Abstract
Object
Transient aphasias are often observed in the first few days in patients who undergo surgical resection in the language-dominant hemisphere. The aims of this prospective study were to characterize the incidence and nature of these aphasias, and to determine whether there are relationships between location of the surgical site and deficits in specific language domains.
Methods
110 patients undergoing resection to the language-dominant hemisphere participated in the study. Patients’ language was evaluated prior to surgery, 2-3 days post-surgery, and 1 month post-surgery using the Western Aphasia Battery and the Boston Naming Test. Voxel-based lesion-symptom mapping was used to identify relationships between the location of the surgical site assessed by MRI, and deficits in fluency, information content, comprehension, repetition, and naming.
Results
71% of patients were classified as aphasic based on the Western Aphasia Battery 2-3 days post-surgery, with deficits observed in each of the language domains examined. Fluency deficits were associated with resection of the precentral gyrus and adjacent inferior frontal cortex. Reduced information content of spoken output was associated with resection of the ventral precentral gyrus and posterior inferior frontal gyrus (pars opercularis). Repetition deficits were associated with resection of the posterior superior temporal gyrus. Naming deficits were associated with resection of ventral temporal cortex, with mid temporal and posterior temporal damage more predictive of naming deficits than anterior temporal damage. By 1 month post-surgery, nearly all language deficits were resolved, and no language measure except for naming differed significantly from pre-surgical levels.
Conclusions
These findings show that transient aphasias are very common after left hemisphere resective surgery, and that the precise nature of the aphasia depends on the specific location of the surgical site. This patient cohort provides a unique window on the neural basis of language, since surgical resections are discrete, their locations are not limited by vascular distribution or patterns of neurodegeneration, and language can be studied prior to substantial reorganization.
Keywords: aphasia, resective surgery, voxel-based lesion-symptom mapping
Introduction
Surgery in the language-dominant hemisphere may be associated with injury to areas involved with language function19,57. Cortical stimulation mapping is typically used to identify and protect language regions, and consequently, long-term language outcomes after dominant hemisphere resective surgery are overall excellent in experienced hands24,28,34–36,43,46,49,50,57,58,63,69. The only negative impact that has been consistently documented is a modest decline in naming, in anterior temporal lobectomy patients whose symptoms began later in life23,44,47,65,67.
However, while long-term language outcomes are positive, many patients present with transient aphasias in the first few days after left hemisphere resective surgery10,27,29,35,41,49,50,57,58,61,63. The reported incidence of aphasia in the immediate post-operative period has ranged from 17% to 100% in these studies. This variability likely reflects differences in presurgical morbidity, the sensitivity of language testing, as well as differences in resection sites in the various case series. While acute deficits may occur in any or all language domains, including naming, comprehension, repetition, reading and writing49, no study has characterized transient post-surgical aphasias with a comprehensive aphasia battery.
The majority of transient aphasias appear to resolve within a month29,49,57,63, with the exception of modest naming deficits, which often persist as mentioned above23,44,47,65,67. The transient nature of the post-surgical language deficits raises the question of what neural mechanism(s) are responsible for the deficits. Proposals have included the generalized effects of surgery (‘neuroparalytic edema’)57, diaschisis (dysfunction of adjacent or functionally connected regions due to lack of normal inputs from the resected region)49, transient edema in regions adjacent to the resection29,49, and resection of functional tissue, with subsequent recovery due to reorganization of functional networks27.
Our study had two primary aims. The first was to determine the incidence and nature of transient aphasias after left-hemisphere resections, by studying a large series of 110 dominant hemisphere resection patients with a wide variety of lesion locations using a comprehensive and validated aphasia battery 2-3 days post-surgery. Our second aim was to determine using voxel-based lesion-symptom mapping (VLSM) whether the specific pattern of transient post-operative language deficits is related to the location of the surgical site. If so, this neurosurgical patient cohort has the potential to provide a unique perspective on the neural organization of language, since surgical resections are discrete and their locations are not limited by vascular distribution or patterns of neurodegeneration in stroke and primary progressive aphasia, respectively.
Methods
Patients
110 patients undergoing left hemisphere resective surgery were included in this study. The inclusion criteria were (1) left hemisphere resection in perisylvian language regions (including the anterior temporal lobe); (2) left hemisphere dominant for language confirmed with Wada test, pre-surgical language deficits, or magnetoencephalography lateralization; (3) fluent in English; (4) availability of post-surgical FLAIR and diffusion-weighted imaging (DWI) MRI scans suitable for delineation of resections and any associated infarction; (5) Western Aphasia Battery (WAB) administered 2-3 days post-surgery.
156 consecutive patients met the first three criteria and were considered for inclusion between September 2010 and October 2013. While most patients received follow-up MRI scans at 2-3 days post-surgery, 33 patients did not receive follow-up scans in the course of their clinical care, and so were excluded. Of the remaining 123 patients, there were 13 to whom the WAB was not administered for various situational reasons, leaving 110 patients included in the study.
Demographic information and the distribution of etiologies are shown in Table 1. In most patients, the goal of the surgery was to resect low or high grade gliomas, epileptogenic foci, or vascular malformations; four patients classified as ‘other’ received surgery for metastases (two patients), meningioma (one patient) and hemorrhage (one patient).
Table 1.
Demographic and etiological information
| Age | 44.2 ± 15.2 years (range: 19 to 81) | |
| Sex | 55 male, 55 female | |
| Handedness | 101 right-handed, 8 left-handed, 1 ambidextrous | |
| Education | 15.2 ± 2.7 years (range 11 to 20) | |
| Native speaker of English | 102 native, 8 non-native but fluent | |
| Time since first symptoms | 1479 ± 3255 days (range 4 days to 40 years) | |
| Etiology | Low grade glioma | 39 |
| High grade glioma | 49 | |
| Epileptogenic focus | 13 | |
| Vascular malformation | 5 | |
| Other | 4 | |
The study was approved by the UCSF Human Research Protection Program, and all participants gave written informed consent. Analysis of deidentified data took place at the University of Arizona.
Surgical procedures
All patients underwent craniotomy using monitored anesthesia care without intubation and general anesthesia. Generous local anesthetic infiltration was applied to create a scalp block. Surgical exposure was tailored for each case, depending upon the target lesion and/or seizure focus. Patients were sedated with either propofol or dexmedetomine at the start of the procedure. Interaoperative language mapping with electrical stimulation was performed in the majority of patients. Patients were awakened fully for language mapping, and intraoperative electrocorticography was used to monitor for stimulation-induced afterdischarges. The intraoperative language tasks included counting, confrontation naming, and occasionally reading57,63. After mapping, patients were re-sedated with either propofol or dexmedetomine for the remainder of the procedure. Essential language sites were identified with stimulation mapping, and defined as those resulting in loss of function in at least 2/3 of stimulations. The majority of sites were at least 1 cm from the resection margin. Resection was usually carried out with ultrasonic aspirator guided by intraoperative neuronavigation. When possible, subpial resection technique was used for anatomic resection.
Language assessments
The Western Aphasia Battery (WAB)42 was administered 2-3 days post-surgery by a speech-language pathologist, neuropsychologist, or a trained research assistant. The WAB is a comprehensive and validated aphasia battery that yields an Aphasia Quotient (AQ) quantifying overall aphasia severity, as well as subscores in five language domains: fluency, information content, comprehension, repetition and naming. Fluency is a subjective measure rated by the clinician based on the patient’s spontaneous speech. The information content measure assesses the functional, communicative value of a patient’s speech, and reflects the patient’s ability to convey correct answers to six basic questions. Comprehension includes yes/no questions, auditory word recognition, and sequential commands, and so captures lexical and syntactic aspects of language comprehension. The repetition measure includes the repetition of words, phrases and sentences. The naming subscore includes object naming, semantic fluency, sentence completion, and responsive speech tasks. In our study, we used the AQ and the fluency, information content, comprehension and repetition measures from the WAB. However we did not use the naming subscore of the WAB, because we considered the object naming task too easy and therefore potentially insensitive (i.e. subject to ceiling effects), whereas the other tasks that contribute to the naming subscore make substantial demands on cognitive and executive processes, and language processes other than naming.
In place of the WAB naming subscore, we administered a 15-item version of the Boston Naming Test (BNT)40 to 106 of the 110 patients. The BNT contains difficult, low-frequency items, such as tripod, sphinx and palette, therefore it is less subject to ceiling effects and so quantifies naming more effectively than the naming component of the WAB.
The WAB and BNT were also administered to most patients a few days prior to their surgery. Of the 110 patients, 84 completed the WAB and 85 completed the BNT prior to their surgery. The limiting factors on acquiring presurgical data were situational rather than systematically related to language function, so the patients for whom data were acquired represented an unbiased sample of the 110 included patients.
Follow-up testing was attempted 1 month post-surgery, except for those patients whose Aphasia Quotient was at least 93.8 and whose BNT score was at least 12 when tested 2-3 days post-surgery. There were 29 patients who met those criteria. Of the remaining 81 patients, 38 were administered the WAB and 44 were administered the BNT at 1 month post-surgery. The main limiting factors on acquiring data at this time point were geographical distance and health concerns other than aphasia, so the patients who were studied 1 month post-surgery again represented an unbiased sample of those who were aphasic when tested 2-3 days post-surgery. To construct an unbiased sample of the whole cohort at 1 month post-surgery, we imputed 1 month post-surgery scores for the first 15 of the 29 patients who were not aphasic at 2-3 days post-surgery, by setting their 1 month post-surgery scores equal to their 2-3 days post-surgery scores. This would tend to underestimate their 1 month post scores, since any modest deficits 2-3 days post-surgery may have resolved over the following month.
Some patients completed a subset of the WAB at the pre-surgical and/or 1 month post-surgical time points. Any subscores that were obtained were included in the analyses of language data, but these patients were not included in the lesion analysis since they lacked an AQ (which was to be used as a covariate).
Paired t-tests were used to compare scores on each language measure between presurgical and 2-3 days post-surgical assessments, between 2-3 days post-surgical and 1 month post-surgical assessments, and between presurgical and 1 month post-surgical assessments. Patients were classified as aphasic according to the WAB when their AQ was less than 93.8, which is the standard cutoff based on the mean of a group of patients with brain damage but, clinically, no aphasia. Aphasia by this definition can range from mild to severe, and in this paper we do not distinguish ‘aphasia’ from ‘dysphasia’ (i.e. a milder language disturbance). We considered WAB subscores and/or BNT scores as abnormal when they were 2 or more standard deviations below means for non-brain damaged control participants40,42. Because of the way that AQ is calculated, it is possible for a patient to be impaired on one or more language domains without being classified as aphasic, so long as their AQ is greater than or equal to 93.8.
Neuroimaging and lesion mapping
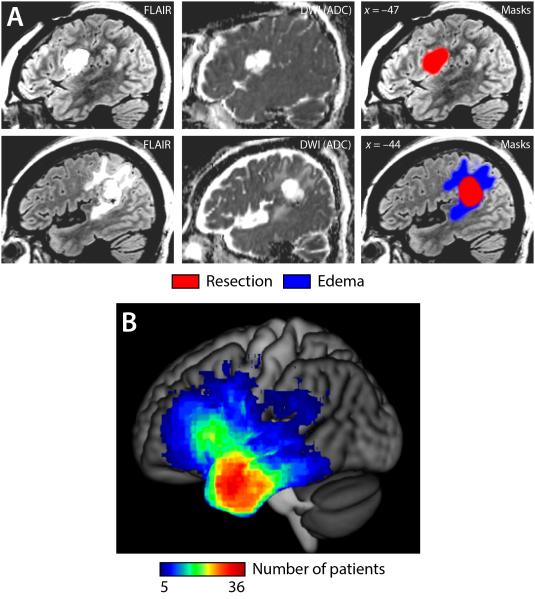
The locations of surgical sites were delineated for each patient based on images acquired 2-3 days post-surgery by a trained research assistant with input from a neurosurgeon and neuroradiologist (Fig. 1A). A mask of the surgical site was manually created by delineating the extent of the resection and any adjacent infarction on each axial slice using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Adjacent infarction was defined as regions showing abnormal FLAIR signal as well as > 10% reduction in apparent diffusion coefficient (ADC) relative to the contralateral region68. These masks were used for lesion-symptom mapping at the 2-3 days post-surgery and 1 month post-surgery time points.
Figure 1.
Lesion masks and overlays. (A) Two example patients. Fluid Attenuated Inversion Recovery (FLAIR) and Diffusion Weighted Imaging (DWI) Apparent Diffusion Coefficient (ADC) images are shown. The first patient had no imaging abnormalities in addition to the resection; the second patient had edema adjacent to the resection. (B) Overlay of surgical sites in 110 patients.
Two additional masks were also created. The first was a mask of the resection only; any adjacent infarction was not included. These masks were used for lesion-symptom mapping at the presurgical time point (to capture relationships between pre-surgical language measures and the intended surgical site). The second was a mask of the surgical site as well as any associated edema, defined as tissue that was abnormal on FLAIR but did not show restricted diffusion. These masks were used only for cost function masking in the registration process, since much of this edema was typically present pre-operatively and its functional status was not known.
All three masks were smoothed with a 4 mm FWHM Gaussian kernel using SPM5 to account for boundary uncertainties due to anatomical variability and imperfect registration. FLAIR images were warped to MNI space using the unified segmentation procedure in SPM54 and cost function masking using the surgical site plus edema image dilated by 4 mm2,14. The surgical site and resection only masks were then warped to MNI space by applying the derived transformation, and resampled with 2 × 2 × 2 mm voxels.
The mean resection volume (including any adjacent infarction) was 33.6 ± 24.4 cm3 (range: 2.7 to 133.1 cm3). Overlays of all surgical site masks (Fig. 1B) showed that the anterior temporal lobe was the most commonly resected region in this patient cohort, followed by the posterior inferior frontal gyrus.
Voxel-based lesion-symptom mapping (VLSM) was carried out to identify any systematic relationships between locations of resection and/or damage, and language measures, using vlsm 2.55 (http://neuroling.arizona.edu/resources.html)8,70. T-maps were created for each of the five language variables (fluency, comprehension, repetition, information content and naming) at each of the three time points (pre-surgery, 2-3 days post-surgery, and 1 month post-surgery).
A general linear model was fit at each voxel with the lesion status of the voxel (0 = intact, 1 = lesioned, with intermediate values near lesion borders due to smoothing) as the independent variable, and the language measure in question as the dependent variable. Total resection volume was included as a covariate, in order to avoid showing spurious effects in voxels that were more likely to be involved in large lesions. Aphasia quotient was also included as a covariate, in order to identify voxels that were differentially important for each specific language measure, rather than associated with global language deficits. Voxels were only included when the sum of the lesion overlay was at least 5, in order to avoid spurious effects driven by small numbers of patients in regions that are not frequently resected. Statistical power in VLSM analyses is not stationary but depends on the ratio of lesioned to intact patients in each voxel; in this cohort, power was greatest in the anterior temporal lobe, followed by the posterior inferior frontal gyrus. No conclusions can be drawn regarding regions where the sum of the overlay was less than 5, since these regions were not included in the analyses.
To determine the statistical significance of the t-maps resulting from each analysis, a permutation procedure was used70. The behavioral scores were randomly reassigned to the patients 1000 times, then each resulting t-map was thresholded at voxelwise p < 0.001, and the cluster size of the largest cluster (if any) was recorded. After thresholding the real data similarly, the p value of any observed clusters was determined with reference to the null distribution of maximum cluster sizes from the permuted datasets. The covariate of resection volume was not permuted, whereas the aphasia quotient covariate was permuted along with each language measure of interest. We also report correlations between resection volume and each language measure of interest as well as the overall AQ.
In order to ensure that VLSM results did not reflect a confound of etiology, we also repeated all VLSM analyses, restricting the dataset to the subset of 88 patients with gliomas, excluding those with epilepsy or other etiologies.
Results
Before surgery, most patients’ language was normal or near-normal (Fig. 2, Table 2). Only 15 of 84 patients (18%) were classified as aphasic based on WAB criteria. In this paper, we use the term ‘aphasia’ in reference to any disturbance in language function (mild or severe) across the domains of fluency, information content, comprehension, repetition and naming, as opposed to distinguishing from the term ‘dysphasia’. Percentages of patients showing impairments on the five language domains—fluency, information content, comprehension, repetition and naming—ranged from 8% on comprehension to 31% on fluency. Of the 15 patients classified as aphasic, 8 were subclassified as anomic and 7 did not meet criteria for any subtype (see Table 2 caption for details on unclassifiable patients).
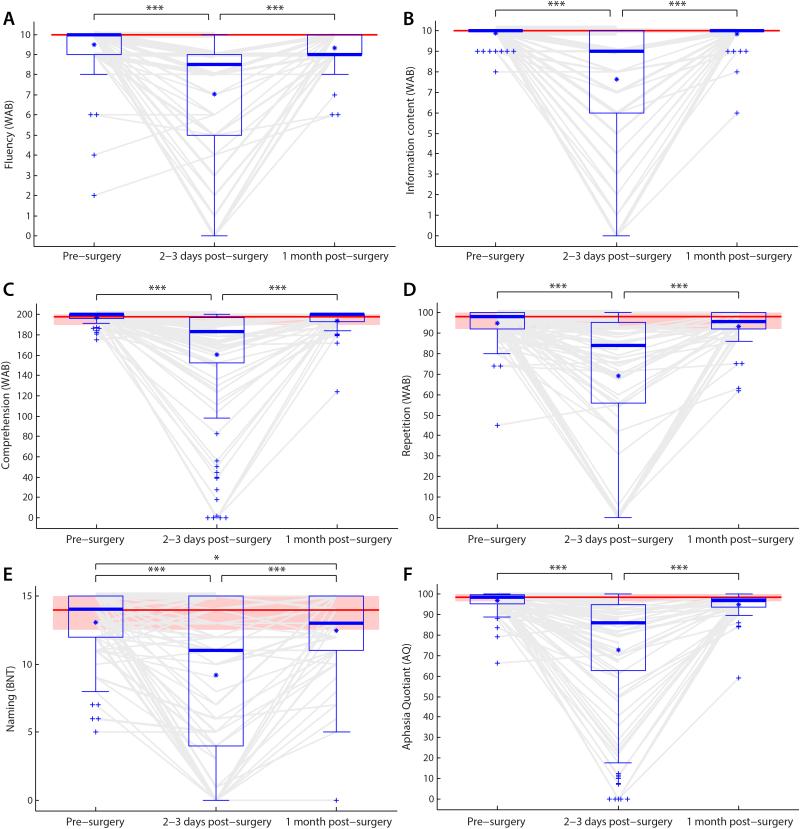
Figure 2.
Language measures before surgery, 2-3 days post-surgery, and 1 month post-surgery. (A) Fluency. (B) Information content. (C) Comprehension. (D) Repetition. (E) Naming. (F) Aphasia Quotient. All measures were significantly impacted at 2-3 days post-surgery, and all measures recovered between two days and one month, such that the only score that differed from pre-surgical scores at 1 month post-surgery was naming. Boxes = interquartile range; whiskers = range not including outliers; plusses = outliers; thick horizontal lines = medians; asterisks = means; *** p < 0.001; ** p < 0.01; * p < 0.05. Light gray lines show the trajectories of each individual patient. Where multiple patients’ lines would coincide, the thickness of lines is proportional to the square root of the number of patients. Distributions at 1 month post-surgery include imputed scores, but lines show only data actually obtained.
Table 2.
Langauge measures pre-surgery, 2-3 days post-surgery, and 1 month post-surgery
| Pre-surgery | 2-3 days post-surgery | 1 month post-surgery | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Abnormal | Mean ± SD | Abnormal | Mean ± SD | Abnormal | |
|
|
||||||
| Fluency | 9.5 ± 1.2 | 27/88 (31%) | 7.0 ± 3.1 | 94/110 (86%) | 9.3 ± 0.9 | 29/56 (52%) |
| Information content | 9.9 ± 0.3 | 8/88 (9%) | 7.6 ± 3.2 | 66/110 (60%) | 9.8 ± 0.6 | 6/56 (11%) |
| Comprehension | 197.2 ± 5.1 | 7/86 (8%) | 160.2 ± 54.8 | 68/110 (62%) | 194.1 ± 12.1 | 10/51 (20%) |
| Repetition | 94.9 ± 8.0 | 19/89 (21%) | 69.2 ± 34.4 | 68/110 (62%) | 93.2 ± 8.3 | 13/54 (24%) |
| Naming (BNT) | 13.1 ± 2.4 | 25/85 (29%) | 9.2 ± 5.7 | 63/106 (59%) | 12.5 ± 3.1 | 22/55 (40%) |
| Aphasia quotiant | 96.6 ± 5.2 | 15/84 (18%) | 72.5 ± 30.0 | 78/110 (71%) | 94.9 ± 6.4 | 13/51 (25%) |
|
| ||||||
| WAB classification | ||||||
| Within normal limits | 69 (82%) | 32 (29%) | 38 (75%) | |||
| Global | – | 10 (9%) | – | |||
| Broca’s | – | 9 (8%) | – | |||
| Transcortical Motor | – | 1 (1%) | – | |||
| Wernicke’s | – | 7 (6%) | – | |||
| Transcortical Sensory | – | – | 1 (2%) | |||
| Conduction | – | 11 (10%) | – | |||
| Anomic | 8 (10%) | 34 (31%) | 8 (16%) | |||
| Unclassifiable | 7 (8%) | 6 (5%) | 4 (8%) | |||
All instances of unclassifiable patients were due to WAB naming subscores being too high for the variant with which the patient would otherwise have been classified. If WAB naming subscores were ignored, pre-surgery there would be an additional 4 cases classified Anomic, 1 Broca’s, 1 Transcortical Motor, and 1 Condition. At 2-3 days post-surgery, there would be an additional 3 Anomic, 2 Broca–s and 1 Conduction. At 1 month post-surgery, there would be an additional 3 Anomic and 1 Conduction.
At 2-3 days post-surgery, many patients showed sharp drops in one or more language domains and in overall language function as assessed by AQ (Fig. 2, Table 2). At the group level, all language domains and overall language function were significantly impaired relative to pre-surgery (all p < 0.001). 78 of 110 patients (71%) were now classified as aphasic based on the WAB, and all five language domains—fluency, information content, comprehension, repetition and naming—were impaired in majorities of patients, ranging from 59% on naming to 86% on fluency. In all, 97 of 110 patients (88%) were either classified as aphasic or impaired on at least one language domain. Of the 78 patients classified aphasic, 34 were Anomic, 11 were Conduction, 10 were Global, 9 were Broca’s, 7 were Wernicke’s, 1 was Transcortical Motor, and 6 did not meet criteria for any subtype.
At 1 month post-surgery, most patients showed substantial improvements in language domains that had been impaired 2-3 days post-surgery, and in overall language function as assessed by AQ (Fig. 2, Table 2). At the group level, all language domains and overall language function (AQ) were significantly improved relative to 2-3 days post-surgery (all p < 0.001). While significant improvements were observed, 13 of 51 patients (25%) still met the cutoff for aphasia based on the WAB criteria. Percentages of patients showing impairments on the five language domains ranged from 11% on information content to 52% on fluency, though dramatically improved in severity (see Fig. 2). Of the 13 patients classified an aphasic, 8 were Anomic, 1 was Transcortical Sensory, and 4 did not meet criteria for any subtype. Compared to pre-surgical evaluations, there was a significant decrease in confrontation naming as assessed by the BNT (t(46) = 2.66, p = 0.011), marginal decreases in AQ (t(43) = 1.70, p = 0.096) and comprehension (t(43) = 1.91, p = 0.063), but no significant changes in fluency (t(50) = 0.82, p = 0.42), information content (t(50) = 0.70, p = 0.49) or repetition (t(48) = 0.62, p = 0.54) scores.
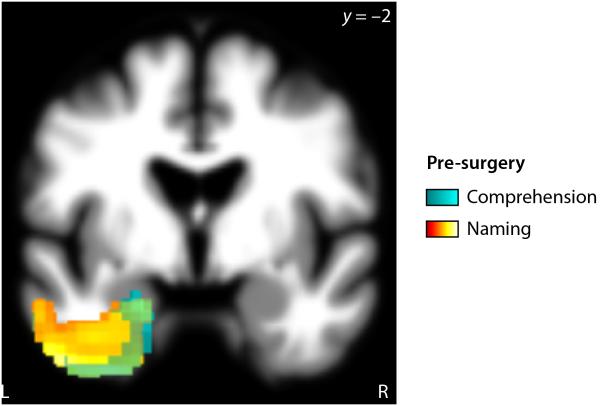
We used voxel-based lesion-symptom mapping (VLSM) to identify relationships between surgical sites and language variables. Although most patients’ language was normal or near-normal before surgery, we first investigated relationships between pre-surgical language measures and the location of resections that were subsequently performed (Fig. 3, Table 3). Naming and comprehension deficits were associated with forthcoming surgery to the anterior temporal lobe (naming p < 0.001; comprehension p = 0.003); there were no significant relationships involving the other language measures. This shows that patients in whom anterior temporal resections were planned already showed poorer naming and comprehension than patients in whom other brain regions were to be resected. The volume of the resection to be subsequently performed was not correlated with AQ or any of the language measures (all p ≥ 0.18).
Figure 3.
Neural correlates of language deficits pre-surgery. Subsequent resection of cyan or hot colored voxels was sigificantly correlated with pre-surgical comprehension or naming measures respectively.
Table 3.
Brain regions showing significant correlations between resection location and
| language variables | |||||||
|---|---|---|---|---|---|---|---|
| Language measure | Brain region(s) | MNI coordinates |
Max t | Extent (mm 3) |
p | ||
| X | y | z | |||||
| Pre-surgery (resection only) | |||||||
| Comprehension | Anterior inferior temporal gyrus, fusiform gyrus, parahippocampal gyrus |
−32 | −5 | −34 | 5.57 | 11 328 | 0.003 |
| Naming | Anterior middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, parahippocampal gyrus |
−38 | −5 | −34 | 5.89 | 25 080 | < 0.001 |
| Two-to-three days post-surgery (resection plus infarction) | |||||||
| Fluency | Precentral gyrus, inferior frontal junction |
−36 | −4 | 33 | 4.08 | 3 192 | 0.019 |
| Information content | Precentral gyrus, posterior inferior frontal gyrus (pars opercularis) |
−51 | 4 | 8 | 4.11 | 2 576 | 0.035 |
| Repetition | Posterior superior temporal gyrus | −52 | −41 | 12 | 5.17 | 4 560 | 0.024 |
| Naming | Middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, parahippocampal gyrus |
−43 | −12 | −30 | 6.09 | 44 456 | < 0.001 |
| One month post-surgery (resection plus infarction) | |||||||
| Fluency | Precentral gyrus | −52 | 6 | 16 | 4.89 | 3 048 | 0.025 |
| Comprehension | Temporal isthmus | −40 | −12 | −8 | 6.65 | 2 648 | 0.023 |
|
| |||||||
| Naming | Middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, parahippocampal gyrus |
−40 | −4 | −32 | 7.93 | 34 088 | < 0.001 |
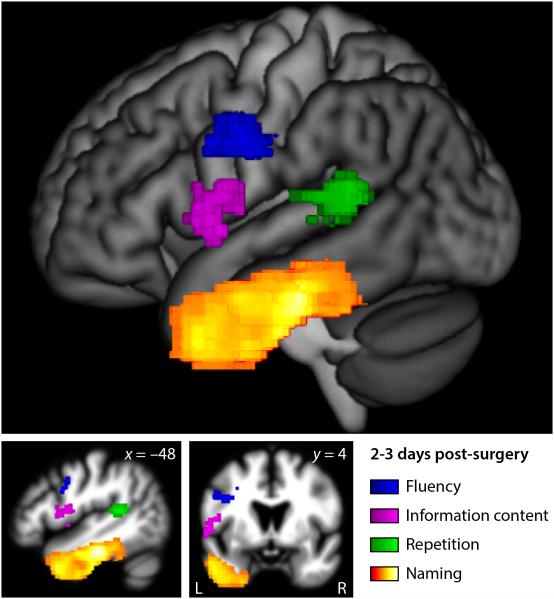
To identify the neural correlates of transient post-surgical aphasias, we next used VLSM to identify relationships between language variables at 2-3 days post-surgery, and the locations of surgical sites (Fig. 4, Table 3). Reduced fluency was associated with resection of the precentral gyrus and adjacent inferior frontal cortex (p = 0.019). Reduced information content was associated with resection of the ventral precentral gyrus and the pars opercularis of the inferior frontal gyrus (p = 0.035). Repetition deficits were associated with resection of the posterior superior temporal gyrus (p = 0.024). Naming deficits were associated with resection of the middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, and parahippocampal gyrus, with the strongest relationship observed in the fusiform gyrus approximately 6 cm posterior to the temporal pole (p < 0.001). There was no significant relationship between comprehension scores and resection location, but the most significant voxel was located in the white matter underlying the temporal lobe (MNI coordinates x = –46, y = –18, z = –12; uncorrected p = 0.0012). The systematic relationships between damage to specific parts of the language network and deficits in particular language domains show that transient post-surgical aphasias reflect localized effects of resection, rather than a global effect on brain function. However, resection volume was modestly negatively correlated with AQ (r = –0.25, p = 0.0087) and every language measure: fluency (r = –0.18, p = 0.058), information content (r = –0.24, p = 0.010), repetition (r = –0.21, p = 0.031), naming (r = –0.27, p = 0.0060) and comprehension (r = –0.27, p = 0.0045).
Figure 4.
Neural correlates of language deficits 2-3 days post-surgery. A lateral projection and two slices are shown. Resection or infarction of colored voxels was significantly correlated with language measures 2-3 days post-surgery: Blue = fluency; magenta = information content; green = repetition; hot = naming.
The cluster associated with naming deficits at 2-3 days post-surgery extended along the axis of the temporal lobe all the way from the temporal pole almost to the occipital lobe, so we divided it into anterior temporal, mid temporal and posterior temporal regions of interest (ROIs) by coronal planes at MNI y = 0 and y = –36. The mid temporal ROI was the most strongly associated with naming deficits as stated above. To determine the relative importance of the anterior temporal and posterior temporal ROIs, we fit two general linear models with naming score (BNT) as the dependent variable. The first model included damage to the mid temporal ROI and damage to the anterior temporal ROI as independent variables, with lesion size, and aphasia quotient as covariates. The mid temporal ROI predicted naming scores (t = 3.83, p < 0.001), but the anterior temporal ROI did not (t = –0.20, p = 0.84). The second model included damage to the mid temporal ROI and damage to the posterior temporal ROI as independent variables, with the same covariates. Again the mid temporal ROI predicted naming scores (t = 5.39, p < 0.001), but in this analysis, the posterior temporal ROI did so also (t = 3.79, p = 0.005). These analyses suggest that mid temporal and posterior temporal regions make independent contributions to naming, whereas anterior temporal damage does not impact naming after mid temporal damage is taken into account.
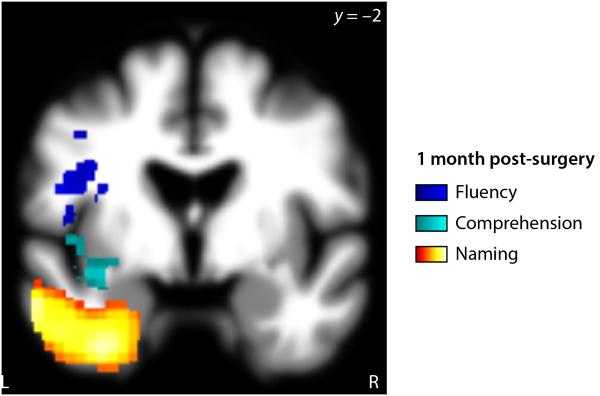
At the relatively early time point of 1 month post-surgery (Fig. 5, Table 3), there continued to be an association between inferior frontal resections and fluency deficits (p = 0.025), though most of these fluency deficits were now mild. There also continued to be an association between ventral temporal resections and naming deficits (p < 0.001). Associations between information content and resection location, and between repetition and resection location, were no longer significant. The temporal isthmus, which had been non-significantly associated with comprehension deficits in the immediate post-surgical period, was now significantly associated with comprehension deficits (p = 0.023). Resection volume was significantly negatively correlated with information content 1 month post-surgery (r = –0.30, p = 0.023), and there were also trends toward negative correlations with AQ (r = –0.27, p = 0.057) and comprehension (r = –0.27, p = 0.055).
Figure 5.
Neural correlates of language deficits 1 month post-surgery. Resection or infarction of colored voxels was significantly correlated with language measures 1 month post-surgery: Blue = fluency; cyan = comprehension; hot = naming.
The VLSM analyses restricted to the 88 patients with gliomas yielded very similar results to the analyses of the whole cohort of patients. In particular, 2-3 days post-surgery, the same regions shown in the main analyses were significantly associated with impaired fluency (p = 0.029), information content (p = 0.025), repetition (p = 0.028) and naming (p = 0.001), and there was no region significantly associated with comprehension deficits.
Discussion
We found that transient aphasias were very common after resective surgery to the language-dominant left hemisphere, that all language domains could be affected, and that all major aphasia types occured. There were systematic relationships between the location of surgical sites and deficits in specific language domains. Recovery was rapid, and by 1 month post-surgery, almost all language deficits had resolved.
Incidence and nature of transient post-operative aphasias
71% of patients were classified as aphasic based on the WAB when tested 2-3 days post-surgery, and the majority of patients were impaired in each language domain investigated, with 88% of patients were classified as aphasic and/or impaired on at least one language domain.
The incidence of language deficits in the immediate post-operative period has varied widely in previous studies10,29,35,41,49,50,57,58,61,63, likely due to sensitivity of aphasia testing, and different distributions of resection sites. The largest series was that of Roberts61, who reported transient aphasias after 144 of 246 (59%) left hemisphere resections to a variety of different brain regions. In another large series, Falconer and Serafetinides29 reported post-operative aphasias in 29 of 56 (52%) patients after surgery to the left temporal lobe. The considerably higher incidence of language deficits that we found in our study is likely a consequence of using a comprehensive aphasia battery, which is more sensitive in detecting milder language deficits.
We found that anomic aphasias were most common, but that Broca’s, Wernicke’s, conduction and global aphasias all occurred quite frequently too. Previous studies have reported deficits in a range of language domains, including fluency10, comprehension49, repetition49,58 and naming49,58, but none have used aphasia batteries, so the precise nature of transient post-surgical aphasias was not clear from previous research.
Neuroanatomical correlates of specific transient post-operative language deficits
Voxel-based lesion-symptom mapping revealed systematic relationships between location of surgical sites, and transient language deficits in specific domains. To our knowledge, our study is the first to show that the specific profiles of transient post-operative aphasias are related to resection site.
Fluency deficits in the immediate post-operative period, 2-3 days post-surgery, were associated with resection of the precentral gyrus and inferior frontal junction, where the inferior frontal sulcus meets the ventral precentral sulcus. Previous studies have reported associations between reduced fluency and left inferior frontal damage in post-stroke aphasia8,26,30,55 and primary progressive aphasia1,3,62,70. Similarly, left inferior frontal regions have been implicated in functional imaging studies of speech production11,12,38. The specific regions reported in these neuropsychological and neuroimaging studies have varied and have included the inferior frontal gyrus and sulcus, middle frontal gyrus, precentral gyrus, rolandic operculum, anterior insula, and underlying white matter (the anterior part of the arcuate fasciculus). The relatively circumscribed resections of the patients in our study, and the presumed lack of any functional reorganization due to the short time frame, may be taken to support our specific finding that a region spanning the precentral gyrus and inferior frontal junction, posterior and dorsal to Broca’s area, is the region most strongly associated with reduced fluency.
Reduced information content was associated with resection of the ventral precentral gyrus and posterior inferior frontal gyrus (pars opercularis). The regions where damage was predictive of reduced information content were ventral to those where damage was associated with fluency deficits. The information content measure assesses the functional, communicative value of a patient’s speech, and reflects the patient’s ability to convey correct answers to six basic questions. Information content is a rather nonspecific measure in that deficits in comprehension, word finding and/or speech production could result in failure to answer basic questions. However it is noteworthy that damage to Broca’s area, long considered one of the most important brain regions for language17, was found to most impact the functional, communicative value of patients’ speech.
Repetition deficits were associated with resection of the posterior superior temporal gyrus. This finding is consistent with studies of repetition deficits in post-stroke aphasia7,16,21 and primary progressive aphasia1,62, functional imaging studies of healthy controls15, and cortical stimulation studies59. Repetition deficits have also been associated with damage or disruption to the adjacent inferior parietal regions31,56,60 and/or the arcuate component of the superior longitudinal fasciculus13,21,45,54, which connects posterior temporal and inferior parietal regions to the frontal lobe.
Naming deficits were associated with resection of the middle temporal gyrus, inferior temporal gyrus, fusiform gyrus and parahippocampal gyrus. The strongest relationship was observed in the fusiform gyrus approximately 6 cm posterior to the temporal pole, in a region referred to as the basal temporal language area (BTLA)51. The BTLA was identified as most predictive of naming deficits, even though the VLSM analysis had more power in the temporal pole due to more patients having resections there. Although the BLTA was the most highly predictive region of naming deficits, the cluster associated with naming deficits extended from the temporal pole all the way along the axis of the temporal lobe, almost reaching the occipital lobe. A follow-up ROI analysis showed that mid temporal and posterior temporal regions made independent contributions to naming deficits, but not the anterior temporal region. Naming involves a series of processing stages including conceptual processing, word selection, phonological retrieval, phonological encoding and articulation48, and hence relies on numerous brain regions and is vulnerable in all types of aphasia9. The mid temporal and posterior temporal regions we identified may be relatively more involved in word selection and phonological retrieval respectively32,39,66. Previous studies have reported associations between naming deficits and damage to a range of left temporal regions in post-stroke aphasia5,20,22,25,37,66. A study of naming deficits in semantic dementia53, and another study of naming deficits in three variants of primary progressive aphasia1, both observed the strongest correlations between atrophy and naming deficits in the anterior fusiform gyrus, likely corresponding to the BTLA.
The only language measure for which we found no association with specific surgical sites in the immediate post-operative period was comprehension. The most significant voxel in the VLSM t-map for comprehension was located in the white matter of the temporal isthmus. Tracts in this region include the inferior longitudinal fasciculus and the extreme capsule fiber system, both of which have been associated with auditory comprehension64,71. A previous VLSM study found that comprehension deficits in chronic stroke patients were most associated with damage to the posterior middle temporal gyrus8. Our study had little to no power in this region (see Fig. 1) because resections to posterior temporal cortex were relatively rare in this set of study patients; this is a likely reason that we did not find any significant lesion correlates of comprehension deficits.
Rapid recovery by one month post-surgery
At 1 month post-surgery, almost all language deficits that had been apparent 2-3 days post-surgery were now resolved in terms of severity and frequency, and no language measure except for naming differed significantly from pre-surgical levels. We must emphasize that 1 month post-surgery does not represent a stable end-point, especially compared to 3 months62 or 1 year time points after surgery examined in previous outcome series. It is certainly likely that those patients who did still show deficits at 1 month post-surgery would subsequently show further improvements, but our study was not designed to examine long-term outcomes.
There was a slight but significant decrease in naming scores relative to pre-surgical levels, and resection of ventral temporal regions continued to be associated with naming deficits. This is consistent with a number of studies documenting persistent naming deficits after anterior temporal lobectomy, in particular in patients with later onset of symptoms23,44,47,65,67. One study showed that naming deficits 6 to 12 months after anterior temporal lobectomy were worse when the basal temporal language area was resected than when it was not, even after accounting for resection volume44. It should be noted that even prior to surgery, forthcoming surgery to anterior ventral temporal regions was correlated with naming deficits. Therefore the association between ventral temporal damage and persistent naming deficits probably reflects a combination of damage due to the tumor (or other abnormality requiring surgery), and damage due to the resection and any associated infarction.
The few deficits that persisted at 1 month post-surgery should not obscure the striking recovery that took place in every language domain. The relatively rapid recovery from these transient post-surgical aphasias that we have documented raises the question of the mechanism(s) of the transient aphasia and its recovery. As mentioned above, there are several possibilities, which are not mutually exclusive.
Penfield and Roberts57 speculated that transient aphasias may be due to nonspecific mechanisms following exposure of the cortex to air and ultraviolet rays, and/or the electrical stimulation carried out for language mapping. They referred to this as ‘neuroparalytic edema’ (p. 141). The specific lesion-deficit correlations that we observed argue against a mechanism such as this, since a global mechanism would presumably affect different domains of language without regard to the specific location of the resection.
A more likely potential mechanism is edema in brain regions adjacent to the resection29,49, with attendant cerebral hypoperfusion and neuronal dysfunction. If brain regions surrounding the resection are dysfunctional in the immediate post-surgical period, then the aphasia in this period would essentially reflect the effect of a larger lesion. The specific relationships we observed between lesion locations and aphasic symptoms would still be compatible with an explanation in terms of edema, since edema is generally localized to regions in the vicinity of the resection. Resolution of edema would result in these surrounding regions returning to normal function, with aphasia resolving accordingly. Note that edema may or may not be visible on MRI, and moreover, many patients already showed substantial edema (probably of a different nature) in the vicinity of tumors prior to surgery. For these reasons, we were not able to quantify the extent of post-surgical edema. However one observation suggests that edema of surrounding regions must be an important mechanism underlying transient aphasias in at least some patients: we observed that many patients’ language was actually less impaired immediately after the surgery than when they were formally assessed after 2-3 days. This time course approximately matches the time course of edema after traumatic brain injury6, and clearly rules out a direct effect of the resection itself.
A third potential mechanism that also relies on the concept of dysfunction of regions other than the resection itself is diaschisis49, that is, abnormalities in metabolism, neuronal function, or connectivity in intact brain regions as a consequence of the lesion. Diaschisis can be identified with functional imaging methodologies, but would not be visible on structural MRI. In this scenario, recovery would involve plasticity in connected regions to adjust for the lack of inputs from the resected region. One argument against this explanation is that it would seem to predict that right hemisphere resective surgeries should result in transient aphasias via diaschitic effects on homotopic brain regions, since transhemispheric diaschisis is the most well-established form of cortico-cortical diaschisis18. However, we have not observed transient aphasias after right hemisphere surgery.
A final factor that may contribute to transient aphasias is the loss of functional tissue in the tumor itself (in the case of low grade gliomas), immediately adjacent to the tumor and within the resection boundaries, or in adjacent areas that are infarcted as a consequence of the surgery27. If this is an important mechanism, then the rapid rate of recovery would be remarkable, and would suggest that there is considerable redundancy within language networks, since the time frame of recovery seems too short for explicit relearning.
While the specific lesion-deficit correlations that we observed argue against a global mechanism, our data are consistent with any of the other explanations we have discussed.
Limitations and future directions
Our study had several notable limitations. First, only routine clinical MRI scans were obtained, and scans were only obtained 2-3 days post-surgery. Pre-surgical scans were not utilized, and follow-up scans 1 month post-surgery were not obtained. It is possible that a more comprehensive battery of scans, including functional MRI in particular, might be capable of more effectively identifying functional abnormalities beyond the resection in the immediate post-operative period. This could enable us to quantitatively define a larger region of transient dysfunction than the resection.
Second, the language assessment was somewhat limited. Although the WAB is a more comprehensive aphasia assessment than the brief assessments that have been used in previous studies that have investigated post-surgical aphasias, the WAB has several weaknesses. In particular, the fluency scale is subjective, maps a multidimensional deficit to an ordinal scale, and does not take into account features such as articulatory agility33. The comprehension score does not discriminate between lexical and syntactic comprehension, and there is no assessment of non-verbal semantic processing. In future work we plan to use quantitative analysis of connected speech samples to better quantify different aspects of fluency. Additionally, domain-specific tests could be added to evaluate aspects of language that are not well captured by the WAB.
Third, our cohort included patients of several different etiologies, including high and low grade gliomas, epilepsy, vascular malformations, and others. The majority of patients had tumors (88 patients had either low grade or high grade gliomas) and we showed that the brain-language relationships observed in the whole group also held in this subset of patients, indicating that our findings are not due to any confound of etiology. However we did not have sufficient patients of any other etiology to determine whether brain-language relationships might differ as a function of etiology33. There is evidence that language reorganization takes place due to epilepsy, tumors, or other abnormalities in language regions64, and may differ as a function of etiology33. Any such reorganization of language regions constitutes a source of noise for our study, which would make it more difficult to identify consistent brain-language relationships. The fact that we found robust relationships between resection location and deficits in specific language domains suggests that language reorganization is generally not so dramatic as to obscure the basic functional neuroanatomy of the language network.
Conclusions
Transient aphasias are very common after resective surgery to the language-dominant left hemisphere, and there are systematic relationships between the location of surgical sites and deficits in specific language domains. Most language deficits resolve within one month. Our study does not attempt to characterize permanent deficits, as this would require significantly longer follow-up with equally careful testing. This patient cohort provides a unique window on the neural basis of language, since surgical resections are discrete and their locations are not limited by vascular distribution8 or patterns of neurodegeneration70, factors which limit the interpretation of many lesion-symptom mapping studies52. Moreover, because we evaluated patients’ language 2-3 days after surgery, we can observe language function before substantial reorganization has taken place.
Acknowledgements
We thank Jessica DeLeon for her assistance with this project, and we thank all of the patients who participated in the research.
Funding: National Institutes of Health (NS065120,DC012379 and OD00862 to EFC, DC010878 and DC013270 to SMW); McKnight Foundation (EFC); New York Stem Cell Foundation (EFC)
Footnotes
Portions of this work were presented in poster form at the Society for the Neurobiology of Language 2013 Annual Meeting, San Diego, California, November 6-8, 2013.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol. 2007;20:203–211. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? NeuroImage. 2010;53:78–84. doi: 10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. J Neurolinguist. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Baldo JV, Arévalo A, Patterson JP, Dronkers NF. Grey and white matter correlates of picture naming: evidence from a voxel-based lesion analysis of the Boston Naming Test. Cortex. 2013;49:658–667. doi: 10.1016/j.cortex.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareyre F, Wahl F, McIntosh TK, Stutzmann JM. Time course of cerebral edema after traumatic brain injury in rats: effects of riluzole and mannitol. J Neurotrauma. 1997;14:839–849. doi: 10.1089/neu.1997.14.839. [DOI] [PubMed] [Google Scholar]
- 7.Bartha L, Benke T. Acute conduction aphasia: An analysis of 20 cases. Brain Lang. 2003;85:93–108. doi: 10.1016/s0093-934x(02)00502-3. [DOI] [PubMed] [Google Scholar]
- 8.Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 9.Benson DF. Neurologic correlates of anomia. In: Whitaker H, Whitaker HA, editors. Studies in Neurolinguistics. Vol. 4. Academic Press; New York: 1979. pp. 293–328. [Google Scholar]
- 10.Benzagmout M, Gatignol P, Duffau H. Resection of World Health Organization Grade II gliomas involving Broca’s area: methodological and functional considerations. Neurosurgery. 2007;61:741–752. doi: 10.1227/01.NEU.0000298902.69473.77. [DOI] [PubMed] [Google Scholar]
- 11.Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. NeuroImage. 2006;32:821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse: an H215O-PET study of narrative production in English and American sign language. Brain. 2001;124:2028–2044. doi: 10.1093/brain/124.10.2028. [DOI] [PubMed] [Google Scholar]
- 13.Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–697. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, et al. Conduction aphasia, sensory-motor integration, and phonological short-term memory – An aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119:119–128. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplan D. Neurolinguistics and linguistic aphasiology: An introduction. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- 18.Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- 19.Chang ED, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122:250–261. doi: 10.3171/2014.10.JNS132647. [DOI] [PubMed] [Google Scholar]
- 20.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 21.Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain. 1980;103:337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- 22.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Davies KG, Bell BD, Bush AJ, Hermann BP, Dohan FC, Jr, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–419. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 24.Davies KG, Maxwell RE, Jennum P, Dhuna A, Beniak TE, Destafney E, et al. Language function following subdural grid-directed temporal lobectomy. Acta Neurol Scand. 1994;90:201–206. doi: 10.1111/j.1600-0404.1994.tb02706.x. [DOI] [PubMed] [Google Scholar]
- 25.DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 26.Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- 27.Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Lopes M, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109:461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- 29.Falconer MA, Serafetinides EA. A follow-up study of surgery in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1963;26:154–165. doi: 10.1136/jnnp.26.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fridriksson J, Guo D, Fillmore P, Holland A, Rorden C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain. 2013;136:3451–3460. doi: 10.1093/brain/awt267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, et al. Impaired speech repetition and left parietal lobe damage. J Neurosci. 2010;30:11057–11061. doi: 10.1523/JNEUROSCI.1120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gesierich B, Jovicich J, Riello M, Adriani M, Monti A, Brentari V, et al. Distinct neural substrates for semantic knowledge and naming in the temporoparietal network. Cereb Cortex. 2012;22:2217–2226. doi: 10.1093/cercor/bhr286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon JK. The fluency dimension in aphasia. Aphasiology. 1998;12:673–688. [Google Scholar]
- 34.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Helmstaedter C, Gleissner U, Zentner J, Elger CE. Neuropsychological consequences of epilepsy surgery in frontal lobe epilepsy. Neuropsychologia. 1998;36:681–689. doi: 10.1016/s0028-3932(97)00134-6. [DOI] [PubMed] [Google Scholar]
- 36.Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74:560–566. doi: 10.3171/jns.1991.74.4.0560. [DOI] [PubMed] [Google Scholar]
- 37.Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman R, Barker PB, et al. Restoring cerebral blood flow reveals neural regions critical for naming. J Neurosci. 2006;26:8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Indefrey P, Brown CM, Hellwig F, Amunts K, Herzog H, Seitz RJ, et al. A neural correlate of syntactic encoding during speech production. Proc Natl Acad Sci U S A. 2001;98:5933–5936. doi: 10.1073/pnas.101118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2 Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 41.Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, et al. Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia. 1989;30:763–771. doi: 10.1111/j.1528-1157.1989.tb05336.x. [DOI] [PubMed] [Google Scholar]
- 42.Kertesz A. Western aphasia battery. Grune and Stratton; New York: 1982. [Google Scholar]
- 43.Kho KH, Indefrey P, Hagoort P, van Veelen CWM, van Rijen PC, Ramsey NF. Unimpaired sentence comprehension after anterior temporal cortex resection. Neuropsychologia. 2008;46:1170–1178. doi: 10.1016/j.neuropsychologia.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–483. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- 45.Kümmerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Klöppel S, et al. Damage to ventral and dorsal language pathways in acute aphasia. Brain. 2013;136:619–629. doi: 10.1093/brain/aws354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–3255. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- 47.Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53:72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- 48.Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- 49.Loring DW, Meador KJ, Lee GP. Effects of temporal lobectomy on generative fluency and other language functions. Arch Clin Neuropsychol. 1994;9:229–238. [PubMed] [Google Scholar]
- 50.Lubrano V, Draper L, Roux F-E. What makes surgical tumor resection feasible in Broca’s area? Insights into intraoperative brain mapping. Neurosurgery. 2010;66:868–875. doi: 10.1227/01.NEU.0000368442.92290.04. [DOI] [PubMed] [Google Scholar]
- 51.Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, et al. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- 52.Mah Y-H, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137:2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- 54.Moritz-Gasser S, Duffau H. The anatomo-functional connectivity of word repetition: insights provided by awake brain tumor surgery. Front Hum Neurosci. 2013;7:405. doi: 10.3389/fnhum.2013.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Palumbo CL, Alexander MP, Naeser MA. CT scan lesion sites associated with conduction aphasia. In: Kohn SE, editor. Conduction aphasia. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- 57.Penfield W, Roberts L. Speech and Brain-Mechanisms. Princeton University Press; Princeton, NJ: 1959. [Google Scholar]
- 58.Peraud A, Ilmberger J, Reulen H-J. Surgical resection of gliomas WHO grade II and III located in the opercular region. Acta Neurochir (Wien) 2004;146:9–17. doi: 10.1007/s00701-003-0165-4. [DOI] [PubMed] [Google Scholar]
- 59.Quigg M, Fountain NB. Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. J Neurol Neurosurg Psychiatry. 1999;66:393–396. doi: 10.1136/jnnp.66.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quigg M, Geldmacher DS, Elias WJ. Conduction aphasia as a function of the dominant posterior perisylvian cortex. Report of two cases. J Neurosurg. 2006;104:845–848. doi: 10.3171/jns.2006.104.5.845. [DOI] [PubMed] [Google Scholar]
- 61.Roberts L. Handedness and cerebral dominance. Trans Am Neurol Assoc. 1955;80:143–148. [PubMed] [Google Scholar]
- 62.Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 64.Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saykin AJ, Stafiniak P, Robinson LJ, Flannery KA, Gur RC, O’Connor MJ, et al. Language before and after temporal lobectomy: specificity of acute changes and relation to early risk factors. Epilepsia. 1995;36:1071–1077. doi: 10.1111/j.1528-1157.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, et al. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia. 2011;52:857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- 68.Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, et al. Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg. 2005;103:428–438. doi: 10.3171/jns.2005.103.3.0428. [DOI] [PubMed] [Google Scholar]
- 69.Whittle IR, Pringle AM, Taylor R. Effects of resective surgery for left-sided intracranial tumours on language function: a prospective study. Lancet. 1998;351:1014–1018. doi: 10.1016/S0140-6736(97)08295-0. [DOI] [PubMed] [Google Scholar]
- 70.Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong FCK, Chandrasekaran B, Garibaldi K, Wong PCM. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. J Neurosci. 2011;31:8780–8785. doi: 10.1523/JNEUROSCI.0999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]