Abstract
Background
In samples from controlled randomized clinical trials, a smoker’s rate of nicotine metabolism, measured by the 3-hydroxycotinine to cotinine ratio (NMR), predicts response to transdermal nicotine. Replication of this relationship in community-based samples of treatment-seeking smokers may help guide the implementation of the NMR for personalized treatment for nicotine dependence.
Methods
Data from a community-based sample of treatment seeking smokers (N = 499) who received 8 weeks of transdermal nicotine and 4 behavioral counseling sessions were used to evaluate associations between the NMR and smoking cessation. Secondary outcomes included withdrawal and craving, depression and anxiety, side effects, and treatment adherence.
Results
The NMR was a significant predictor of abstinence (OR = .58, 95% CI: 0.35-0.98, p = .04), with faster metabolizers showing lower quit rates than slower metabolizers (24% vs. 33%). Faster nicotine metabolizers exhibited significantly higher levels of anxiety symptoms over time during treatment, vs. slower metabolizers (NMR × Time interaction: F[3,357] = 3.29, p = .02). NMR was not associated with changes in withdrawal, craving, depression, side effects, and treatment adherence (p’s > .05).
Conclusions
In a community-based sample of treatment-seeking smokers, faster nicotine metabolizers were significantly less likely to quit smoking and showed higher rates of anxiety symptoms during a smoking cessation treatment program, vs. slower nicotine metabolizers. These results provide further evidence that transdermal nicotine is less effective for faster nicotine metabolizers and suggest the need to address cessation-induced anxiety symptoms among these smokers to increase the chances for successful smoking cessation.
Keywords: smoking cessation, nicotine dependence, pharmacogenetics, biomarker, anxiety, nicotine metabolite ratio
1. Introduction
Several studies suggest that a genetically-informed biomarker that characterizes smokers by their rate of nicotine metabolism may be useful for selecting pharmacotherapy to maximize treatment efficacy for smokers interested in cessation.1,2 This biomarker, referred to as the nicotine metabolite ratio (NMR) and formed using a ratio of the two primary metabolites of nicotine (3-hydroxycotinine and cotinine), is a surrogate marker for the rate of nicotine clearance, representing genetic (CYP2A6) and demographic influences (e.g., race, gender) on the rate of nicotine metabolism.3 Three independent randomized clinical trials4-6 have shown that smokers characterized as having faster nicotine metabolism (higher NMR values) are significantly less likely to quit smoking using transdermal nicotine than smokers with slower nicotine metabolism (lower NMR values); in a fourth clinical trial,7 bupropion offset the relapse risk among faster nicotine metabolizers, suggesting that bupropion may help fast nicotine metabolizers to quit smoking. Most recently, a randomized clinical trial that prospectively stratified allocation to transdermal nicotine or varenicline showed that varenicline can effectively treat nicotine dependence among fast nicotine metabolizers.8
Should the NMR be used clinically to determine treatment selection to maximize therapeutic benefit, the relationship between the NMR and response to transdermal nicotine should be replicated among community samples of smokers who may differ in important ways (e.g., higher rates of comorbid psychiatric conditions) from the participants in the efficacy clinical trials which form the basis for knowledge regarding the effects of the NMR on treatment response. In this study, data from a community-based effectiveness trial of transdermal nicotine patches were used to assess the replication of the relationship previously found between NMR and response to transdermal nicotine.4-6 In addition, this study examined how the rate of nicotine metabolism predicted secondary outcomes relevant to cessation outcomes: withdrawal, craving, depression and anxiety symptoms, side effects, and treatment adherence.9 Assessment of secondary outcomes between slow and fast nicotine metabolizers may elucidate potential mechanisms through which the NMR influences response to treatments for nicotine dependence and offer insight into targets for adjunctive treatment to help offset the heightened relapse risk documented among fast nicotine metabolizers. The results from this study may provide further support for, and enhance understanding of, the potential use of the NMR to guide the personalization of treatment for nicotine dependence.
2. Methods
Data for this study were from an effectiveness study designed to evaluate long-term use of transdermal nicotine for nicotine dependence (ClinicalTrials.gov Identifier: NCT01047527). Community smokers were recruited through media advertisements and the trial was conducted at the University of Pennsylvania and Northwestern University, which provided Institutional Review Board oversight and approval. Potential participants called to inquire about the study; an evaluation of study interest and initial eligibility was completed by phone. An in-person visit confirmed eligibility and participants were randomly selected for either 8, 24, or 52 weeks of transdermal nicotine patch therapy; all participants received 12 standardized, manual-based behavioral smoking cessation counseling sessions based on established guidelines.10 For the present analysis, only data up to 8 weeks were used to standardize treatment across participants (i.e., all participants received 8 weeks of nicotine patch treatment and four behavioral counseling sessions) and to remain consistent with previous studies.4-6
2.1 Participants
Consistent with an effectiveness trial, inclusion and exclusion criteria were limited to increase generalizability of findings (e.g., 20% of the present sample had current or past major depression and 5% had current substance abuse). Eligible participants were ≥ 18 years of age, reported smoking ≥ 10 cigarettes/day, and were interested in quitting smoking. Participants were excluded if they had a current medical problem for which transdermal nicotine is contraindicated (e.g., allergy to latex), had a lifetime DSM-IV diagnosis of psychosis or bipolar disorder, reported current suicidality as identified by the Mini International Neuropsychiatric Interview (MINI),11 or were unable to communicate in English. Female participants were excluded if they were pregnant, planning a pregnancy, or lactating.
2.2 Procedures
All participants received 8 weeks of 21mg transdermal nicotine patches (Nicoderm CQ; GlaxoSmithKline, Research Triangle Park, NC). Participants received one in-person pre-quit counseling session at week −2 (baseline), which focused on preparing for cessation, and then set a quit date for week 0, at which time they were instructed to start using the nicotine patch. At weeks 0, 4, and 8, participants received behavioral counseling over the telephone. These sessions were based on standard smoking cessation behavioral treatment guidelines10 focusing on managing urges and triggers to smoking and developing strategies to avoid relapse. Assessments, described below, were conducted at baseline (in-person) and at weeks 0, 4, and 8 (by telephone). Week 8 self-reports of smoking cessation (for the 7 days preceding this assessment) were biochemically confirmed using breath carbon monoxide (CO).
2.3 Measures
2.3.1 Covariates
At baseline, participants self-reported demographics (e.g., age, race, sex) and smoking history and behavior (e.g., cigarettes per day; the Fagerström Test for Nicotine Dependence [FTND]).12
2.3.2 Nicotine Metabolite Ratio
Saliva samples (5ml) were collected during eligibility assessment to determine NMR using liquid chromatography with tandem mass spectrometry.13 Individual NMR values remain stable over time, and saliva NMR results correlate strongly with plasma NMR results.14
2.3.4 Nicotine Withdrawal
The Minnesota Nicotine Withdrawal Scale15 assesses 7 DSM-IV items of nicotine withdrawal (e.g., restlessness, irritability) and items were summed.
2.3.5 Nicotine Craving
The 10-item brief Questionnaire of Smoking Urges (QSU)16 contains 2 subscales (anticipation of reward, relief from negative affect).
2.3.6 Depression
The Inventory of Depressive Symptomatology (IDS) is a 30-item self-report measure used to assess the severity and frequency (past 7-days) of depressive symptoms, consistent with the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders – 4th Edition diagnosis of a major depressive episode.17-19
2.3.7 Anxiety
The 21-item Beck Anxiety Inventory (BAI) assessed anxiety symptoms over the past week.20 Items were summed to obtain a total score.
2.3.8 Side Effects
A checklist of nicotine patch-related side effects used in past studies6 was administered. The occurrence and severity of side effects (e.g., nausea, rash) were rated by subjects from 0 (none) to 4 (severe) and a summary score is used.
2.3.9 Treatment Adherence
Daily patch use from weeks 0-8 was assessed at each session using a timeline follow-back measure as done previously.21,22 Total adherence across the 8-week treatment, defined as wearing the patch for 42 out of 56 days (i.e., 75% or 6/7 days per week), was utilized, as done previously.23
2.3.10 Smoking Cessation
Self-reports of smoking were obtained at each session using the timeline follow-back approach.21 At week 8, participants were asked to provide a breath sample for biochemical verification of smoking status. Participants were considered abstinent at week 8 if they self-reported abstinence for 7 days prior to the assessment and provided a breath sample with CO ≤ 10ppm.24 Participants who withdrew from the study, failed to provide a sample, or provided a CO breath sample >10ppm were considered smokers.
2.4 Data Analysis
The sample was characterized in terms of demographic and smoking-related data (e.g., age, race, nicotine dependence, smoking rate). Pearson correlation and analysis of variance (ANOVA) were used to examine the relationship between NMR and demographic and smoking-related data and variables related to NMR (p < .10) were included as covariates in subsequent analyses. We conducted a Receiver Operator Characteristic (ROC) analysis to determine the optimal cut-point using the NMR for distinguishing subjects who quit smoking (i.e., week 8 abstinence), vs. those who did not, and examined this categorical measure of nicotine metabolism as a predictor of week 8 quit rates, withdrawal and craving, anxiety and depression, and side effects and treatment adherence. Determining a cut-point in ROC analysis involves use of the point on the curve closest to (0,1) criterion.25,26 The conceptual basis for this criterion is that this point on the curve represents the place in the distribution with the highest sensitivity and specificity, thus minimizing misclassification.27 This approach was used in a previous study of the NMR.6 Logistic regression was used to examine the relationship between the categorical NMR measure (controlling for covariates) and week 8 abstinence and multivariate repeated measures ANOVA was used to examine the effects of the categorical NMR and time on withdrawal and craving, anxiety and depression, and side effects and treatment adherence.
3. Results
3.1 Sample Characteristics
The intent-to-treat (ITT) sample was 525 but, for the present study, the subset of 499 participants who agreed to provide saliva for the NMR was analyzed. One-half of the sample was female, 49% were African American and 49% were Caucasian, 31% were married or living as married, and 32% of the sample had a high school education or less. The average age of the sample was 46.5 years (SD = 12.1) and the average BMI was 28.6 (SD = 6.5). The average age at which participants started to smoke was 16.3 years (SD = 5.0) and, on average, participants had been smoking for 28.9 years (SD = 12.7). The average FTND score was 5.1 (SD = 2.0) and, on average, participants smoked 17.2 cigarettes/day (SD = 8.4) at baseline. The average NMR was .35 (SD = .21). Among variables in Table 1, attrition was significantly associated with age, BMI, and number of years smoked (p’s < .05).
Table 1.
Demographic and Smoking-related Variables for Slow and Fast Metabolizers
| Characteristic |
Slow
(N = 386) |
Fast
(N = 113) |
Overall
(N = 499) |
|---|---|---|---|
| Gender | |||
| Female | 74.1 | 25.9 | 49.5 |
| Male | 81.0 | 19.0 | 50.5 |
| Race | |||
| African American | 81.1 | 18.9 | 49.5 |
| Caucasian | 75.3 | 24.7 | 50.5 |
| Education | |||
| GED or less | 75.3 | 24.7 | 31.7 |
| Some College or More | 78.6 | 21.4 | 68.3 |
| Age (Mean, SD) | 45.6 (12.5) | 49.6 (9.9) | 46.5 (12.1) |
| BMI (Mean, SD) | 28.6 (6.4) | 28.4 (6.9) | 28.6 (6.5) |
| FTND (Mean, SD) | 5.1 (1.9) | 5.1 (2.4) | 5.1 (2.0) |
| Cigarettes per Day (Mean, SD) | 16.9 (8.1) | 18.5 (9.4) | 17.2 (8.4) |
| Age Started Smoking (Mean, SD) | 16.3 (5.0 | 16.4 (5.3) | 16.3 (5.0) |
| Years Smoking (Mean, SD) | 28.3 (13.0) | 31.1 (11.3) | 28.9 (12.7) |
| 3-HC/Cotinine | 0.25 (0.11) | 0.65 (0.18) | 0.34 (0.21) |
Note. Slow and fast metabolizers based on cut-off of 0.47 from ROC analysis.
3.2 The NMR and Demographic and Smoking Data
Caucasians had significantly higher NMR than African Americans (M = .38 vs. M = .32; F[1,479] = 10.62, p = .001). The relationship between gender and NMR approached significance (F[1,497] = 2.90, p = .09), with females showing a higher NMR than men (M = .36 vs. M = .33). Age was positively correlated with NMR (r = .15, p = .001). NMR was not associated with other demographic or smoking-related data, including FTND, baseline smoking rate, age started smoking, years smoked, or BMI (p’s > .10).
3.3 ROC Analysis
The results of the ROC analysis indicated a cut-point, based on the closest-to-(0,1) criterion, at 0.47 for the NMR distribution (close to the cut-off for the top quartile of the NMR distribution which was 0.45). Based on this result, a binary NMR variable was created at this cut-point for subsequent analysis with slow metabolizers (N = 386) defined as NMR values < 0.47 and fast metabolizers (N = 113) defined as NMR values ≥ 0.47. Demographic and smoking-related characteristics of these two groups are presented in Table 1.
3.4 The NMR and Abstinence at Week 8
Using this cut-point for the NMR, and controlling for covariates (gender, race, and age, BMI, FTND, years smoked), the rate of nicotine metabolism was a significant predictor of point-prevalence, CO-confirmed abstinence at week 8 (OR = .58, 95% CI: 0.35-0.98, p = .04; Table 2). Among slow nicotine metabolizers (NMR < 0.47), 33% were confirmed abstinent at week 8, vs. 24% of the fast nicotine metabolizers (NMR ≥ 0.47).ii
Table 2.
Multivariate Logistic Regression Analysis of Smoking Abstinence at Week 8 by NMR, Controlling for Covariates (n = 499)
| Predictor | OR | 95% CI | p |
|---|---|---|---|
| Sex (Reference = Female) | 0.82 | 0.55 - 1.23 | 0.34 |
| Race (Reference = Caucasian) | 0.95 | 0.62 – 1.46 | 0.83 |
| Age | 1.04 | 1.01 - 1.08 | 0.02 |
| BMI | 1.01 | 0.98 - 1.04 | 0.53 |
| FTND | 0.85 | 0.77 - 0.94 | < 0.01 |
| Years Smoked | 0.97 | 0.94 - 1.00 | 0.07 |
| NMR (Reference = Slow) | 0.56 | 0.33 - 0.95 | 0.03 |
Note. NMR based on cut-off of 0.47 from ROC analysis. FTND was included as a covariate since it is a common predictor of cessation outcomes and was included as a covariate in prior studies.
3.5 The NMR and Withdrawal, Craving, Depression, Anxiety, Adherence, and Side Effects
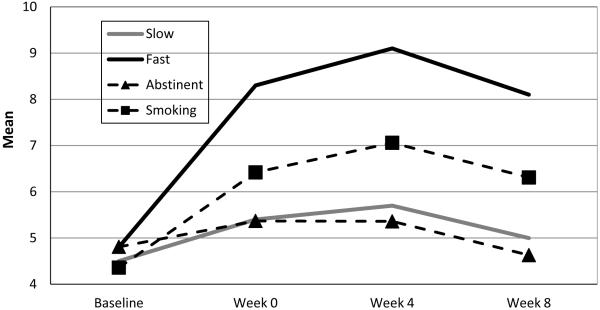
Models that examined variation in withdrawal, craving, depression, side effects and adherence, controlling for covariates across slow and fast nicotine metabolizers based on the NMR cut-off from the ROC analysis were not statistically significant (all p’s > .05). Only the model that assessed variation in anxiety symptoms across slow and fast metabolizers was statistically significant (NMR × Time interaction: F[3,357] = 3.29, p = .02). Controlling for covariates, slow metabolizers showed very little increase in anxiety symptoms from baseline to week 8, whereas the fast metabolizers showed a substantial increase in anxiety symptoms over this time (Figure 2). Post-hoc tests indicated that, while slow and fast metabolizers were not significantly different in their anxiety symptoms at baseline and week 0 (p > .05), faster metabolizers showed a significant increase in anxiety at week 4 and 8, vs. slow metabolizers (p’s < .01). Parenthetically, controlling for covariates, participants who were smoking at week 8 exhibited increased anxiety symptoms from baseline to week 8, vs. participants who were abstinent, but this relationship only approached significance (F[3, 357) = 2.43, p = .07; Figure 2).
Figure 2.
Variation in Anxiety Symptoms Over Time for Slow and Fast Nicotine metabolizers and for Week 8 Abstainers and Smokers
4. Discussion
The primary aims of this study were to replicate the finding that the nicotine metabolite ratio (NMR) predicts response to transdermal nicotine and behavioral counseling in a community-based sample of treatment-seeking smokers and to explore secondary smoking cessation outcomes that may also relate to variation in rate of nicotine metabolism. This study provides additional support for the predictive value of the NMR for smoking cessation outcomes. As two studies5,6 found previously, faster nicotine metabolizers were significantly less likely than slower nicotine metabolizers to achieve smoking abstinence after 8 weeks of transdermal nicotine and behavioral counseling. Furthermore, the present study demonstrated that faster nicotine metabolizers show a greater increase in anxiety symptoms during treatment, relative to slower metabolizers. Whereas previous studies of the NMR have focused on differences in withdrawal, craving, and treatment-related side effects,4-6 this study suggests that individual differences between fast and slow metabolizers in anxiety symptoms – particularly after the first 2 weeks of treatment – may also differentiate responses to smoking cessation treatment across these groups of smokers.
The primary finding – that faster nicotine metabolizers show significantly lower rates of smoking cessation when treated with transdermal nicotine and counseling – lends further support to the potential use of the NMR as a method for selecting treatments for individual smokers. This is the fourth independent study to document this finding. The present study replicates this relationship in a community-based sample, which better reflects the characteristics of the smoking population than previous controlled trials. Compared with past trials, the present study had far fewer inclusion and exclusion criteria, particularly with regard to co-occurring substance abuse or psychiatric illness. Therefore, the present findings offer replication and enhanced generalizability of the relationship between NMR and response to transdermal nicotine. Indeed, the present findings may be more reflective of individuals attempting to quit smoking by accessing transdermal nicotine over-the-counter or individuals seeking access to transdermal nicotine through a healthcare provider.
While there were few additional differences noted between slow and fast nicotine metabolizers across the eight weeks of treatment, faster nicotine metabolizers did show a fairly stark increase in anxiety symptoms between the first counseling session (week −2) and initiation of the nicotine patch (week 0) relative to slow metabolizers; these symptoms persisted across the 8 weeks of treatment. There is substantial documentation of the relationship between smoking and anxiety,28 and several studies have implicated anxiety as a risk factor for relapse during a smoking cessation attempt. For example, one trial found that 16% of smokers felt anxious or tense prior to an initial lapse, and those who lapsed due to anxiety or stress resumed smoking more quickly than those who lapsed for other reasons.29 Post-quit negative affect—including stress or anxiety—has been identified as a strong predictor of relapse.30 Smokers prone to anxiety sensitivity (i.e., those who fear becoming anxious or experiencing negative affect) are more likely to lapse during a cessation attempt.31 Lastly, one large randomized trial testing several medications for nicotine dependence found that anxious smokers had the lowest long-term quit rates.32 The present study found that faster nicotine metabolizers may be at greater risk for experiencing anxiety symptoms, which heightens the individual’s risk for relapsing back to smoking. In fact, while participants who were abstinent at week 8 showed very little change in anxiety symptoms from baseline to week 8 (mean reduction of .01), participants who were smoking at week 8 showed a substantial increase in anxiety symptoms over this period (mean increase of 1.82, p = .02).
These results have clinical implications for improving response to treatments for nicotine dependence. Given that faster nicotine metabolizers do not benefit as much as slow metabolizers from transdermal nicotine, other cessation aids should be considered for this sub-group of smokers. To date, one study7 has examined bupropion treatment for faster nicotine metabolizers. This placebo-controlled randomized trial assessed the predictive value of the NMR for cessation rates among smokers using bupropion versus placebo. Participants in the highest quartile of the NMR distribution in this study (i.e., the fastest nicotine metabolizers) benefited significantly from bupropion: 34% of participants from the active medication group had quit by the end of treatment, vs. 10% of participants using placebo. In addition, participants in the lowest quartile of the NMR distribution in this study (i.e., the slowest nicotine metabolizers) showed no benefit from bupropion over counseling alone (quit rates of 32%). Further, a recently-completed placebo-controlled clinical trial, which prospectively stratified slow and fast nicotine metabolizers prior to randomization to nicotine patch or varenicline, showed that varenicline is also an efficacious treatment for fast nicotine metabolizers.8 Since slow metabolizers are adequately treated with either behavioral counseling or transdermal nicotine – with no additional benefit from bupropion or varenicline – and faster metabolizers are more effectively treated with bupropion or varenicline, the NMR may serve as a useful biomarker for selecting treatments for individual smokers.
These results also suggest the need to assess the potential effect of a behavioral intervention specifically designed to address cessation-induced anxiety symptoms among fast nicotine metabolizers. One ongoing trial33 is evaluating the role of exercise as a behavioral adjunct to treating nicotine dependence among anxious smokers; another line of research is focused on a behavioral intervention designed to reduce anxiety sensitivity among smokers in order to increase cessation rates (ClinicalTrials.gov Identifier: NCT01789125). Promising results have emerged concerning evaluation of behavioral interventions tailored to address depression symptoms,34 so perhaps a similar approach for anxiety symptoms could also prove efficacious.
The present study must be considered in the context of its limitations. First, although a relationship was observed between the NMR and anxiety levels during a cessation attempt, the nature of the relationship between nicotine metabolism, anxiety, and treatment response remains unclear. Post-hoc analysis indicated that fast metabolizers do not initiate cessation treatment with higher anxiety symptoms but, rather, experience increased anxiety early during treatment. As such, heightened anxiety symptoms may be a dimension of the withdrawal experience for fast metabolizers, or it may be a consequence of early failure in treatment. Notably, participants who had returned to smoking at week 8 showed a prominent increase in anxiety symptoms over time compared to participants who remained abstinent at week 8, indicating that the experience of high levels of anxiety is clinically relevant. Second, this study did not include long-term assessment of outcomes since after week 8 one-third of participants no longer used transdermal nicotine. Consequently, it is unclear from this study if the benefit of transdermal nicotine for slow metabolizers persists beyond 8 weeks or interacts with treatment duration.iii Third, while this study improved generalizability by including a more diverse sample than prior studies of the NMR,6 it is impossible to generalize these results to all smokers interested in quitting. Finally, in the present study, the NMR was assessed using saliva rather than plasma, as seen in previous trials.5-7 This meant that the cut-off used for the NMR to distinguish slow and fast nicotine metabolizers in the present study was based on the ROC analysis from the current data and varies from cut-offs used in previous studies. Since there are currently no established cut-points for distinguishing slow and fast nicotine metabolizers, this is a priority for future research. While the present methods have been used in past studies,6 the authors are hopeful that an accumulation of studies such as this one will help facilitate the development of an established cut-point. This work will facilitate the provision of treatment recommendations using the three primary methods for determining NMR: plasma, urine, and saliva.
The present findings advance understanding of the potential use of the NMR for personalizing the selection of treatments for nicotine dependence. This trial provides important validation in a community-based sample of treatment-seeking smokers that transdermal nicotine treatment is best suited for slow nicotine metabolizers. The NMR, therefore, may help determine suitable candidates for treatment with transdermal nicotine in the real world. The present study also expands the current NMR literature by highlighting anxiety as a potential risk for fast nicotine metabolizers undergoing a quit attempt. While previous studies have provided evidence of a correlation between nicotine metabolism and trait anxiety,31 the data from this study suggest a relationship also exists between the NMR and state anxiety. Further research could be directed toward assessing the role of anxiety symptoms in mediating the relationship between NMR and response to cessation treatments and determining if anxiety-reduction techniques are uniquely helpful for fast nicotine metabolizers undergoing a cessation attempt. Increased understanding of the potential use of the NMR as the first biomarker for individualizing treatment for nicotine dependence may offer a critical tool for helping to reduce overall population smoking rates. This biomarker, like other possible genetic markers for success in smoking cessation,4,35 may usher in a new evolution in personalized medicine for nicotine dependence.
Highlights.
Nicotine metabolism and smoking cessation was examined in a community sample
Faster nicotine metabolizers showed lower quit rates than slower metabolizers
Faster metabolizers reported higher anxiety levels than slower metabolizers
Rate of nicotine metabolism can individualize treatment for nicotine dependence
Figure 1.
CONSORT Diagram
Note. *A list of the reasons for participant ineligibility can be provided by the authors upon request.
Acknowledgments
Role of Funding Sources
This research was supported by grants from the National Institute on Drug Abuse (R01 DA025078 and R01 DA033681) and from the National Cancer Institute (R01 CA165001 and P30 CA16520). Neither organization had a role in study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
While separating the sample by race reduces power substantially, in addition to a model that controlled for race in predicting week 8 abstinence, we separated the sample by race and ran two separate regression models. Results were significant for Caucasian only (resembling the overall sample). For African-Americans, NMR was not significantly related to primary outcomes.
The authors are currently analyzing the long-term outcome data from the current trial to evaluate the interaction between NMR and treatment duration.
Contributors
Amanda Kaufmann led the preparation of this manuscript. Brian Hitsman assisted with the ascertainment of funding for this study, collaborated on the study design, and served as Principal Investigator at Northwestern. Patricia Goelz and Anna Veluz-Wilkins oversaw participant recruitment, data collection, and data management at the University of Pennsylvania and Northwestern University sites, respectively. Sonja Blazekovic and Lindsay Powers oversaw the provision of treatment and the collection of data at the University of Pennsylvania and Northwestern University sites, respectively. Frank Leone and Peter Gariti oversaw participant eligibility and safety monitoring. Rachel Tyndale oversaw the evaluation of the NMR. Robert Schnoll designed the study and ascertained the funding for this trial. All authors approved the final version of this manuscript.
Conflicts of Interest
Dr. Schnoll receives medication (Chantix) and matching placebo free of charge from Pfizer and has provided consultation to Pfizer and GlaxoSmithKline. Dr. Hitsman receives medication (Chantix) and matching placebo free of charge from Pfizer. These companies had no involvement in this study. All other authors declare that they have no conflicts of interest.
References
- 1.Bough KJ, Lerman C, Rose JE, et al. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93(6):526–38. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnoll RA, Leone FT. Biomarkers to optimize the treatment of nicotine dependence. Biomark Med. 2011;5(6):745–61. doi: 10.2217/bmm.11.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- 4.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson F, Schnoll RA, Wileyto EP, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(3):320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 8.Lerman C, Schnoll RA, Hawk LW, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. doi: 10.1016/S2213-2600(14)70294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West O, Hajek P, McRobbie H. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology. 2011;21:313–322. doi: 10.1007/s00213-011-2341-1. [DOI] [PubMed] [Google Scholar]
- 10.Fiore MC, Jaen CR, Baker TB, et al. Clinical Practice Guideline. US Public Health Service; Rockville, MD: 2008. Treating tobacco use and dependence: 2008 Update. [Google Scholar]
- 11.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 12.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.St Helen D, Novalen M, Heitjan DF, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1105–14. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 16.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 18.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 20.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 21.Brown RA, Burgess ES, Sales SD, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1998;12:101–112. [Google Scholar]
- 22.Goelz PM, Audrain-McGovern JE, Hitsman B, et al. The association between changes in alternative reinforcers and short-term smoking cessation. Drug Alcohol Depend. 2014;138:67–74. doi: 10.1016/j.drugalcdep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnoll RA, Patterson F, Wileyto EP, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–51. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 25.Coffin M, Sukhatme S. Receiver operating characteristic studies and measurement errors. Biometrics. 1997;5:823–37. [PubMed] [Google Scholar]
- 26.Sharir T, Berman DS, Waechter PB, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: normal heterogeneity and criteria for abnormality. J Nuc Med. 2001;42:1630–8. [PubMed] [Google Scholar]
- 27.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epi. 2006;163:670–5. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychol Bull. 2007;133(2):245–72. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- 29.Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15(2):105–14. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 30.Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70(1):216–27. [PubMed] [Google Scholar]
- 31.Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine Tob Res. 2009;11(3):323–31. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper ME, Smith SS, Schlam TR, et al. Psychiatric disorders in smokers seeking treatment for tobacco dependence: relations with tobacco dependence and cessation. J Consult Clin Psychol. 2010;78(1):13–23. doi: 10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits JA, Zvolensky MJ, Rosenfield D, et al. The efficacy of vigorous-intensity exercise as an aid to smoking cessation in adults with elevated anxiety sensitivity: study protocol for a randomized controlled trial. Trials. 2012;13:207. doi: 10.1186/1745-6215-13-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78(1):55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beirut LJ, Johnson EO, Saccone NL. A glimpse into the future – Personalized medicine for smoking cessation. Neuropharmacology. 2014;76:592–9. doi: 10.1016/j.neuropharm.2013.09.009. Pt B. [DOI] [PMC free article] [PubMed] [Google Scholar]