Abstract
cAMP-PKA protein kinase is a key nodal signaling pathway that regulates a wide range of heart pacemaker cell functions. These functions are predicted to be involved in regulation of spontaneous action potential (AP) generation of these cells. Here we investigate if the kinetics and stoichiometry of increase in PKA activity match the increase in AP firing rate in response to β-adrenergic receptor (β-AR) stimulation or phosphodiesterase (PDE) inhibition, that alter the AP firing rate of heart sinoatrial pacemaker cells. In cultured adult rabbit pacemaker cells infected with an adenovirous expressing the FRET sensor AKAR3, the EC50 in response to graded increases in the intensity of β-AR stimulation (by Isoproterenol) the magnitude of the increases in PKA activity and the spontaneous AP firing rate were similar (0.4±0.1nM vs. 0.6±0.15nM, respectively). Moreover, the kinetics (t1/2) of the increases in PKA activity and spontaneous AP firing rate in response to β-AR stimulation or PDE inhibition were tightly linked. We characterized the system rate-limiting biochemical reactions by integrating these experimentally derived data into mechanistic-computational model. Model simulations predicted that phospholamban phosphorylation is a potent target of the increase in PKA activity that links to increase in spontaneous AP firing rate. In summary, the kinetics and stoichiometry of increases in PKA activity in response to a physiological (β-AR stimulation) or pharmacological (PDE inhibitor) stimuli match those of changes in the AP firing rate. Thus Ca2+-cAMP/PKA-dependent phosphorylation limits the rate and magnitude of increase in spontaneous AP firing rate.
Keywords: Computational modeling, Coupled-clock system, Pacemaker, Phosphorylation
1. Introduction
Protein kinase A (PKA) is a key nodal signaling molecule that regulates a wide range of cellular functions [1]. However, its magnitude, dynamic and spatiotemporal regulation are not known in heart pacemaker cells. Recent live-imaging provides direct measurements of protein kinase dynamics in intact cells from different organs [2, 3], and specifically in the heart [4–6].
In heart pacemaker cells, integrated functions of molecular mechanisms on cell membrane surface and in the sarcoplasmic reticulum (SR) create a system of coupled biological clocks that determine the heart rate [7]. PKA plays and important role in regulating molecular function of variety of cellular locations: e.g. cell membrane (L-type [8], K-channels [9]) and Ca2+ storage in the SR (phospholamban (PLB) [10] and ryanodine receptors (RyR) [11]) (Fig. 1). Ca2+-activated adenylyl cyclase (AC) expressed in pacemaker cells generates high cAMP activity [12, 13]. The high basal rate of cAMP production controls PKA-dependent phosphorylation, and is coupled to high basal phosphodiesterase (PDE) activity, which degrades cAMP and limits the extent to which basal cAMP levels increase, and the extent of pacemaker clock protein phosphorylation [14–16]. In response to β-adrenergic receptor (β-AR) stimulation or PDE inhibition, the coupled-clock intrinsic mechanisms, i.e., cAMP/PKA-dependent phosphorylation levels, become further activated [15], leading to increased action potential (AP) firing rate [17] [18]. Although PKA-dependent mechanisms are linked to AP firing rate, real-time measurements of PKA signaling in the context of heart pacemaker function are lacking. Specifically, it has never been demonstrated that the kinetics and stoichiometry of increases in PKA activity and protein phosphorylation in individual pacemaker cell are similar to and sufficient to account for changes in the kinetics and stoichiometry of increases in AP firing rate that occur in response to physiological (β-AR stimulation) or PDE inhibition. To address this question, we measured changes in kinetics and magnitude of PKA activity and of the spontaneous AP firing rate in response to β-AR stimulation or PDE inhibition. We characterized kinetics of rate-limiting biochemical reactions by integrating these and other experimental data into mechanistic computational model simulations. We found that the kinetics and stoichiometry of increases in PKA activity in response to a physiological (β-AR stimulation) or pharmacological (PDE inhibitor) match those of changes in the AP firing rate. Thus Ca2+-cAMP/PKA-dependent phosphorylation limits the rate and magnitude of changes in spontaneous AP firing rate in response to these stimuli.
Figure 1. Schematic illustration of the coupled-clock pacemaker cell system.
Basal Ca2+-calmodulin activation of adenylyl cyclases (AC) initiates cAMP-PKA-dependent phosphorylation signaling, and in parallel to AC, activates calmodulin-dependent kinase II (CaMKII) phosphorylation signaling. cAMP positively shifts the f-channel activation curve. Phosphodiesterases (PDE) degrade cAMP production, while protein phosphatase (PPT) degrades phosphorylation. PKA and CaMKII phosphorylate: sarcoplasmic reticulum (SR) Ca2+ cycling proteins (ryanodine receptor (RyR), phospholamban (PLB), which bind to and inhibit the sarcoplasmic reticulum Ca2+-ATPase (SERCA)); surface membrane ion channel proteins (L-type, K channel); and mitochondrial ATP production proteins to activate ATP production and sarcomeres to modulate contraction.
2. Materials and Methods
Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The animal protocols have been approved by the Animal Care and Use Committee of the National Institutes of Health (protocol #034LCS2013). To test the hypothesis that the AP firing rate is regulated by PKA activity, we imaged PKA activity and measured AP firing rate of sinoatrial node pacemaker cells (SANC) in response to β-AR stimulation (isoproterenol, ISO), PDE inhibitor (IBMX), and PKA inhibitor (H-89). We characterized their kinetics by calculating the drug concentration that lead to 50% max response (EC50) and the half time to reach steady state response (t1/2). To measure PKA activity, we cultured adult rabbit pacemaker cells for 24 hours, infected them with a 3rd-generation of a genetically encoded A-Kinase activity receptor (AKAR3), and monitored PKA activity and its response to β-AR stimulation or PDE inhibition 24 hours later (see expended view for additional data on the probe properties). Prior experiments in cardiomyocyes [4] and in primary neurons [19] have demonstrated the ability and specificity of this probe to monitor PKA activity. We measured AP firing rate under the same experimental conditions in another subset of cultured cells.
To simulate the expected rate of cAMP production and the kinetics and magnitude of protein phosphorylation, we formulated a novel numerical model that integrated our previously experimentally measured levels of steady-state AC activity, cAMP and phosopholamban phosphorylation in response to perturbations that influenced cAMP/PKA signaling and the changes in the kinetics and magnitude of PKA activity determined experimentally in the present study.
Data are presented as mean±SEM. When the means of paired samples are to be compared, a paired t-test was employed. In cases where the samples are independent, a two-sample t-test was applied. P < 0.05 was taken to indicate statistical significance.
A detailed, expanded description of experimental and numerical methods is available in the on-line supplement.
3. Results
3.1 Imaging PKA FRET probe fluorescence and establishing its dynamic range for reporting changes in PKA activity in response to β-AR stimulation or PDE inhibition
The activation deactivation kinetics and the working range of the FRET probe
To estimate the real-time kinetics and magnitude of change in PKA activity in live single SANC, we measured real-time PKA activity in 48-hour cultured pacemaker cells using a FRET-based A-kinase activity receptor 3 (AKAR3) probe [20] (Fig. 2A). To verify that introduction of the FRET probe did not affect pacemaker function, we determined whether the FRET probe infection alters the spontaneous AP firing rate of cultured SANC. The AP firing rate in the presence and absence of FRET probe did not change (104±10 (n=15) and 101±9 (n=12) beats/min), respectively). Note that we used here only pacemaker cells that were spontaneously beating. For comparison of spontaneously beating to quiescent cells, see online supplement.
Figure 2. FRET based measurements of PKA activity.
(A) Schematic of AKAR3. Phosphorylation of a PKA-specific substrate (LRRATLVD) by PKA leads to its association with the Forkhead association 1 (FHA1) domain bringing the cyan florescent protein (CFP) closer to yellow fluorescent protein (YFP) which results in increased FRET from YFP to CFP and hence an increase in the yellow (FRET)/cyan emission ratio. (B) Changes in PKA activity in response to the PDE inhibitor (IBMX) or to the PKA inhibitor (H-89) in a single cultured SANC. (C) Representative example of the PKA activity (AKAR3 emission ratio Y/C) in a single cultured SANC in response to cumulative increases in the ISO concentration. (D) Representative example of the increase in spontaneous AP firing in response to cumulative increases in the ISO concentration.
To characterize the PKA activation kinetics and its maximum response that could be elicited in these cells, we treated the cells with the PDE inhibitor IBMX (100 µM). PDE inhibition induced a marked and rapid significant increase in PKA activity (R/R0=1.4±0.02, t1/2=116±27s n=7) (Fig. 2B, C). Application of an AC activator (forskolin, 50µM) or β-AR activation (isoproterenol, 100nM) in the presence of IBMX did not further increase PKA activity (Fig. S1). Hence, the average PKA activity elicited by 100 µM IBMX was taken as the maximum PKA activity in these cultured pacemaker cells. To determine the deactivation kinetics and the minimum response of PKA, we applied the PKA inhibitor, H-89 (10 µM). H-89 significantly reduced the IBMX-induced increase in PKA activity (R/R0=1.02±0.03, t1/2=254±25s n=5). Direct application of 10µM H-89, in the absence of PDE inhibition, significantly reduced the basal PKA activity to a level below baseline (R/R0=0.95±0.02, n=4). Because higher concentrations of H-89 did not further decrease the PKA activity, we defined the average PKA activity in response to H-89 alone as the minimum PKA activity. Thus, the working range of the FRET probe in adult cultured rabbit SANC extends from an R/R0 of 0.95 to 1.4.
Response to β-AR stimulation
The aforementioned characterization of the maximum and minimum bounds of PKA activity was achieved through pharmacological agents that inhibit PDE or PKA. Under physiological conditions, however, an increase in AP firing rate is achieved through β-AR stimulation. In order to quantify the kinetics and magnitudes of the increase in PKA activity in SANC in response to a physiologic stimulus, we applied graded increases in the concentration of ISO, a β-AR agonist to the cells. Graded increases in ISO concentrations were accompanied by graded increases in PKA activity (Fig. 2C). Specifically, in response to a low concentration of ISO (< 10nM), there was a rapid increase in PKA activity, which subsequently decayed to a steady-state level. As the concentration of ISO increased, the initial overshoot in PKA activity was reduced, and in response to 100 nM ISO, the maximal initial increase in PKA activity was close to the steady-state level (Fig. 2C). These results of PKA dynamics have been observed in other systems [5]. The EC50 of the steady-state PKA activity increase in response to ISO was 0.4±0.1nM. Interestingly, adding IBMX in the presence of the maximal response to ISO alone only slightly increased PKA activity (R/R0=1.32±0.2 in response to ISO only vs. 1.4±0.02 in response to both ISO and IBMX, p<0.05). This suggests that in cultured SANC physiological stimulation increases the PKA activity almost to its maximal level.
3.2 The changes in kinetics and magnitude of PKA activity in response to graded increases in β-AR stimulation or PDE inhibition are tightly linked to concurrent changes in kinetics and magnitude of the spontaneous AP firing rate
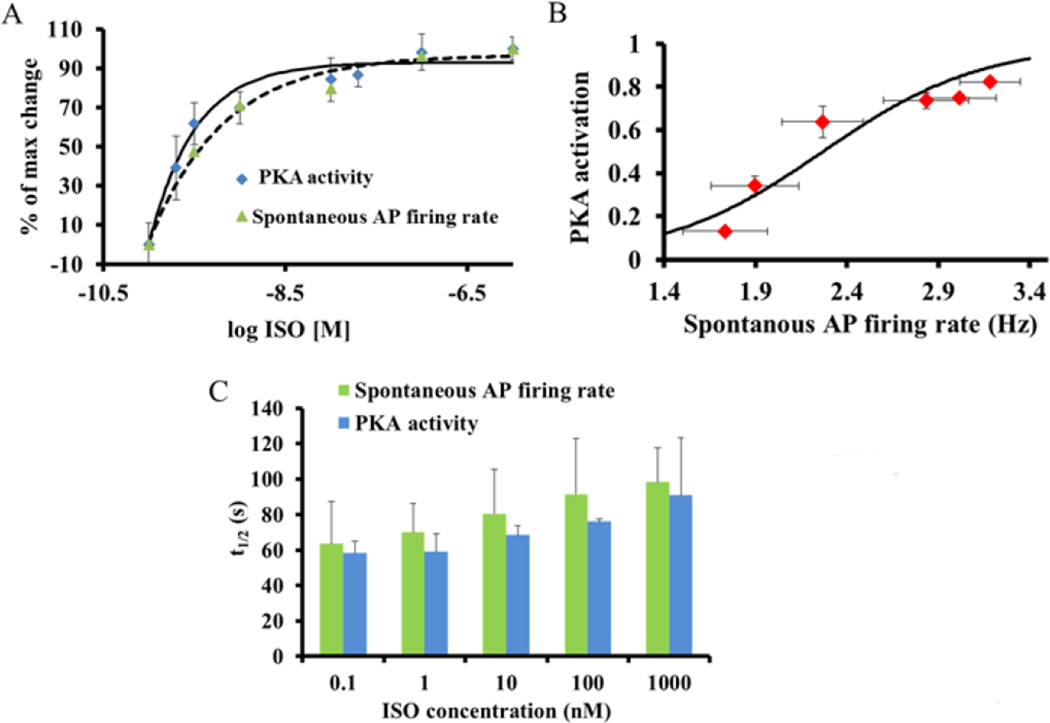
We employed graded increases in the concentrations of ISO to quantify the relationship between changes in PKA activity and spontaneous AP firing rate. We found that these changes led to a progressive increase in the spontaneous AP firing rate (Fig. 2D, from 104±14 to 173±5 beats/min). Furthermore, over the entire range of [ISO] the graded increases in PKA activity and AP firing rate are strikingly similar (Fig. 3A), yielding similar values of the EC50 of the increase in PKA activity (0.4±0.1nM) and increase in spontaneous AP firing rate (0.6±0.15nM) in response to β-AR stimulation.
Figure 3. PKA activity in response to β-AR stimulation.
(A) Average graded changes in PKA activity (emission ratio Y/C) and spontaneous AP firing rate in response to different ISO concentrations. (B) The relationship between the increase in PKA activity and the spontaneous AP firing rate. PKA activity was normalized to the minimum activity (the decrease in PKA in response to 10 µM H-89) and to the maximal activity (the increase in PKA in response to 100 µM IBMX). The dashed line represents the estimated basal level of PKA activity and spontaneous AP firing rate in freshly isolated SANC. (C) Half times (t1/2) of the increase in PKA activity and spontaneous AP firing rate in response to different single concentrations of ISO.
To compare the kinetics of the increases in PKA activity and spontaneous AP firing rate, only single concentrations of ISO were administrated to each cell. Note that, the experiments to quantify PKA activity and spontaneous AP firing rate were not performed on the same cell (see limitation section below). In these cells, the increases in PKA activity and AP firing rate magnitudes were similar to those in response to ISO concentration achieved by cumulative increase of ISO (Fig. 3C). The kinetics of the increase in PKA activity and the increase in spontaneous AP firing rate over the entire range of single ISO concentrations (Fig. 3C) do not statistically differ (by ANCOVA). Moreover, statistically the t1/2 of PKA activity and spontaneous AP firing over the entire range of single ISO concentrations are similar. Because PKA and AP firing dynamic do not statistically differ, one can claim that both are tightly linked.
To prove that neither time nor the presence of AKAR3 affects the changes in PKA activity and spontaneous AP firing rate in response to ISO we performed two types of experiments: (i) in response to drug vehicle application, neither PKA activity (Fig. S2A) nor spontaneous AP firing rate (Fig. S2B) significantly changed; (ii) furthermore, the presence of AKAR3 did not affect the response of AP firing rate to ISO (Fig. S3).
PKA activity and AP firing rate in response to PDE inhibition
To characterize the AP firing rate at the maximum PKA activity, we compared the responses of PKA activity and AP firing rate to the maximal PDE inhibition by IBMX (100 µM). The steady-state spontaneous AP firing rate increased from 103±10 to 188±10 beats/min. The t1/2 of the increase in PKA activity in response to PDE inhibition (116±27s, n=7) was strikingly similar to the t1/2 of the increase in spontaneous AP firing rate (123±14s, n=5). Interestingly, the kinetics of the increase in PKA activity and AP firing rate in response to IBMX are significantly slower than those in response to ISO (p<0.05).
To determine the kinetics of PKA inactivation and reduction in AP firing rate in response to a reduction in PKA activity, we applied H-89 in the presence of IBMX. PKA activity was reduced from 1.4±0.02 to 1.02±0.02. The spontaneous AP firing rate was reduced from 100±5 to 26±5 beats/min. The t1/2 of the reduction in PKA activity in response to H-89 in the presence of IBMX was 254±25s (n=5), and that of the spontaneous AP firing rate was 175±22s (n=5). Note that the kinetics of the reduction of PKA activity and AP firing rate toward the baseline (by H-89) are slower than those of the increase in PKA activity in response to either maximal β-AR stimulation or to PDE inhibition (Fig. 2B). In summary, in response to PDE inhibition or β-adrenergic stimulation, the beating rate magnitude and kinetics are tightly linked to these changes in PKA activity.
3.3 Spatiotemporal synchronization of PKA activity in response to perturbations of cAMP
Spatio-temporal gradients of molecular dynamics enable a cell to expand its repertoire of functional outcomes [21]. In ventricular myocytes, compartmentalized cAMP/PKA signaling has been documented and studied through the use of AKAR variants and other genetically encoded biosensors [6]. To assess changes in spatio-temporal gradients of PKA activity in response to graded β-AR stimulation, we measured the magnitudes of changes in PKA activity amplitude and its t1/2 within different random regions of 1µm radius across the cell. In response to graded β-AR stimulation, changes in the spatio-temporal variability in PKA activity were quantified by calculating the coefficients of variance (CV) of changes in PKA activity amplitude and its t1/2 measured at different regions within the cells. In order to gain a robust estimate of the spatial dynamics, only cells large enough to accommodate at least ten regions were analyzed.
In response to graded increases in ISO concentration (from 0.1 to 10nM), as the average PKA activity amplitude increased, local spatiotemporal (CV of PKA activity amplitude and its t1/2) gradients in PKA activity become reduced (Fig 4 A–D). Specifically, graded increases in average PKA activity are accompanied by reductions in the coefficients of variance of PKA activity amplitude and t1/2 gradients. Coefficients of variance of both changes in the amplitude of PKA activity and its t1/2 gradients are linked (Fig. 4E).
Figure 4. Distribution of PKA amplitude and time response to β-adrenergic receptor stimulation.
(A) Distributions of PKA activity gradients amplitude among different spatial regimes within the same cell and (B) the relationship between average PKA activity amplitude and PKA coefficient of variation in response to graded increases in isoproterenol (ISO) concentration. (C) Distributions of the t1/2 among different spatial regimes within the same cell and (D) the relationship between average t1/2 and t1/2 coefficient of variation in response to graded increases in isoproterenol (ISO) concentration. (E) Coefficient of variation of PKA activity and t1/2 gradients at different ISO concentrations.
Conversely application of H-89 in the presence of IBMX to lower the average PKA activity is associated with increase in spatiotemporal desynchronization of changes in PKA activity and t1/2 (Fig. 5A–B). For comparison of spontaneously beating to quiescent cells, see online supplement (Fig. S4).
Figure 5. Distribution of PKA amplitude and time response to PDE inhibition or PKA inhibition.
Distributions of the (A) PKA amplitudes and (B) the time response to achieve the activity in different spatial regimes within the same cell in response to PDE inhibition (IBMX) following PKA inhibition (H-89).
3.4 Elevating PKA activity in cultured sinoatrial node pacemaker cells to mimic the PKA activity in fresh cells
Prior observations indicate that after 48 hours in culture, the spontaneous AP firing rate of cultured pacemaker cells becomes reduced [22]; Our observations were similar. The beating rate was reduced from 152±7 beats/min in fresh SANC to 101±9 beats/min in cultured SANC (p<0.05). This reduction in AP firing rate is commonly accompanied by a reduction in phosphorylation of both phospholamban and RyR [22]. Furthermore, in response to a maximal β-AR stimulation, the spontaneous AP firing rate, and phospholamban and RyR phosphorylation levels in cultured SANC become similar to those in fresh isolated SANC [22]. We found that exposure of cultured SANC to 1nM of ISO increased the spontaneous AP firing rate in these cells to the value similar to the basal level AP firing rate of freshly isolated SANC (Fig. 3B). Therefore, we estimated that adding 1 nM of ISO to cultured cells mimics the basal phosphorylation level of freshly isolated SANC (Fig. 3B dashed line). We next assessed PKA activity and AP firing rate of cultured SANC responses to physiological stimulation (β-AR stimulation) or pharmacological intervention (PDE inhibitor) in the presence of 1.0 nM ISO to mimic basal PKA activity in freshly isolated SANC. β-AR stimulation (100 nM ISO) in the presence of 1 nM of ISO increased the PKA activity by 35% (Fig. 6A) with t1/2= 38±8s (n=4). PDE inhibition (100 µM IBMX) in the presence of 1 nM of ISO (Fig. 6B) increased the PKA activity by 43% with t1/2= 43±6s (n=6). We also directly measured the kinetics of the change in AP firing rate in response to drug stimuli in freshly isolated SANC. In response to β-AR stimulation or PDE inhibition the kinetics of the AP firing rate do not significantly differ from those kinetics of PKA activity: t1/2 of the change in AP firing rate in response to ISO was 47±15s (n=7) and t1/2 of the change in AP firing rate in response to IBMX was 58±14s (n=5).
Figure 6. PKA activity in response to β-AR stimulation or PDE inhibition.
Representative example of time course of change in PKA activity (AKAR3 emission ratio Y/C) in response to (A) β-adrenergic receptor stimulation (ISO 100 nM) in the presence of 1 nM ISO and (B) PDE inhibition (IBMX 100 µM) in the presence of 1 nM ISO.*The estimated basal level of PKA activity (see text for details).
3.5 Numerical model simulation of cAMP and PKA-mediated phosphorylation targets
Pacemaker function is regulated by a complex array of signaling molecules, including Ca2+, cAMP and PKA, which interact with each other in a complicated non-linear fashion. Although our experiments quantified the magnitude and rate of change in PKA activity in response to physiological and drug interventions, and compared these to changes corresponding to AP firing rate, current experimental methods do not permit simultaneous quantification of changes in phosphorylation of target proteins and changes in AP firing rates. In the absence of such crucial dynamic experimental information, it is difficult to interpret the interplay between Ca2+, cAMP, PKA activation and phosphorylation level in determining SANC AP firing rate. Therefore, we developed a computational model that integrated previously measured parameters of steady-state AC activity, cAMP and phospholamban phosphorylation levels in response to perturbations that influence cAMP/PKA signaling, and changes in the kinetics and magnitude of PKA activity measured in the present experiments (we used the experimental results of PKA activity under the conditions that mimics the PKA activity in fresh cells, see above). When intracellular Ca2+ changes, the model predicts changes in the spontaneous AP firing rate that result from the Ca2+-dependent downstream changes in cAMP or the PKA-dependent phosphorylation of ion channels (f-channel, L-type channel and slow-activated potassium channels) and SR Ca2+ cycling kinetics (PLB and RyR phosphorylation) that are affected by changes in the ion channels. The model assumptions and equations are described in the expended view.
Numerical model simulation of the relative roles of M clock and Ca2+ clock protein phosphorylation in the maintenance of SANC automaticity
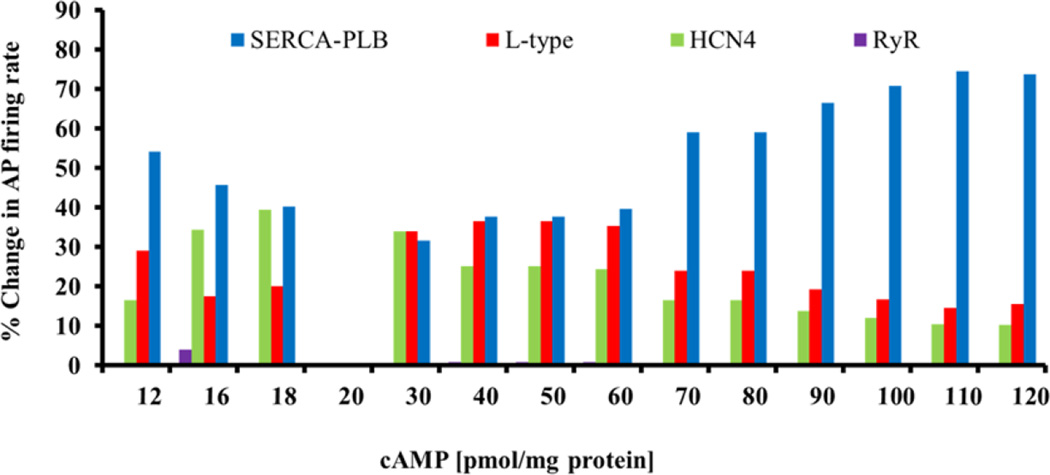
It is unclear which PKA target components of the clocks are crucial for maintaining an AP firing rate. We made use of our model to address this question. We estimated the relative contributions of changes in phosphorylation of surface membrane protein (L-type and funny current) and Ca2+ mechanisms (PLB and RyR) in maintenance of SANC automaticity at a constant cAMP, by calculating the changes in AP firing rate when only a single of the above mechanisms sensed the changes in cAMP (i.e., cAMP was “clamped” to basal level for the remaining other mechanisms) and comparing the change in AP firing rate to that when all mechanisms were functional (Fig. 7). As illustrated in Fig. 7, in the mid-range of cAMP (35–60 pmol/mg protein) L-type current, PLB phosphorylation together with HCN4 have substantial impact on the changes in spontaneous AP firing rate. When cAMP begins to drop below the basal level (16–18 pmol/ mg protein), however, PLB and HCN4 contribute equally to the changes in spontaneous AP firing rate. As cAMP level further drops, PLB phosphorylation dominates the changes in spontaneous AP firing rate. As cAMP progressively increases above the basal level, PLB phosphorylation progressively dominates and the contributions of both L-type current and HCN4 become progressively reduced. Thus, at cAMP levels higher or lower than basal conditions (i.e., 20 pmol/ mg proteins) the relative role of phosphorylated intracellular proteins (i.e., PLB) in maintaining SANC periodicity remains higher than the other targets. Therefore the model simulations predict that PLB is the most potent cAMP/PKA target over the entire range of [cAMP] (Fig. 7).
Figure 7. Model simulations of the synergism of different mechanisms in mediating SANC spontaneous AP firing rate.
The relative role of each mechanism was tested at a constant cAMP, by determining the changes in AP firing rate when only the tested mechanism sensed the changes in cAMP (cAMP was “clamped” to basal level for the remaining other mechanisms) and comparing the resultant change in AP firing rate to that when all mechanisms (direct cAMP and PKA-dependent phosphorylation) were functional.
Numerical model simulations of cAMP and PKA-mediated phosphorylation targets in response to β-AR stimulation or PDE inhibition
Cardiac rhythm is a dynamic process. Although steady state simulations of SANC automaticity as detailed above are instructive of the general mechanistic features, kinetic information is more crucial to an understanding of how changes in pacemaker function from the basal state are regulated. Specifically, the temporal changes in cAMP and PLB phosphorylation in response to perturbations that increase cAMP have not been quantified previously. We modeled the kinetics of changes in cAMP, PKA, PLB phosphorylation (the dominant phosphorylation target; see above) and AP firing rate in response to acute β-AR. Simulations of concurrent steady-state cAMP and PLB phosphorylation levels in response to β-AR stimulation or PDE inhibition (Fig. 8) employ values similar to the levels experimentally quantified previously [15, 23], and the PKA activity measured in the present study. The t1/2 of the increase in PKA activity in response to maximal β-AR stimulation in the model simulation is 20 s (Fig. 8A), i.e. similar to the experimental results of the present study under conditions that mimic fresh cells (excluding direct and instant effect of the drug in the model). That PKA activity kinetics are similar to the experimental results presented above also validates the ability of the model to virtually approximate the kinetics of intracellular PKA signaling. The kinetics of the increase in the spontaneous AP firing rate in response to β-AR stimulation were similar to that of PLB phosphorylation, and lag behind the increases in cAMP and PKA activity (Fig. 8D). Therefore, the model simulation predicts that the kinetics of PKA activation are closely linked to the kinetics of the change in spontaneous AP firing rate in response to β-AR stimulation.
Figure 8. Kinetics and magnitude of cAMP/PKA-phospholamban phosphorylation and spontaneous AP firing rate in response to maximal β-adrenergic receptor stimulation or PDE inhibition.
Model simulations in response to maximal β-adrenergic stimulation predict the rate and extent of changes in (A) PKA activity, (B) cAMP, and (C) PLB phosphorylation in response to maximal β-adrenergic receptor stimulation. (D) The simulations predict that the kinetics of the increase in PLB phosphorylation are similar to those of the increase in spontaneous AP firing rate. Model simulations in response to PDE inhibition predict the rate and extent of changes in (E) PKA activity, (F) cAMP, and (G) PLB phosphorylation in response to PDE inhibition. (H) The simulations predict that the kinetics of the increase in PKA are similar to those of the increase in spontaneous AP firing rate.
Simulated dynamic changes in cAMP, PKA, PLB phosphorylation and AP firing rate in response to PDE inhibition are illustrated in Fig. 8 E–H. PKA activity, cAMP and PLB phosphorylation increased in response to PDE inhibition. cAMP and PLB phosphorylation levels were similar to the steady levels quantified experimentally previously [14, 15], and the PKA activity kinetics were similar to the experimental results in the present study. In particular, t1/2 of the increase in PKA activity in response to PDE inhibition in the model simulations is 30 s (Fig. 8E), i.e., similar to the presented experimental results under conditions that mimic fresh cells (excluding direct and instant effect of the drug in the model). Note that the simulated kinetics of changes in cAMP and PLB in response to PDE inhibition were slower than in response to β-AR stimulation. In contrast to β-AR stimulation, in response to PDE inhibition the kinetics of the change in spontaneous AP firing rate were more closely related to the kinetics of changes in PKA activity than the rate at which PLB phosphorylation increases (Fig. 8). Therefore, the model simulation predicts that in response to PDE inhibition phosphorylation states of cAMP/PKA-dependent targets other than PLB (i.e., If, L-type and ryanodine receptor phosphorylation) play more dominant role than in response to β-AR stimulation. The experiential PKA dynamics measured here were critical to understand by using numerical model how molecular clock mechanisms operate the AP firing rate.
4. Discussion
While it has been hypothesized that pacemaker function is tightly regulated by the overall phosphorylation levels of various signaling molecules that constitute the M and Ca2+ clocks, and that PKA is one of the important kinases that effect this phosphorylation process, experimental measurements of dynamics of PKA activity required to quantify cAMP-PKA signaling in SANC to substantiate this hypothesis have been lacking. Here we present, for the first time, direct real-time measurements of PKA activity dynamics in single heart pacemaker cells using a technique similar to that used previously in ventricular myocytes [6, 24]. The most novel findings of our study are (1) that substantial PKA activity is present in SANC under basal conditions and (2) that, in response to perturbations that increase cAMP, the magnitude and kinetics of the increases in PKA activity and spontaneous AP firing rate are correlated. The significance of these findings is that changes in the kinetics and stoichiometry of PKA activity in response to drug interventions tightly regulates changes in SANC AP firing rate, validating the hypothesis that PKA-dependent phosphorylation regulates the coupled-clock system that drives the SANC AP firing rate [7]. We used cultured pacemaker cell model to explore the PKA activity dynamics. The use of this model has two limitations: 1) the number of cultured cell (and therefore proteins) is small and therefore it is particularly challenging to genetically to knock down or overexpress molecules of interest to test mechanistic hypotheses related to chrontropic stimuli. 2) After 48 hours in culture, the spontaneous AP firing rate of cultured and their electrophysiological characteristics pacemaker cells becomes reduced17, accompanied by a reduction in phosphorylation of both phospholamban and RyR [22]. In response to a maximal ISO concentration, however, the spontaneous AP firing rate, AP characteristics and phospholamban and RyR phosphorylation levels in cultured SANC are restored to those levels in fresh isolated SANC [22] making them a valid model of sinoatrial node pacemaker activity in the rabbit heart.
The use of the genetically encoded PKA activity sensor enabled us to detect changes in PKA activity dynamics in response to β-AR stimulation or PDE inhibition which would have been difficult to observe using traditional techniques. For example, we found that the kinetics of the increase in PKA activity and AP firing rate in response to PDE inhibition are significantly slower than those in response to the maximal β-AR stimulation. Moreover, the kinetics of the reduction of PKA activity and AP firing rate to baseline levels when PKA is inhibited are slower than those of the increase in PKA activity in response to either maximal β-AR stimulation or to PDE inhibition. Such temporal differences suggest that different mechanisms by which PKA is regulated may have a significant bearing on how PKA activity regulates cardiac rate in response to different biochemical signaling. Moreover, by normalizing the PKA activity to minimal (H-89) and maximal (IBMX) PKA activity, it becomes clear that the pacemaker cells bear an intrinsic ability to produce high rates of formation and degradation of cAMP. Similar results have been documented previously [14]. Although the baseline beating rate is similar in isolated, intact rabbit sinoatial node tissue and isolated single SANC [25], the change in spontaneous AP firing rate in response to β-AR stimulation is lower in freshly isolated SANC than in intact sinoatial node tissue [26]. Therefore, it is possible that during the isolation procedure of single SANC some mechanisms that regulate the response to ISO become altered. Moreover, in cultured rabbit pacemaker cells half maximal concentration to stimulate PKA activation and AP firing are by ISO are in the range of nM (Fig. 3) where in freshly isolated pacemaker cells half maximal stimulation concentration is higher in the range of µM [23]. A similar trend was documented in cultured vs. fresh rat ventricular myocytes [27]. Therefore, it is possible that during culture some mechanisms that regulate receptor affinity, or mechanisms down stream of receptor stimulation are altered. Additionally, in response to prolonged exposure to ISO, receptor desensitization can occur [28]. Therefore, during consecutive increases in ISO concentration, the higher concentration may have a desensitizing effect.
While spatial heterogeneity within the baseline PKA activity cannot be interpreted from heterogeneity in baseline PKA activity because the distribution of the FRET probe may not be equal in all cell loci. But, changes of fluorescence intensity (i.e., amplitude and t1/2) at a given locus can be interpreted as related to the corresponding local changes in PKA activity, in a relative, dynamic sense, supporting the experimental analysis of local PKA kinetics presented here. Another novel finding of our study is that the local spatiotemporal gradients of changes in PKA activity occur in response to stimuli that activate PKA and that the integral of these gradients generates the average cell change in the magnitude and kinetics of the PKA activity, the averages of which in PKA activity are linked to the kinetics and magnitudes of the change in AP firing rate. As the intensity of β-AR stimulation increases, the variation in spatial gradients in PKA amplitude and the variation in temporal gradients of kinetics of PKA activity changes become reduced (Fig. 4). Conversely, when PKA is inhibited, spatiotemporal gradients of PKA activity within the cell increase (Fig. 5). Prior studies in SANC demonstrated that cAMP and PKA-dependent phosphorylation of SR Ca2+ cycling proteins in response to β-AR stimulation or PDE inhibition effect spatiotemporal synchronization of spontaneous local diastolic Ca2+ releases generated within the coupled clock system [29]. The synchronization of these local Ca2+ releases is linked to changes in the AP firing rate. Therefore it is tempting to speculate that local oscillatory in Ca2+ releases in SANC are associated with local oscillations in cAMP and PKA activity that have been documented in other cell types [2]. That the graded reductions in PKA spatial amplitude gradients and in the acceleration of t1/2 gradients occur in parallel may suggest that PKA activity may oscillate locally, and that such local spatiotemporal heterogeneity oscillations are synchronized by β-AR stimulation. In the coupled-clock system, synchronization of local oscillations in PKA activity would lead to a larger average PKA signal that evolves rapidly and synchronizes local diastolic Ca2+ oscillations. Synchronization of local Ca2+ signals generates a larger ensemble Ca2+ signal, leading to increased diastolic depolarization current of the M clock, resulting in acceleration of the AP firing rate. Although we demonstrate here key spatiotemporal PKA activity using epifluorescence microscopy, future experiments using specific modalities that provide higher spatial resolution are required to provide more detailed insights. PKA signaling heterogeneity may originate from diffusion mechanisms, differential kinetics in PKA activation and/or inactivation at the level of protein complexes, e.g., compartmentalization of AKAPS which binds PKA. Indeed, such compartmentalized signaling may also be the source of differences in the kinetics of PKA activation between β-AR stimulation and PDE inhibition responses that have been observed in other cell types [5]. Our experiments, however, cannot link spatiotemporal heterogeneity of changes in PKA activity dynamics to specific subcellular compartments (e.g. cell membrane vs. nucleus vs. intracellular) that may occur in the context of compartmentalization of PKA activity, as in ventricular myocytes.32
Employing a PKA biosensor in the present study also enabled us to extract precise quantitative information regarding the activation and deactivation kinetics of PKA. This enabled us to construct a computational model that interfaces the coupled-clock system (i.e., ion channels and SR Ca2+ cycling kinetics) with a cAMP/PKA signaling module. An AC-cAMP/PKA module has been explored by computational models in prior studies in ventricular myocytes (e.g., Saucerman-McCulloch model [30], Heihman-Rudy model [31]) and in other cell types [2]. However, due to the unique properties of pacemaker cells, e.g., the fundamental importance of Ca2+-activated AC, the ability to spontaneously generate APs, different mechanisms coupling ATP supply to ATP demand, a novel model design is required for pacemaker cells.
A previous mathematical model in SANC has integrated ionic mechanisms together with biochemical reactions that underlie the response of pacemaker cells to β-AR stimulation [32]. This model, however, lacks the ability to define the kinetics of signaling initiated by Ca2+-activated AC [12, 13], and its coupling to other mechanisms that regulate changes in AP firing rate. In the present study, integration of our novel experimental data on PKA activity and kinetics into SANC models of ionic mechanisms enabled simulation of kinetics of cAMP accumulation, increases in PKA activation and PKA-mediated phosphorylation in response to drugs that increases AC-cAMP signaling.
Our novel integrated model of biochemical and biophysical mechanisms not only correctly simulated our experimental results, but also generated predictions providing an insight into the finer details of how pacemaker cells are regulated by PKA. The first prediction of our numerical model is that the level of PKA activity-dependent PLB phosphorylation in spontaneously beating SANC is higher than the numerically predicted level in ventricular myocytes beating at similar rates (~3 Hz) [33]. This prediction is in accord with high cAMP levels documented in pacemaker cells [34], and may be related to Ca2+ activation of AC [12, 13]. The second prediction of our numerical model simulations is that the changes of kinetics of PKA-dependent mechanisms are tightly linked to changes in AP firing rate. The third prediction of our numerical model is that PKA activity in response to β-AR stimulation is different than in response to PDE inhibition. This result is consistent with an interpretation of studies in ventricular cells suggesting that different phosphorylation targets have different sensitivities to PDE inhibition or β-AR stimulation, or that PKA in SANC is localized within microdomains and different stimuli may differently affect different compartments [5, 35]. Although our numerical model simulates the changes in cAMP kinetics and magnitude cAMP was not directly measured, novel insight generated by our model simulations is that kinetics of PKA-dependent phosphorylation are more closely linked to the control of the spontaneous AP firing rate than direct cAMP-dependent mechanisms. Our numerical model, however, is a “common-pool” type of model, and cannot delineate differences in cAMP and PKA signaling in different compartments (SR, cytosol, membranes), taking into account consideration of localization and co-localization of the various constituents. Future experiments that measure PKA and cAMP within different compartments are required for the development of next-generation models of local Ca2+ activation of cAMP/PKA signaling in pacemaker cells.
The delayed rectifier potassium current in ventricular myocytes is modulated by PKA-mediated phosphorylation [9] and is an essential component in primary cardiac pacemaker activity in the rabbit SAN. Sodium current that has a role on cardiac peacemaking in “follower” cells is modulated by PKA-mediated phosphorylation [36]. Unfortunately, no experimental data exist on the PKA-mediated phosphorylation of these channels in pacemaker cells. Future experimental results on PKA-mediated phosphorylation of delayed rectifier potassium current and sodium current are required for the development of modeling of full description of cAMP/PKA signaling in pacemaker cells. Additionally, Ca2+ also activates Ca2+/calmodulin-dependent protein kinase II (CaMKII) protein phosphorylation in parallel with Ca2+-calmodulin-activated AC-cAMP/PKA signaling [37]. There is crosstalk in rabbit pacemaker cells between CaMKII and PKA activity and a reduction in CaMKII activity indirectly reduces steady-state cAMP levels [38]. Because of PKA and CaMKII crosstalk, our experimental and numerical model results reporting on PKA activity and AP firing rate indirectly include a CaMKII activity effect. The level of CaMKII and specific relative roles of CaMKII and PKA activation in pacemaker cells are not established. Future experiments, like those performed in ventricular myocytes [39] and employment of novel developed techniques to visualize CaMKII and calmodulin activities [40] may precisely define the relative role of CaMKII in protein phosphorylation cascades in regulation of SANC AP firing rate.
Limitations
In this paper, we measured PKA activity and spontaneous AP firing rate characteristics in a separate group of cells. Technical limitations prevented dual measurements in the same cell: (i) the equipment available to us limited the recording time of PKA activity to 10 sec; in contrast, to resolve spontaneous AP firing characteristics a recording time in the order of tens of msec is required. Therefore, we could not use the same camera to record both PKA and AP signals. (ii) The membrane of pacemaker cells in culture is deformed and it is difficult to implement a perforated patch recording technique. Because in each culture dish approximately 4–5 cells exhibited spontaneous beating and there was less than 10% chance to successfully patch the cell, measuring AP firing rate by patch recording simultaneously with PKA activity within same cell is very technically challenging. Future studies are required to overcome these limitations. Note that the high standard errors around PKA activity and spontaneous AP firing derived from beat-to-beat variability between cells. When EC50 or t1/2 were each calculated within the same cell there was small standard error and similar values between the variables.
Prior to performing our experiments, we developed a numerical model based on previously integrated measured parameters of steady-state AC activity, cAMP and phospholamban phosphorylation levels in response to perturbations that influence cAMP/PKA signaling. We chose PKA activities that are close to 25, 50 and 75% response based on the model prediction, and therefore did not spread the ISO concentrations evenly. Thereafter, we trained the model with the new data to predict the changes in PKA activity in response to the entire physiological range of ISO concentrations. Therefore, although we did not directly measure PKA activity in response to the entire physiological range of ISO concentrations, we can predict it based on our novel mathematical model.
In pacemaker cells, in addition to PKA activation by β-AR stimulation and PDE inhibition, phosphatase (PPT) inhibition can increase the spontaneous AP firing rate [15]. Additional information on cellular mechanisms affected by PPT and the identity of which dominate PPT isoforms exist in pacemaker cells are required to determine the potential effect of PPT inhibition on PKA dynamics.
5. Conclusions
Our experimental results show that changes in the kinetics and magnitude of the spontaneous AP firing rate in response to β-AR stimulation or PDE inhibition are attributable to changes in kinetics and amplitude of PKA activity. Changes in average PKA are attributable to changes in spatiotemporal synchronization of local gradients changes. We interpret our model simulations to indicate that basal PKA activity in SANC is higher than in ventricular myocytes cell, and that the rate of PKA-dependent phosphorylation can limit the rate and magnitude of changes in spontaneous AP firing rate in response to perturbations that change the cAMP level. Future experiments employing live-cell imaging of pacemaker cells with localized PKA probes and numerical modeling are required for a more precise definition of compartmentalization of intracellular PKA activity.
Supplementary Material
Acknowledgments
Sources of Funding
The work was supported partially by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Public Health Service Grants NIH R01-DK073368 (J.Z., A.L.) and GM07024 (A.L.), AHA Predoctoral Fellowship (A.G) as well as Technion V.P.R Fund-Mallat Family Research Fund, Technion E.V.P.R Fund-Elias Fund for Medical research, and by NSFC-ISF joint research program, No. 398/14 (Y.Y).
Non-standard abbreviations and acronyms
- AC
adenylyl cyclases
- AKAR
A-kinase activity receptor 3
- AP
action potential
- β-AR
β-adrenergic receptor
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- ISO
isoproterenol
- M
membrane
- PDE
phosphodiesterase
- PKA
protein kinase A
- PLB
phospholamban
- RyR
ryanodine receptors
- SANC
sinoatrial node cells
Footnotes
Disclosers
None.
References
- 1.Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nature reviews Molecular cell biology. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 2.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, et al. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nature chemical biology. 2011;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology (Bethesda) 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Zhang J, Xiang YK. FRET-based direct detection of dynamic protein kinase A activity on the sarcoplasmic reticulum in cardiomyocytes. Biochemical and biophysical research communications. 2011;404:581–586. doi: 10.1016/j.bbrc.2010.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, et al. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circulation research. 2004;95:67–75. doi: 10.1161/01.RES.0000134629.84732.11. [DOI] [PubMed] [Google Scholar]
- 6.Saucerman JJ, Zhang J, Martin JC, Peng LX, Stenbit AE, Tsien RY, et al. Systems analysis of PKA-mediated phosphorylation gradients in live cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12923–12928. doi: 10.1073/pnas.0600137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaniv Y, Sirenko S, Ziman BD, Spurgeon HA, Maltsev VA, Lakatta EG. New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: bradycardic effects of ivabradine are linked to suppression of intracellular Ca(2)(+) cycling. Journal of molecular and cellular cardiology. 2013;62:80–89. doi: 10.1016/j.yjmcc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trautwein W, Cavalie A, Flockerzi V, Hofmann F, Pelzer D. Modulation of calcium channel function by phosphorylation in guinea pig ventricular cells and phospholipid bilayer membranes. Circulation research. 1987;61:I17–I23. [PubMed] [Google Scholar]
- 9.Freeman LC, Kwok WM, Kass RS. Phosphorylation-independent regulation of cardiac IK by guanine nucleotides and isoproterenol. Am J Physiol. 1992;262:H1298–H1302. doi: 10.1152/ajpheart.1992.262.4.H1298. [DOI] [PubMed] [Google Scholar]
- 10.Toyofuku T, Kurzydlowski K, Tada M, MacLennan DH. Identification of regions in the Ca(2+)-ATPase of sarcoplasmic reticulum that affect functional association with phospholamban. The Journal of biological chemistry. 1993;268:2809–2815. [PubMed] [Google Scholar]
- 11.Takasago T, Imagawa T, Shigekawa M. Phosphorylation of the cardiac ryanodine receptor by cAMP-dependent protein kinase. Journal of biochemistry. 1989;106:872–877. doi: 10.1093/oxfordjournals.jbchem.a122945. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Lyashkov AE, Graham D, Sheydina A, Volkova MV, Mitsak M, et al. Ca(2+) -stimulated basal adenylyl cyclase activity localization in membrane lipid microdomains of cardiac sinoatrial nodal pacemaker cells. The Journal of biological chemistry. 2008;283:14461–1448. doi: 10.1074/jbc.M707540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattick P, Parrington J, Odia E, Simpson A, Collins T, Terrar D. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. The Journal of physiology. 2007;582:1195–1203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinogradova TM, Sirenko S, Lyashkov AE, Younes A, Li Y, Zhu W, et al. Constitutive phosphodiesterase activity restricts spontaneous beating rate of cardiac pacemaker cells by suppressing local Ca2+ releases. Circulation research. 2008;102:761–769. doi: 10.1161/CIRCRESAHA.107.161679. [DOI] [PubMed] [Google Scholar]
- 15.Yaniv Y, Spurgeon HA, Ziman BD, Lyashkov AE, Lakatta EG. Mechanisms that match ATP supply to demand in cardiac pacemaker cells during high ATP demand. Am J Physiol Heart Circ Physiol. 2013;304:H1428–H1438. doi: 10.1152/ajpheart.00969.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon H, Sollott SJ, Lakatta EG. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. Journal of molecular and cellular cardiology. 2011;51:740–748. doi: 10.1016/j.yjmcc.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. Faseb J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 18.Lehnart SE, Marks AR. Phosphodiesterase 4D and heart failure: a cautionary tale. Expert Opin Ther Tar. 2006;10:677–688. doi: 10.1517/14728222.10.5.677. [DOI] [PubMed] [Google Scholar]
- 19.Nunes AR, Sample V, Xiang YK, Monteiro EC, Gauda E, Zhang J. Effect of oxygen on phosphodiesterases (PDE) 3 and 4 isoforms and PKA activity in the superior cervical ganglia. Advances in experimental medicine and biology. 2012;758:287–294. doi: 10.1007/978-94-007-4584-1_39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto D, De Arcangelis V, Zhang J, Xiang Y. Dynamic Protein Kinase A Activities Induced by beta-Adrenoceptors Dictate Signaling Propagation for Substrate Phosphorylation and Myocyte Contraction. Circulation research. 2009;104:770–U121. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes JS, Brunton LL, Mayer SE. Selective Activation of Particulate Camp-Dependent Protein-Kinase by Isoproterenol and Prostaglandin-E1. Journal of Biological Chemistry. 1980;255:5113–5119. [PubMed] [Google Scholar]
- 22.Yang D, Lyashkov AE, Li Y, Ziman BD, Lakatta EG. RGS2 overexpression or G(i) inhibition rescues the impaired PKA signaling and slow AP firing of cultured adult rabbit pacemaker cells. Journal of molecular and cellular cardiology. 2012;53:687–694. doi: 10.1016/j.yjmcc.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinogradova TM, Bogdanov KY, Lakatta EG. beta-Adrenergic stimulation modulates ryanodine receptor Ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circulation research. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 24.Warrier S, Belevych AE, Ruse M, Eckert RL, Zaccolo M, Pozzan T, et al. Beta-adrenergic-and muscarinic receptor-induced changes in cAMP activity in adult cardiac myocytes detected with FRET-based biosensor. American journal of physiology. 2005;289:C455–C461. doi: 10.1152/ajpcell.00058.2005. [DOI] [PubMed] [Google Scholar]
- 25.Yaniv Y, Ahmet I, Liu J, Lyashkov AE, Guiriba TR, Okamoto Y, et al. Synchronization of sinoatrial node pacemaker cell clocks and its autonomic modulation impart complexity to heart beating intervals. Heart Rhythm. 2014;11:1210–1219. doi: 10.1016/j.hrthm.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang D, Petrov V, Lou Q, Osipov G, Efimov IR. Spatiotemporal control of heart rate in a rabbit heart. Journal of electrocardiology. 2011;44:626–634. doi: 10.1016/j.jelectrocard.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JH, Polanowska-Grabowska RK, Smith JS, Shields CWt, Saucerman JJ. PKA catalytic subunit compartmentation regulates contractile and hypertrophic responses to beta-adrenergic signaling. Journal of molecular and cellular cardiology. 2014;66:83–93. doi: 10.1016/j.yjmcc.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff WP, Bouvier M, O’Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. The Journal of biological chemistry. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 29.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circulation research. 2010;106:659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saucerman JJ, Brunton LL, Michailova AP, McCulloch AD. Modeling beta-adrenergic control of cardiac myocyte contractility in silico. The Journal of biological chemistry. 2003;278:47997–48003. doi: 10.1074/jbc.M308362200. [DOI] [PubMed] [Google Scholar]
- 31.Heijman J, Volders PG, Westra RL, Rudy Y. Local control of beta-adrenergic stimulation: Effects on ventricular myocyte electrophysiology and Ca(2+)-transient. Journal of molecular and cellular cardiology. 2011;50:863–871. doi: 10.1016/j.yjmcc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Himeno Y, Sarai N, Matsuoka S, Noma A. Ionic mechanisms underlying the positive chronotropy induced by beta1-adrenergic stimulation in guinea pig sinoatrial node cells: a simulation study. J Physiol Sci. 2008;58:53–565. doi: 10.2170/physiolsci.RP015207. [DOI] [PubMed] [Google Scholar]
- 33.Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and beta-adrenergic signaling in regulation of cardiac myocyte Ca(2+) handling. Biophysical journal. 2010;99:2038–2047. doi: 10.1016/j.bpj.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinogradova TM, Lyashkov AE, Zhu W, Ruknudin AM, Sirenko S, Yang D, et al. High basal protein kinase A-dependent phosphorylation drives rhythmic internal Ca2+ store oscillations and spontaneous beating of cardiac pacemaker cells. Circulation research. 2006;98:505–514. doi: 10.1161/01.RES.0000204575.94040.d1. [DOI] [PubMed] [Google Scholar]
- 35.Mika D, Leroy J, Vandecasteele G, Fischmeister R. PDEs create local domains of cAMP signaling. Journal of molecular and cellular cardiology. 2012;52:323–329. doi: 10.1016/j.yjmcc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Emerick MC, Shenkel S, Agnew WS. Regulation of the eel electroplax Na channel and phosphorylation of residues on amino- and carboxyl-terminal domains by cAMP-dependent protein kinase. Biochemistry. 1993;32:9435–9444. doi: 10.1021/bi00087a023. [DOI] [PubMed] [Google Scholar]
- 37.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovascular research. 2005;68:366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Yaniv Y, Spurgeon HA, Ziman BD, Lakatta EG. Ca(2)+/calmodulin-dependent protein kinase II (CaMKII) activity and sinoatrial nodal pacemaker cell energetics. PLoS One. 2013;8:e57079. doi: 10.1371/journal.pone.0057079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson JR, Patel R, Ferguson A, Bossuyt J, Bers DM. Fluorescence resonance energy transfer-based sensor Camui provides new insight into mechanisms of calcium/calmodulin-dependent protein kinase II activation in intact cardiomyocytes. Circulation research. 2011;109:729–738. doi: 10.1161/CIRCRESAHA.111.247148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bossuyt J, Bers DM. Visualizing CaMKII and CaM activity: a paradigm of compartmentalized signaling. Journal of molecular medicine (Berlin, Germany) 2013;91:907–916. doi: 10.1007/s00109-013-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.