Abstract
Background
Hypertension is often preceded by cardiac structural abnormalities. Thus, we assessed whether high-sensitivity cardiac troponin-T (hs-cTNT), a marker of chronic subclinical myocardial damage, can identify persons at risk for hypertension or left ventricular hypertrophy (LVH).
Methods and Results
We studied 6,516 ARIC Study participants, free of prevalent hypertension and cardiovascular disease at baseline (1990–1992). We examined the association of baseline hs-cTNT categories with incident diagnosed hypertension (defined by self-report of a diagnosis or medication use during a maximum of 19.9 years of follow-up) and with incident visit-based hypertension (defined by self-report, medication use, or measured BP >140/90 mmHg over 6 years). Relative to hs-cTNT <5ng/L, adjusted hazard ratios for incident diagnosed hypertension were 1.16 (95% CI 1.08, 1.25) for persons with hs-cTNT 5–8ng/L, 1.29 (1.14, 1.47) for hs-cTNT 9–13ng/L, and 1.31 (1.07, 1.61) for hs-cTNT ≥14ng/L (p-trend <0.001). Associations were stronger for incident visit-based hypertension. These associations were driven by higher relative hazard in normotensive persons (compared to those with prehypertension, p-interaction=0.001). Baseline hs-cTNT was also strongly associated with incident LVH by electrocardiography over 6 years (e.g. adjusted HR 5.19 [1.49–18.08] for hs-cTNT ≥14ng/L vs <5ng/L). Findings were not appreciably changed after accounting for competing deaths or adjustment for baseline BP levels or NT-proBNP.
Conclusions
In an ambulatory population with no history of cardiovascular disease, hs-cTNT was associated with incident hypertension and risk of LVH. Further research is needed to determine whether hs-cTNT can identify persons who may benefit from ambulatory BP monitoring or hypertension prevention lifestyle strategies.
Keywords: Hypertension, Hypertrophy, Tests, Epidemiology, Prevention
Introduction
Hypertension remains a major cause of heart disease and stroke, with approximately 1 in 3 U.S. adults currently diagnosed as hypertensive and a further 6–10% of Americans estimated to have undiagnosed hypertension. 1 In addition, a significant proportion of those with diagnosed hypertension have poorly controlled blood pressure (BP). 2, 3 Thus, there is a need to identify persons at risk either for progressing to clinically overt hypertension or for developing complications of this disease. In particular, BP control can be improved by more aggressive lifestyle and dietary changes in those with both prehypertension and overt hypertension. 4
The onset of hypertension is an insidious process that typically occurs over many years and is often preceded by altered diurnal BP patterns and prehypertension. 5, 6 While hypertension is diagnosed using defined BP thresholds, risks associated with elevated BP occur on a continuous spectrum. Indeed, even early, pre-hypertensive, BP abnormalities can induce structural heart changes7, potentially affording the opportunity both to detect persons at risk for overt hypertension and to initiate timely preventive strategies. 8 Prior studies have focused on the association between subclinical structural heart disease, particularly left ventricular hypertrophy (LVH), and subsequent development of hypertension. While these studies have confirmed that baseline LVH, measured by both echocardiography9, 10 and by cardiac MRI11, is associated with incident hypertension, these imaging modalities are often impractical and too costly for routine screening.
New highly sensitive cardiac troponin T (hs-cTNT) assays have great potential as noninvasive laboratory-based markers of subclinical myocardial damage. 12 These assays can detect subclinical myocardial damage in a significant proportion of persons who are free of known cardiovascular disease in the community. 13, 14 Interestingly, detectable levels of hs-cTNT appear to be more strongly linked to structural heart disease and its risk factors than to epicardial coronary artery disease. 14–16 Thus, hs-cTNT screening may be of particular use in persons at risk for clinically apparent hypertension and other cardiovascular endpoints. Hs-cTNT may also help identify individuals who are at risk of developing hypertensive end-organ damage, such as LVH.
Thus, we sought to determine if hs-cTNT could identify persons at risk for subsequent hypertension or LVH in a large U.S. community-based cohort, the Atherosclerosis Risk in Communities (ARIC) Study.
METHODS
Study Population
The ARIC Study is a prospective cohort of 15,792 participants enrolled between 1987 and 1989 from four U.S. communities (Forsyth Country, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland). 17 We measured hs-cTNT in samples collected from all participants at ARIC visit 2, which took place from 1990 to 1992 and represents the baseline for this current analysis. Of the 14,348 persons who attended visit 2, we excluded all persons with prevalent diagnosed hypertension (n=6,801), those who had coronary heart disease (CHD, including silent myocardial infarction detected by electrocardiogram [ECG]), stroke, or heart failure at or prior to visit 2 (n=194), or those who were missing variables of interest (n=837) (eFigure 1). Thus, 6,516 persons were included in the main analysis evaluating our primary outcome of incident diagnosed hypertension (self-report of diagnosis or medication use during annual telephone follow-up). In our secondary analysis of incident visit-based hypertension, we excluded 599 persons with measured BP at baseline >140/90 mmHg (elevated blood pressure) and 242 persons without available BP measurement data, for an analytic study sample of 5,675 persons. In our analysis of incident LVH, we further excluded 44 persons with baseline LVH by ECG for an analytic sample of 5,631 (eTable 1). Institutional review boards at each clinical site reviewed the study and informed consent was obtained from all participants.
Measurement of hs-cTNT and other exposure variables
We measured hs-cTNT in stored serum samples, collected at visit 2, using the Roche high-sensitivity Troponin T reagent kit, a sandwich immunoassay performed on a Roche Elecsys 2010 Analyzer (Roche Diagnostics, Indianapolis, Indiana) at the University of Minnesota in 2012–2013. Intra-assay coefficients of variation (CVs) are 2.1% at a mean hs-cTNT concentration of 26 ng/L and 1.0% at 1990 ng/L. Inter-assay CVs are 6.0% at a mean hs-cTNT concentration of 25 ng/L and 3.7% at 1940 ng/L. The measurement range of the assay is 3– 100,000 ng/L, the limit of blank is 3 ng/L and the lower limit of detection is 5 ng/L. The intra-individual reliability of hs-cTNT values have been demonstrated to be high among ARIC study participants (laboratory reliability r value=0.94, six week biological reliability r value=0.94). 18 Participants self-reported smoking status. Plasma lipid concentrations and body weight and height for body mass index (BMI) were determined using standardized protocols. Glucose was measured using the hexokinase method.19 Diagnosed diabetes was defined as a self-reported physician diagnosis of diabetes or current use of diabetic medications. Glomerular filtration rate was estimated using serum creatinine and the CKD-EPI 2009 equation.20 N-terminal pro-brain natriuretic peptide (NT-proBNP) was measured in stored serum samples from visit 2 on a Roche Elecsys 2010 Analyzer using a sandwich immunoassay method (Roche Diagnostics, Indianapolis, Indiana).
Follow-up for outcomes of interest
Participants were contacted annually via telephone, with follow-up currently available through March 2012. Incident diagnosed hypertension was assessed during these annual telephone calls using the following questions: “Has a doctor ever said you had high blood pressure?”, or “Since we last contacted you has a doctor said you had high blood pressure?”, and “Did you take any medications during the past two weeks for high blood pressure?”. After visit 2, ARIC participants also completed two further follow-up study examinations, visit 3 (1992–1995) and visit 4 (1996–1998). During these follow-up study visits, BP was recorded as the mean of at least 2 seated measurements using a manual random-zero sphygmomanometer.
We also evaluated the association between hs-cTNT and a well-known sequela of hypertension, LVH. This secondary outcome was assessed at visits 3 and 4, in persons free of both hypertension and LVH at visit 2, using resting 12-lead electrocardiograms and defined by Cornell criteria. 21
Statistical Analyses
Characteristics for the study population were presented according to categories of hs-cTNT (<5, 5–8, 9–13, or ≥14 ng/L) at baseline (visit 2, 1990–1992). While the hs-cTNT assay used can measure concentrations of troponin as low as 3 ng/L14, values <5 ng/L are measured with reduced precision and 5 ng/L represents the typical lower cut-point of detection in categorical analyses. Values ≥14ng/L represent approximately the 90th percentile of the ARIC population and correspond to the 99th percentile value for a “healthy” reference group of persons aged 20–70 years. 22 We further divided persons with values between 5–13 ng/L into two categories using a cutoff at the mid-point of this range. 15
The primary outcome of interest was incident diagnosed hypertension during a maximum of 19.9 and a median of 12 years of follow-up. Incident cases were identified at ARIC study visits 3 and 4 and, thereafter, by annual telephone contact with all participants. Cases were defined as a self-reported diagnosis of hypertension or antihypertensive medication use after visit 2. Time of incident diagnosed hypertension was the date when participants first reported diagnosis of or treatment for hypertension. We evaluated visit-based hypertension during a maximum of 8.7 and a median of 6 years follow-up as a secondary outcome. Visit-based cases of hypertension were identified by mean systolic BP ≥140mmHg or mean diastolic BP ≥90mmHg, self-reported physician diagnosis, or medication use at visits 3 (1993–1995) or 4 (1996–1998). For both hypertension endpoints, we also conducted analyses stratified by baseline BP category (normotensive [BP ≤120 mmHg systolic and ≤80 mmHg diastolic] or pre-hypertensive [BP >120 mmHg <140 mmHg systolic and/or >80 mmHg <90 mmHg]). Finally, we evaluated the association between baseline hs-cTNT and incident LVH at visit 3 (1993–1995) or visit 4 (1996–1998) among persons free from both hypertension and LVH at visit 2 (1990–1992).
We used Cox proportional hazards regression models for the primary outcome of diagnosed hypertension and discrete proportional hazards (cloglog) regression models for both secondary outcomes (visit-based incident hypertension or incident LVH) to estimate adjusted hazard ratios (HRs) for the association between baseline hs-cTNT and these outcomes. We modeled hs-cTNT as a categorical (<5 [reference], 5–8, 9–13, or ≥14 ng/L) and continuous exposure (log-transformed). In analyses with hs-cTNT as a continuous exposure, visit 2 hs-cTNT was modeled as untransformed or log-transformed and either truncated at <3ng/L (the limit of measurement) or with unmeasurable levels assigned a value of half the lower limit of measurement (1.5ng/L). 23 Using Martingale residuals to assess fit, the log-transformed hs-cTNT without truncating fit the continuous models best. P-values for linear trend among hs-cTNT categories were obtained by assigning the median hs-cTNT value in the above categories of hs-cTNT and modeling this ordinal variable continuously. For all models, we verified the proportionality of the hazards with Schoenfeld residuals.
Models were adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), smoking (current; former; never), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current lipid-lowering medication use (yes or no), LVH (yes or no), and diagnosed diabetes (yes or no). We tested for interactions by age, sex, and race. Model discrimination was assessed using Harrell’s C-statistic24, and we evaluated improvement in the C-statistic for the addition of hs-cTNT as a log-transformed continuous variable to the fully adjusted model. Furthermore, for the primary outcome of diagnosed hypertension, we calculated the continuous net reclassification index (NRI) statistic 25, also based on the addition of hs-cTNT to the full model.
In sensitivity analyses we additionally adjusted for baseline (visit 2) BP or NT-proBNP levels. Because baseline hs-cTNT is associated with all-cause mortality, cardiovascular death, and sudden cardiac death14, 16, 26–28, we also conducted sensitivity analyses with a competing risk regression method (Fine-Gray approach) using a cumulative incidence function to account for intervening deaths. 29
In addition, we also modeled hs-cTNT using linear splines in fully-adjusted Cox models for both incident diagnosed and incident visit-based hypertension, with knots at hs-cTNT concentrations of 5, 8 and 13 ng/L (figures truncated at the 99th percentile), These models are shown graphically and overlaid on histograms showing the distribution of hs-cTNT in the study population. Finally, we repeated the analyses evaluating the outcome of incident diagnosed hypertension, after further excluding the 599 persons with elevated blood pressure at baseline. All analyses were performed using Stata version 13.0 (College Station, TX: StataCorp LP).
Results
In our community-based study sample of subjects without a history of cardiovascular disease and free from baseline hypertension, persons with higher baseline hs-cTNT were more likely to be older, male, black, pre-hypertensive, obese, diabetic, and have reduced kidney function (Table 1). Persons excluded due to missing values were not markedly different but were somewhat older, more likely to be black, and had a slightly more adverse health profile compared to those who were included in our study (eTable2).
Table 1.
Characteristics of the Study Population (ARIC participants without cardiovascular disease or diagnosed hypertension): Overall and According to categories of high sensitivity cardiac troponin T (ng/L) at baseline (1990–1992).
| Overall | Stratified by hs-cTNT | ||||
|---|---|---|---|---|---|
| <5 ng/L | 5–8 ng/L | 9–13 ng/L | ≥14 ng/L | ||
| Number (%) | 6516 | 4681 (72%) | 1317 (20%) | 384 (6%) | 134 (2%) |
| Age, years | 56 (6) | 55 (5) | 58 (6) | 59 (6) | 59 (6) |
| Male % | 44 | 36 | 61 | 74 | 76 |
| Black % | 17 | 16 | 18 | 25 | 24 |
| Current smoker % | 23 | 25 | 18 | 17 | 20 |
| Systolic blood pressure mmHg | 115 (15) | 114 (15) | 118 (16) | 120 (15) | 124 (19) |
| Diastolic blood pressure mmHg | 70 (9) | 69 (9) | 71 (9) | 71 (9) | 71 (10) |
| Hypertension categories (%) | |||||
|
Normotension (BP <120/80 mmHg) |
66 | 70 | 60 | 55 | 49 |
|
Prehypertension (BP 120-139/80-89 mmHg) |
27 | 25 | 32 | 35 | 35 |
|
Undiagnosed hypertension (BP >140/90mmHg)* |
6 | 5 | 8 | 10 | 16 |
| LVH % | 1 | 1 | 1 | 2 | 3 |
| BMI, kg/m2 | |||||
| Normal weight % (< 25) | 39 | 41 | 33 | 33 | 30 |
| Overweight % (25 – 30) | 41 | 40 | 45 | 43 | 43 |
| Obese % (> 30) | 20 | 19 | 22 | 25 | 28 |
| Total cholesterol, mg/dL | 207 (38) | 208 (38) | 206 (37) | 203 (38) | 206 (41) |
| LDL-cholesterol, mg/dL | 132 (36) | 132 (36) | 133 (35) | 131 (36) | 131 (37) |
| HDL-cholesterol, mg/dL | 52 (17) | 53 (17) | 48 (16) | 47 (16) | 49 (19) |
| Triglyceride, mg/dL | 121 (61) | 119 (59) | 126 (64) | 123 (67) | 126 (68) |
| Lipid Medicines % | 3 | 3 | 3 | 2 | 1 |
| Diagnosed diabetes % | 4 | 3 | 5 | 10 | 15 |
| eGFR <60 mL/min/1.73m2 (%) | 1 | 0 | 1 | 1 | 4 |
Estimates are mean (SD) or %, unless otherwise indicated. Hs-cTNT= high-sensitivity Troponin-T, BP= Blood Pressure, LVH= left ventricular hypertrophy, BMI= body mass index, LDL= Low Density Lipoprotein, HDL= High Density Lipoprotein, eGFR= estimated Glomerular Filtration Rate.
This group was excluded for the visit-based hypertension outcome analysis
During a maximum of 19.9 and a median 12 years of follow-up, 68% (n=4421) of the study sample developed our primary endpoint; incident diagnosed hypertension. Of these, 70% (n=3108) had hs-cTNT <5 ng/L at baseline, 21% (n=936) had hs-cTNT 5–8 ng/L, 6% (n=281) had hs-cTNT 9–13 ng/L, and 2% (n=96) had hs-cTNT ≥14 ng/L (Table 2). Crude incidence rates (per 1,000 person/years) of diagnosed hypertension were 54 in those with hs-cTNT <5 ng/L, 64 with hs-cTNT 5–8 ng/L, 73 with hs-cTNT 9–13 ng/L, and 74 in persons with baseline hs-cTNT ≥14 ng/L.
Table 2.
Crude incidence rates and adjusted* hazard ratios (95% confidence intervals) for incident hypertension outcomes, according to baseline high sensitivity cardiac troponin T.
| Proportional Hazards Regression† |
Competing Risks Regression‡ |
||||
|---|---|---|---|---|---|
| Baseline hs-cTnT | N | Events (n) |
Incidence rate, per 1,000 person years (95% CI) |
HR (95% CI) |
HR (95% CI) |
| Incident Diagnosed Hypertension | |||||
| Categories | |||||
| <5 ng/L | 4,681 | 3,108 | 54.0 (52.2–56.0) |
1 (reference) |
1 (reference) |
| 5–8 ng/L | 1,317 | 936 | 64.0 (60.0–68.3) |
1.16 (1.08–1.25) |
1.15 (1.06–1.24) |
| 9–13 ng/L | 384 | 281 | 72.6 (64.6–81.6) |
1.29 (1.14–1.47) |
1.21 (1.05–1.38) |
| ≥14 ng/L | 134 | 96 | 73.9 (60.5–90.2) |
1.31 (1.07–1.61) |
1.15 (0.91–1.44) |
| p-value for linear trend | <0.001 | <0.001 | |||
| Continuous | |||||
| Log(hs-cTnT) | 6,516 | 4421 | 57.2 (55.5–58.9) |
1.14 (1.09–1.19) |
1.11 (1.06–1.16) |
| Incident Visit-based Hypertension | |||||
| Categories | |||||
| <5 ng/L | 4,139 | 1,059 | 47.1 (44.4–50.0) |
1 (reference) |
1 (reference) |
| 5–8 ng/L | 1,130 | 351 | 58.6 (52.8–65.1) |
1.15 (1.01–1.30) |
1.14 (1.00–1.29) |
| 9–13 ng/L | 306 | 120 | 74.9 (62.6–89.5) |
1.32 (1.08–1.61) |
1.38 (1.13–1.67) |
| ≥14 ng/L | 100 | 41 | 77.8 (57.3–105.6) |
1.49 (1.09–2.05) |
1.47 (1.07–2.01) |
| p-value for linear trend | <0.001 | <0.001 | |||
| Continuous | |||||
| Log(hs-cTnT) | 5,675 | 1,571 | 51.3 (48.9–53.9) |
1.14 (1.06–1.22) |
1.15 (1.07–1.24) |
Adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), smoking (current; former; never), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current lipid-lowering medication use (yes or no), left ventricular hypertrophy (yes or no), diagnosed diabetes (yes or no). Abbreviations as per Table 1.
Cox regression for diagnosed hypertension outcome, cloglog regression for visit-based hypertension
Fine-Gray regression model. 29 There were 532 interval deaths for the diagnosed hypertension outcome and 57 interval deaths for the visit-based hypertension outcome prior to administrative censoring.
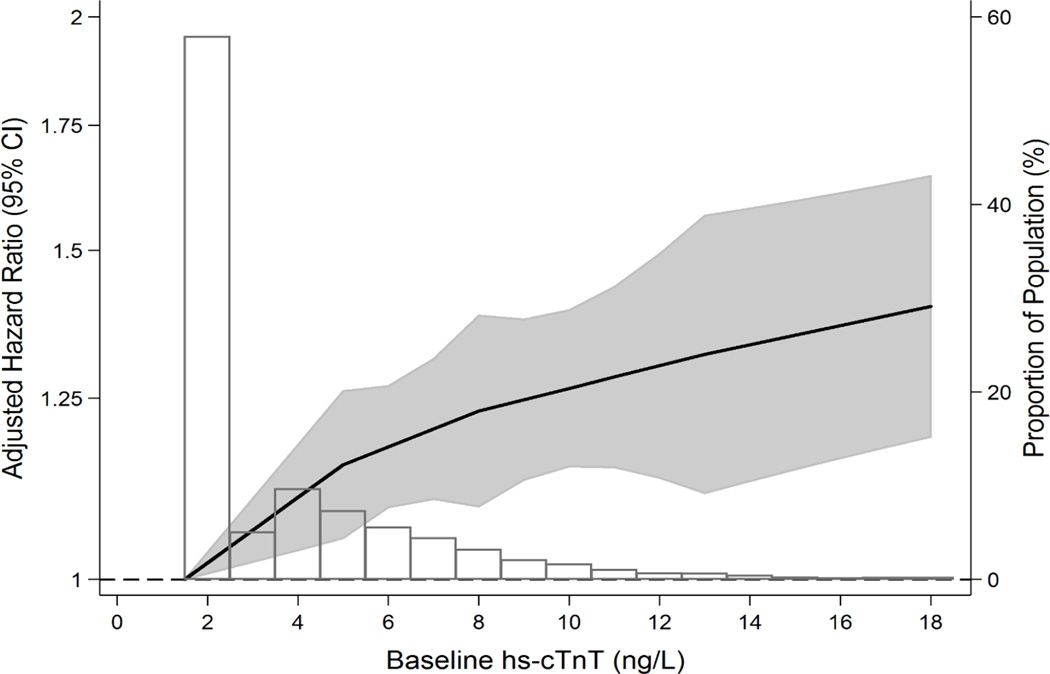
After multivariable adjustment, baseline hs-cTNT remained significantly associated with incident diagnosed hypertension (Table 2). Specifically, relative to those with undetectable hs-cTNT at baseline, persons in the higher categories of hs-cTNT had a higher adjusted risk of hypertension. Similar results were found for log-hs-cTNT modeled as a continuous variable. A continuous and roughly linear association between baseline hs-cTNT and incident diagnosed hypertension is shown graphically in Figure 1a. Furthermore, for the primary outcome of incident diagnosed hypertension, the addition is hs-cTNT as a log-transformed continuous variable to our fully adjusted base model resulted in a significant increase in the C-statistic from 0.609 to 0.613 (p=0.003) and an improvement in continuous NRI of 20% (p=0.001). Results for the incident diagnosed hypertension outcome were not appreciably different in the sensitivity analysis that excluded persons with elevated BP at baseline (eTable 3).
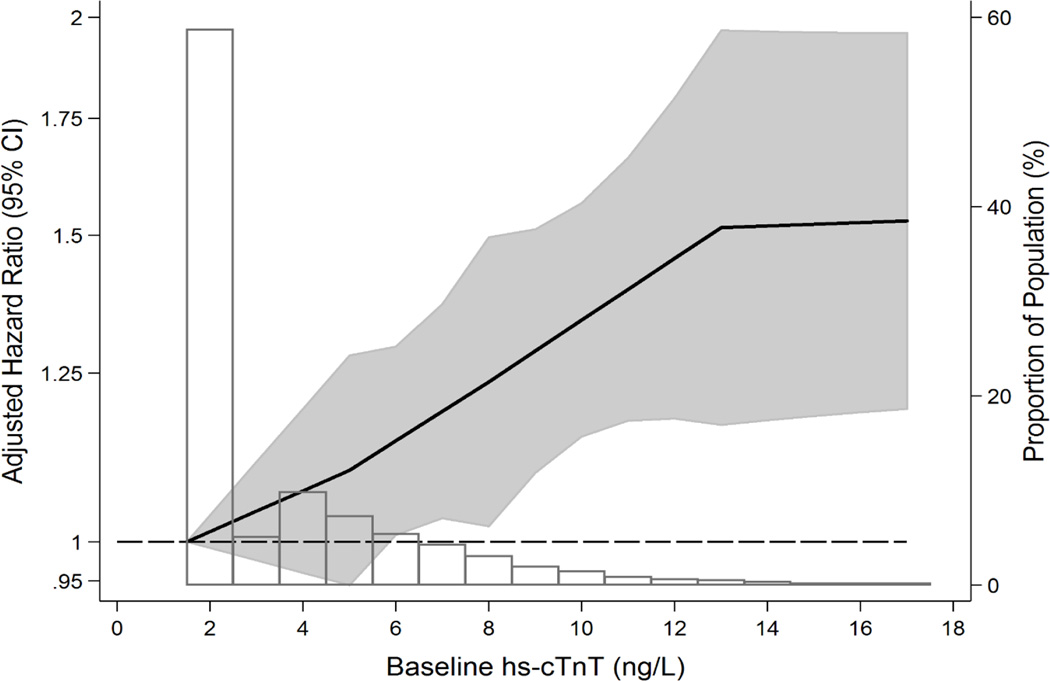
Figure 1.
Adjusted* hazard ratio (95% confidence interval) of A) incident diagnosed hypertension and B) incident visit-based hypertension, according to baseline hs-cTnT modeled as linear splines (knots at 5, 8 and 13 ng/L) with background histogram of hs-cTNT in the study sample of ARIC subjects without baseline cardiovascular disease or hypertension. *Adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), smoking (current; former; never), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current lipid-lowering medication use (yes or no), left ventricular hypertrophy (yes or no), diagnosed diabetes (yes or no). Abbreviations as per Table 1. Linear splines of hazard for hypertension with background distributional histogram of baseline hs-cTNT levels, truncated at the 99th percentile of hs-cTnT (18 ng/L). Persons with hs-cTNT <3 ng/L were imputed values of 1.5 ng/L. Note that the "Proportion of the Population" axis label identifies the percentage of the ARIC analytic sample at each point on this background histogram. The shaded area around the regression line represents the 95% confidence interval.
The association between baseline hs-cTNT (both by category and as a continuous exposure) and our secondary outcome of incident visit-based hypertension was stronger than that for incident diagnosed hypertension (Table 2, Figure 1b). Further, in analyses stratified by baseline BP category (normotensive or pre-hypertensive), we observed that the overall association between hs-cTNT and both incident hypertension outcomes appeared to be largely driven by more robust associations in persons who were normotensive at baseline (p-for-interaction=0.04 for diagnosed hypertension and p-for-interaction=0.001 for visit-based hypertension) (Table 3 and eTable 4). Associations between hs-cTNT and both incident diagnosed and visit-based hypertension were largely unchanged after further adjusting for baseline BP or NT-proBNP (eTable 5). Interactions for the association of hs-cTNT and hypertension by age, gender, and race were all non-significant (all p-values-for-interaction >0.10).
Table 3.
Crude incidence rates and adjusted* hazard ratios (95% confidence intervals) for incident diagnosed hypertension, according to baseline categories of high sensitivity cardiac troponin T: further stratified by baseline blood pressure (N=6,516).
| Proportional Hazards Regression† |
Competing Risks Regression‡ |
||||
|---|---|---|---|---|---|
| Baseline hs-cTNT | N | Events (n) |
Incidence rate, per 1,000 person years (95% CI) |
HR (95% CI) |
HR (95% CI) |
| NORMOTENSIVE SUBGROUP (BP ≤120 mmHg systolic and ≤80 mmHg diastolic) | |||||
| Categorical | |||||
| <5 ng/L | 3,259 | 1,892 | 42.4 (40.6–44.4) | 1 (reference) | 1 (reference) |
| 5–8 ng/L | 785 | 480 | 47.9 (43.8–52.3) | 1.13 (1.02–1.25) |
1.12 (1.01–1.25) |
| 9–13 ng/L | 213 | 134 | 53.1 (44.8–62.9) | 1.24 (1.03–1.48) |
1.18 (0.97–1.43) |
| ≥14 ng/L | 65 | 41 | 54.1 (39.9–73.5) | 1.39 (1.01–1.90) |
1.29 (0.93–1.79) |
| p-value for linear trend | <0.001 | 0.006 | |||
| Continuous | |||||
| Log(hs-cTnT) | 4,322 | 2,547 | 44.0 (42.3–45.7) | 1.13 (1.06–1.20) |
1.11 (1.04–1.18) |
| PREHYPERTENSION SUBGROUP (BP >120 <140 mmHg systolic and/or >80<90 mmHg diastolic) | |||||
| Categorical | |||||
| <5 ng/L | 1,179 | 988 | 87.4 (82.1–93.0) | 1 (reference) | 1 (reference) |
| 5–8 ng/L | 424 | 356 | 90.6 (81.7–100.6) | 1.11 (0.98–1.26) |
1.11 (0.98–1.25) |
| 9–13 ng/L | 133 | 113 | 99.7 (82.9–119.9) | 1.28 (1.05–1.58) |
1.20 (0.97–1.48) |
| ≥14 ng/L | 47 | 33 | 78.6 (55.9–110.6) | 0.88 (0.61–1.25) |
0.72 (0.48–1.08) |
| p-value for linear trend | 0.125 | 0.667 | |||
| Continuous | |||||
| Log(hs-cTnT) | 1,783 | 1,490 | 88.8 (84.4–93.4) | 1.07 (0.99–1.15) |
1.03 (0.96–1.11) |
| ELEVATED BLOOD PRESSURE SUBGROUP (BP ≥140 mmHg systolic and/or ≥90mmHg diastolic) | |||||
| Categorical | |||||
| <5 ng/L | 243 | 228 | 138.1 (121.3–157.3) | 1 (reference) | 1 (reference) |
| 5–8 ng/L | 108 | 100 | 150.1 (123.4–182.6) | 1.14 (0.89–1.46) |
1.10 (0.84–1.44) |
| 9–13 ng/L | 38 | 34 | 158.5 (113.2–221.8) | 1.31 (0.90–1.93) |
1.12 (0.76–1.65) |
| ≥14 ng/L | 22 | 22 | 180.1 (118.6–273.5) | 1.45 (0.91–2.32) |
1.53 (1.04–2.26) |
| p-value for linear trend | 0.045 | 0.088 | |||
| Continuous | |||||
| Log(hs-cTnT) | 411 | 384 | 144.7 (130.9–159.9) | 1.14 (1.00–1.30) |
1.10 (0.96–1.26) |
Adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), smoking (current; former; never), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current lipid-lowering medication use (yes or no), left ventricular hypertrophy (yes or no), diagnosed diabetes (yes or no). Abbreviations as per Table 1.
Cox regression for diagnosed hypertension outcome
Fine-Gray regression model. 29 There were 532 interval deaths for the diagnosed hypertension outcome prior to administrative censoring.
There were 532 competing deaths for the primary outcome of incident diagnosed hypertension over 12-years median follow-up, but only 57 competing deaths for the secondary outcome of incident visit-based hypertension over 6-years. In competing risk models, the association between hs-cTNT and incident diagnosed hypertension remained significant but was somewhat weakened in the hs-cTNT 5–8 ng/L and 9–13 ng/L categories and was no longer significant in those with baseline hs-cTNT ≥ 14 ng/L (Table 2). In contrast, results for the 6-year incident visit-based hypertension were similar in the Fine-Gray competing-risk models
Finally, we found that, relative to hs-cTNT of <5ng/L, persons in higher categories of hs-cTNT had a highly significant 6-year risk of incident LVH by ECG: HR, 2.29 (95% CI 1.24–4.26) for hs-cTNT 5–8ng/L, HR, 2.94 (95% CI 1.14–7.58) for hs-cTNT 9–13ng/L and HR, 5.19 (95% CI 1.49–18.08) for hs-cTNT ≥14ng/L (Table 4).
Table 4.
Adjusted* Hazard Ratios (95% confidence intervals) for the secondary outcome of incident left ventricular hypertrophy over 6 years of follow-up by baseline high sensitivity cardiac troponin T among persons free of baseline cardiovascular disease or hypertension (N=5,631
| Proportional Hazards Regression† | |||
|---|---|---|---|
| Baseline hs-cTnT | N | Events (n) | HR (95% CI) |
| Categorical | |||
| <5 ng/L | 4109 | 30 | 1 (reference) |
| 5–8 ng/L | 1121 | 17 | 2.29 (1.24–4.26) |
| 9–13 ng/L | 302 | 6 | 2.94 (1.14–7.58) |
| ≥14 ng/L | 99 | 3 | 5.19 (1.49–18.08) |
| p-value for trend | <0.001 | ||
| Continuous | |||
| Log(hs-cTnT) | 5,631 | 56 | 1.79 (1.27–2.52) |
Adjusted for age (years), race-center (whites-Washington County; whites-Minneapolis; blacks-Jackson; blacks-Forsyth County, whites-Forsyth County), sex (male or female), body mass index (kg/m2), smoking (current; former; never), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), current lipid-lowering medication use (yes or no), diagnosed diabetes (yes or no). Abbreviations as per Table 1.
Cloglog regression for incident LVH by ECG
Discussion
In this large community-based study of U.S. adults, free of baseline hypertension and cardiovascular disease, we found that hs-cTNT was independently associated with subsequent development of hypertension, defined either by diagnosed cases (self-reported diagnosis or medication use) or by objective office measurement of BP, and also with incident LVH by ECG. Findings were similar by age, race and gender categories. Our results suggest that chronic subclinical myocardial damage, detected by elevated hs-cTNT, may precede the development of hypertension in the general population and that this novel biomarker of cardiac damage may have utility for identifying persons at future risk for hypertension and hypertensive end-organ damage.
Prior studies have reported the cross-sectional association between established hypertension and prevalent hs-cTNT elevations16, 30–32. However, to our knowledge, this is the first study to evaluate the association between preceding subclinical myocardial damage, as measured by hs-cTNT, and subsequent risk of hypertension over prospective follow-up. This association was strongest for persons who had baseline normal BP readings (in contrast to those with pre-hypertension). Notably, the vast majority of individuals with prehypertension go on to develop hypertension, irrespective of baseline hs-cTNT. Therefore, the absolute risk of hypertension is very high in this entire sub-group, helping to explain why the relative impact of hs-cTNT for prediction of hypertension appeared non-significant in those with prehypertension.
While the exact pathway linking hs-cTNT to incident hypertension is unclear, a number of considerations are worth discussing. Elevated BP develops over time along a spectrum from normotension, to abnormal diurnal patterns of BP, to pre-hypertension, and then, ultimately, to clinical hypertension5. It is known that abnormalities in cardiac structure can occur long before the diagnosis of overt hypertension is made in the clinic5, 9, 33. Based on our results, it is possible that these early BP aberrations cause myocardial damage, leading to elevated hs-cTNT, before clinically overt hypertension is recognized. Compatible with this hypothesis, it has been shown elsewhere that “non-dippers” (an abnormal diurnal BP pattern where sleep systolic BP fails to decrease >10% from daytime systolic BP and a known risk-factor for subsequent hypertension6), are more likely to have prevalent detectable troponin levels by hs-cTNT assays34.
In addition, BP is highly variable and its true hemodynamic load cannot easily be captured by single office measurements (which are subject to misclassification error). Thus, it is also possible that the association between hs-cTNT and subsequent hypertension in ARIC could be a manifestation of the well-known phenomenon of “masked hypertension”, the clinical condition in which a patient’s office BP level is <140/90 mm Hg but home or ambulatory readings are in the hypertensive range35. Masked hypertension occurs in over 10% of the adult population35, 36 and is known to result in end organ damage37, 38.
Further, we found that elevated hs-cTNT was also a risk factor for the development of LVH in persons without baseline hypertension or clinical cardiovascular disease (including silent MI). This suggests that hs-cTNT may also predict future sequelae of hypertension and is consistent with our hypothesis that elevated hs-cTNT may reflect occult BP abnormalities in some individuals. Indeed, prior case-control data suggest that hs-cTNT is also associated with subsequent albuminuria in hypertensive patients. 39 It is also worth noting that hs-cTNT could potentially be contributing more directly to the development of hypertension. For example, elevated hs-cTNT has been linked with arterial stiffness in diabetics40.
Our results were robust to multiple sensitivity analyses. Standard Cox proportional-hazards models assume that persons censored (including those censored for death) prior to the end of follow-up have the same probability of incident hypertension as similar persons who remain under-observation. However, in persons who die, the development of hypertension cannot be observed. In this context, the Fine-Gray regression results, which account for interval deaths, are important to determine the ‘real-world’ impact of hs-cTNT screening in persons at risk for hypertension. In these Fine-Gray models, we found that the association between hs-cTNT and incident diagnosed hypertension (an endpoint with longer follow-up and increased interval accrual of deaths) was attenuated, particularly in the ≥14 ng/L category.
Our findings may have implications for future clinical practice. Specifically, normotensive persons with elevated hs-cTNT are at risk for subsequent hypertension and may benefit from evaluation for masked hypertension and from more intensive lifestyle BP interventions to reduce the likelihood of developing this morbid disease. Further research is necessary to confirm whether hs-cTNT could be useful in screening those at risk for hypertension.
This analysis has some limitations. This study was observational and, thus, may be subject to residual confounding. The small proportion of persons who were excluded for missing values (12%) had a slightly worse health profile than those included in our study. We do not have ambulatory BP recordings to determine whether or not masked hypertension or abnormal diurnal values were present in persons with elevated hs-cTNT. Cases of diagnosed hypertension were identified by self- report of diagnoses or medication used during annually follow-up; however, similar results were obtained in our analyses of visit-based hypertension (incorporating objectively elevated BP obtained during the clinical visit). Strengths of the study include the large sample size, bi-racial population, and rigorous measurement of cardiovascular risk factors.
In conclusion, elevated hs-cTNT in the general population, particularly among persons with normal blood pressure, identifies persons at risk for subsequent hypertension or LVH. Further studies are necessary to determine whether subclinical myocardial damage in these settings is due to masked hypertension or abnormal diurnal variability in BP. Elevated hs-cTNT in low-risk ambulatory populations may prove to be clinically useful in identifying persons at risk for hypertension, affording the opportunity both to consider ambulatory blood pressure monitoring and to initiate more intensive preventive strategies.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions. Dr McEvoy is supported by the Pollin Cardiovascular Prevention Fellowship and the P.J. Schafer fund for early career investigators. Reagents for the high-sensitivity cardiac troponin-T and C-reactive protein assays were donated by Roche Diagnostics.
Funding Sources: This research was supported by NIH/NIDDK grant R01DK089174 to Dr. Selvin. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Dr Ballantyne has received grant support from Roche Diagnostics (and the National Institutes of Health). Drs. Ballantyne and Nambi are co-investigators on a provisional patent filed by Roche for use of biomarkers in heart failure prediction. Drs. Ballantyne and Selvin have served on an advisory board for Roche Diagnostics. Dr. Matsushita has received an honorarium from Mitsubishi Tanabe Pharma, Kyowa Hakko Kirin, and Merck Sharp & Dohme.
Footnotes
Disclosures: The other authors declare no commercial conflicts of interest (but receive National Institutes of Health grant funding).
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Egan BM, Zhao Y, Axon RN. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 3.Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, Kazmi K, Lanas F, Wei L, Lopez-Jaramillo P, Fanghong L, Ismail NH, Puoane T, Rosengren A, Szuba A, Temizhan A, Wielgosz A, Yusuf R, Yusufali A, McKee M, Liu L, Mony P, Yusuf S, investigators PS. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 esh/esc guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of hypertension (esh) and of the european society of cardiology (esc) Eur Heart J. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viera AJ, Zhu S, Hinderliter AL, Shimbo D, Person SD, Jacobs DR., Jr Diurnal blood pressure pattern and development of prehypertension or hypertension in young adults: The cardia study. J Am Soc Hypertens. 2011;5:48–55. doi: 10.1016/j.jash.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stabouli S, Kotsis V, Rizos Z, Toumanidis S, Karagianni C, Constantopoulos A, Zakopoulos N. Left ventricular mass in normotensive, prehypertensive and hypertensive children and adolescents. Pediatr Nephrol. 2009;24:1545–1551. doi: 10.1007/s00467-009-1165-2. [DOI] [PubMed] [Google Scholar]
- 8.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The trials of hypertension prevention, phase ii. The trials of hypertension prevention collaborative research group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 9.Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The framingham heart study. Circulation. 1994;90:179–185. doi: 10.1161/01.cir.90.1.179. [DOI] [PubMed] [Google Scholar]
- 10.De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Russell M, Howard BV, Devereux RB. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension: The strong heart study. Hypertension. 2009;54:974–980. doi: 10.1161/HYPERTENSIONAHA.109.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimbo D, Muntner P, Mann D, Barr RG, Tang W, Post W, Lima J, Burke G, Bluemke D, Shea S. Association of left ventricular hypertrophy with incident hypertension: The multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;173:898–905. doi: 10.1093/aje/kwq509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy JW, Lazo M, Chen Y, Shen L, Nambi V, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Patterns and determinants of temporal change in high-sensitivity cardiac troponin-t: The atherosclerosis risk in communities cohort study. Int J Cardiol. 2015;187:651–657. doi: 10.1016/j.ijcard.2015.03.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lemos JA. Increasingly sensitive assays for cardiac troponins: A review. JAMA. 2013;309:2262–2269. doi: 10.1001/jama.2013.5809. [DOI] [PubMed] [Google Scholar]
- 14.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin t measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, Hoogeveen RC, Sharrett AR, Ballantyne CM, Coresh J. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130:1374–1382. doi: 10.1161/CIRCULATIONAHA.114.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin t detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, Sharrett AR, Coresh J, Heiss G, Hoogeveen RC. Sources of variability in measurements of cardiac troponin t in a community-based sample: The atherosclerosis risk in communities study. Clin Chem. 2011;57:891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of hba1c from stored whole blood samples in the atherosclerosis risk in communities study. J Diabetes. 2010;2:118–124. doi: 10.1111/j.1753-0407.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 22.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin t assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 23.D'Angelo G, Weissfeld L, Gen IMSI. An index approach for the cox model with left censored covariates. Stat Med. 2008;27:4502–4514. doi: 10.1002/sim.3285. [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin t using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eggers KM, Al-Shakarchi J, Berglund L, Lindahl B, Siegbahn A, Wallentin L, Zethelius B. High-sensitive cardiac troponin t and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J. 2013;166:541–548. doi: 10.1016/j.ahj.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, deFilippi C, Dickfeld T, Deo R, Siscovick D, Stein PK, Lloyd-Jones D. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: The cardiovascular health study. J Am Coll Cardiol. 2013;62:2112–2120. doi: 10.1016/j.jacc.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 30.Ucar H, Gur M, Kivrak A, Koyunsever NY, Seker T, Akilli RE, Turkoglu C, Kaypakli O, Sahin DY, Elbasan Z, Tanboga HI, Cayli M. High-sensitivity cardiac troponin t levels in newly diagnosed hypertensive patients with different left ventricle geometry. Blood Pressure. 2014;23:240–247. doi: 10.3109/08037051.2013.840429. [DOI] [PubMed] [Google Scholar]
- 31.Sato Y, Yamamoto E, Sawa T, Toda K, Hara T, Iwasaki T, Fujiwara H, Takatsu Y. High-sensitivity cardiac troponin t in essential hypertension. J Cardiol. 2011;58:226–231. doi: 10.1016/j.jjcc.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Beatty AL, Ku IA, Christenson RH, DeFilippi CR, Schiller NB, Whooley MA. High-sensitivity cardiac troponin t levels and secondary events in outpatients with coronary heart disease from the heart and soul study. JAMA Intern Med. 2013;173:763–769. doi: 10.1001/jamainternmed.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redwine KM, Falkner B. Progression of prehypertension to hypertension in adolescents. Curr Hypertens Rep. 2012;14:619–625. doi: 10.1007/s11906-012-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cayli M, Gur M, Elbasan Z, Seker T, Turkoglu C, Kaypakli O, Sahin DY, Ucar H, Kivrak A, Koyunsever NY, Sen O. High-sensitivity cardiac troponin t predicts nondipper hypertension in newly diagnosed hypertensive patients. J Clin Hypertens. 2013;15:731–736. doi: 10.1111/jch.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulos DP, Makris TK. Masked hypertension definition, impact, outcomes: A critical review. J Clin Hypertens. 2007;9:956–963. doi: 10.1111/j.1524-6175.2007.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickering TG, Eguchi K, Kario K. Masked hypertension: A review. Hypertens Res. 2007;30:479–488. doi: 10.1291/hypres.30.479. [DOI] [PubMed] [Google Scholar]
- 37.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131:564–572. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 38.Leitao CB, Canani LH, Kramer CK, Boza JC, Pinotti AF, Gross JL. Masked hypertension, urinary albumin excretion rate, and echocardiographic parameters in putatively normotensive type 2 diabetic patients. Diabetes Care. 2007;30:1255–1260. doi: 10.2337/dc06-2131. [DOI] [PubMed] [Google Scholar]
- 39.Hellemons ME, Lambers Heerspink HJ, Gansevoort RT, de Zeeuw D, Bakker SJ. High-sensitivity troponin t predicts worsening of albuminuria in hypertension, results of a nested case-control study with confirmation in diabetes. J Hypertens. 2013;31:805–812. doi: 10.1097/HJH.0b013e32835eb5e8. [DOI] [PubMed] [Google Scholar]
- 40.Yiu KH, Zhao CT, Chen Y, Siu CW, Chan YH, Lau KK, Liu S, Lau CP, Tse HF. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:94. doi: 10.1186/1475-2840-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.