heart was excised and placed in ice-cold saline. Subepicardial arterioles (1 mm in length; 40–80 μm in internal diameter in situ) were dissected out for in vitro study [23]. Coronary arterioles were cannulated with glass micropipettes and pressurized to 60 cmH2O intraluminal pressure and bathed in physiological salt solution (PSS) at 37°C. The internal diameter of the coronary arteriole was measured using videomicroscopic techniques [23].
2.2 Functional Assessment of Isolated Coronary Arterioles
To characterize the vasomotor response to ET-1, concentration-dependent constriction of isolated coronary arterioles to ET-1 (1 pmol/L to 10 nmol/L) was assessed at 5 minutes after ET-1 administration. To examine the impact of a sub-vasomotor concentration of ET-1 on NO-mediated vasodilation, concentration-dependent responses of coronary arterioles to endothelium-dependent NO-mediated vasodilators serotonin (0.1 nmol/L to 0.1 μmol/L) [24] and adenosine (0.1 nmol/L to 10 μmol/L) [24] were examined in the absence (vehicle treatment) and presence of a normal (1 pmol/L) or clinical relevant (10 pmol/L) concentration of ET-1 for 60 minutes. Our preliminary data indicated that treatment of ET-1 for 30 minutes did not consistently inhibit coronary arteriolar dilations to serotonin and adenosine. In contrast, ET-1 treatment for 60 minutes consistently reduced coronary arteriolar dilations to serotonin and adenosine and this inhibitory effect was not further enhanced at 120 minutes. Therefore, a 60-minute treatment was chosen for further study. To test whether ET-1 has a direct effect on the vasodilator machinery of smooth muscle, the ET-1-treated vessels were challenged with the endothelium-independent vasodilator sodium nitroprusside (SNP), which has been shown to lack reactivity with superoxide [25] and to elicit vasodilation via activation of guanylyl cyclase through formation of nitrosonium cation [25–27].
The roles of ET receptor subtypes, ETA and ETB, in mediating the ET-1 effect were assessed by co-incubating the vessels with ET-1 (10 pmol/L) and ETA receptor blocker BQ-123 and ETB receptor blocker BQ-788, respectively, for 60 minutes. The vasodilations to serotonin and adenosine were subsequently assessed. To examine whether the effect of ET-1 is affected by the inhibition of NO synthase (NOS), the vasodilations to serotonin and adenosine were examined in the presence of ET-1 and NOS inhibitor L-NAME (10 μmol/L) [24]. The involvements of superoxide, hydrogen peroxide (H2O2) and peroxynitrite in the adverse effect of ET-1 were also examined by co-incubating the vessels with ET-1 plus superoxide scavengers Tempol (1 mmol/L) [28, 29] or MnTBAP (10 μmol/L) [30], H2O2 scavenger catalase (1,000 units/mL) [31], and peroxynitrite scavenger urate (0.1 mmol/L) [29] for 60 minutes, respectively. To determine the contribution of superoxide generation enzyme NADPH oxidase (NOX) in mediating the ET-1 effect, vasodilations to serotonin and adenosine were studied in separate groups of vessels treated with ET-1 (0.1 nmol/L) in combination with NOX inhibitor apocynin (100 μmol/L) [28], VAS2870 (10 μmol/L) [32], NOX2-based assembly inhibitor gp91 ds-tat (10 μmol/L) [33] or Rac1/NOX2 inhibitor mycophenolic acid (10 μmol/L, Santa Cruz Biotechnology) [34]. The role of xanthine oxidase was investigated using its inhibitor allopurinol (10 μmol/L) [35]. The involvements of protein kinase C (PKC) and c-Jun N-terminal kinase (JNK) were examined by treating another groups of vessels with ET-1 combined with inhibitors for PKC (Gö 6983, 1 μmol/L) [36] and JNK (SP600125, 5 μmol/L; Calbiochem) [37], respectively. The p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (0.1 μmol/L; Calbiochem) [38] and Rho kinase (ROCK) inhibitors Y27632 (0.1 μmol/L; Calbiochem) [39] or H1152 (0.1 μmol/L; Tocris Bioscience) [40] were administered to examine the roles of these kinases in mediating the effects of ET-1. The SP600125, VAS2870, SB203580, H1152, and Gö 6983 were dissolved as stock solutions in dimethyl sulfoxide, which had final concentrations of 0.01%, 0.03%, 0.001%, 0.01%, and 0.01% by volume in the vessel bath, respectively. The mycophenolic acid was dissolved in ethanol (0.05% final bath concentration). The final concentrations of these solvents had no effect on vasomotor function. All drugs, unless otherwise stated, were obtained from Sigma and were dissolved in PSS.
2.3 Immunohistochemical Analysis of Isolated Coronary Arterioles
To identify and localize vascular ETA receptors, coronary arterioles were embedded and frozen in OCT compound (Tissue-Tek) [41]. Frozen sections (10-μm thick) were fixed in 4% paraformaldehyde, and then immunolabeled with specific primary antibodies for ETA receptors (Sigma) and endothelial NOS (eNOS; Santa Cruz Biotechnology). Afterwards, the slides were incubated with rhodamine red-labeled (Jackson Laboratories) and FITC-labeled (Jackson Laboratories) secondary antibodies. Staining control tissues were exposed for the same duration to non-immune serum (Jackson Laboratories) in place of primary antibody. Slides were observed for red (rhodamine red for eNOS) and green (FITC for ETA) images, and analyzed using a fluorescence microscope (Axiovert 200, Zeiss). Merged images were created with ImageJ software.
2.4 Detection of Vascular Superoxide
Superoxide production in isolated coronary arterioles was evaluated with the fluorescent dye dihydroethidium (DHE) as described previously [28, 38]. In this series of studies, coronary arterioles (40 to 100 μm in diameter and 1.5 mm in length) were incubated with PSS vehicle or ET-1 (10 pmol/L) in combination with Tempol (1 mmol/L), apocynin (100 μmol/L), VAS2870 (10 μmol/L), SB203580 (0.1 μmol/L) or Y27632 (0.1 μmol/L), and then stained with DHE (4 μmol/L) for 30 minutes. After being washed, arterioles were embedded in OCT compound (Tissue-Tek) for cryostat sections. The embedded arterioles were cut into 10-μm-thick sections and placed on glass slides. The DHE fluorescence image was taken at excitation/emission wavelength of 360/460 nm with a fluorescence microscope (Axiovert 200, Zeiss). Control and experimental tissues were placed on the same slide and processed under the same conditions. Settings for image acquisition were identical for control and experimental samples.
2.5 Data Analysis
As described previously, coronary vasomotor response to the ET-1 was normalized to resting vessel diameter following development of basal tone [42]. Vascular response to vasodilators was normalized to the maximal diameter changes in response to 100 μmol/L SNP and expressed as a percentage of maximal dilation [43]. The vascular DHE fluorescence images were quantitatively analyzed after subtracting the background fluorescence using ImageJ software (National Institutes of Health) [38]. Statistical comparisons of data were performed by Student’s t test or by one- or two-way ANOVA followed by the Bonferroni multiple range test, as appropriate. Data are expressed as mean±SEM. A value of P < 0.05 was considered significant.
3. Results
3.1 Effect of ET-1 on Endothelium-dependent NO-Mediated Vasodilation
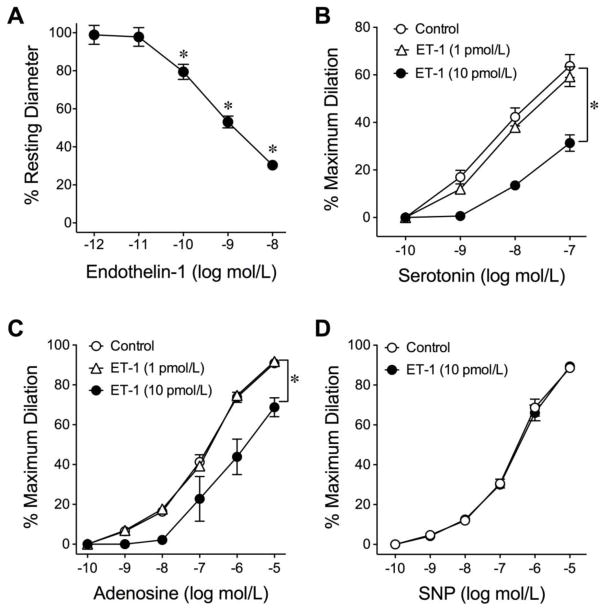
All isolated coronary arterioles developed a similar level of basal tone to about 60±3% of maximal diameter (100±5 μm, range from 64 μm to 115 μm; n=113) within 40 minutes after pressurizing to 60 cmH2O lumenal pressure at 37°C. ET-1 caused a concentration-dependent constriction of coronary arterioles with significant reduction in diameter at concentrations greater than 10 pmol/L (Fig. 1A). The resting vascular diameter was not altered by 10 pmol/L ET-1 (Control: 65±8 μm vs. ET-1: 65±9 μm). To determine whether sub-vasomotor concentrations of ET-1 can modulate vasodilation, the vasomotor responses to the endothelium-dependent NO-mediated vasodilators serotonin[24] and adenosine[24] were assessed. Both serotonin and adenosine elicited concentration-dependent dilation of an isolated coronary arteriole and these vasodilations were attenuated after treating the vessels with ET-1 (10 pmol/L) for 60 minutes (Supplementary Fig. I). The vasodilations to serotonin and adenosine and the adverse effect of ET-1 are summarized in Figs. 1B and 1C, respectively. On the other hand, the vasodilation to SNP, an endothelium-independent smooth muscle relaxation agent was not altered by 10 pmol/L ET-1 (Fig. 1D). The lower concentration of ET-1, 1 pmol/L, had no effect on the vasodilations to serotonin and adenosine (Figs. 1B and 1C).
Fig. 1.
Response of isolated coronary arterioles to ET-1, serotonin, adenosine, and SNP. The low concentrations of ET-1 (1 and 10 pmol/L) had no effect on vascular tone, but higher concentrations (> 10 pmol/L) caused significant vasoconstriction (A) (8 vessels; *P < 0.05 vs. 1 pmol/L ET-1, one-way ANOVA followed by the Bonferroni multiple range test). Dilations of coronary arterioles to serotonin (B) and adenosine (C) were examined before and after incubation with 1 or 10 pmol/L ET-1 for 60 minutes. ET-1 at 10 pmol/L, but not 1 pmol/L, significantly inhibited dilations to serotonin (5 vessels for each set of experiments) and adenosine (5 vessels for each set of experiments). D, A sub-vasomotor concentration of ET-1 (10 pmol/L) did not affect coronary arteriolar dilation to sodium nitroprusside (SNP; 5 vessels for each set of experiments). *P < 0.05 vs. Control, two-way ANOVA followed by the Bonferroni multiple range test.
3.2 Roles of NOS and ET-1 Receptors
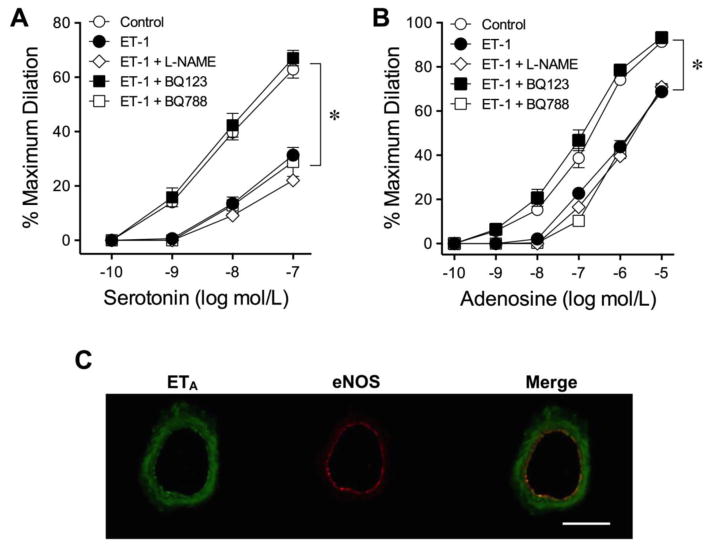
As reported previously, vasodilations of porcine coronary arterioles to serotonin and adenosine are sensitive to NO synthase (NOS) inhibitor L-NAME (10 μmol/L) [24]. However, the attenuated vasodilations to serotonin (Fig. 2A) and adenosine (Fig. 2B) in the presence of ET-1 were not enhanced by L-NAME (10 μmol/L). ET-1 (10 pmol/L) also had no effect on the vasodilations elicited by a calcium-activated potassium channel activator H2O2 [31] and an ATP-sensitive potassium channel opener pinacidil [44] (Supplementary Fig. II).
Fig. 2.
Role of NOS and ET receptors in the adverse action of ET-1. Administration of NOS inhibitor (L-NAME) did not affect the adverse action of ET-1 (10 pmol/L) on vasodilations to serotonin (A) and adenosine (B). However, blockade of ETA receptors by BQ-123 (1 μmol/L) prevented ET-1-induced reduction of vasodilations in response to serotonin and adenosine. The ETB receptor antagonist BQ-788 (0.1 μmol/L) had no impact on the adverse effect of ET-1. 5 vessels for each set of experiments. *P < 0.05 vs. Control, two-way ANOVA followed by the Bonferroni multiple range test. The ETA receptors (green staining) were detected in the vascular wall (C; n = 3). The expression of eNOS protein (red staining) was used to localize the endothelium. Scale bar indicates 50 μm.
The roles of ET receptor subtypes ETA and ETB in mediating the adverse effect of ET-1 were evaluated by treating the vessels with their respective antagonists BQ123 (1 μmol/L) and BQ788 (0.1 μmol/L). As shown in Fig. 2, the vasodilations to both serotonin (Fig. 2A) and adenosine (Fig. 2B) were preserved in the presence of BQ123 but not BQ788. The expression of ETA receptors was detected in both smooth muscle and endothelial cells (Fig. 2C, merged image).
3.3 Roles of Superoxide, H2O2 and Peroxynitrite
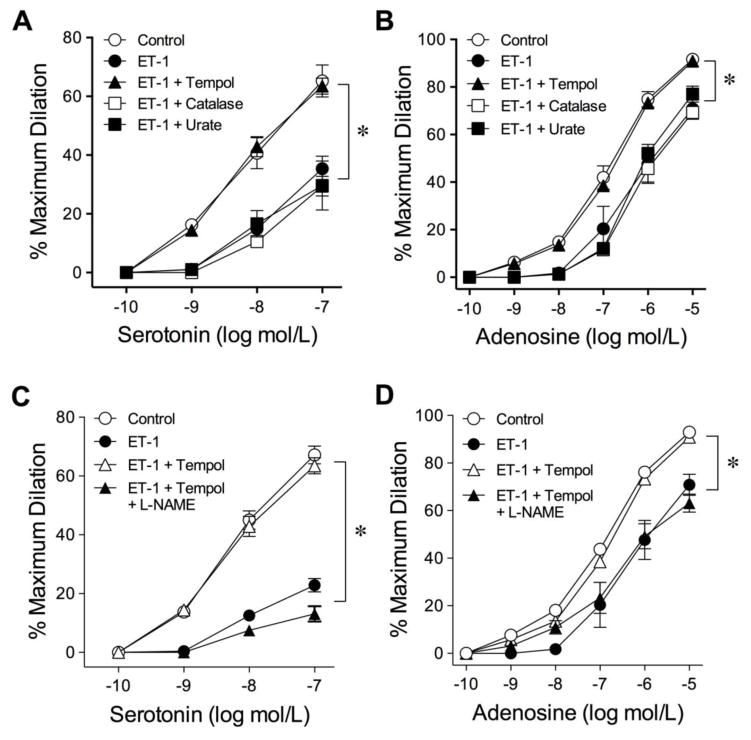
To determine whether reactive oxygen/nitrogen species is involved in the attenuation of endothelium-dependent NO-mediated vasodilation, vessels were treated with ET-1 in the presence of either superoxide scavenger Tempol (1 mmol/L), H2O2 scavenger catalase (1,000 units/mL) or the peroxynitrite scavenger urate (100 μmol/L). Among these scavengers, only Tempol preserved the vasodilations to serotonin (Fig. 3A) and adenosine (Fig. 3B). Administration of another membrane-permeable superoxide dismutase mimetic, MnTBAP, also prevented the ET-1-induced vascular impairment (Supplementary Fig. III). These scavengers did not alter resting tone of the arterioles. To investigate whether the protective effect of Tempol is mediated by the preservation of endothelial NO, the isolated vessels were treated with Tempol and ET-1 in the presence of L-NAME. As shown in Fig. 3C and 3D, the protective effect of Tempol on vasodilations to serotonin and adenosine, respectively, was abolished by L-NAME.
Fig. 3.
Effect of superoxide, hydrogen peroxide and peroxynitrite scavengers on the adverse action of ET-1. The attenuated vasodilations to serotonin (A) and adenosine (B) by ET-1 were prevented by superoxide scavenger Tempol (1 mmol/L). The hydrogen peroxide scavenger catalase (1,000 units/mL) or peroxynitrite scavenger urate (100 μmol/L) had no impact on the adverse effect of ET-1. The preserved vasodilations to serotonin (C) and adenosine (D) by Tempol were abolished in the presence of L-NAME. 5 vessels for each set of experiments. *P < 0.05 vs. Control, two-way ANOVA followed by the Bonferroni multiple range test.
3.4 Roles of NOX and Xanthine Oxidase
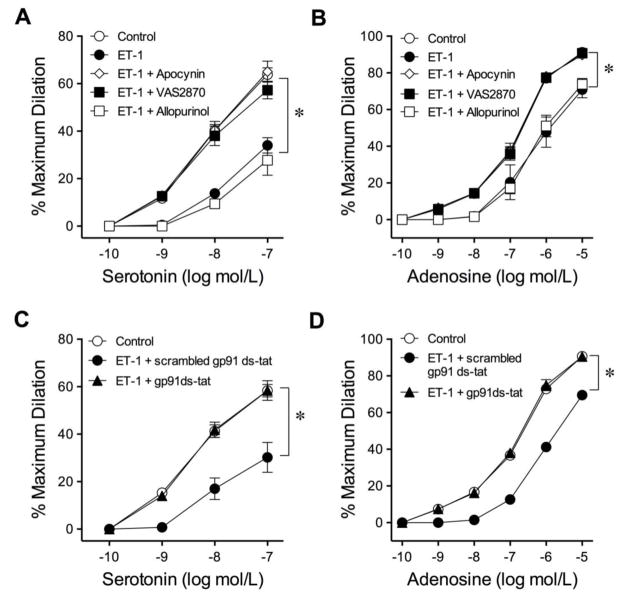
To determine whether NOX or xanthine oxidase contributes to the observed adverse effect, vessels were treated with ET-1 in the presence of inhibitors for NOX (apocynin100 μmol/L, VAS2870 10 μmol/L, or mycophenolic acid 1 μmol/L), or the xanthine oxidase inhibitor allopurinol (10 μmol/L). The inhibitory effects of ET-1 on serotonin- and adenosine-induced dilations were prevented in the vessels treated with apocynin or VAS2870 but not with allopurinol (Fig. 4A and 4B). The NOX2-based assembly inhibitor gp91 ds-tat (Figs. 4C and 4D) and the Rac1/NOX2 inhibitor mycophenolic acid (Supplementary Fig. IV) also preserved the vasodilator function in response to serotonin and adenosine. In contrast, the scrambled gp91 ds-tat did not exert protection on vasodilations (Fig. 4C and 4D). The resting vascular tone was not affected by these inhibitors.
Fig. 4.
Roles of xanthine oxidase and NOX in the adverse action of ET-1. The attenuated vasodilations to serotonin (A) and adenosine (B) by ET-1 were prevented by NOX inhibitors apocynin (100 μmol/L) and VAS2870 (10 μmol/L), but not by xanthine oxidase inhibitor allopurinol (100 μmol/L). Blockade of NOX2 with gp91 ds-tat peptides (100 μmol/L) but not scrambled gp91 ds-tat peptides (10 μmol/L) prevented ET-1-induced reduction of coronary arteriolar dilations to serotonin (C) and adenosine (D). 5 vessels for each set of experiments. *P < 0.05 vs. Control, two-way ANOVA followed by the Bonferroni multiple range test.
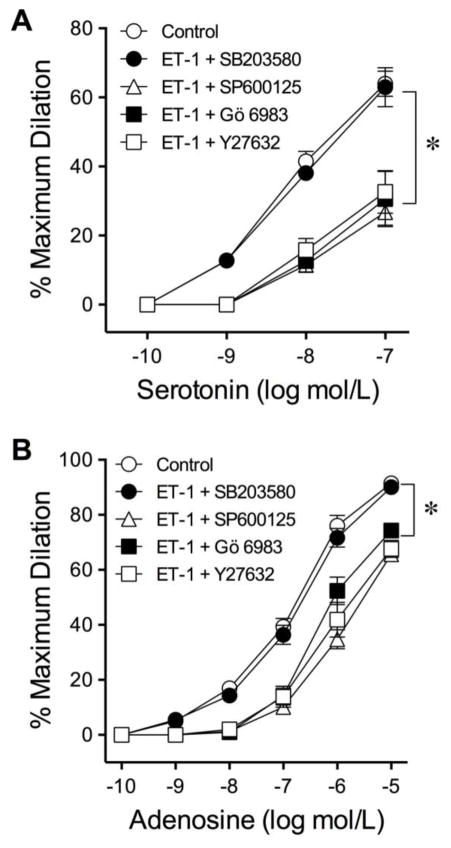
3.5 Roles of Protein Kinases
The involvements of PKC and JNK were examined by treating vessels with ET-1 combined with inhibitor Gö 6983 (1 μmol/L) and SP600125 (5 μmol/L), respectively. SB203580 (0.1 μmol/L) and Y27632 (0.1 μmol/L) were administered to examine the roles of p38 MAPK and ROCK, respectively, in mediating the effects of ET-1. Although administration of PKC inhibitor Gö 6983 prevented the vasoconstriction induced by the PKC activator PDBu (45±3% reduction in resting diameter; n=5), Gö 6983 failed to protect the serotonin- and adenosine-induced vasodilations in the vessels challenged with ET-1 (Fig. 5). The inhibitor of JNK did not alter the adverse effect of ET-1 on vasodilations to serotonin (Fig. 5A) and adenosine (Fig. 5B). Administration of ROCK inhibitor Y27632 (Fig. 5) or H1152 (Supplementary Fig. IV) also had no impact on the effect of ET-1. However, p38 MAPK inhibitor SB203580 preserved the vasodilator responses (Fig. 5). No change of vascular tone was detected in the presence of these inhibitors alone.
Fig. 5.
Roles of PKC, ROCK, JNK and p38 MAPK in the adverse action of ET-1. Blockade of p38 MAPK by SB203580 (0.1 μmol/L) prevented ET-1-induced reduction of coronary arteriolar dilations to serotonin (A) and adenosine (B). Administration of inhibitors for PKC (Gö 6983, 1 μmol/L), JNK (SP600125, 5 μmol/L), or ROCK (Y27632, 0.1 μmol/L) had no impact on the adverse effect of ET-1. 5 vessels for each set of experiments. *P < 0.05 vs. Control, two-way ANOVA followed by the Bonferroni multiple range test.
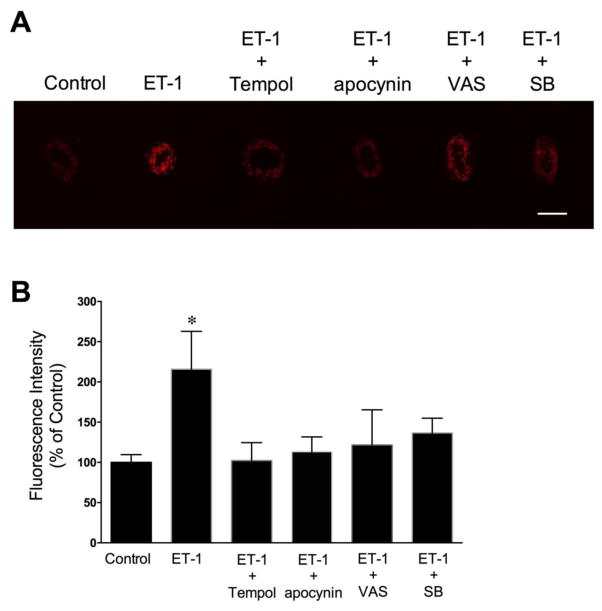
3.6 Effect of ET-1 on Vascular Superoxide Production
The level of superoxide generation in isolated coronary arterioles was assessed by dihydroethidium (DHE) histochemical staining. In the absence of ET-1 (i.e., vehicle control), DHE fluorescence revealed minimal levels of superoxide in the vessel wall (Fig. 6A). In contrast, incubation of vessels with ET-1 (10 pmol/L; 60 minutes) markedly increased superoxide production in the vascular wall (Fig. 6A). Tempol, apocynin, VAS2870 and SB203580 markedly reduced the ET-1-induced fluorescent signals for superoxide (Fig. 6A and 6B). However, the elevated superoxide production by ET-1 was not affected by the ROCK inhibitor Y27632 (0.1 μmol/L) (Supplementary Fig. V).
Fig. 6.
Dihydroethidium imaging of superoxide production in coronary arterioles. (A) The elevated superoxide level by ET-1 (10 pmol/L) was prevented by superoxide scavenger Tempol (1 mmol/L), NOX inhibitors apocynin (100 μmol/L) and VAS2870 (VAS; 10 μmol/L), and the p38 MAPK inhibitor SB203580 (SB; 0.1 μmol/L). (B) The corresponding quantitative analysis of DHE fluorescence signals is shown. 4 vessels for each set of experiments. Scale bar indicates 100 μm. *P < 0.05 vs. Control, Student’s t test.
4. Discussion
The present study suggests that a pathophysiological level of ET-1 at a sub-vasomotor concentration modulates coronary arteriolar function by influencing endothelium-dependent vasodilation. This was revealed by the attenuation of endothelium-dependent NO-mediated dilations to adenosine and serotonin after incubation of the vessel with 10 pmol/L ET-1 for 60 minutes. The mechanism underlying the inhibitory effect of ET-1 involves activation of ETA receptors leading to downstream activation of p38 kinase and generation of superoxide from NOX independent of PKC, JNK and ROCK signaling.
ET-1 is a potent vasoconstrictor released mainly from endothelial cells and has been implicated in the development of coronary vascular pathophysiology. In the coronary circulation, the small resistance arterioles (<100 μm in diameter) are more sensitive and responsive to ET-1 than the upstream large arteries [8, 9]. In anesthetized dogs, topical application of ET-1 to the beating heart causes constriction of epicardial arterioles (~100 μm diameter) with EC50 about 1 nmol/L [45], which is slightly higher than our finding of 0.36 nmol/L (pEC50 = 9.5±0.05) in the isolated vessel preparation (Fig. 1A). This discrepancy might be due to differences in experimental model and animal species. It has been shown that the plasma level of ET-1 is significantly elevated in patients with atherosclerosis [46], cardiac syndrome X [47], coronary heart disease [48], and hypertension [49], which all are known to closely associate with microvascular dysfunction. The plasma concentration of ET-1 in healthy subjects is around 1–3 pmol/L (2–8 pg/mL) [10–15] and may increase up to 10 pmol/L [48] in patients with coronary heart diseases. The level of ET-1 (10 pmol/L) that was found to cause endothelial dysfunction in the present study is consistent with that reported under pathophysiological conditions. It should be noted that this concentration of ET-1 did not produce significant vasomotor activity of coronary arterioles (Fig. 1A). It is likely that the underlying mechanism responsible for the development of vascular dysfunction is different from the pathways involved in the activation of contractile machinery by ET-1 in the coronary microvasculature.
We have previously characterized the component of endothelium-dependent NO-mediated dilation of porcine coronary arterioles in response to serotonin and adenosine [38, 43, 50–52]. These vasodilations were selectively reduced by substances known to reduce the bioavailability of NO such as NOS inhibitors, C-reactive protein, activation of L-arginine consuming enzyme arginase, and the endothelial disruption [38, 43, 50–52]. Both adenosine and serotonin are important endogenous vasodilators for maintaining coronary perfusion, in view that adenosine contributes to the metabolic regulation of coronary blood flow and the released serotonin is known to facilitate coronary microvascular flow during platelet aggregation. Incubation of coronary arterioles with ET-1 (10 pmol/L) led to a reduction in vasodilations to these agonists comparable to that observed in the presence of NOS inhibitor L-NAME [38, 43, 50, 51]. Moreover, the inhibitory effect of ET-1 was not further enhanced by L-NAME (Fig. 2A and 2B), suggesting that the component of NO-mediated vasodilation is compromised by ET-1. On the other hand, the vasodilations to the endothelium-independent vasodilator SNP (Fig. 1D) and potassium channel openers (H2O2 [31] and pinacidil [44]) independent of NO were not affected by ET-1 (Supplementary Fig. II), suggesting that the relaxing capability of the smooth muscle was not compromised. Collectively, these results indicate that ET-1 exerts an adverse vascular effect by selectively impairing endothelial NO function. A previous study reported that a 24-hour treatment of cultured venous endothelial cells with 10–100 nmol/L ET-1, concentrations that are noted to induce maximum constriction of coronary arterioles in the present study, caused inhibition of NO production.[53] Although the finding of the above study is consistent with our result, it appears that the intact microvessel is more sensitive than those cultured cells in view that a sub-vasomotor level of ET-1 detected in the patients is sufficient to exert microvascular dysfunction related to endothelial NO deficiency. It is worth noting that our study was performed in the juvenile pigs, so extrapolating the present finding to the adult animal should be cautious because vascular susceptibility to ET-1 might vary with age.
Both vascular endothelial [54] and smooth muscle [55] cells have been shown to express ETA and ETB receptors with a predominant role of ETA receptors for smooth muscle contraction and endothelial ETB receptors for activation of NO and prostacyclin release [56]. The population of ETA receptors is also far more dominate than that of ETB receptors in the vasculature [55]. Although the diminished NO production by ET-1 (0.1 μmol/L), linking to PKC signaling and endothelial NOS protein downregulation, has been suggested in cultured (24 hours) venous endothelial cells, the specific receptor subtype exerting the effect was not identified [53]. In the atherogenesis of apolipoprotein E-deficient mice, the diminished NO-mediated aortic relaxation and reduced plasma level of nitrate correlate with increased vascular ET-1 and ET receptor-binding capacity [57]. The observed vascular deficiency was restored by chronic treatment of an ETA receptor antagonist for 30 weeks [57], suggesting the close association between ETA receptor activation and reduced NO production. In the present study, the ETA receptor expression is detected in both endothelial and smooth muscle layers of the coronary arterioles. The inhibitory effect of ET-1 on NO-mediated dilations appears to be mediated by the activation of ETA receptors because its action was prevented by the ETA receptor blocker BQ123 but not by the ETB receptor blocker BQ788 (Fig. 2A and 2B). However, the underlying mechanism responsible for the adverse effect of ET-1 in the present study appears to be different from that observed in cell culture (24 hours) with PKC activation [53] and in an intact animal with upregulated tissue ET-1 (30 weeks) [57], because the current ET-1 effect was not altered by a broad spectrum PKC inhibitor (Fig. 5) but was readily prevented by blocking oxidative stress (Fig. 3) linking to NOX (Fig. 4 and 6).
In certain tissues/organs, ET-1 appears to increase reactive oxygen/nitrogen species, including superoxide, peroxynitrite and H2O2. The released superoxide, following ET-1 stimulation, can be converted to H2O2 by superoxide dismutase or to peroxynitrite after reaction with NO. Since both H2O2 and peroxynitrite have been shown to impair endothelium-dependent NO-mediated vasodilation in coronary arterioles [29, 51], the observed adverse effect of ET-1 in the present study might be the consequence of the formation of H2O2 and/or peroxynitrite. However, administration of reported effective concentrations of scavengers for H2O2 (i.e., catalase) [51] and peroxynitrite (i.e., urate) [29] did not protect the vessels from ET-1 insult (Fig. 3). It is likely that the concentration of H2O2 and peroxynitrite might not reach the level sufficient to exert an adverse effect on vascular function in the present study. Nevertheless, the impaired NO-mediated vasodilation by ET-1 was prevented by a membrane-permeable superoxide scavenger, Tempol, suggesting the important role of superoxide in mediating the adverse effect of ET-1. Tempol itself had no effect on resting vascular tone or vasodilations to SNP, serotonin or adenosine (data not shown), but effectively reduced ET-1-elicited superoxide level in the vascular wall (Fig. 6), suggesting the specificity of its superoxide scavenging and vascular protection. It appears that the effect of Tempol was mediated by the preservation of endothelial NO because L-NAME effectively abolished its protective action (Fig. 3C and 3D). Concurrent to Tempol, another membrane-permeable SOD mimetic, MnTBAP [30], also prevented the ET-1-induced vascular impairment on serotonin- and adenosine-induced vasodilations (Supplementary Fig. III).
Several pathways have been proposed for ET-1-induced superoxide production in various vasculatures. Previous studies suggested that xanthine oxidase and mitochondrial oxidative enzymes partly contribute to ET-1-stimulated vascular production of superoxide in DOCA-salt hypertensive rat [58]. However, the role of xanthine oxidase becomes prominent in small mesenteric arteries compared with aortic tissue in this hypertensive animal model, suggesting the tissue-dependent activation of specific oxidative pathways [58]. It is worth noting that the increased vascular tone by ET-1 can be a consequence of superoxide production by NOX and NOS uncoupling in rat aortic rings [59]. However, our study shows that in the absence of changing vascular tone the ET-1-induced endothelial dysfunction was prevented by apocynin and VAS2870 but not by allopurinol, suggesting the prominent role of NOX in mediating the adverse effect of ET-1. This notion is consistent with data obtained with DHE staining showing that apocynin and VAS2870 prevented the ET-1-enhanced superoxide production in the vascular wall (Fig. 6). The activation of vascular NOX is dependent upon the assembly of multi-subunit protein complexes composed of two membrane catalytic subunits (NOX1-5 and DUOX1-2) [60], at least three cytosolic subunits (p67phox, p47phox, and p40phox), and the regulatory small GTPase Rac1 or Rac2 [61]. In the present study, the effect of ET-1 was sensitive to apocynin [62] and VAS2870 [32], which are non-selective NOX inhibitors by interfering with the enzyme subunit assembly. Interestingly, application of NOX2-based peptide inhibitor gp91 ds-tat [33] (Fig. 4) or Rac1/NOX2 inhibitor mycophenolic acid [34] (Supplementary Fig. IV) prevented the coronary arteriolar dysfunction mediated by ET-1. It appears that NOX2 (gp91phox) is a major NOX subunit exerting the adverse action of ET-1 in isolated coronary arterioles.
Previous studies have shown that various protein kinases, e.g., PKC [40, 42], ROCK [40, 42] and stress-activated kinases p38 MAPK [63] and JNK [64] can participate in the signaling of ET-1-induced vasoconstriction. Although the concentration of ET-1 employed in the present study was not sufficient to activate vasomotor activity, the contribution of the above signaling molecules to the adverse effect of ET-1 cannot be excluded because these kinases have also been shown to associate with oxidative stress in some vascular cells [35, 38, 65, 66]. In the present study, the compromised vasodilations by ET-1 were not affected by inhibitors of PKC, ROCK and JNK (Fig. 5) but were prevented by SB203580, suggesting the involvement of p38 MAPK in vascular impairment. The relationship between p38 MAPK and NOX is controversial. The activation of p38 MAPK by NOX-derived superoxide has been reported in various types of cells in culture, including endothelium [67, 68] and vascular smooth muscle cells [69]. However, in-vivo infusion of angiotensin II causes p38 MAPK-dependent production of superoxide from NOX in cardiac and aortic tissues [70]. The hypoxia-induced superoxide production from NOX was found to be blunted by p38 MAPK inhibitors in human pulmonary endothelial cells [71]. We found that the increased DHE staining for superoxide in the vascular wall was blocked not only by apocynin and Tempol, but also by SB203580 (Fig. 6), supporting the idea that ET-1, at a sub-vasomotor concentration, is capable of generating superoxide in the vascular wall from NOX through p38 MAPK activation.
5. Conclusions
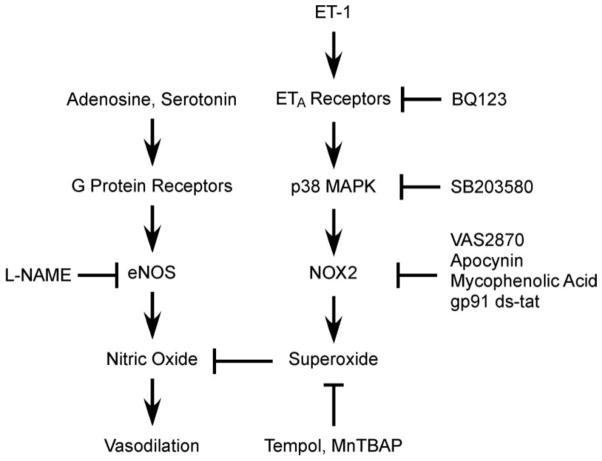
Our study has revealed for the first time that ET-1, at a level found in patients with coronary heart diseases [48], or known to predict vascular disease [17], inhibits the endothelium-dependent NO-mediated dilation of isolated coronary arterioles. The global increase in oxidative stress by ET-1 in the vascular wall (both endothelial and smooth muscle layers) contributes to the reduction of NO bioavailability and consequently leads to vascular dysfunction (Fig. 7). Elevated ET-1 in the heart during the disease state has been proposed to cause vasoconstriction and diminish responses to metabolic stress and thus aggravate myocardial ischemia and reduce survival [17–20]. However, systemic acute infusion of ET-1 to raise its plasma level by 3–12 fold causes inhibition of cardiac function but does not elicit ischemia in human hearts [72–74]. It is worth noting that the ET-1 in patients with coronary diseases is only increased 2–4 fold [10–12, 14], which is unlikely to elicit significant vasoconstriction and consequently compromise coronary perfusion. Interestingly, the arterial and coronary sinus ET-1 levels did not change significantly in the patients who manifest close association between higher resting ET-1 level and the severity of reduced myocardial perfusion [75]. It appears that the reduction in myocardial blood flow in the aforementioned clinical study is not related to the increased discharge of ET-1, if there is any. Instead, the chronic elevation of ET-1 above resting level might have contributed to the observed deficiency of coronary flow. In view that endothelial NO plays important roles in coronary flow regulation, we suggest that the compromised endothelium-dependent NO-mediated vasodilation by NOX-derived superoxide following ETA receptor activation and p38 MAPK signaling may likely trigger clinical events during metabolic stress, although the increased plasma ET-1 per se is not sufficient to elicit vasoconstriction.
Figure 7.
The diagram shows the proposed signaling pathway exerting the adverse effect of ET-1 on endothelium-dependent, nitric oxide-mediated vasodilation. The bioavailability of nitric oxide, following activation of eNOS by G protein-coupled adenosine and serotonin receptors, is reduced by the elevated level of superoxide in the vascular wall. A sub-vasomotor level of ET-1 (10 pmol/L) activates NOX2 for superoxide production through ETA receptor-dependent p38 MAPK activation and consequently compromises vasodilation to nitric oxide. Alleviating the overproduction of superoxide by agents that inhibit the activation of ETA receptors, p38 MAPK and NOX2 or that reduce superoxide level directly protects the vascular insult from ET-1.
Supplementary Material
Highlights.
A subvasomotor level of ET-1 inhibits endothelial NO-mediated coronary dilation.
Blocking ETA receptors, p38 MAPK, or O2− production from NOX2 prevents ET-1 effect.
ET-1-elevated O2− release is sensitive to the inhibition of O2−, NOX, and p38 MAPK.
ET-1 activates ETA receptor and leads to p38 MAP-mediated O2− production from NOX2.
Acknowledgments
We thank Wenjuan Xu and Xin Xu for their technical support. This work was supported by the Scott & White Hospital Kruse Endowment Fund [to L.K.]; and the National Institutes of Health [R01EY018420 and R01EY023335 to T.W.H.].
Appendix A. Supplementary Data
Supplementary Data are available at the Journal of Molecular and Cellular Cardiology online.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bender SB, Klabunde RE. Altered role of smooth muscle endothelin receptors in coronary endothelin-1 and α1-adrenoceptor-mediated vasoconstriction in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H2281–H2288. doi: 10.1152/ajpheart.00566.2007. [DOI] [PubMed] [Google Scholar]
- 2.Miki S, Takeda K, Kiyama M, Hatta T, Moriguchi J, Kawa T, et al. Modulation of endothelin-1 coronary vasoconstriction in spontaneously hypertensive rats by the nitric oxide system. Am J Hypertns. 2000;13:83–87. doi: 10.1016/s0895-7061(99)00100-4. [DOI] [PubMed] [Google Scholar]
- 3.Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, et al. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ushio-Fukai M, Nishimura J, Kobayashi S, Kanaide H. Endothelin-1 and endothelin-3 regulate differently vasoconstrictor responses of smooth muscle of the porcine coronary artery. Br J Pharmacol. 1995;114:171–179. doi: 10.1111/j.1476-5381.1995.tb14922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J App Physiol. 1998;84:884–889. doi: 10.1152/jappl.1998.84.3.884. [DOI] [PubMed] [Google Scholar]
- 6.Wiley KE, Davenport AP. Comparison of the effects of atherosclerosis and nitrate therapy on responses to nitric oxide and endothelin-1 in human arteries in vitro. Clin Sci. 2002;103 (Suppl 48):124S–127S. doi: 10.1042/CS103S124S. [DOI] [PubMed] [Google Scholar]
- 7.Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamping KG, Clothier JL, Eastham CL, Marcus ML. Coronary microvascular response to endothelin is dependent on vessel diameter and route of administration. Am J Physiol. 1992;263:H703–H709. doi: 10.1152/ajpheart.1992.263.3.H703. [DOI] [PubMed] [Google Scholar]
- 9.Homma S, Miyauchi T, Sugishita Y, Goto K, Sato M, Ohshima N. Vasoconstrictor effects of endothelin-1 on myocardium microcirculation studied by the Langendorff perfusion method: differential sensitivities among microvessels. Microvasc Res. 1992;43:205–217. doi: 10.1016/0026-2862(92)90017-j. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann E, Assennato P, Donatelli M, Colletti I, Valenti TM. Plasma endothelin-1 levels in patients with angina pectoris and normal coronary angiograms. Am Heart J. 1998;135:684–688. doi: 10.1016/s0002-8703(98)70286-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaski JC, Elliott PM, Salomone O, Dickinson K, Gordon D, Hann C, et al. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J. 1995;74:620–624. doi: 10.1136/hrt.74.6.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaski JC, Cox ID, Crook JR, Salomone OA, Fredericks S, Hann C, et al. Differential plasma endothelin levels in subgroups of patients with angina and angiographically normal coronary arteries. Coronary Artery Disease Research Group. Am Heart J. 1998;136:412–417. doi: 10.1016/s0002-8703(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda M, Kohno M, Tahara A, Itagane H, Toda I, Akioka K, et al. Circulating immunoreactive endothelin in ischemic heart disease. Am Heart J. 1990;119:801–806. doi: 10.1016/s0002-8703(05)80315-1. [DOI] [PubMed] [Google Scholar]
- 14.Fujii H, Takiuchi S, Kamide K, Horio T, Niizuma S, Tanaka N, et al. Clinical implications of assessing coronary flow velocity reserve and plasma endothelin-1 in hypertensive patients. Hypertens Res. 2005;28:911–916. doi: 10.1291/hypres.28.911. [DOI] [PubMed] [Google Scholar]
- 15.Shichiri M, Hirata Y, Ando K, Emori T, Ohta K, Kimoto S, et al. Plasma endothelin levels in hypertension and chronic renal failure. Hypertension. 1990;15:493–496. doi: 10.1161/01.hyp.15.5.493. [DOI] [PubMed] [Google Scholar]
- 16.Wackenfors A, Emilson M, Ingemansson R, Hortobagyi T, Szok D, Tajti J, et al. Ischemic heart disease induces upregulation of endothelin receptor mRNA in human coronary arteries. Eu J Pharmacol. 2004;484:103–109. doi: 10.1016/j.ejphar.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Eitel I, Nowak M, Stehl C, Adams V, Fuernau G, Hildebrand L, et al. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J. 2010;159:882–890. doi: 10.1016/j.ahj.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Katayama T, Yano K, Nakashima H, Takagi C, Honda Y, Suzuki S, et al. Clinical significance of acute-phase endothelin-1 in acute myocardial infarction patients treated with direct coronary angioplasty. Circ J. 2005;69:654–658. doi: 10.1253/circj.69.654. [DOI] [PubMed] [Google Scholar]
- 19.Omland T, Lie RT, Aakvaag A, Aarsland T, Dickstein K. Plasma endothelin determination as a prognostic indicator of 1-year mortality after acute myocardial infarction. Circulation. 1994;89:1573–1579. doi: 10.1161/01.cir.89.4.1573. [DOI] [PubMed] [Google Scholar]
- 20.Yip HK, Wu CJ, Chang HW, Yang CH, Yu TH, Chen YH, et al. Prognostic value of circulating levels of endothelin-1 in patients after acute myocardial infarction undergoing primary coronary angioplasty. Chest. 2005;127:1491–1497. doi: 10.1378/chest.127.5.1491. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, et al. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 22.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- 23.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991;261:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 24.Hein TW, Zhang C, Wang W, Chang CI, Thengchaisri N, Kuo L. Ischemia-reperfusion selectively impairs nitric oxide-mediated dilation in coronary arterioles: counteracting role of arginase. FASEB J. 2003;17:2328–2330. doi: 10.1096/fj.03-0115fje. [DOI] [PubMed] [Google Scholar]
- 25.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 26.Butler AR, Flitney FW, Williams DL. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist’s perspective. Trends Pharmacol Sci. 1995;16:18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- 27.Severina IS, Bussygina OG, Grigorjev NB. Effect of nitroso complexes of some transition metals on the activity of soluble guanylate cyclase. Biochem Int. 1992;26:695–705. [PubMed] [Google Scholar]
- 28.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 29.Hein TW, Qamirani E, Ren Y, Kuo L. C-reactive protein impairs coronary arteriolar dilation to prostacyclin synthase activation: role of peroxynitrite. J Mol Cell Cardiol. 2009;47:196–202. doi: 10.1016/j.yjmcc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Liao D, Bharadwaj U, Li M, Yao Q, Chen C. C-reactive protein inhibits cholesterol efflux from human macrophage-derived foam cells. Arterioscler Thromb Vasc Biol. 2008;28:519–526. doi: 10.1161/ATVBAHA.107.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 32.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, et al. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 33.Ren Y, D’Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;298:H1769–H1775. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lassegue B, Griendling KK. Mycophenolic acid is a new Nox2 inhibitor. Hypertension. 2007;49:25–26. doi: 10.1161/01.HYP.0000251161.93696.d0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:247–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Young LH, Balin BJ, Weis MT. Gö 6983: a fast acting protein kinase C inhibitor that attenuates myocardial ischemia/reperfusion injury. Cardiovasc Drug Rev. 2005;23:255–272. doi: 10.1111/j.1527-3466.2005.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 37.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 39.Nagaoka T, Kuo L, Ren Y, Yoshida A, Hein TW. C-reactive protein inhibits endothelium-dependent nitric oxide-mediated dilation of retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2008;49:2053–2060. doi: 10.1167/iovs.07-1387. [DOI] [PubMed] [Google Scholar]
- 40.Potts LB, Bradley PD, Xu W, Kuo L, Hein TW. Role of endothelium in vasomotor responses to endothelin system and protein kinase C activation in porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2013;54:7587–7594. doi: 10.1167/iovs13-13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hein TW, Ren Y, Yuan Z, Xu W, Somvanshi S, Nagaoka T, et al. Functional and molecular characterization of the endothelin system in retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3329–3336. doi: 10.1167/iovs.08-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potts LB, Ren Y, Lu G, Kuo E, Ngo E, Kuo L, et al. Constriction of retinal arterioles to endothelin-1: requisite role of rho kinase independent of protein kinase C and L-type calcium channels. Invest Ophthalmol Vis Sci. 2012;53:2904–2912. doi: 10.1167/iovs.12-9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and KATP channels. Circ Res. 1999;85:634–642. doi: 10.1161/01.res.85.7.634. [DOI] [PubMed] [Google Scholar]
- 44.Hein TW, Zhang C, Wang W, Kuo L. Heterogeneous β2-adrenoceptor expression and dilation in coronary arterioles across the left ventricular wall. Circulation. 2004;110:2708–2712. doi: 10.1161/01.CIR.0000134962.22830.CF. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Kanatsuka H, Akai K, Sugimura A, Kumagai T, Komaru T, et al. Effects of low doses of endothelin-1 on basal vascular tone and autoregulatory vasodilation in canine coronary microcirculation in vivo. Jpn Circ J. 1999;63:617–623. doi: 10.1253/jcj.63.617. [DOI] [PubMed] [Google Scholar]
- 46.Ihling C, Bohrmann B, Schaefer HE, Technau-Ihling K, Loeffler BM. Endothelin-1 and endothelin converting enzyme-1 in human atherosclerosis--novel targets for pharmacotherapy in atherosclerosis. Curr Vasc Pharmacol. 2004;2:249–258. doi: 10.2174/1570161043385718. [DOI] [PubMed] [Google Scholar]
- 47.Desideri G, Gaspardone A, Gentile M, Santucci A, Gioffre PA, Ferri C. Endothelial activation in patients with cardiac syndrome X. Circulation. 2000;102:2359–2364. doi: 10.1161/01.cir.102.19.2359. [DOI] [PubMed] [Google Scholar]
- 48.Chai SB, Li XM, Pang YZ, Qi YF, Tang CS. Increased plasma levels of endothelin-1 and urotensin-II in patients with coronary heart disease. Heart Vessels. 2010;25:138–143. doi: 10.1007/s00380-009-1178-6. [DOI] [PubMed] [Google Scholar]
- 49.Saito Y, Nakao K, Mukoyama M, Imura H. Increased plasma endothelin level in patients with essential hypertension. New Engl J Med. 1990;322:205. doi: 10.1056/nejm199001183220315. [DOI] [PubMed] [Google Scholar]
- 50.Hein TW, Kuo L. LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ Res. 1998;83:404–414. doi: 10.1161/01.res.83.4.404. [DOI] [PubMed] [Google Scholar]
- 51.Thengchaisri N, Hein TW, Wang W, Xu X, Li Z, Fossum TW, et al. Upregulation of arginase by H2O2 impairs endothelium-dependent nitric oxide-mediated dilation of coronary arterioles. Arterioscler Thromb Vasc Biol. 2006;26:2035–2042. doi: 10.1161/01.ATV.0000233334.24805.62. [DOI] [PubMed] [Google Scholar]
- 52.Hein TW, Qamirani E, Ren Y, Xu X, Thengchaisri N, Kuo L. Selective activation of lectin-like oxidized low-density lipoprotein receptor-1 mediates C-reactive protein-evoked endothelial vasodilator dysfunction in coronary arterioles. Circ Res. 2014;114:92–100. doi: 10.1161/CIRCRESAHA.114.301763. [DOI] [PubMed] [Google Scholar]
- 53.Ramzy D, Rao V, Tumiati LC, Xu N, Sheshgiri R, Miriuka S, et al. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation. 2006;114:I319–326. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura J, Aoki H, Chen X, Shikasho T, Kobayashi S, Kanaide H. Evidence for the presence of endothelin ETA receptors in endothelial cells in situ on the aortic side of porcine aortic valve. Br J Pharmacol. 1995;115:1369–1376. doi: 10.1111/j.1476-5381.1995.tb16625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bacon CR, Davenport AP. Endothelin receptors in human coronary artery and aorta. Br J Pharmacol. 1996;117:986–992. doi: 10.1111/j.1476-5381.1996.tb15292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 57.Barton M, Haudenschild CC, d’Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viel EC, Benkirane K, Javeshghani D, Touyz RM, Schiffrin EL. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H281–H288. doi: 10.1152/ajpheart.00304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther. 2005;315:1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- 60.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Physiol Resp Cell Mol Physiol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 63.Yamboliev IA, Hedges JC, Mutnick JL, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP27 pathway. Am J Physiol Heart Circ Physiol. 2000;278:H1899–H1907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- 64.Zhou MS, Schulman IH, Chadipiralla K, Raij L. Role of c-Jun N-terminal kinase in the regulation of vascular tone. J Cardiovasc Pharmacol Ther. 2010;15:78–83. doi: 10.1177/1074248409354603. [DOI] [PubMed] [Google Scholar]
- 65.Huang A, Yan C, Suematsu N, Cuevas A, Yang YM, Kertowidjojo E, et al. Impaired flow-induced dilation of coronary arterioles of dogs fed a low-salt diet: roles of ANG II, PKC, and NAD(P)H oxidase. Am J Physiol Heart Circ Physiol. 2010;299:H1476–H1483. doi: 10.1152/ajpheart.01250.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–519. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mo Y, Wan R, Chien S, Tollerud DJ, Zhang Q. Activation of endothelial cells after exposure to ambient ultrafine particles: the role of NADPH oxidase. Toxicol Appl Pharmacol. 2009;236:183–193. doi: 10.1016/j.taap.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 68.Dandapat A, Hu C, Sun L, Mehta JL. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler Thromb Vasc Biol. 2007;27:2435–2442. doi: 10.1161/ATVBAHA.107.152272. [DOI] [PubMed] [Google Scholar]
- 69.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al. Novel gp91phox homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 70.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, et al. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007;49:362–368. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]
- 71.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, et al. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 72.Talbot NP, Balanos GM, Robbins PA, Dorrington KL. Can intravenous endothelin-1 be used to enhance hypoxic pulmonary vasoconstriction in healthy humans? Br J Anaesth. 2008;101:466–472. doi: 10.1093/bja/aen214. [DOI] [PubMed] [Google Scholar]
- 73.Kaasjager KA, van Rijn HJ, Koomans HA, Rabelink TJ. Interactions of nifedipine with the renovascular effects of endothelin in humans. J Pharmacol Exp Ther. 1995;275:306–311. [PubMed] [Google Scholar]
- 74.Kiely DG, Cargill RI, Struthers AD, Lipworth BJ. Cardiopulmonary effects of endothelin-1 in man. Cardiovasc Res. 1997;33:378–386. doi: 10.1016/s0008-6363(96)00219-2. [DOI] [PubMed] [Google Scholar]
- 75.Cox ID, Botker HE, Bagger JP, Sonne HS, Kristensen BO, Kaski JC. Elevated endothelin concentrations are associated with reduced coronary vasomotor responses in patients with chest pain and normal coronary arteriograms. J Am Coll Cardiol. 1999;34:455–460. doi: 10.1016/s0735-1097(99)00224-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.