Abstract
Numerous clinical investigations have reported that children with cerebral palsy (CP) have tactile discrimination deficits that likely limit their ability to plan and manipulate objects. Despite this clinical awareness, we still have a substantial knowledge gap in our understanding of the neurological basis for these tactile discrimination deficits. Previously, we have shown that children with CP have aberrant alpha-theta (4–14 Hz) oscillations in the somatosensory cortices following tactile stimulation of the foot. In this investigation, we evaluated if these aberrant alpha-theta oscillations also extend to the hand. Magnetoencephalography was used to evaluate event-related changes in the alpha-theta and beta (18–34 Hz) somatosensory cortical oscillations in groups of children with CP and typically developing (TD) children following tactile stimulation of their hands. Our results showed that the somatosensory alpha-theta oscillations were relatively intact in the children with CP, which is in contrast to our previous results for foot tactile stimulations. We suspect that these inter-study differences may be related to the higher probability that the neural tracts serving the lower extremities are damaged in children with CP, compared to those serving the upper extremities. This inference is plausible since the participating children with CP had Manual Ability Classification System (MACS) levels between I-II. In contrast to the alpha-theta results, the children with CP did exhibit a sharp increase in beta activity during the same time period, which was not observed in TD children. This suggests that the children with CP still have deficits in the computational aspect of somatosensory processing.
Keywords: Sensory, MEG, Magnetoencephalography, tactile, fingers, beta, alpha, theta
1. Introduction
Cerebral palsy (CP) is one of the most prevalent and costly pediatric neurologic impairments diagnosed in the United States (Christensen et al., 2014). Historically, CP has been largely recognized as a motor impairment that results from damage to the developing brain. However, the reclassification of CP now includes other characteristics such as sensation, and perception deficits that may arise from the primary insult or secondarily from restricted perception-action experiences (Rosenbaum et al., 2007). The inclusion of sensory deficits in the updated classification of CP has been supported by numerous clinical reports of proprioception, stereognosis and tactile discrimination deficits in these children (Cooper et al., 1995; Clayton et al., 2003; Sanger and Kukke, 2007; Wingert et al., 2007; Auld et al., 2012; Robert et al., 2013).
The acknowledgement of the presence of sensory deficits has sparked considerable interest in identifying how the perinatal brain insult may affect the structural organization of the somatosensory cortices, and how this structural damage may be related to the degree of sensory impairments noted in children with CP. Several studies have shown that ipsilateral motor cortices will often assume the roles of the damaged contralateral homologue in children with CP that have extensive cortical damage (Carr et al., 1993; Staudt et al., 2002; Holmstrom et al., 2010). However, this has not been shown to be the case for the somatosensory cortices. Several investigations have shown that the thalamocortical somatosensory tracts often terminate in adjacent non-damaged neural tissues, which allows for the somatosensory processing to remain within the lesioned hemisphere (Staudt et al., 2006; Guzzetta et al., 2007; Wilke et al., 2009). Despite these neural adaptations, diffusion tensor imaging (DTI) shows that the redirected tracts are not as dense and often still have significant injury (Rose et al., 2007; Trivedi et al., 2008; Hoon et al., 2009; Trivedi et al., 2010; Papadelis et al., 2014). The current working hypothesis is that the poor integrity of these tracts disrupts the fidelity of the sensory information that is transmitted along these pathways.
Several magnetoencephalography (MEG) and electroencephalography (EEG) studies have further explored how the noted structural damage may impact the activation of the somatosensory cortices. These studies have identified that the somatosensory evoked-potentials/fields for the hand are diminished, and in some cases latent in children with CP (Kulak et al., 2005; Kulack et al., 2006; Riquelme and Montoya, 2010; Teflioudi et al., 2011; Maitre et al., 2012; Pihko et al., 2014; Papadelis et al., 2014). We have previously reported similar results for the foot (Kurz and Wilson, 2011; Kurz et al., 2012). Far fewer studies have explored how the perinatal brain injury may also affect the oscillatory somatosensory activity that occurs after peripheral somatosensations are applied to the hand. A preliminary study conducted by Pihko and colleagues (2014) suggested that the alpha and beta oscillatory activity within the hand region of somatosensory cortices might be diminished in the damaged hemisphere of children with CP that have a hemiplegic presentation. While these exploratory results are enlightening, the electric median nerve stimulation paradigm employed in this initial investigation causes a broad stimulation of the somatosensory receptors, which does not allow for quantifying how the stimulation of specific sensory receptors found in the hand (i.e., mechanoreceptors, vibrotactile, etc.) promote event-related changes in the alpha and beta cortical oscillations.
The mechanoreceptors found in the palmar surface of the hand provide unique information about the physical properties of an object and the precision of the hand contact forces (Johansson & Flanagan, 2009). It is now well appreciated that many children with CP lack the ability to properly register and discriminate different tactile stimuli that are provided to the hand mechanoreceptors (Cooper et al., 1995; Clayton et al., 2003; Sanger and Kukke, 2007; Wingert et al., 2007; Auld et al., 2012; Robert et al., 2013). Furthermore, it has been shown that theses tactile discrimination deficits likely impact the child’s ability to properly plan and control their grip forces (Gordon and Duff, 1999; Auld et al., 2012). Despite this clinical awareness, we still have a substantial knowledge gap in our understanding of the neural basis of the tactile discrimination deficits seen in children with CP. In our previous studies, we used high-density MEG and advanced beamforming methodology to show that children with CP have aberrant alpha-theta (4–14 Hz) oscillations in the somatosensory cortices following tactile stimulation of the foot (Kurz et al., 2014; 2015). In this investigation, we used the same experimental design to evaluate if similar oscillatory changes occur after a mechanoreceptor-specific tactile stimulation was applied to the hands of a group of children with CP and a cohort of typically developing (TD) children.
2. Experimental Procedures
2.1 Participants
Eight children (Age = 14.5 ± 0.7 yrs.) with a diagnosis of CP and motor ability classification system (MACS) levels between I-II participated in this investigation. MACS levels between I-II indicates that the children were able to manipulate objects with their hands, but the quality and speed of their hand dexterity was somewhat reduced (Eiliasson et al., 2006). None of the participating children had large white or grey matter lesions that would have affected the integrity of the cortical surface. Further description of the children with CP is provided in Table 1. An additional eight TD children (Age = 14.1 ± 0.7 yrs.) served as a control group. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Informed consent was acquired from the parents and the children assented to participate in the experiment.
Table 1.
Description of the participating children with cerebral palsy. MACS = Motor Ability Classification System, GMFCS = Gross Motor Function Classification System
| Subject | Gender | Age | MACS | GMFCS | Presentation Type |
|---|---|---|---|---|---|
| 1 | Male | 14 | I | II | Spastic Diplegia |
| 2 | Female | 13 | II | III | Spastic Diplegia |
| 3 | Male | 17 | I | I | Hemiplegia |
| 4 | Male | 13 | I | I | Spastic Diplegia |
| 5 | Male | 12 | I | I | Hemiplegia |
| 6 | Male | 13 | II | III | Spastic Quadraplegia |
| 7 | Male | 18 | I | II | Spastic Diplegia |
| 8 | Male | 16 | II | II | Spastic Quadraplegia |
2.2 MEG Acquisition & Experimental Paradigm
Throughout the somatosensory experiment, the children were seated in a custom-made nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array and their eyes closed. A unilateral tactile stimulation was applied using a small airbladder that was affixed to the distal portion of palmar surface of the index finger of the hand. The airbladder consisted of a thin elastic membrane that was surrounded by a plastic outer shell that was taped to the finger. The pressure of the compressed air was 172.4 kPa and was held constant for all subjects. For each child, more than 135 trials were collected using an inter-stimulus interval that varied randomly between 2900 and 3300 ms. The more affected side was stimulated for the children with CP, while the non-dominant hand was stimulated for the TD children. The dominant hand of the TD children was determined by examining which hand they used to sign the assent form.
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio-video feeds from inside the shielded room. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system (Helsinki, Finland) with 306 sensors, including 204 planar gradiometers and 102 magnetometers. Each MEG data set was individually corrected for head motion during task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu et al. 2005; Taulu and Simola, 2006).
2.3 MEG Coregistration & Structural MRI Processing
Four coils were affixed to the head of the subject and were used for continuous head localization during the experiment. Prior to the experiment, the location of these coils, three fiducial points and the scalp surface were digitized to determine their three-dimensional position (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the child was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data was coregistered with structural T1-weighted MRI data prior to source reconstruction. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into a standardized space (Talairach and Tournoux 1988) using BrainVoyager QX version 2.2 (Brain Innovations, The Netherlands).
2.4 MEG Pre-Processing
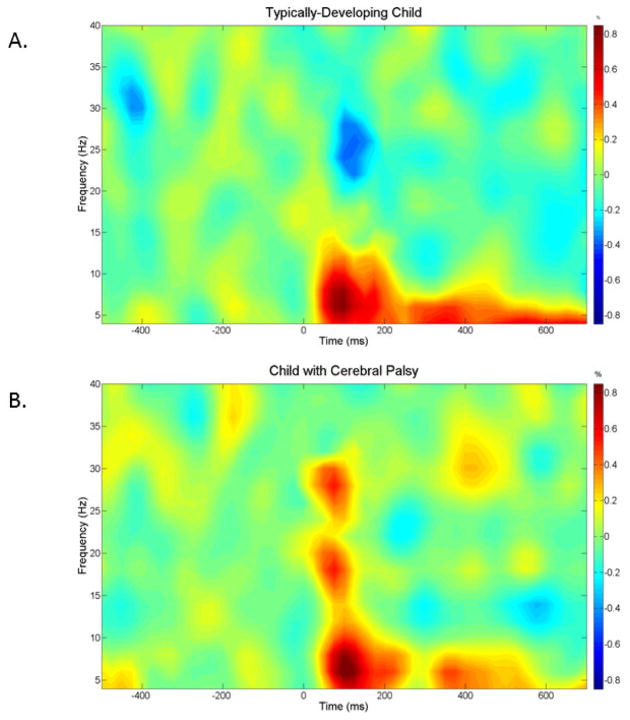
Artifact rejection was based on a fixed threshold method, supplemented with visual inspection. The data analysis epochs were a total duration of 1.2 s (−0.5 s to +0.7 s), with the onset of the mechanical stimulation defined as time 0.0 s, and the baseline defined as −0.5 s to 0.0 s. Artifact-free epochs for each sensor were transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms) and averaged over the respective trials. The power of each time-frequency bin was normalized by dividing it by the amount of power present in the respective frequency’s baseline period. This normalization procedure allowed event-related oscillatory responses to be easily discernible in sensor space, and visual inspection of the sensor-level spectrograms from the fronto-parietal region revealed clear alpha-theta (4–14 Hz) and beta (18–34 Hz) oscillations during the 25–225 ms time window (see Figure 1). This alpha-theta response was consistent in time and frequency with that observed in our previous studies of foot tactile somatosensation (Kurz et al., 2014; 2015), and thus we focused on this response and the slightly faster beta response in the beamforming analyses of the current study.
Figure 1.
Time-frequency spectrograms for a representative typically developing child (A) and a child with cerebral palsy (B). Frequency (Hz) is shown on the y-axis and time (ms) is denoted on the x-axis (with 0 ms defined as stimulation onset). The event-related spectral changes during the hand stimulation task are expressed as percent difference from baseline (−500 to 0 ms). In each case, the MEG sensor with the greatest response amplitude that was located near the sensorimotor cortices, contralateral to the hand stimulated, was chosen. As shown, the TD child had a strong increase in 4–14 Hz alpha-theta band but also a decrease in 18–34 Hz beta band during the same time period. The child with cerebral palsy exhibited an increase in 4–14 Hz alpha-theta band and an increase in the 18–34 Hz beta band during the 25–225 ms time window. The color scale bar for both plots is shown to the far right.
2.5 MEG Source Image Reconstruction
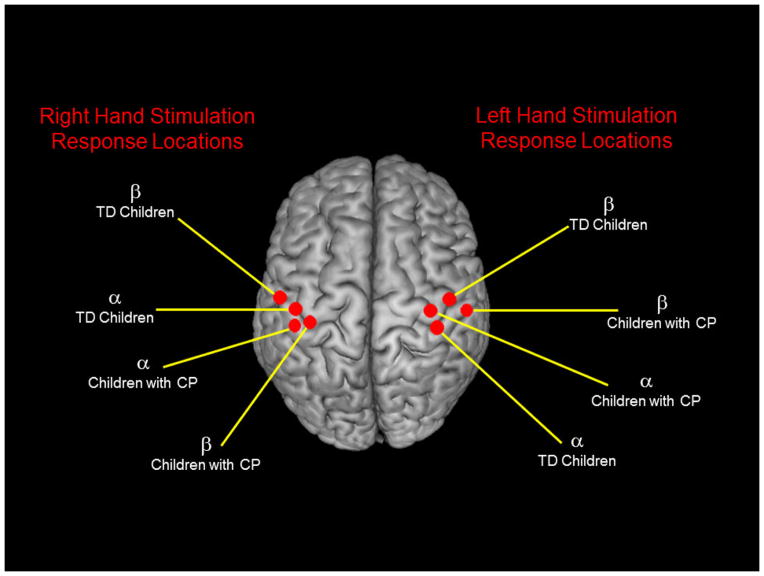
A minimum variance vector beamforming algorithm was employed to calculate the source power across the entire brain volume (Gross et al., 2001; van Veen et al., 1997). The single images were derived from the cross spectral densities of all combinations of MEG sensors within the time-frequency ranges of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per subject using a separately averaged pre-stimulus noise period of equal duration and bandwidth (van Veen et al., 1997; Hillebrand et al., 2005). Thus, the normalized power per voxel was computed separately for alpha-theta and beta band over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Each subject’s functional images were then transformed into standardized space using the transform previously applied to the structural MRI volume, and the entire volume was then spatially resampled. Since the tactile stimulation was not applied to the same hand for all subjects, we extracted the peak voxel amplitude for each time-frequency component of interest (i.e., 4–14 Hz from 25–225 ms; 18–34 Hz from 25–225 ms) from the hand-specific group mean beamformer images per group. The centroid for each oscillatory response (alpha-theta/beta), hand (left/right), and group (CP/TD) from these images is shown in Figure 2. The amplitude values of the peak alpha-theta and beta voxels per participant were subsequently used to evaluate differences in the somatosensory cortical oscillations between the children with CP and the TD children. The magnitude and sign of the peak voxel were used to describe the strength of the event-related synchronization (ERS) or event-related desychronization (ERD) of activity in the neuronal groups that represent the hand in the somatosensory cortices. A negative sign indicated a suppression of the power at the particular frequency relative to the baseline period (i.e., ERD). Conversely, a positive sign indicated an amplification of the power at the particular frequency relative to the baseline (i.e., ERS; Pfurtscheller, 2001; Neuper and Pfurtscheller, 2001; Klimesch et al., 2007). MEG pre-processing and imaging were performed with the BESA software (BESA version 6.0).
Figure 2.
Centroids of the group-mean beamformer images for each frequency bin (alpha-theta/beta), stimulated hand (left/right), and group (cerebral palsy/typically-developing). The amplitude of the peak voxel was extracted from each centroid of the alpha-theta and beta responses for the typically developing (TD) children and children with cerebral palsy (CP) for the respective hand that was stimulated. All statistics were conducted using these peak voxel amplitude values. For this experiment, the side of the body with the greatest impairment was stimulated for the children with CP, while the non-dominate hand was stimulated for the TD children.
2.6 Statistical Analysis
Prior to any statistical analysis, the amplitudes extracted from the peak voxel of the group-level images were log transformed to insure that they were normally distributed. Subsequently, separate independent t-tests were performed to determine if the alpha-theta and beta peak amplitudes were significantly different between the groups within the respective time periods. We further interrogated the data by performing Pearson product moment correlations between the alpha-theta and beta peak amplitudes during the respective time windows to evaluate if the neural oscillations at the respective frequencies were related. These correlations were performed for the entire group of participants, as well as the TD children and children with CP separately. All statistical calculations were performed at the 0.05 alpha level. The data in the text and figures are not log transformed, and are presented as the mean ± standard error of the mean.
3. Results
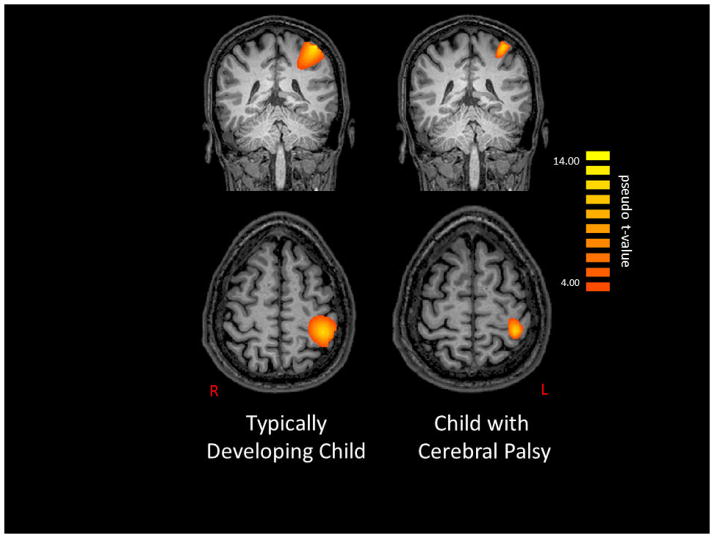
Figure 3 shows alpha-theta band activity during the 25–225 ms time window for a TD child and a child with CP that were representative of the group results. As can be seen in the figure, both the TD child and child with CP demonstrated an alpha-theta ERS after the tactile stimulus was applied to the hand. The magnitude of the peak voxel extracted from the group-level images at the alpha-theta frequency during this time window confirmed that both groups of children generated an alpha-theta ERS (CP = 3.3 ± 2.0 pseudo-t; TD = 5.5 ± 2.2 pseudo-t; P=0.25). However, the magnitude of the ERS was not significantly different between the children with CP and the TD children.
Figure 3.
Noise-normalized beamformer output image of the alpha-theta (4–14 Hz) response from 25 to 225 ms post-stimulation for a representative typically-developing (TD) child (left) and a child with cerebral palsy (CP; right). Both maps show voxels with pseudo-z values > 4.0. As can be discerned, both the children with CP and the TD children exhibited activity clustered in the central sulcus and postcentral gyrus of the hemisphere contralateral to stimulation. In both cases, this activity was maximal just posterior to the motor hand-knob feature of the precentral gyrus. As shown, both children had a strongly synchronized response (orange) to the external tactile stimulus. The images are displayed following the radiological convention (R=L).
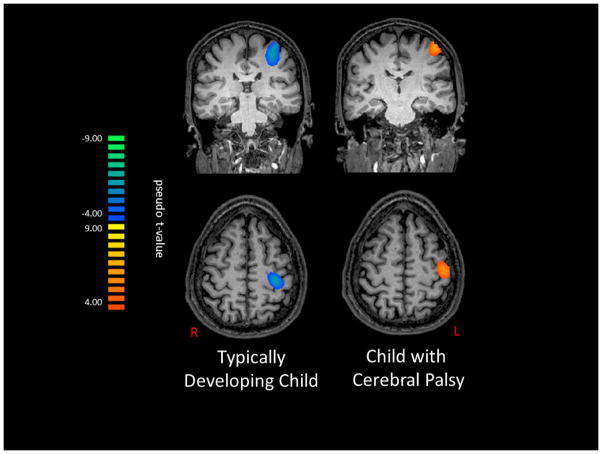
Figure 4 depicts beta band activity during the 25–225 ms time window for a TD child and a child with CP that were representative of the group-level images. As can be seen in the figure, the TD child generated a beta ERD, while the child with CP generated a beta ERS after the tactile stimulus was applied to the hand. The magnitude of the peak voxel extracted from the group-level images at the beta frequency confirmed these observations, and our statistical analysis revealed that the beta oscillations were quite different for the TD children and the children with CP (CP= 2.8 ± 2.2 pseudo-t; TD= −1.7 ± 1.2 pseudo-t; P=0.04).
Figure 4.
Noise-normalized beamformer out image of the beta (18–34 Hz) response from 25 to 225 ms post-stimulation for a representative typically-developing (TD) child (left) and a child with cerebral palsy (CP; right). The map on the left shows voxels with pseudo-z values < −4.0, whereas as that on the right shows pseudo-z values > 4.0. As can be discerned, both the children with CP and the TD children exhibited activity clustered around the central sulcus and the postcentral gyrus, near the motor hand-knob feature in the hemisphere contralateral to stimulation. As shown, the TD children had a desynchronized response (blue) to the external tactile stimulation, while the children with CP had a synchronized response (orange). The images are displayed following the radiological convention (R=L).
Based on the data from all of the participating children, the magnitude of the peak voxel at the alpha-theta frequency was positively correlated with the magnitude of the peak voxel at the beta frequency (r= 0.51; P=0.04). This suggested that stronger alpha-theta oscillations within the somatosensory cortices were associated with stronger beta oscillations. However, inspection of the scatterplots suggested a more complicated story, therefore these data were split by group and the correlations were re-computed. Based on the data from the TD children alone, the magnitude of the peak voxel at the alpha-theta frequency was positively correlated with magnitude of the peak voxel at the beta frequency (r= 0.81; P=0.01). This relationship suggests that the TD children that had strongest alpha-theta oscillations also had the weakest beta oscillations (i.e., smallest ERD – closer to 0). In the children with CP, the magnitude of the peak voxel at the alpha-theta and beta frequencies were not significantly correlated (r=0.21; P=0.36). This lack of a correlation suggests that alpha-theta and beta oscillations may not be well linked in children with CP.
4. Discussion
The primary goal of this investigation was to provide further insight on the neural basis for the hand tactile sensory deficits often reported in the clinic for children with CP (Cooper et al., 1995; Clayton et al., 2003; Sanger and Kukke, 2007; Wingert et al., 2007; Auld et al., 2012; Robert et al., 2013). To this end, we used high-density MEG to identify the alpha-theta and beta oscillatory differences between children with CP and TD children in the somatosensory cortices following tactile stimulation of the hand. Our results indicate that the somatosensory cortical oscillations in the beta frequency range were abnormal in the children with CP, while alpha-theta responses seemed relatively intact. Similar exploratory results for the beta frequency were recently reported for a cohort of children with hemiplegic CP that underwent a median nerve stimulation paradigm (Pihko et al., 2014). Together these results imply that aberrations in somatosensory beta oscillatory activity may play a role in the tactile discrimination deficiencies often seen in children with CP.
The alpha-theta oscillatory responses were relatively normal in the participating children with CP, as both groups exhibited a strong ERS slightly after stimulus onset. Conversely, in our previous studies we have noted that children with CP exhibited a strong alpha-theta ERD within the medial postcentral gyrus/paracentral lobule immediately following tactile stimulation of the foot, while TD children had a strong alpha-theta ERS within the same location (Kurz et al., 2014; 2015). We were expecting to see similar activation deficiencies for the hand at the alpha-theta frequency for the children with CP, and suspect that the discrepancies between the two studies may be related to differences in the integrity of the fiber tracts serving the foot and hand. This interpretation is based on recent preliminary work that suggests that the magnitudes of the tactile evoked hand somatosensory related potentials for children with CP might be related to the quantity and quality of the thalamocortical projections to the post-central gyrus (Papadelis et al., 2014). This suggests that the alpha-theta oscillations might be normal in the participating children with CP because the thalamocortical projections that terminate at the hand cortical area were adequately intact. This interpretation is additionally supported by prior results that indicate that children with CP are more likely to have damage to the neural fiber tracts that serve the lower extremities than the hands (Aicardi & Bax, 1992). All of the children participating in this investigation had noticeable impairments of the lower extremity that limited their mobility; yet, they all had MACS levels between I-II, which indicates that they had fairly adequate hand dexterity. This clinical observation fuels our speculation that the participating children may not have had sufficient damage to the fiber tracts serving the hand to perturb the alpha-theta oscillations. However, we recognize that these statements are speculative and should be challenged by future studies that use a multi-modal imaging approach of diffusion tensor imaging and MEG to evaluate the integrity of the somatosensory processing networks seen across the respective MACS levels.
Despite the normal alpha-theta response, the tactile stimulation resulted in a beta ERS for the children with CP and a beta ERD for the TD children. These stark differences suggest that the somatosensory processing networks at the beta frequency were aberrant in the children with CP, and may better capture the uncharacteristic hand tactile stimulation deficits of children with CP that have a MACS level between I-II. The current scientific trends suggest that the neural processing of sensory information likely involves the dynamic interaction between groups of neurons that are oscillating at different frequencies (Palva & Palva, 2007). Based on this perspective, we suspect that the aberrant beta oscillations seen in the children with CP may be partially linked with the alpha-theta oscillations that were occurring during the same time frame. This notion is based on our finding that there was a correlation between the magnitudes of the beta and alpha-theta oscillations for the TD children, but not for the children with CP. The lack of a relationship for the children with CP implies that there may be uncharacteristic interactions between the neuronal groups of the somatosensory cortices that are oscillating at the alpha-theta and beta frequencies. A more thorough investigation of the potential interactions of the neuronal groups within the somatosensory cortices that are oscillating at the alpha-theta and beta frequencies may provide unique information about the neural basis for the tactile discrimination deficits seen in children with CP with a wide range of MACS levels.
5. Conclusions
To summarize, our results showed that following tactile stimulation of the hand, event-related somatosensory cortical oscillations in the alpha-theta frequency range during the 25–225 ms time frame were not different between the children with CP and the TD children. This result is different from our prior investigations that have shown that TD children and children with CP have different somatosensory cortical oscillations at the alpha-theta frequency after a tactile stimulus is applied to the foot (Kurz et al., 2014; 2015). Despite the lack of differences at alpha-theta frequency, there were blunt differences at the beta frequency range between the TD children and the children with CP. These aberrant beta oscillations might be linked with the alpha-theta oscillations that occur during at the same time frame since our results show that the magnitude of the beta oscillations were related with the magnitude of the alpha-theta oscillations for the TD children, but were not for the children with CP. This implies that synergy between the neural oscillations at the respective frequencies may be important for processing somatosensory information. Altogether these results have identified new vistas for quantifying the neurophysiological underpinnings for the tactile discrimination deficits often reported for children with CP.
Highlights.
Children with cerebral palsy (CP) have tactile discrimination deficits.
MEG was used to evaluate the somatosensory cortical oscillations after a tactile stimulus was applied to the hand.
Alpha-theta oscillations (4–14 Hz) were similar between the children with CP and typically developing (TD) children
Stark differences between the respective groups were found for the beta oscillations (18–34 Hz).
Acknowledgments
Funding for this project was provided by the Hattie B. Munroe Foundation and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (5R21HD077532).
Abbreviations
- CP
Cerebral palsy
- ERS
Event related synchronization
- ERD
Event related desynchronization
- MEG
Magnetoencephalography
- MACS
Manual Ability Classification System
- TD
Typically Developing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aicardi J, Bax M. Diseases of the Nervous System in Childhood. In: Aicardi J, editor. Diseases of the Nervous System in Childhood. 2. Cambridge, UK: Mac Keith Press; 1992. pp. 210–239. [Google Scholar]
- 2.Auld ML, Boyd RN, Moseley GL, Ware RS, Jonston LM. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehab. 2012;93:696–702. doi: 10.1016/j.apmr.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 4.Christensen D, Van Naarden BK, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R, Yeargin-Allsopp M. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56:59–65. doi: 10.1111/dmcn.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton K, Fleming JM, Copley J. Behavioral responses to tactile stimuli in children with cerebral palsy. Phys Occup Ther Ped. 2003;23:43–62. [PubMed] [Google Scholar]
- 6.Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. Journal of Child Neurology. 1995;10:300–309. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- 7.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The manual ability classification system (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–554. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 8.Gordon AM, Duff SV. Relationship between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999;41:586–591. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- 9.Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Nat Acad Sci. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzzetta A, Bonanni P, Biagi L, Tosetti M, Monanaro D, Guerrini R, Cioni G. Reorganization of the somatosensory system after early brain damage. Clin Neurophys. 2007;118:1110–1121. doi: 10.1016/j.clinph.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Map. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, Forssberg H, Eliasson AC. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52:145–152. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoon AH, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell M, Levey E, Mori S, Johnston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nature Rev Neurosci. 2009;10:345–359. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- 15.Klimesch W, Sauseng P, Hanslmayr EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and visual evoked potentials in children with cerebral palsy: correlations and discrepancies with MRI findings and clinical picture. Ped Rehab. 2006;9:201–209. doi: 10.1080/13638490500343179. [DOI] [PubMed] [Google Scholar]
- 17.Kulak W, Sobaniec W, Solowiej E, Bockowski L. Somatosensory and MRI findings in children with cerebral palsy: correlations and discrepancies with clinical practice. J Ped Neurol. 2005;3:77–78. doi: 10.1080/13638490500343179. [DOI] [PubMed] [Google Scholar]
- 18.Kurz MJ, Wilson TW. Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett. 2011;490:1–5. doi: 10.1016/j.neulet.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 19.Kurz MJ, Wilson TW, Corr B, Volkman KG. Neuromagnetic activity of the somatosensory cortices associated with body weight-supported treadmill training in children with cerebral palsy. J Neuro Phys Ther. 2012;36:166–172. doi: 10.1097/NPT.0b013e318251776a. [DOI] [PubMed] [Google Scholar]
- 20.Kurz MJ, Heinrichs-Graham E, Becker KM, Wilson TW. The magnitude of somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J Neurophys. 2015;113:3143–3150. doi: 10.1152/jn.00602.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurz MJ, Heinrichs-Graham E, Becker KM, Wilson TW. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophys. 2014;111(3):573–579. doi: 10.1152/jn.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maitre NL, Barnett ZP, Key APF. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol. 2012;27:1276–1283. doi: 10.1177/0883073811435682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostry DJ, Darainy M, Mattar AAG, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci. 2010;30:5384–5393. doi: 10.1523/JNEUROSCI.4571-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadelis C, Ahtam B, Nazarova M, Nimec D, Snyder B, Grant PE, Okada Y. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neurosci. 2014;8:1–15. doi: 10.3389/fnhum.2014.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palva S, Palva JM. New vistas for a-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Pfurtscheller G. Functional brain imaging based on ERD/ERS. 2001;41:1257–1260. doi: 10.1016/s0042-6989(00)00235-2. [DOI] [PubMed] [Google Scholar]
- 27.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophys. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 28.Pihko E, Nevalaninen P, Vaalto S, Laaksonen K, Maenpaa, Valanne L, Lauronen L. Reactivity of sensorimotor oscillations is altered in children with hemiplegic cerebral palsy: a magnetoence phalographic study. Hum Brain Map. 2014;35:4105–4117. doi: 10.1002/hbm.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophys. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- 30.Riquelme I, Montoya P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurol. 2010;121:1314–1320. doi: 10.1016/j.clinph.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Robert MT, Guberek R, Sveistrup H, Levin MF. Motor learning in children with hemiplegic cerebral palsy and the role of sensation in short-term motor training of goal-directed reaching. Dev Med Child Neurol. 2013;55:1121–1128. doi: 10.1111/dmcn.12219. [DOI] [PubMed] [Google Scholar]
- 32.Rose J, Mirmiran M, Butler EE, Lin CY, Barnes PD, Kermoian R, Stevenson DK. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum P, Paneth N, Leviton A, Goldstein N, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 34.Sakamoto T, Arissian K, Asanuma H. Functional role of the sensory cortex in learning motor skill in cats. Brain Res. 1989;503:258–264. doi: 10.1016/0006-8993(89)91672-7. [DOI] [PubMed] [Google Scholar]
- 35.Sanger TD, Kukke SN. Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol. 2007;22:289–293. doi: 10.1177/0883073807300530. [DOI] [PubMed] [Google Scholar]
- 36.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 37.Staudt M, Braun C, Gerloff C, Erb M, Grodd W, Kragelhoh-Mann I. Developing somatosensory projections bypass periventricular brain lesions. Neurology. 2006;67:522–525. doi: 10.1212/01.wnl.0000227937.49151.fd. [DOI] [PubMed] [Google Scholar]
- 38.Talairach G, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1998. [Google Scholar]
- 39.Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEE Trans Signal Proc. 2005;53:3359–3372. [Google Scholar]
- 40.Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- 41.Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E, Tsikoulas I. Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatric Neurol. 2011;44:177–182. doi: 10.1016/j.pediatrneurol.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RKS, Gupta RK. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiol. 2010;52:759–765. doi: 10.1007/s00234-010-0703-8. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi R, Gupta RK, Shah V, Tripathi M, Rathore RKS, Kumar M, Pandey CM, Narayana PA. Treatment-induced plasticity in cerebral palsy: a diffusion tensor imaging study. Ped Neurol. 2008;39:341–349. doi: 10.1016/j.pediatrneurol.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Uswatte G, Taub E. Neuroplasticity to treat motor disorders. Prog Brain Res. 2013;207:379–401. doi: 10.1016/B978-0-444-63327-9.00015-1. [DOI] [PubMed] [Google Scholar]
- 45.van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- 46.Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol Learning Memory. 2010;93:532–539. doi: 10.1016/j.nlm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Wilke M, Staudt M, Juenger H, Grodd W, Braun C, Krageloh-Mann I. Somatosensory system in two types of motor reorganization in congenital hemiparesis: topography and function. Hum Brain Map. 2009;30:776–788. doi: 10.1002/hbm.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wingert JR, Burton H, Sinclair RJ, Brunstrom JE, Damiano EL. Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol. 2008;50:832–838. doi: 10.1111/j.1469-8749.2008.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]