SUMMARY
Background
Treatment of IBS and lower functional gastrointestinal disorders is still based predominantly on symptoms; biomarkers that reflect the mechanism or pathophysiology have been identified. Given the diverse mechanisms that result in the same clinical phenotype of IBS, it is hypothesized that identification of biomarkers may lead to individualization of medical therapy.
Aim
To review the biomarkers that have been appraised in IBS.
Methods
A single author reviewed the published literature on biomarkers appraised in IBS.
Results
The current literature suggests that these biomarkers are insufficiently sensitive or specific to differentiate IBS from health or from other diseases causing similar symptoms, such as celiac disease or inflammatory bowel disease. Most of the proposed biomarkers are not actionable, that is, they do not lead to an efficacious therapy based on the biological inference of the biomarker itself. However, among proposed biomarkers in IBS, some are actionable, as they specifically reflect a quantitative difference in a mediator of dysfunction or result in a quantifiable disturbance of function that can be specifically treated. Such biomarkers may potentially identify relevant subgroups that respond to specific therapy. The most promising actionable biomarkers are measurement of colonic transit (leading to treatments that reverse the abnormal transit) and measurements of bile acid diarrhea to identify responders to bile acid sequestrants.
Conclusions
Therefore, although biomarkers are not ready for prime time as diagnostic tests in IBS, some biomarkers could identify subgroups of patients with IBS for inclusion in clinical trials that target specific dysfunctions. Such an approach may enhance treatment efficacy, and may ultimately help reduce costs in drug development and in the management of patients in clinical practice.
INTRODUCTION
Irritable bowel syndrome (IBS) is a highly prevalent disease in most countries1 that is generally identified on the basis of symptoms2 and exclusion of organic diseases. Symptoms may vary between subtypes of IBS, in particular, bowel function; thus, consensus criteria discern IBS with constipation, diarrhea, mixed bowel function or unspecified. In addition, the symptom complex may vary over time in the same patient with functional gastrointestinal disorders.3
The identification of perturbations of gastrointestinal motor, immune, barrier and sensory functions in IBS provides potential biomarkers based on those functions, rare genetic mutations (as in GUCY2C) associated with functional diarrhea, changes in tissue expression, and therapeutic responses.4 These biomarkers may provide an opportunity to diagnose, prevent or reverse symptoms of IBS.
Definition and Required Characteristics of a Biomarker
A valid biomarker is defined as ‘a characteristic that is measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacological responses to a therapeutic intervention’.5
A useful IBS biomarker would be expected to enhance one or more of the following: improve diagnosis, predict prognosis, discriminate patients with IBS from healthy individuals and from other organic diseases, reduce disease-related costs, identify relevant subgroups responding to specific therapy, identify subgroups of patients for inclusion in clinical trials, enhance drug development and monitor drug efficacy.6 Based on current evidence, it was recently suggested that biomarkers for IBS were not ready for prime time.7 One of the challenges in appraising the validity of biomarkers in IBS is that there is no current gold standard for the diagnosis of IBS; in addition, the heterogeneity of the condition implies that any biomarker is unlikely to have optimal sensitivity and specificity in all phenotypic subgroups.
Mechanisms Underlying the Irritable Bowel Syndrome
Several peripheral and central mechanisms may initiate the perturbations of gastrointestinal motor and sensory functions and lead to IBS symptoms. Research has identified genetic traits, central mechanisms, peripheral irritants, quantitative traits of motor, barrier, immune or sensory functions, or alterations in tissue expression in IBS.
This review addresses the potential for these diverse traits to identify ways to diagnose, prevent or reverse symptoms of IBS,4,6–9 akin to the biomarker revolution in oncology. The need for developing and validating biomarkers in IBS stems from the weak pooled sensitivity and specificity for symptoms in diagnosing IBS9 and the relatively small proportion of responders to approved therapies, based on trials anchored by bowel function phenotype. For example, Sood et al.9 estimated the specificity of lower abdominal pain is 32% (95% CI 21–44) and the specificity for sense of incomplete rectal evacuation is 45% (95% CI 31–60). In addition, the “diagnostic” symptom-based Rome III criteria had sensitivity of 68.8% (95% CI 63.8–73.3) and specificity of 79.5% (95% CI 77.4–81.5). In addition, in IBS therapeutic trials, active treatments are associated with at most 70% efficacy with a background of ~50% response to placebo. Thus, an important question arises: Are there valid biomarkers that could be applied in IBS to improve diagnosis or enhance therapy to prevent or reverse symptoms of IBS, like the biomarker revolution in oncology? This review concludes that there are actionable biomarkers that are ready for application to identify IBS subgroups and optimize their treatment.
Single or Combination Biomarkers for Diagnosis of IBS?
To date, a wide spectrum of individually appraised markers based on serological, immune, or sensation traits has not had sufficient sensitivity or specificity to achieve the discriminatory goals required of a valid biomarker for diagnostic purposes.7,9 Therefore, one approach has been to pool different markers. For example, the diagnostic performance of a pool of 10 serum biomarkers [including markers of pain, 5-hydroxytryptamine (5-HT) metabolism, mast cell activation and inflammation] was disappointingly poor and improved only when the original list was extended to 34 serological and gene expression markers for differentiating IBS from health (AUC = 0.81), or IBS-C (constipation) vs. IBS-D (diarrhea) (AUC = 0.92).10 This differentiation of IBS from health improved further with the addition of four psychological markers (combined AUC = 0.93);10 however, psychological variables provided almost no incremental discrimination for identifying IBS-C compared to IBS-D.
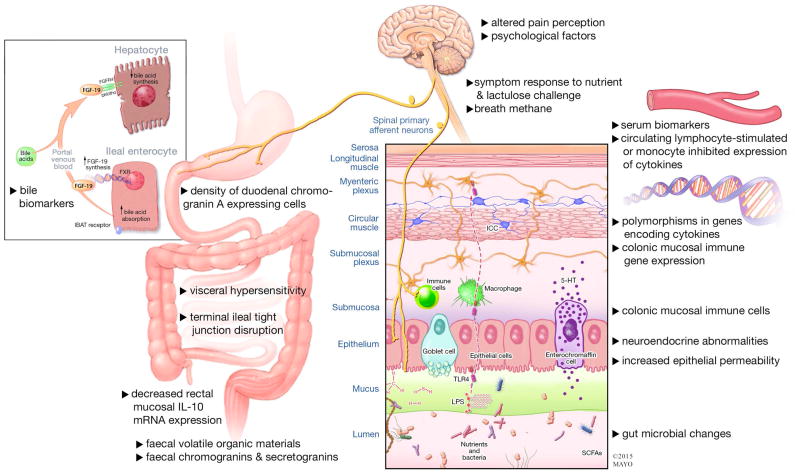
Figure 1 shows a summary of potential etiologic mechanisms or quantitative variables that have been proposed as diagnostic biomarkers in IBS (reviewed in references 7–9,11). The sensitivity and specificity of these markers to differentiate IBS from health or other functional gastrointestinal diseases (FGIDs) or inflammatory bowel diseases have been reviewed elsewhere.9 Unfortunately, for all these markers, specificity is relatively low at high sensitivity and vice versa.
Figure 1.
Summary of potential etiologic mechanisms or quantitative variables proposed as diagnostic biomarkers in IBS
Three recently published studies propose diverse approaches.
El-Salhy et al. suggested that densities of rectal peptide YY and somatostatin cells may be useful biomarkers for the diagnosis of IBS. In an analysis of 101 patients with IBS based on Rome III criteria, and 62 healthy subjects who underwent rectal biopsy immunostaining for PYY and somatostatin, the density of PYY cells was significantly lower in IBS patients, with an area under the ROC curve (AUC) of 0.99, whereas somatostatin cell density was higher in IBS patients than in the controls (AUC of 0.86).12
Based on 2375 patients with IBS-D based on Rome criteria participating in a large multicenter clinical trial, Pimentel et al. suggested that serum anti-cytolethal distending toxin B (CdtB) and anti-vinculin titers were significantly higher in IBS-D compared to inflammatory bowel disease (IBD), healthy controls, and celiac disease, with AUCs of 0.81 and 0.62 respectively for diagnosis of IBS-D versus IBD, lower AUCs of 0.77 and 0.62 for differentiating IBS-D from celiac disease, and AUCs of 0.77 and 0.68 for differentiating IBS-D from healthy controls.13
In a third recent paper, Casen et al. examined 54 DNA probes targeting ≥300 bacteria in fecal samples on different taxonomic levels based on ability to distinguish between healthy controls and IBS patients. Thus, 165 healthy controls (normobiotic reference collection) were used to develop a dysbiosis model with a bacterial profile and Dysbiosis Index Score output. Dysbiosis was detected in 73% of IBS patients, in 70% of treatment-naïve IBD patients, and in 80% of IBD patients in remission, versus 16% of healthy individuals. The authors claimed that the test provides insight into a patient’s intestinal microbiota, and they speculate that evaluating microbiota as a diagnostic strategy may allow monitoring of prescribed treatment regimens and improvement in new therapeutic approaches.14 Unfortunately, no data were available to support this speculative claim.
It is relevant to note that, for most of the markers, there is no specific or validated treatment that, when directed at the marker (such as tight junction disruption or fecal secretogranin or serum levels of anti-CdtB), would result in restoration of normal function or relief of symptoms.
Although measurements of small bowel permeability improved with gluten withdrawal from the diet, and this was associated with a reduced frequency of bowel movements in a randomized controlled trial of 45 IBS-D patients, especially in HLA-DQ2/8 carriers,15 measurement of intestinal permeability alone or in combination with other traits (colonic transit and fecal bile acid excretion) did not significantly contribute to the discrimination between health and IBS or IBS-C from IBS-D.11
Is the Biomarker Prevalent and Actionable?
The relative prevalence of a biomarker will clearly influence its performance from a diagnostic perspective (in the discrimination of health and organic diseases), but even if not omnipresent, it may identify a subgroup of patients with an actionable phenotype. Thus, even though the prevalence in IBS of visceral sensitivity ranges, in published series from tertiary centers, from ~20 to ~95%, the identification of rectal hypersensitivity would be highly relevant if there was an effective visceral analgesic therapy. Similarly, although only 20% of IBS-C and 45% of IBS-D patients have, respectively, significantly delayed or accelerated colonic transit,16 this trait may identify best candidates for treatment with agents that accelerate or retard colonic transit. A classic example is provided by the 5-HT3 antagonist, alosetron, which retards ascending colon emptying in patients with IBS-D17 and reduces pain ratings in patients with non-constipated IBS.18 Alosetron also reduces regional blood flow in the brain’s emotional system and increases flow in the periacqueductal grey after noxious sigmoid stimulation in patients with IBS. Several clinical trials show that 5-HT3 antagonists are efficacious in the treatment of IBS-D.19 Alosetron has been associated with serious complications of constipation or ischemic colitis; it is indicated only for women with severe diarrhea-predominant IBS who have not responded adequately to conventional therapy.20
Genetic Mutations and Variants Associated with IBS Symptom Phenotype
Genetic susceptibility to IBS has also been investigated among non-Mendelian genetic variants. For example, 30 of the main susceptibility genes for Crohn’s disease were investigated because there are etiopathogenetic mechanisms (e.g. immune activation, abnormal intestinal mucosal barrier function) that are common to Crohn’s disease and IBS. In an analyses of 30 susceptibility genes associated with Crohn’s disease, variation in TNFSF15 (related to immune function) has been associated with IBS in cohorts from Sweden, USA and Britain,21,22 and results have been recently confirmed in a separate cohort from Germany.23 The latter report includes a meta-analysis of all published data showing the significant association of TNFSF15 with IBS. However, despite the association of TNFSF15 with IBS, it is unclear whether this constitutes an actionable biomarker, since it is unclear whether the downstream products of the gene can be altered by currently available therapies.
Recent studies of associations between large numbers of genes and IBS symptom phenotype have been published. Thus, a study of 384 SNPs covering 270 genes in ~1600 people identified association of rs2349775 (NXPH1, which is associated with neuroticism) in IBS-D and rs17837965 (CDC42, involved in brain development and intestinal stem cell differentiation and proliferation) in IBS-C.24 In addition, in the first full paper utilizing GWAS in a multicenter study of about 8,000 individuals based on the binary analysis (IBS present or absent), Ek et al. identified two genes showing risk of IBS: KDELR2 (endoplasmic reticulum protein retention receptor 2) and GRID2IP (glutamate receptor ionotropic delta 2 interacting protein).25 The biological relevance of these two genes in IBS mechanisms is unknown, and their role as diagnostic, actionable or therapeutic biomarkers is unproven to date.
Tissue Gene/Protein Expression in Colorectal Mucosa
RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with IBS-D compared to mucosa from the same region in healthy controls,26 particularly upregulation of genes associated with enterocyte secretion [such as GUCA2B, PDZD3 (also known as NHERF4)], tight junction proteins [such as fibronectin 1 and retinol-binding protein 2 (RBP2)], and neuronal function (such as VIP and P2RY4). Most of these findings on RNA sequencing were confirmed on RT-PCR. In addition, in a study of rectosigmoid mucosa from 47 patients with IBS-D compared to 17 healthy controls, mRNA expression of GUC2AB and PDZD3 (involved in ion secretion) was increased, whereas, mRNA expression of CLDN1 and FN1 (both tight junction proteins) was decreased. These changes were not identified in mucosal biopsies from 10 patients with IBS-C in whom there were increased fold expressions of P2RY4, VIP and occludin relative to the healthy controls.27
In a study of mRNA expression in colonic mucosa in 12 patients with chronic constipation and 12 healthy controls (all female), there were no significant group differences in expression of the GC-C receptor or endogenous GC-C receptor agonists (guanylin and uroguanylin), or expression of the cGMP transporter proteins. However, there was significant negative correlation between levels of expression of guanylin protein (endogenous ligand of guanylate cyclase C receptor resulting in chloride and water secretion) and current overall GI symptom severity in patients with chronic constipation (r= −0.701, p=0.024).28
These differential mucosal expressions represent potential biomarkers of mechanisms resulting in the common symptom phenotype, such as increased expression of uroguanylin presumed from the increased mRNA expression of GUCA2B in IBS-D, and reduced protein levels of guanylin protein in patients with chronic constipation. Further validation of these biomarkers may provide targets for individualized therapy in patients with IBS-C or IBS-D, such as guanylate cyclase C agonists or chloride channel activators for IBS-C with reduced guanylin protein levels, or tenapanor, a minimally absorbed small molecule inhibitor of NHE3 which is a transporter of sodium in patients with IBS-C that bypasses the chloride secretory pathway in the enterocyte (reviewed in ref. 29).
Genetic Influence of Response to IBS Therapy: Biomarker in Pharmacogenetics
There are a few examples in the published literature suggesting that genetic variants may serve as biomarkers to identify patients with differential responses to pharmacological therapies.
-
There is evidence that circulating 5-HT levels are increased in patients with IBS-D or post-infectious IBS, but reduced in patients with IBS-C.30,31 Since 95% of the body serotonin is located in the gut, it is considered that the circulating levels reflect the gut tissue levels of serotonin. Genetic variation in 5-HTTLPR, the gene that determines the level of 5-HT at serotonergic synapses, has been demonstrated to affect responses to therapy in IBS. Thus, reduced 5-HT at synapse associated with the long polymorphic repeat (LL genotype) which is associated with high levels of transcription of the 5-HT reuptake protein, SERT, results in enhanced colonic transit response (slowing) to the 5-HT3 antagonist, alosetron, in IBS-D. The interpretation of these data is that, in the presence of high levels of SERT, there is greater re-uptake of 5-HT in the synapse and, therefore, less endogenous 5-HT that needs to be inhibited from activating the post-synaptic receptor.32 There is also evidence that the 5–HTTLPR genotype impacts the response to another 5-HT3 receptor antagonist, ondansetron. Thus, the SL genotype was borderline associated (p=0.07) with effects on change in stool consistency and whole gut transit time in an analysis of 87 patients in a placebo-controlled, crossover study of 5 weeks of ondansetron, 4mg (dose titration allowed).33
Conversely, the short (by 44bp) polymorphic repeat in 5-HTTLPR, which results in reduced transcription of SERT protein, is associated with greater efficacy, measured as symptomatic benefit of tegaserod in IBS-C. The interpretation of these data is that, with less SERT transcribed, less 5-HT is taken up in the synapse, leaving more 5-HT available to stimulate serotonergic receptors such as 5-HT3 and 5-HT4 receptors. Stimulation of these receptors results in the release of acetyl choline from cholinergic neurons, enhancing the effects of the exogenous 5-HT4 receptor agonist, tegaserod.34
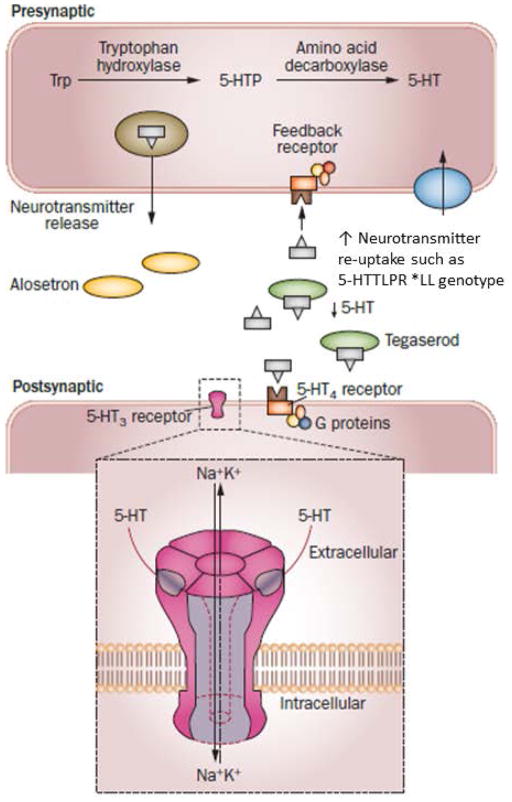
These pharmacogenomics interactions with 5-HTTLPR are illustrated in Figure 2.
Klotho-ß (KLB) and fibroblast growth factor receptor 4 (FGFR4) are proteins that mediate feedback inhibition of hepatocyte bile acid (BA) synthesis by fibroblast growth factor 19 (FGF-19) reaching the liver from the portal circulation. Variants of KLB and FGFR4 influence colonic transit in patients with IBS-D;35 in addition, FGFR4 rs351855 and KLB rs497501 coding variants influenced the colonic transit response to the BA sequestrant, colesevelam, 1.875g b.i.d., compared to placebo in 24 female IBS-D patients. Thus, FGFR4 rs351855 and KLB rs497501 were associated with differential effects of colesevelam on ascending colon half-emptying time (t1/2) and on overall colonic transit at 24 hours, suggesting that these SNPs may identify a subset of IBS-D patients with beneficial response to colesevelam.36
CNR1 (cannabinoid type 1 receptor) rs806378 CT/TT genotype is associated with a modest delay in colonic transit at 24 hours compared with CC (P=0.13) for differential treatment effects on post- minus pre-treatment changes in colonic transit by genotype.37
Figure 2.
Proposed explanations for the association of alosetron treatment with slower colonic transit in patients with IBS-D who are 5-HTTLPR*LL carriers with the worst clinical response to tegaserod in patients with IBS-C who are 5-HTTLPR*LL carriers with constipation-predominant IBS. The 5-HTTLPR*LL homozygous carrier status is associated with optimal function of the reuptake serotonin transporter protein (SERT, also called SLC6A4 [solute carrier family 6 [neurotransmitter transporter, member 4]). This results in less 5-HT in the synapse that requires inhibition by alosetron at the 5-HT3 receptor. Therefore the same dose of alosetron is more effective at slowing colonic transit in IBS-D. Conversely, with less 5-HT at the synapse in LL carriers, the same dose of the 5-HT4 receptor agonist, tegaserod, will be less effective at stimulating the 5-HT4 receptors, resulting in lower impact of tegaserod on symptoms in IBS-C.
Reproduced with permission from ref. 64, Camilleri M. The role of pharmacogenetics in nonmalignant gastrointestinal diseases. Nature Rev Gastroenterol Hepatol 9:173–184, 2012
Brain Imaging as a Potential Biomarker
The literature on brain or regional (e.g. insula) connectivity in patients with IBS or chronic abdominal pain suggests that there are significant associations with symptoms.38–40 Structural and functional alterations in brain regions of the salience, emotional arousal, and sensorimotor networks, as well as in the prefrontal region, show moderate correlations with behavioral and clinical measures.41 In addition, although not currently generalizable or easily applied in clinical practice, brain imaging can relate to targets for therapy, can be actionable, and may predict responsiveness to therapy. Thus, the association of resting connectivity with psychological disturbances (including early life events42 and emotional stress) could be targets for psychological or psychotropic treatment.
Responsiveness in the brain imaging biomarker to brain-directed treatments is illustrated by several examples:
Improvement with hypnosis treatment is associated with differential brain imaging effects in hypnosis responders;43
Brain imaging abnormalities in the cingulate region reverted to normal in response to behavioral and psychotropic treatment that was associated with clinical improvement;44
Neurodegeneration observed in stress, depression or brain trauma45,46 in patients with chronic pain and IBS can potentially reverse with psychotropic treatment.
These preliminary observations on brain imaging require replication and would be most impactful if less expensive and more accessible methods are developed for use in patients with lower FGID.
Are Any Biomarkers Ready for Prime Time in Diagnosis or Treatment of Lower FGID?
Barbara and Stanghellini suggested that there are no biomarkers ready for application in routine clinical care in IBS.7 The literature certainly supports this conclusion relative to diagnostic biomarkers, that is, the ability of markers to differentiate health from IBS or IBS from other gastrointestinal diseases.7
However, there are biomarkers that characterize or quantify a trait that is the target for treatment and, therefore, such biomarkers may facilitate selection of patients for inclusion in clinical trials, potentially facilitating drug development and reducing risks of adverse effects by only exposing patients who have a potential to respond to personalized therapy. With further validation, these biomarkers may also conceivably serve as surrogate endpoints5 to appraise treatment efficacy in the clinical trials.
A logical approach to develop biomarkers in IBS focuses on important mechanisms or traits with relatively high prevalence (e.g. 25% or more) and selection of biomarkers that are actionable, that is, the trait can be altered or normalized by currently available or approved medications. The four most relevant classes of actionable targets are: serotonin, inflammation, colonic transit and bile acids. These principles will be described briefly.
Serotonin is a signaling molecule involved in sensation, secretion, motor and platelet functions. Importantly, 95% of the body’s serotonin is produced by gastrointestinal entero-endocrine cells, and there is a local reuptake mechanism [serotonin transporter protein (SERT), also called solute carrier family 6 (neurotransmitter transporter), member 4 (SLC6A4)] that inactivates 5-HT after its release. Plasma levels of 5-HT in the postprandial period are increased in IBS-D or post-infectious IBS (that usually manifests as diarrhea-predominant) and are lower in IBS-C compared to controls.30,31 The increased 5-HT in IBS-D provides the rationale for use of the 5-HT3 receptor antagonist class of drugs, such as alosetron47 and ondansetron.33 The lower 5-HT in IBS-C provides the rationale for treatment with 5-HT4 receptor agonists.48
The role of immune activation and neuroimmune interactions in IBS has been reviewed elsewhere.49 However, the prevalence of each of the immune activation mechanisms among IBS patients is unclear, and therapeutic approaches such as mast cell stabilizers,50 corticosteroids,51 and 5-ASA compounds52,53 have not been successful when used in IBS patients. Further studies are needed to establish whether these therapies would be more successful in highly selected patients with activated immune mechanisms that respond to those treatments.
-
Colonic transit is abnormal in about 30% of IBS patients.16,54,55 In the largest available study of 287 patients with lower FGIDs, colonic transit at 24 hours was abnormal (GC24 slow <1.50 or fast >3.86) in 29.7% (delayed in 24.5 of IBS-C/functional constipation or IBS-M; accelerated in 33.3% of IBS-D/functional diarrhea) and delayed at 48 hours in 22.9% of IBS-C/FC and 6.7% of IBS-M patients.54 Abnormal colonic transit is significantly associated with clinical symptoms such as the stool consistency based on Bristol stool form scale and the frequency of bowel movements.56
Radiopaque marker transit measurement also detects abnormal transit in patients with functional diarrhea and constipation. Thus, the proportion of patients with abnormal colonic transit in relation to IBS subgroups (Rome III) was slow transit in 12% of those with IBS-C and 27% of the subgroup with IBS-D.57 Table 1 summarizes the literature demonstrating the application of scintigraphic colonic transit measurement to correctly predict the efficacy of the same medication in phase IIB or III clinical trials.
Bile acid malabsorption (BAM) affects 25–40% of patients with IBS-D or functional diarrhea. In a systematic review of 18 studies that used 75SeHCAT retention to identify BAM, there were 31% of 1223 patients with <10% isotope retention, and 80% and 96% of patients, respectively, in the <10% and <5% retention groups responding to bile acid sequestrants therapy.58 A recent analysis based on all methods available to detect bile acid diarrhea [BAD (75SeHCAT retention, fasting serum C4, fasting serum FGF-19 and total fecal bile acid excretion over 48 hours)] showed that, in 30 studies enrolling at least 4249 patients, approximately 25% (average) of patients with lower FGIDs and diarrhea had evidence of idiopathic BAD.59
Table 1.
Evidence of Clinical Efficacy Predicted by Colonic Transit Measured by Scintigraphy (updated from ref. 60, Camilleri M. Clin Pharmacol Ther. 2010;87:748–53)
| Drug Class | Pharmacodynamics (intestine or colon) | Clinical Efficacy: Phase IIB or III Studies |
|---|---|---|
| 5-HT3-antagonist, alosetron | 1 mg bid delayed colonic transit diarrhea in IBS-D | IIB, III studies in thousands of patients with non-C-IBS or D-IBS adequate relief of pain and discomfort of IBS, bowel dysfunction (including diarrhea) and urgency |
| 5-HT4-agonist, Tegaserod | 2mg bid accelerated SB transit and colonic transit in healthy and C-IBS (without evacuation disorder) | IIB, III studies in several thousands of patients with C-IBS and CC experienced relief of pain and discomfort of IBS, and bowel dysfunction |
| 5-HT4-agonist, Prucalopride | Increases SB, colon transit in healthy and patients with CC | IIB and III in CC (thousands of pts): BM frequency and satisfaction with bowel function both improved |
| 5-HT4-agonist, Velusetrag | Dose-related increase in SB and colon transit in healthy | A IIB, dose-ranging study in 401 CC patients increased BM frequency and proportion with adequate relief |
| 5-HT4-agonist, YKP10811 | Accelerates colonic transit and improves stool consistency in CC | ClinicalTrials.gov: NCT01989234; study completed, no posted results |
| Bisacodyl | Accelerates colon transit in healthy | Relief of constipation after acute administration and CC |
| Recombinant human neurotrophin (NT)-3 | NT3 accelerates colonic transit in CC | NT-3, administered TTW, increased stool frequency, accelerated colon transit, and improved symptoms of chronic constipation. |
| Cl–C2 channel activator, lubiprostone | Accelerates SB and colonic transit in healthy controls | Several phase III in several hundred CC and IBS-C patients: efficacious in relief of pain and bowel dysfunction |
| Guanylate cyclase-C agonist, linaclotide | Accelerated AC transit and induced looser bowel function in 36 women with IBS-C | Several IIA, IIB and III studies in CC or C-IBS (several hundred) patients: increased BM frequency, relief of bloating, and abdominal discomfort |
| GLP-1 analog, ROSE-010 | Accelerated colonic transit at 48h | Relieved severity of pain attacks and enhanced satisfaction score in IBS patients |
| Ileal bile acid transport inhibitor, Elobixibat | Accelerates colonic transit and loosens stool consistency in functional constipation patients | One phase IIB study showed improved stool frequency, and improved constipation-related symptoms in idiopathic CC |
| Bile acid sequestrant colesevelam | Retards ascending colon emptying, | Improves stool consistency in IBS-D with high fecal BA excretion (phase IV study) |
| VSL-III combination probiotic | Retards colonic transit in IBS-D, improves flatulence and bloating in IBS-D | Meta-analyses demonstrate symptom relief of multiple symptoms in IBS: global IBS, abdominal pain, bloating, and flatulence scores |
| κ-opioid agonist, asimadoline | No significant effect on colonic transit in healthy volunteers | On-demand dosing not effective in reducing severity of abdominal pain in 100 IBS patients; a phase IIB, dose-ranging in 596 IBS patients, post-hoc analysis: benefit in moderate pain in D-IBS and Alt-IBS |
| CCK1-antagonist, dexloxiglumide | Slower AC emptying with no effect on overall colonic transit in IBS-C | Two initial IIB or III trials: not efficacious in IBS-C; a randomized withdrawal design trial showed longer time to loss of therapeutic response for dexloxiglumide |
| CRH1-antagonist, Pexacerafont | No effect on colonic transit and bowel function in IBS-D | One phase IIB study showed GW876008 had no significant difference from placebo in the global improvement scale, daily self-assessment of IBS pain/discomfort or individual lower GI symptoms |
| β-3 adrenergic agonist, solabegron | No significant effect on gastrointestinal or colonic transit | One phase IIB study showed no significant change in bowel symptoms, though there is significant effect on adequate relief of IBS pain and discomfort |
AC=ascending colon; BA=bile acid; BM=bowel movements; CC=chronic constipation; CCK=cholecystokinin; Cl–C2=chloride channel type 2; CRH=corticotrophin releasing hormone; GLP-1=glucagon-like peptide 1; 5-HT=5-hydroxytryptamine; IBS=irritable bowel syndrome; ITT=intention to treat; SB=small bowel; TTW=three times per week
Thus, biomarkers with the greatest current potential in IBS identify relevant subgroups responding to specific therapy. This principle has been applied successfully for scintigraphic measurement of colonic transit and 48-hour fecal bile acid excretion.11 For example, colonic transit measurement has correctly predicted efficacy or lack of efficacy of 18 medications in development or use for lower FGIDs associated with bowel dysfunction (ref. 60 and Table 1). Such approaches have the potential to help identify homogeneous groups of patients for inclusion in clinical trials, reduce costs in drug development, and identify relevant subgroups responding to specific therapy. Similarly, several studies now demonstrate the utility of bile acid sequestrants and farnesoid X receptor agonist (obeticholic acid) for the management of BAM in IBS-D/functional diarrhea,61–63 including a first study that selected patients based on elevated total fecal BA excretion over 48 hours.62
CONCLUSION
Treatment of IBS and lower functional gastrointestinal disorders is still based predominantly on symptoms; however, with greater understanding of the biology of the diseases and identification of biomarkers that reflect either the mechanism or manifestations of the pathophysiology, it is likely that significant advances will be made, leading to a renaissance in the field of IBS4 and the individualization of medical therapy.
Among the proposed biomarkers in IBS, none of those proposed to date fulfill the expectations of diagnostic biomarkers, but, among proposed biomarkers in lower FGID, some are actionable, as they specifically reflect a quantitative difference in a mediator of dysfunction or result in a quantifiable disturbance of function that can be specifically treated. Such biomarkers may potentially identify relevant subgroups that respond to specific therapy. This principle of biomarker identification and use as target for treatment has been applied successfully for the mediator, serotonin, and the class of secretagogues (lubiprostone, linaclotide) in scintigraphic measurement of colonic transit. Similarly, studies with 75SeHCAT retention and 48-hour fecal bile acid excretion have identified responders to bile acid sequestrants. Therefore, some biomarkers are ready for prime time because they have potential to identify subgroups of patients for inclusion in clinical trials targeting specific dysfunctions. Further experience with the use of these biomarkers to optimize efficacy in clinical trials and effectiveness in clinical practice will more firmly establish the concept of actionable biomarkers in IBS. By identifying subgroups with optimal responsiveness, these actionable biomarkers may ultimately help reduce costs in drug development and in the management of patients in clinical practice.
Acknowledgments
The author thanks Mrs. Cindy Stanislav for excellent secretarial assistance.
Funding: Dr. M. Camilleri is supported by R01-DK92179 from National Institutes of Health and the Atherton and Winifred W. Bean Professorship at Mayo Clinic.
Footnotes
Authorship Statement
Guarantor of the article: Dr. M. Camilleri is the guarantor of this article. He takes responsibility for the integrity of the work as a whole, from inception to published article.
Specific author contributions: Dr. M. Camilleri collected and analyzed the data and wrote the paper. He approves the final version of the manuscript.
CONFLICT OF INTEREST/DISCLOSURE
The author has no relevant disclosures pertaining to this article.
References
- 1.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–21. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–35. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 5.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 6.Barbara G, Stanghellini V. Biomarkers in IBS: when will they replace symptoms for diagnosis and management? Gut. 2009;58:1571–5. doi: 10.1136/gut.2008.169672. [DOI] [PubMed] [Google Scholar]
- 7.Barbara G. IBS: Biomarkers for IBS: ready for prime time? Nat Rev Gastroenterol Hepatol. 2015;12:9–10. doi: 10.1038/nrgastro.2014.217. [DOI] [PubMed] [Google Scholar]
- 8.Corsetti M, Van Oudenhove L, Tack J. The quest for biomarkers in IBS-where should it lead us? Neurogastroenterol Motil. 2014;26:1669–76. doi: 10.1111/nmo.12475. [DOI] [PubMed] [Google Scholar]
- 9.Sood R, Law GR, Ford AC. Diagnosis of IBS: symptoms, symptom-based criteria, biomarkers or ‘psychomarkers’? Nat Rev Gastroenterol Hepatol. 2014;11:683–91. doi: 10.1038/nrgastro.2014.127. [DOI] [PubMed] [Google Scholar]
- 10.Jones M, Chey WD, Singh S, et al. A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Aliment Pharmacol Ther. 2014;39:426–37. doi: 10.1111/apt.12608. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Shin A, Busciglio I, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:1677–85. doi: 10.1111/nmo.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides. 2015;67:12–9. doi: 10.1016/j.peptides.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Pimentel M, Morales W, Rezaie A, et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One. 2015;10:e0126438. doi: 10.1371/journal.pone.0126438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casén C, Vebø HC, Sekelja M, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42:71–83. doi: 10.1111/apt.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in irritable bowel syndrome-diarrhea: effect on bowel frequency and intestinal functions. Gastroenterology. 2013;144:903–11. e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–81. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;92:2671–6. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]
- 18.Mayer EA, Berman S, Derbyshire SW, et al. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther. 2002;16:1357–66. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 19.Andresen V, Montori VM, Keller J, West C, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and metaanalysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021107s013lbl.pdf).
- 21.Zucchelli M, Camilleri M, Andreasson AN, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;60:1671–7. doi: 10.1136/gut.2011.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swan C, Duroudier NP, Campbell E, et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut. 2013;62:985–94. doi: 10.1136/gutjnl-2011-301213. [DOI] [PubMed] [Google Scholar]
- 23.Czogalla B, Schmitteckert S, Houghton LA, et al. A meta-analysis of immunogenetic case-control association studies in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:717–27. doi: 10.1111/nmo.12548. [DOI] [PubMed] [Google Scholar]
- 24.Wouters MM, Lambrechts D, Knapp M, et al. Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut. 2014;63:1401–9. doi: 10.1136/gutjnl-2013-304570. [DOI] [PubMed] [Google Scholar]
- 25.Ek WE, Reznichenko A, Ripke S, et al. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut. 2014 Sep 23; doi: 10.1136/gutjnl-2014-307997. pii: gutjnl-2014-307997. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M, Carlson P, Acosta A, et al. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol. 2014;306:G1089–98. doi: 10.1152/ajpgi.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Carlson P, Acosta A, Busciglio IA. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol. 2015 Apr 30; doi: 10.1152/ajpgi.00080.2015. ajpgi.00080.2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Videlock E, Mahurkar-Joshi S, Hoffman J, Shih W, Alberto M, Chang L. Guanylate cyclase-C receptor and ligand expression in colonic mucosa in chronic constipation. Am J Gastroenterol. 2014;109:S540. [Google Scholar]
- 29.Camilleri M. Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin Gastroenterol Hepatol. 2015;13:1051–7. doi: 10.1016/j.cgh.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Atanasova E, Carlson PJ, et al. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–32. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 33.Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617–25. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Nie Y, Xie J, et al. The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci. 2007;52:2942–9. doi: 10.1007/s10620-006-9679-y. [DOI] [PubMed] [Google Scholar]
- 35.Wong BS, Camilleri M, Carlson PJ, et al. A klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–42. doi: 10.1053/j.gastro.2011.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong BS, Camilleri M, Carlson PJ, et al. Pharmacogenetics of the effects of colesevelam on colonic transit in irritable bowel syndrome with diarrhea. Dig Dis Sci. 2012;57:1222–6. doi: 10.1007/s10620-012-2035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong BS, Camilleri M, Eckert D, et al. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol Motil. 2012;24:358–65. doi: 10.1111/j.1365-2982.2011.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci. 2014;34:14252–9. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irimia A, Labus JS, Torgerson CM, Van Horn JD, Mayer EA. Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2015 May 7; doi: 10.1111/nmo.12586. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labus JS, Gupta A, Coveleskie K, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154:2088–99. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156 (Suppl 1):S50–63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, Kilpatrick L, Labus J, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychos Med. 2014;76:404–12. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowén MB, Mayer EA, Sjöberg M, et al. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment Pharmacol Ther. 2013;37:1184–97. doi: 10.1111/apt.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drossman DA, Ringel Y, Vogt BA, et al. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology. 2003;124:754–61. doi: 10.1053/gast.2003.50103. [DOI] [PubMed] [Google Scholar]
- 45.Han X, Tong J, Zhang J, et al. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J Neurotrauma. 2011;28:995–1007. doi: 10.1089/neu.2010.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perera TD, Coplan JD, Lisanby SH, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–40. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 48.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–43. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- 49.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–73. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 50.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–21. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 51.Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18:77–84. doi: 10.1046/j.1365-2036.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 52.Barbara G, Cremon C, Annese V, et al. Randomised controlled trial of mesalazine in IBS. Gut. 2014 Dec 22; doi: 10.1136/gutjnl-2014-308188. pii: gutjnl-2014-308188. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam C, Tan W, Leighton M, et al. A multi-centre, parallel group, randomised placebo controlled trial of mesalazine for treatment of diarrhoea-predominant irritable bowel syndrome (IBS-D) Gastroenterology. 2014;146:S123–4. [Google Scholar]
- 54.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293–e82. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadik R, Stotzer PO, Simrén M, Abrahamsson H. Gastrointestinal transit abnormalities are frequently detected in patients with unexplained GI symptoms at a tertiary centre. Neurogastroenterol Motil. 2008;20:197–205. doi: 10.1111/j.1365-2982.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 56.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–23. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol. 2012;107:754–60. doi: 10.1038/ajg.2012.5. [DOI] [PubMed] [Google Scholar]
- 58.Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–17. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 59.Valentin N, Camilleri M, Altayar O, et al. Biomarkers for bile acid diarrhea in lower functional gastrointestinal disorders with diarrhea: a systematic review and meta-analysis. Am J Gastroenterol. submitted 2015. [Google Scholar]
- 60.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–53. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39:923–39. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]
- 62.Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015 Jan 16; doi: 10.1111/apt.13065. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 64.Camilleri M. The role of pharmacogenetics in nonmalignant gastrointestinal diseases. Nat Rev Gastroenterol Hepatol. 2012;9:173–84. doi: 10.1038/nrgastro.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]